SUMMARY
Protein phosphatase activity acts as a primary determinant of the extent and duration of phosphorylation of cellular proteins in response to physiological stimuli. Ser/Thr protein phosphatase-1 (PP1) belongs to the PPP superfamily, and is associated with regulatory subunits that confer substrate specificity, allosteric regulation and subcellular compartmentalization. In addition, all eukaryotic cells contain multiple heat-stable proteins that originally were thought to inhibit phosphatase catalytic subunits released from the regulatory subunits, as a fail-safe mechanism. However, discovery of CPI-17 required fresh thinking about the endogenous inhibitors as specific regulators of particular phosphatase complexes, acting in addition to, not instead of, regulatory subunits. The cellular actions of the endogenous inhibitors are controlled by phosphorylation, connecting them to kinase pathways. More recent progress has unveiled additional functions of PP1 inhibitor-2 (I-2), including regulation of protein kinases. Transcriptional mechanisms govern the expression levels of CPI-17 in response to stimuli. If true for other inhibitor proteins, they have the potential of being diagnostic markers for pathological conditions. We discuss specific examples of PP1 inhibitor proteins regulating particular cellular functions and the rationale for incorporating phosphatase inhibitor proteins in development of new therapeutic strategies.
Keywords: CPI-17, inhibitor-2, PP2A, ROCK, PKC, Aurora, Pin1
I. INTRODUCTION
Protein phosphatases modulate stimulus-response
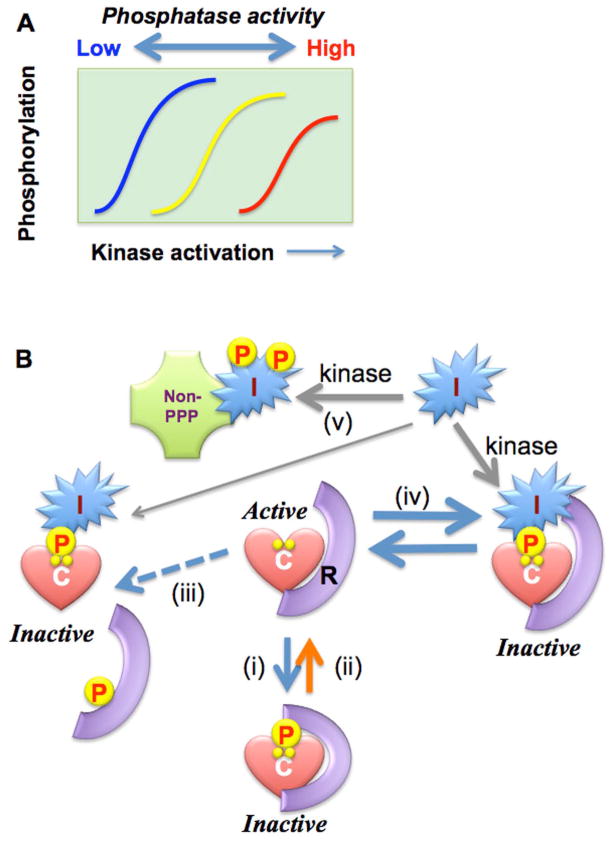
Over the past 50 years protein phosphorylation has been firmly established as a fundamental mechanism for cell signaling, vital to every aspect of biology and pathophysiology. Protein phosphatases as well as kinases are positively and negatively regulated in response to hormonal and neuronal inputs, as well as environmental stresses (1). Figure 1A shows kinase activation in response to a cellular stimulus that increases protein phosphorylation. When protein phosphatase activity is coincidently suppressed this stimulus-response curve is shifted to the left, and relatively less kinase activity is needed to produce phosphorylation (a process called sensitization, left curve in Figure 1A). An outstanding example is the Ca2+ dependent myosin light chain phosphorylation and smooth muscle contraction that are controlled through the negative regulation of the myosin light chain phosphatase (MLCP) (2). By contrast, higher phosphatase activity requires more kinase activity to achieve phosphorylation of substrates, causing desensitization in the response to stimuli (right-shift of the activation curve in Figure 1A). For example, dexamethasone induction of dual specificity phosphatases (called DUSP or MKP) suppresses responses to stimuli by limiting the phosphorylation and activation of MAPKs. (3). Thus, there are examples where changes in phosphatase activity modify cellular responses to stimuli. Kinases have become a focus for drug discovery, but phosphatase regulation needs to be taken into account for a better understanding of the responses and resistance to these new pharmaceuticals.
Figure 1. Regulation of Ser/Thr phosphatases.
A. Phosphatase Regulation of cellular responses. Phosphorylation is increased by the strength of stimulation that activates a kinase. Inhibition and activation of the opposing phosphatase causes left and right shifts of the stimulation-phosphorylation curve (sensitization and de-sensitization), respectively. B. Schemes in phosphatase regulation. I: PPP inhibitor protein. Scheme: i) phosphorylation of regulatory subunit causes allosteric inhibition of the holoenzyme, dissociation/scavenger model, ii) regulatory factor, such as CaM for CaN, relieves inhibition holoenzyme, iii) phosphorylation or degradation of regulatory subunit releases catalytic subunit, which is inhibited by an endogenous inhibitor protein, iv) An inhibitor protein directly binds to holoenzyme without subunit dissociation, and v) An PPP inhibitor protein regulates other, non-phosphatase proteins.
Diversity of Ser/Thr phosphatases
There are at least three distinct superfamilies of Ser/Thr phosphatases based on structures and mechanisms (1). The major family is called PPP, with three predominant phosphatases PP1, PP2A and Ca2+/calmodulin-dependent calcineurin (CaN, a.k.a. PP3), in addition the PPP family has other members including PP4, PP5 and PP6. Activities of PPP in cells are restricted primarily through interaction with regulatory subunits that are responsible for compartmentalization, allosteric regulation and substrate specificity. Our favorite example is the myosin-binding PP1 regulatory subunit (MYPT1) that tethers the catalytic subunit of PP1 (PP1C) to actomyosin filaments for the dephosphorylation of myosin regulatory light chain MLC20 in smooth muscle and non-muscle cells, such as fibroblasts. Phosphorylation of MYPT1 at Thr696 and Thr853 causes inhibition of the myosin phosphatase (Figure 1B, scheme (i)), allowing for multiple kinase inputs to alter phosphatase activity. Because each PPP catalytic subunit interacts with a variety of regulatory subunits, including >100 regulatory subunits for PP1, and three or four families of B regulatory subunits for PP2A, the number of individual Ser/Thr phosphatase combinatorial complexes approaches the number of Ser/Thr kinases, encoded by approximately 400 genes in human genome.
Inhibitor Proteins for PPP Ser/Thr phosphatases
In addition to regulatory subunits, PPP phosphatases are regulated by endogenous polypeptide inhibitors. Table 1 lists inhibitor proteins for PP1, PP2A and CaN in mammals. Most PP1 inhibitor proteins are phosphorylated at multiple sites and phosphorylation governs their functions. For example, I-1, DARPP32, CPI-17, and its homologs PHI-1, KEPI and GBPI all are phosphorylation-dependent inhibitors for PP1. Likewise, α-endosulfine (Ensa) and ARPP19 were identified as selective inhibitors of PP2A due to phosphorylation at Ser67 by Greatwall kinase (4, 5). On the other hand, I-2, I-3, I-4, NIPP1, SET/I2PP2A, cain/cabin1, and RCAN1 spontaneously bind to their phosphatase target without phosphorylation, even though functions are modulated by phosphorylation (Table 1) (6–9). The phosphorylation dependence of the inhibitor proteins is partly how kinase activation transduces into changes in protein phosphorylation, by coincident reduction in phosphatase activity. Phosphorylation-dependent modulation of the inhibitor proteins is key to accurate mapping and modeling of signaling networks.
Table 1.
Mammalian endogenous inhibitor proteins for PPP superfamily
| Inhibitor | Phosphorylation site | Kinase | Function | Reference |
|---|---|---|---|---|
| PP1 inhibitors | ||||
| Inhibitor-1 (I-1) | Thr35 | PKA | Inhibition of PP1 | (53) |
| Ser67 | Cdk5/PKC | Inhibition of PKA | (54) | |
| DARPP32 | Thr34 | PKA | Inhibition of PP1 | reviewed in (55) |
| Thr75 | Cdk5 | Inhibition of PKA | (55) | |
| Ser97 | CK-II | Exposing Thr34 | (55) | |
| Ser130 | CK-I | Inhibition of CaN | (55) | |
| Inhibitor-2 (I-2) | Thr72 | GSK3, cdc2, MAPK | Activation of PP1C•I-2 complex | (6) |
| Ser86/120/121 | CK-II | Recognition of Thr72 by GSK3 | (6) | |
| Tyr | NDa | (56) | ||
| Inhibitor-4 (I-4) | I-2 homolog | |||
| Inhibitor-3 (I-3) | ND | PKA, PKC, CK-II | Dis-inhibition | (7) |
| NIPP-1 | Ser199 | PKA | Activation of PP1C•NIPP-1 complex | (8) |
| Ser204 | CK-II | Activation of PP1C•NIPP-1 complex | (57) | |
| CPI-17 | Thr38 | PKC, ROCK, ZIPK, ILK | Inhibition of PP1∂-MYPT1 complex | reviewed in (11) |
| Ser12 | PKC, ZIPK | ND | (11) | |
| Ser128 | ND | ND | (11) | |
| PHI-1 | Thr57 | PKC, ROCK, ILK | Inhibition of PP1 | (58) |
| Serb | PKC, ROCK | ND | (58) | |
| KEPI | Thr73 | PKC, ILK | Inhibition of PP1 | (59) |
| Serb | PKC | ND | (60) | |
| GBPI | Thr58 | PKC | Inhibition of PP1 | (18) |
| Serb | PKA | Dis-inhibition | (18) | |
| PP2A inhibitor | ||||
| SET/I2PP2A | Upregulated in cancer cells | |||
| CIP2A | Upregulated in cancer cells | |||
| α-endosulfine (Ensa) | Ser67 | Greatwall | Inhibition of PP2A-B55∂ complex | (5) |
| ARPP19 | Ser67 | Greatwall | Inhibition of PP2A-B55∂ complex | (4) |
| CaN/PP2B inhibitors | ||||
| CAIN/CABIN | Inhibition of CaN | |||
| RCAN1 | Ser163 | ND | Inhibition of CaN | (9) |
ND, not determined.
Phosphorylation site(s) are not identified.
The first PP1 inhibitors discovered, I-1 and I-2, proved relatively ineffective as inhibitors in biochemical assays with PP1C complexed with glycogen-targeting subunit (GM) or myosin-targeting subunit (MYPT1), compared to monomeric PP1 catalytic subunit. In 1993, a model illustrated in Figure 1B(iii) was proposed where dissociation of regulatory subunit releases PP1C, making it available for inhibition by PP1 inhibitors (10). The model was based on PKA phosphorylation of Ser67 in the RVSF PP1 binding motif of GM, which released PP1C. The model involved simultaneous phosphorylation of GM and I-1 by PKA with net transfer of PP1C from the former to the latter binding partner (Figure 1B, scheme (iii)) (10). Rapid and reversible binding of PP1C with multiple regulatory subunits is vital to this “scavenger” model for the action of PP1 inhibitors, which was popular and even made its way into textbooks. However, the scavenger model has some problems. There was no evidence for formation of the phospho-I-1::PP1C complex in response to PKA activation. The model has no path for return to the initial state of the PP1 bound to GM. Later, a new inhibitor CPI-17 was discovered that has no RVxF motif for tethering to PP1C, but interacts with PP1C that is bound to the regulatory subunit MYPT1. The example of CPI-17 showed that PP1 inhibitors bind in addition to, not instead of, regulatory subunits. Contacts of CPI-17 with both subunits (PP1C and MYPT1) allows this protein to selectively inhibit one subset of PPP holoenzymes (Figure 1B, scheme (iv)) (11). This new model explains how specific phenotypes could be caused by the ablation, mutation, or pathological overexpression of particular endogenous inhibitor protein genes.
II. Specificity of Individual PP1 inhibitors
CPI-17, a selective inhibitor of PP1C::MYPT1
CPI-17 (C-kinase-activated PP1 inhibitor, Mr of 17kDa) was discovered by biochemical purification using an assay for phosphorylation-dependent inhibition of the MLCP complex (reviewed in (11)). Inhibition occurs without dissociation of PP1C and the IC50 is enhanced over 1,000-fold (μM to nM) when CPI-17 is phosphorylated at Thr38. Neither Asp- nor Glu-substitution of Thr38 can mimic phosphorylation to produce the inhibitory effects. Thr38 is phosphorylated by multiple kinases, such as PKC, ROCK, ILK, ZIPK, and PAK, making it a hub for inputs to increase myosin phosphorylation and contractility (11). Upon G-protein activation CPI-17 is phosphorylated and selectively inhibits the MLCP, promoting an increase in the phosphorylation of myosin and also other MLCP substrates such as merlin (12, 13). CPI-17 expression is restricted in specific cell types, where it contributes to the regulation of specific cell functions, such as smooth muscle contraction, platelet activation, Purkinje cell long-term depression, endothelium function, cancer cell growth and gene regulation (11, 13, 14).
Phospho-CPI-17 inhibition is restricted to PP1C monomer or PP1C associated with MYPT1. When phospho-CPI-17 binds to PP1C complexed with other regulatory subunits (e.g. PP1C::GM), it gets dephosphorylated, thereby losing its inhibitory potential, making CPI-17 an inhibitor AND/OR a substrate(15). We propose inhibition is a function of the relative rate of Thr38 dephosphorylation. This concept also provides a mechanism for relief of inhibition, and resetting to basal conditions, by dephosphorylation of the bound CPI-17. Yet another phosphatase is not required for inactivation of the phosphoinhibitor.
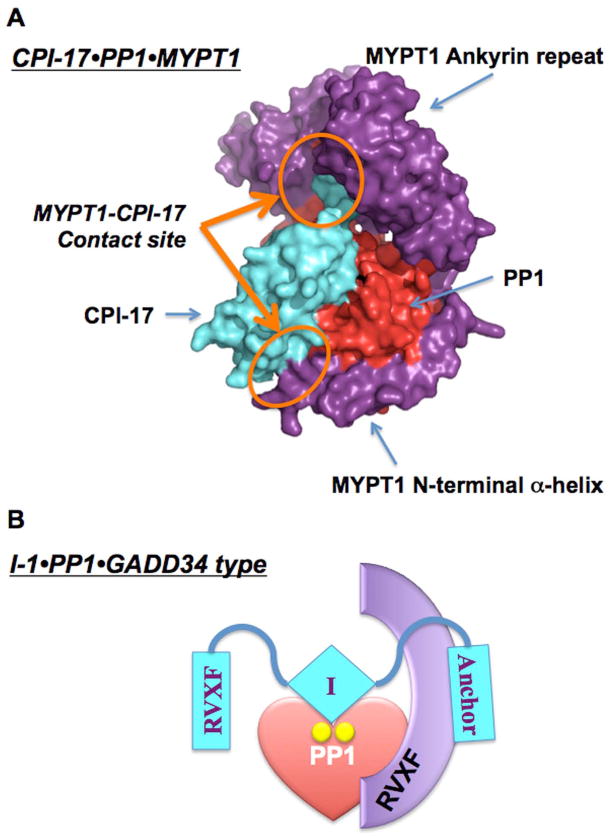
Figure 2A shows an in silico structural model of the complex of phospho-CPI-17 (cyan) binding to MYPT1 (purple)•PP1 (red) (16). The solution NMR structure of CPI-17 reveals a flexible loop region with the phosphorylation site, followed by a bundle of four α-helices, which undergo realignment upon Thr38 phosphorylation (16). In this 3D model, phospho-CPI-17 docks at the PP1 active site, and simultaneously contacts MYPT1 at the ankyrin-repeat domain and the N-terminal helix domain (Figure 2A orange circles). In fact, deletion of the MYPT1 N-terminal helix greatly diminishes binding to phospho-CPI-17. We propose that simultaneous contacts with different subunits of PPP holoenzymes account for the specificity of the endogenous PPP inhibitor proteins (Table 1) (16).
Figure 2. Models of PP1 holoenzymes with PP1 inhibitors.
A. MYPT1•PP1•CPI-17, B. GADD34•PP1•I-1. In the new PP1 heterotrimer model, PP1 inhibitor protein (cyan) directly binds to a complex of PP1 catalytic subunit (red) and regulatory subunit (purple). The direct contact between PP1 inhibitor protein and regulatory subunit may contribute to the specific recognition of PP1 holoenzymes.
The CPI-17 Family
Following the discovery of CPI-17, related proteins PHI-1, KEPI and GBPI were characterized as inhibitors of PP1 holoenzymes, dependent on phosphorylation at a site corresponding to CPI-17 Thr38 (Table 1) (11). The primary structure of CPI-17 family members is highly conserved within the inhibitory domain, including the phosphorylation loop, therefore it is easy to imagine that each family member shares the same overall architecture. Despite this, each CPI-17 family member is projected to play unique roles in cell signaling. For example, PHI-1 cannot mimic CPI-17 action in permeabilized smooth muscle, but it does contribute to regulation of cell motility, without affecting myosin phosphorylation. KEPI is involved in mechanisms of morphine addiction (17), and its inhibitory potency toward MLCP is lower, compared to PP1C, distinguishing it from CPI-17 (11). A clear difference in the sequence of PHI-1, KEPI and GBPI compared to CPI-17 is the presence of a RVXF motif in the N-terminal tail domain. Unphosphorylated KEPI and GBPI associate with PP1, probably using this RVXF sequence, although structural insights into the interaction have yet to be revealed (18).
Inhibitor-1 regulates cardiac β-agonist signaling to selected targets
I-1 has actions in the regulation of protein synthesis and Ca2+ signaling. I-1 is phosphorylated at Thr35 by PKA to become a potent PP1 inhibitor (Table 1) (19). Phospho-Thr35 directly docks at the active site of PP1 as a pseudosubstrate (20). In addition, the N-terminal KIQF motif, which is conserved in another inhibitor protein called DARPP32, can anchor to the PP1 RVxF-binding pocket, if the pocket is not occupied by a regulatory subunit. This may be why phospho-I-1 is in general less potent toward PP1 complexes, compared with monomeric PP1C. However, yeast two-hybrid screening with I-1 as bait identified GADD34, which binds to the C-terminal region of I-1 (21). GADD34 encoded by the PPP1R15A gene is a regulatory subunit that targets PP1 to translation initiation factor eIF-2alpha via an RVxF motif. The results support formation of a heterotrimeric complex of phospho-I-1, PP1C and GADD34 (Figure 2B) (21). The specific recognition of a subset of PP1 holoenzymes by I-1 may explain the selective regulation of the phosphorylation of phospholamban at Ser16 and ryanodine receptor at Ser2814, compared to troponin I and myosin-binding C-protein in cardiac β-adrenergic signaling (see review(22)).
Inhibitor-2 is a mitotic phosphoprotein with multiple targets/partners
The physiological function of phosphatase I-2 has been a mystery for decades. Different groups purified a I-2::PP1C heterodimer that was studied intensively for many years, with the presumption that it represented a major form of cytosolic PP1 in cells and tissues. The inactive heterodimer was named MgATP-dependent phosphatase, because the phosphatase activity could be evoked by the phosphorylation of I-2 at Thr72 in the PSTP sequence by an activating factor FA, a reaction shown later to be catalyzed by Pro-directed kinases GSK3, MAPKs or CDKs (see (23)). Deletion and mutation analysis showed I-2 interacts with PP1C through multiple sites. The 3D structure of the crystallized heterodimer visualized only parts of I-2, one long helical segment across the active site, and other short segments in contact with PP1 (24). Other portions of I-2 apparently were too flexible to give an electron density map. Recent studies of I-2 binding to PP1C by NMR showed I-2 associated with PP1C already complexed with neurabin, forming a heterotrimer of I-2::PP1C::Nrb (25). Based on analysis of NMR spectra different regions of I-2 were used to contact PP1C in the heterodimer, compared to the heterotrimer. These results reinforced a previous proposal that I-2 function involves heterotrimers containing PP1C and other partners. Those other partners were found by yeast two-hybrid screening, and validated individually, including NIMA-related kinase 2 (Nek2), the kinase LMTK2, and neurabin 2 (a.k.a. spinophilin) (1). These proteins each form a trimeric complex with I-2 and PP1C. Other studies found I-2 directly binds and activates Aurora A kinase (26) and forms heterotetramers with the prolyl isomerase Pin1 (27), which alters Pin1 specificity for mitotic phosphoproteins (28). These complexes do not involve PP1C at all. Taken together, results indicate that I-2 forms heterotrimers with PP1C and also has other potential targets in cells, not just PP1C.
Genetics and cell biology point to a conserved biological function of I-2 in control of chromosome segregation during mitosis, probably involving Aurora B. First, in yeast the I-2 ortholog GLC8 regulates PP1 (GLC7) and Aurora (IPL1) for proper chromosome segregation (29). Second, a phospho-specific antibody exposed a >25-fold increase in phosphorylation of the conserved PxTP motif in HeLa cells blocked in mitosis (30). Third, in growth synchronized cells going from G2 through mitosis the rise and fall of phospho-I-2 exactly paralleled the phosphorylation of histone H3 Ser10 and the inhibitory phospho-Thr in PP1, consistent with these being common substrates of CDK1::cyclinB1 (31). Fourth, knockdown of I-2 in human epithelial cells caused a failure of cytokinesis and accumulation of cells with multipolar mitotic spindles and defective nuclei (32). Knockdown and overexpression of I-2 shifted the dose-response for Aurora B inhibitors. Lastly, in vivo and genetic evidence from Drosophila revealed maternal expression and essential function (33). Using a species-specific antibody, Dm-I-2 was visualized by immunofluorescent microscopy as a maternal protein loaded into oocytes during maturation. Phospho-I-2 was localized in vivo in bands of mitotic cells during gastrulation. Genetic crosses produced hypomorphic embryos that showed loss of mitotic synchrony, defective mitotic spindles with lagging chromosomes, and loss of nuclei in syncytial blastoderms, resulting in reduced viability. Transgenic expression of Dm-I-2 gave dose-dependent rescue of the maternal effect lethality (33). Thus, I-2 is a mitotic phosphoprotein that has an essential role in chromosome segregation.
III. Longer-term Changes in Expression Levels of Inhibitor Proteins
Transcriptional regulation of CPI-17
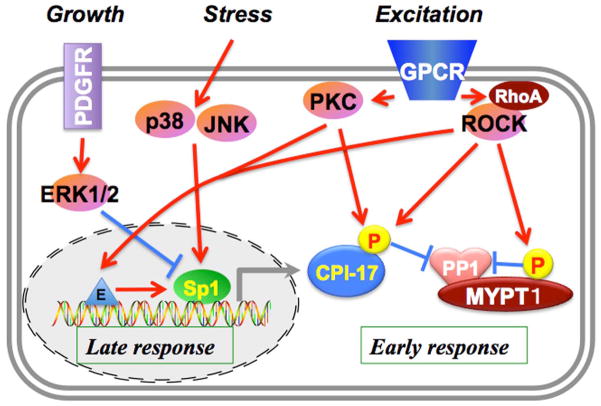
Responses of cells to physiological stimuli and pathological stresses involve both early responses by post-translational modification of the PPP inhibitor proteins (Figure 1B) plus later transcriptional regulation of the genes. For example, CPI-17 is involved in the early and late responses in smooth muscle contraction (Figure 3). GPCR activation triggers quick activation of conventional PKC through Ca2+ release from SR in smooth muscle cells. ROCK is activated through Rho-GEF signaling, causing sustained phosphorylation of CPI-17 and parallel inhibition of MLCP by MYPT1 phosphorylation (11). CPI-17 expression levels are altered during embryonic development and perturbed under pathological conditions, as listed in Table 2. CPI-17 expression increases during the course of development of smooth muscle cells in the embryo (34). Elevation of CPI-17 expression is linked to hyper-responsiveness of smooth muscle contraction, such as in the pregnant myometrium, diabetes mellitus and bronchus of asthma models and patients (35–38). Hypoxia, androgen treatment, subarachnoid hemorrhage and mechanical obstruction also lead to CPI-17 upregulation (39–42). On the other hand, CPI-17 expression can be down-regulated. In intestinal smooth muscle, inflammatory cytokine stimulation causes reduction of CPI-17 expression and loss of smooth muscle tone (43). Furthermore, even in the same organ (urinary bladder) the response of CPI-17 expression is different for mouse vs. rabbit (44) (Table 2). Thus, the regulatory network for controlling CPI-17 expression seems to be a specific adaptation to environmental changes in different smooth muscles. The regulation of CPI-17 expression is mediated in part though kinase signaling (Figure 3) (45). A potent smooth muscle growth factor, platelet derived growth factor (PDGF) ceases the CPI-17 gene promoter, which is regulated by Sp1/Sp3 binding (45). This is consistent to the downregulation of CPI-17 expression at vascular lesions (34). Interestingly, the CPI-17 promoter is independent of KLF4/myocardin signaling, which is a dominant regulator of smooth muscle genes. Osmotic stress and inflammatory cytokine signals can cause upregulation of the CPI-17 gene through p38/JNK pathways, which likely dominate in bronchial smooth muscle, where inflammation upregulates CPI-17 expression. On the other hand, ERK1/2 signals or other inhibitory pathways are likely involved in downregulation of CPI-17 in inflamed intestine. PKC and ROCK positively regulate the CPI-17 promoter, indicating dual roles of these kinases in the early and late response in the CPI-17 signaling (45). These results suggest coupling of CPI-17 activation and expression to enhance smooth muscle responsiveness to stimuli. A growing body of evidence also suggests that fluctuations occur in the expression of a subset of the PPP inhibitor proteins, such as RCAN1, SET/I2PP2A, DARPP32 and CIP2A, under pathological conditions. Thus, changes in gene expression of endogenous inhibitor proteins are part of adaptation in the disease state. Therefore the PPP inhibitor proteins have potential as diagnostic markers and possibly as therapeutic targets.
Figure 3. Multi-phase regulation of MLCP through CPI-17.
GPCR activation triggers activation of PKC and ROCK, which leads to a sequential phosphorylation of CPI-17 and a quick and robust phosphorylation of myosin light chain in smooth muscle. In addition to the early response, PKC/ROCK, as well as p38/JNK activated under pathological stresses upregulates the CPI-17 promoter, and the growth stimulation with PDGF negatively regulates the expression through ERK1/2, adjusting MLCP signaling to environmental changes.
Table 2.
Fluctuations in CPI-17 expression under physiological and pathological conditions
| Tissue | Treatment/Condition | Reference |
|---|---|---|
| UPREGULATION | ||
| Human myometrium | Pregnancy | (35) |
| Pig pulmonary artery | Hypoxia | (39) |
| Rat bronchus | Antigen challenge | (37) |
| Human bronchus | Asthma | (38) |
| Rabbit bladder | Hyperglycemia | (36) |
| Mouse bladder | Partial urinary outlet obstruction | (42) |
| Rabbit cerebral artery | Subarachonoid hemorrhage | (41) |
| Rat renal cortex | Testosterone treatment | (40) |
| Cancer cells | (13) | |
| DOWNREGULATION | ||
| Rat artery | Neointima | (34) |
| Mouse ileum | IL1β, inflammatory bowel disease | (43) |
| Human colon | Chronic colitis | (43) |
Alternative splicing of PP1 inhibitor proteins
Splicing variants and isoforms of PP1 inhibitor proteins with disparate activities are expressed under different pathophysiological conditions. A truncated version of CPI-17 called β includes Thr38, but lacks 27 residues in the middle region encoded by exon 2, which includes the hydrophobic residues necessary for the four-helix bundle. This would be predicted to lack structural stability and inhibitory potency (16, 46). A shorter isoform of I-2 called β is encoded by an independent gene expressed in testis. This I-2β gene product lacks both N-terminal and C-terminal regions of the conventional I-2 and fails to inhibit PP1C (47). I-1 splicing variants, I-1α and I-1β, are generated due to loss of exon 4 and exon 5 that might alter target recognition, even though these shorter versions have the inhibitory phosphorylation site Thr35 and retain the inhibitory potency with PP1C (48, 49). One study using yeast suggests that the physiological functions of the truncated I-1α/β are distinguishable from the conventional I-1 (49). Alternative splicing that deletes functional regions can change the target oPP1 inhibitors and alter the cellular response. It is possible that some variants that do not inhibit PP1C still possess phosphorylation sites and may function as decoys or competitors in cells. Physiological significance of these PP1 inhibitor variants is underestimated.
IV. Prospects for future study of endogenous PPP inhibitor proteins
Our view is that the primary action of each endogenous inhibitor protein is to provide regulation of individual PPP phosphatase holoenzymes by simultaneous interaction with the catalytic and regulatory subunits. In this way the phospho-regulation of a subset of the PPP inhibitors provides a way for kinases to fine-tune the activity of particular phosphatase holoenzymes in response to signaling stimuli. Natural products such as okadaic acid, calyculin A, and microcystin-LR, inhibit multiple PPP phosphatases by binding at the active sites, and pose limited translational potential, due to toxicity. In contrast, as discussed, many endogenous inhibitor proteins potentially regulate a narrow subset of PPP enzymes, at particular times and locations, contributing to temporal and spatial control of cell signaling. Cellular PPP inhibitor proteins can be regulated by transcriptional and post-translational mechanisms. We confidently can predict there are more gene products to be discovered as PPP phosphatase inhibitors. Because the levels of the endogenous inhibitor change in different pathophysiological situations, they may serve as diagnostic markers. These PPP inhibitors may even be therapeutic targets. Interfering with the PP2A inhibitor called SET/I2PP2A to increase PP2A phosphatase activity is already being exploited as a possible therapeutic approach (50, 51). Guanabenz is a compound recently characterized as a specific inhibitor of the GADD34-PP1 complex, which gives resistance to ER-stress by maintaining eIF2alpha phosphorylation, in effect mimicking one action of I-1 (52). Structural insights into how the endogenous inhibitors generate specificity by recognition of PPP could be applied to the design of new antagonists that recognize individual PPP holoenzymes to elicit specific physiological responses.
Acknowledgments
This work was supported by NIH R01HL083261, R01DK088905, research grants from Brandywine Hemophilia Foundation and Pennsylvania CURE (to ME), R01 GM56362 (to D.L.B.) and CA142823 (supporting DLB).
References
- 1.Brautigan DL. Protein Ser/Thr Phosphatases : The Ugly Ducklings of Cell Signaling. FEBS J. 2012 doi: 10.1111/j.1742-4658.2012.08609.x. [DOI] [PubMed] [Google Scholar]
- 2.Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1991;88:9307–910. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20:7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gharbi-Ayachi A, Labbé J, Burgess A, Vigneron S, Strub J, Brioudes E, et al. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 5.Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 6.Park IK, Roach P, Bondor J, Fox SP, DePaoli-Roach AA. Molecular mechanism of the synergistic phosphorylation of phosphatase inhibitor-2. Cloning, expression, and site-directed mutagenesis of inhibitor-2. J Biol Chem. 1994;269:944–54. [PubMed] [Google Scholar]
- 7.Zhang L, Qi Z, Gao Y, Lee EYC. Identification of the interaction sites of Inhibitor-3 for protein phosphatase-1. Biochem Biophys Res Commun. 2008;377:710–713. doi: 10.1016/j.bbrc.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beullens M, Van Eynde A, Bollen M, Stalmans W. Inactivation of nuclear inhibitory polypeptides of protein phosphatase- 1 (NIPP-1) by protein kinase A. J Biol Chem. 1993;268:13172–1317. [PubMed] [Google Scholar]
- 9.Genesca L, Aubareda A, Fuentes JJ, Estivill X, De La Luna S, Perez-Riba M. Phosphorylation of calcipressin 1 increases its ability to inhibit calcineurin and decreases calcipressin half-life. Biochem J. 2003;374:567–575. doi: 10.1042/BJ20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- 11.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009;284:35273–35277. doi: 10.1074/jbc.R109.059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- 13.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–579. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- 14.Pagiatakis C, Gordon JW, Ehyai S, McDermott JC. A novel RhoA/ROCK-CPI-17-MEF2C signaling pathway regulates vascular smooth muscle cell gene expression. J Biol Chem. 2012;287:8361–8370. doi: 10.1074/jbc.M111.286203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eto M, Kitazawa T, Brautigan DL. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphatase-1 by regulatory subunits. Proc Natl Acad Sci U S A. 2004;101:8888–8893. doi: 10.1073/pnas.0307812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eto M, Kitazawa T, Matsuzawa F, Aikawa S, Kirkbride JA, Isozumi N, et al. Phosphorylation-induced conformational switching of CPI-17 produces a potent myosin phosphatase inhibitor. Structure. 2007;15:1591–1602. doi: 10.1016/j.str.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drgonova J, Zimonjic DB, Hall FS, Uhl GR. Effect of KEPI (Ppp1r14c) deletion on morphine analgesia and tolerance in mice of different genetic backgrounds: when a knockout is near a relevant quantitative trait locus. Neuroscience. 2010;165:882–895. doi: 10.1016/j.neuroscience.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu QR, Zhang PW, Lin Z, Li QF, Woods AS, Troncoso J, et al. GBPI, a novel gastrointestinal- and brain-specific PP1-inhibitory protein, is activated by PKC and inactivated by PKA. Biochem J. 2004;377:171–181. doi: 10.1042/BJ20030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang FL, Glinsmann WH. Separation and Characterization of Two Phosphorylase Phosphatase Inhibitors from Rabbit Skeletal Muscle. Eur J Biochem. 1976;70:419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- 20.Desdouits F, Cheetham JJ, Huang HB, Kwon YG, da Cruz e Silva EF, Denefle P, et al. Mechanism of inhibition of protein phosphatase 1 by DARPP-32: studies with recombinant DARPP-32 and synthetic peptides. Biochem Biophys Res Commun. 1995;206:652–68. doi: 10.1006/bbrc.1995.1092. [DOI] [PubMed] [Google Scholar]
- 21.Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–650. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wittkopper K, Dobrev D, Eschenhagen T, El-Armouche A. Phosphatase-1 inhibitor-1 in physiological and pathological beta-adrenoceptor signalling. Cardiovasc Res. 2011;91:392–401. doi: 10.1093/cvr/cvr058. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Satinover DL, Brautigan DL. Phosphorylation and functions of inhibitor-2 family of proteins. Biochemistry. 2007;46:2380–2389. doi: 10.1021/bi602369m. [DOI] [PubMed] [Google Scholar]
- 24.Hurley TD, Yang J, Zhang L, Goodwin KD, Zou Q, Cortese M, et al. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J Biol Chem. 2007;282:28874–83. doi: 10.1074/jbc.M703472200. [DOI] [PubMed] [Google Scholar]
- 25.Marsh JA, Dancheck B, Ragusa MJ, Allaire M, Forman-Kay JD, Peti W. Structural diversity in free and bound states of intrinsically disordered protein phosphatase 1 regulators. Structure. 2010;18:1094–1103. doi: 10.1016/j.str.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satinover DL, Leach CA, Stukenberg PT, Brautigan DL. Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc Natl Acad Sci U S A. 2004;101:8625–8630. doi: 10.1073/pnas.0402966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sami F, Smet-Nocca C, Khan M, Landrieu I, Lippens G, Brautigan DL. Molecular basis for an ancient partnership between prolyl isomerase Pin1 and phosphatase inhibitor-2. Biochemistry. 2011;50:6567–6578. doi: 10.1021/bi200553e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Stukenberg PT, Brautigan DL. Inding of phosphatase inhibitor-2 to prolyl isomerase Pin1 modifies specificity for mitotic phosphoproteins. Biochemistry. 2008;47:292–300. doi: 10.1021/bi701819k. [DOI] [PubMed] [Google Scholar]
- 29.Tung HY, Wang W, Chan CS. Regulation of chromosome segregation by Glc8p, a structural homolog of mammalian inhibitor 2 that functions as both an activator and an inhibitor of yeast protein phosphatase 1. Mol Cell Biol. 1995;15:6064–674. doi: 10.1128/mcb.15.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leach C, Shenolikar S, Brautigan DL. Phosphorylation of phosphatase inhibitor-2 at centrosomes during mitosis. J Biol Chem. 2003;278:26015–26020. doi: 10.1074/jbc.M300782200. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Stefansson B, Wang W, Schaefer EM, Brautigan DL. Phosphorylation of the Pro-X-Thr-Pro site in phosphatase inhibitor-2 by cyclin-dependent protein kinase during M-phase of the cell cycle. Cell Signal. 2006;18:1318–1326. doi: 10.1016/j.cellsig.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Todd Stukenberg P, Brautigan DL. Phosphatase inhibitor-2 balances protein phosphatase 1 and aurora B kinase for chromosome segregation and cytokinesis in human retinal epithelial cells. Mol Biol Cell. 2008;19:4852–4862. doi: 10.1091/mbc.E08-05-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Cronmiller C, Brautigan DL. Maternal phosphatase inhibitor-2 is required for proper chromosome segregation and mitotic synchrony during Drosophila embryogenesis. Genetics. 2008;179:1823–1833. doi: 10.1534/genetics.108.091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JI, Young GD, Jin L, Somlyo AV, Eto M. Expression of CPI-17 in smooth muscle during embryonic development and in neointimal lesion formation. Histochem Cell Biol. 2009;132:191–198. doi: 10.1007/s00418-009-0604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozaki H, Yasuda K, Kim Y, Egawa M, Kanzaki H, Nakazawa H, et al. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol. 2003;140:1303–1312. doi: 10.1038/sj.bjp.0705552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang S, Hypolite JA, DiSanto ME, Changolkar A, Wein AJ, Chacko S. Increased basal phosphorylation of detrusor smooth muscle myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase beta and CPI-17. Am J Physiol Renal Physiol. 2006;290:F650–6. doi: 10.1152/ajprenal.00235.2005. [DOI] [PubMed] [Google Scholar]
- 37.Sakai H, Chiba Y, Hirano T, Misawa M. Possible involvement of CPI-17 in augmented bronchial smooth muscle contraction in antigen-induced airway hyper-responsive rats. Mol Pharmacol. 2005;68:145–151. doi: 10.1124/mol.104.004325. [DOI] [PubMed] [Google Scholar]
- 38.Morin C, Fortin S, Cantin AM, Rousseau E. Docosahexaenoic acid derivative prevents inflammation and hyperreactivity in lung: implication of PKC-Potentiated inhibitory protein for heterotrimeric myosin light chain phosphatase of 17 kD in asthma. Am J Respir Cell Mol Biol. 2011;45:366–375. doi: 10.1165/rcmb.2010-0156OC. [DOI] [PubMed] [Google Scholar]
- 39.Dakshinamurti S, Mellow L, Stephens NL. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Pediatr Pulmonol. 2005;40:398–407. doi: 10.1002/ppul.20290. [DOI] [PubMed] [Google Scholar]
- 40.Song J, Eyster KM, Kost CK, Kjellsen B, Martin DS. Involvement of protein kinase C-CPI-17 in androgen modulation of angiotensin II-renal vasoconstriction. Cardiovasc Res. 2010;85:614–621. doi: 10.1093/cvr/cvp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kikkawa Y, Matsuo S, Kameda K, Hirano M, Nakamizo A, Sasaki T, et al. Mechanisms underlying potentiation of endothelin-1-induced myofilament Ca2+ sensitization after subarachnoid hemorrhage. J Cerebral Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boberg L, Poljakovic M, Rahman A, Eccles R, Arner A. Role of Rho-kinase and protein kinase C during contraction of hypertrophic detrusor in mice with partial urinary bladder outlet obstruction. BJU Int. 2012;109:132–140. doi: 10.1111/j.1464-410X.2011.10435.x. [DOI] [PubMed] [Google Scholar]
- 43.Ohama T, Hori M, Fujisawa M, Kiyosue M, Hashimoto M, Ikenoue Y, et al. Downregulation of CPI-17 contributes to dysfunctional motility in chronic intestinal inflammation model mice and ulcerative colitis patients. J Gastroenterol. 2008;43:858–865. doi: 10.1007/s00535-008-2241-2. [DOI] [PubMed] [Google Scholar]
- 44.Chang S, Hypolite JA, Mohanan S, Zderic SA, Wein AJ, Chacko S. Alteration of the PKC-mediated signaling pathway for smooth muscle contraction in obstruction-induced hypertrophy of the urinary bladder. Lab Invest. 2009;89:823–832. doi: 10.1038/labinvest.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JI, Urban M, Young GD, Eto M. Reciprocal regulation controlling the expression of CPI-17, a specific inhibitor protein for the myosin light chain phosphatase in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2012 doi: 10.1152/ajpcell.00118.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamawaki K, Ito M, Machida H, Moriki N, Okamoto R, Isaka N, et al. Identification of human CPI-17, an inhibitory phosphoprotein for myosin phosphatase. Biochem Biophys Res Commun. 2001;285:1040–105. doi: 10.1006/bbrc.2001.5290. [DOI] [PubMed] [Google Scholar]
- 47.Osawa Y, Nakagama H, Shima H, Sugimura T, Nagao M. Identification and characterization of three isotypes of protein phosphatase inhibitor-2 and their expression profiles during testis maturation in rats. Eur J Biochem. 1996;242:793–798. doi: 10.1111/j.1432-1033.1996.0793r.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu HT, Lin TH, Kuo HC, Chen YC, Tsay HJ, Jeng HH, et al. Identification of the alternative splice products encoded by the human protein phosphatase inhibitor-1 gene. Biochem Biophys Res Commun. 2002;291:1293–1296. doi: 10.1006/bbrc.2002.6602. [DOI] [PubMed] [Google Scholar]
- 49.Weiser DC, Sikes S, Li S, Shenolikar S. The inhibitor-1 C terminus facilitates hormonal regulation of cellular protein phosphatase-1: functional implications for inhibitor-1 isoforms. J Biol Chem. 2004;279:48904–48914. doi: 10.1074/jbc.M404416200. [DOI] [PubMed] [Google Scholar]
- 50.Christensen DJ, Chen Y, Oddo J, Matta KM, Neil J, Davis ED, et al. SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target. Blood. 2011;118:4150–4158. doi: 10.1182/blood-2011-04-351072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Switzer CH, Cheng RYS, Vitek TM, Christensen DJ, Wink DA, Vitek MP. Targeting SET/I2 PP2A oncoprotein functions as a multi-pathway strategy for cancer therapy. Oncogene. 2011;30:2504–2513. doi: 10.1038/onc.2010.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 53.Aitken A, Bilham T, Cohen P. Complete primary structure of protein phosphatase inhibitor-1 from rabbit skeletal muscle. Eur J Biochem. 1982;126:235–246. doi: 10.1111/j.1432-1033.1982.tb06771.x. [DOI] [PubMed] [Google Scholar]
- 54.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 55.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 56.Williams JP, Jo H, Hunnicutt RE, Brautigan DL, McDonald JM. Tyrosine phosphorylation of phosphatase inhibitor 2. J Cell Biochem. 1995;57:415–422. doi: 10.1002/jcb.240570307. [DOI] [PubMed] [Google Scholar]
- 57.Van Eynde A, Beullens M, Stalmans W, Bollen M. Full activation of a nuclear species of protein phosphatase-1 by phosphorylation with protein kinase A and casein kinase-2. Biochem J. 1994;297:447–49. doi: 10.1042/bj2970447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J. 2002;367:517–524. doi: 10.1042/BJ20020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu QR, Zhang PW, Zhen Q, Walther D, Wang XB, Uhl GR. KEPI, a PKC-dependent protein phosphatase 1 inhibitor regulated by morphine. J Biol Chem. 2002;277:13312–1320. doi: 10.1074/jbc.M107558200. [DOI] [PubMed] [Google Scholar]
- 60.Erdodi F, Kiss E, Walsh MP, Stefansson B, Deng JT, Eto M, et al. Phosphorylation of protein phosphatase type-1 inhibitory proteins by integrin-linked kinase and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 2003;306:382–387. doi: 10.1016/s0006-291x(03)00976-8. [DOI] [PubMed] [Google Scholar]