Abstract
Dendritic spines are the principal recipients of excitatory synaptic inputs and the basic units of neural computation in the mammalian brain. Alterations in the density, size, shape, turnover of mature spines, or defects in how spines are generated and establish synapses during brain development, could all result in neuronal dysfunction and lead to cognitive and/or behavioral impairments. That spines are abnormal in fragile X syndrome (FXS) and in the best-studied animal model of this disorder, the Fmr1 knockout mouse, is an undeniable fact. But the trouble with spines in FXS is that the exact nature of their defect is still controversial. Here, we argue that the most consistent abnormality of spines in FXS may be a subtle defect in ac tivity-dependent spine plasticity and maturation. We also propose some future directions for research into spine plasticity in FXS at the cellular and ultrastructural levels that could help solve a two-decade-long riddle about the integrity of synapses in this prototypical neurodevelopmental disorder.
Keywords: dendritic spine, filopodia, Fmr1, fragile X mental retardation protein, mGluR, motility, synaptic plasticity, turnover, two-photon
Fragile X syndrome (FXS) is the most common single-gene cause of autism and mental impairment (Hagerman et al., 2010). In this disorder, transcriptional silencing of the Fmr1 gene causes loss of Fragile X mental retardation protein (FMRP), a synaptic RNA-binding protein with regulatory roles in both neuronal growth and plasticity (Bassell and Warren, 2008, De Rubeis and Bagni, 2010). Despite what are often disabling behavioral problems and profound intellectual dysfunction, the brains of individuals with FXS are normal in appearance, at least at the level of routine neuroimaging or gross inspection at autopsy. In fact, only a rather subtle, microscopic neuropathological abnormality has been identified in FXS: abnormal dendritic spines in the brain. In contrast, other developmental brain disorders such as neurofibromatosis or tuberous sclerosisare characterized by gross neuroanatomical defects that are readily apparent to the naked eye, including brain tumors, atrophy, or widespread gliosis, all of which contribute to neuronal dysfunction. Importantly, a similar defect in dendritic spines has been identified in the best-studied animal model of FXS, the Fmr1 knockout (KO) mice (Dutch-Belgian Fragile X Consortium, 1994). These mutant mice have reproducible problems with learning and memory and anxiety-like behaviors that are reminiscent of what is seen in humans with FXS (Penagarikano et al., 2007). This makes FXS an ideal neurodevelopmental disorder in which to study how altered signaling in certain molecular pathways leads to synaptic defects and dysfunctional circuits. Based on the symptoms of affected individuals, including cognitive impairment, sensory integration deficits, learning disability, anxiety, and autistic traits, scientists have focused their studies on three brain regions: the cerebral cortex, the hippocampus, and the amygdala. Here, we review what is known about Fmr1 KO mice and humans with FXS in terms of the density, size and shape, and baseline dynamics of dendritic spines, as well as their role in activity-dependent synaptic plasticity. For a discussion of a different dilemma, whether spine defects are directly caused by loss of FMRP or are an epiphenomenon of circuit dysfunction in FXS, please refer to a related review (Portera-Cailliau, 2011).
1. Spine Density
Spine density is an important aspect of network function. As the number of spines increases, so do the number of neuronal connections and the computational power of the brain (Yuste, 2010). It follows, then, that alterations in the numbers of spines would result in significant network dysfunction. This concept prompted neuroscientists, several decades ago, to begin exploring whether the numbers of spines were altered in neuropsychiatric disorders, including those leading to mental retardation and autism (Purpura, 1974).
Thus far, the majority of studies examining dendritic spines in FXS have relied on Golgi staining of pyramidal neurons from the neocortex and hippocampus in either autopsy material from adult individuals with FXS or in Fmr1 KO mice. The first publications from FXS subjects described an overabundance of immature spines in the dendrites of Layer 3 and Layer 5 pyramidal cells, but did not quantify spine density (Rudelli et al., 1985, Hinton et al., 1991). In a subsequent human FXS autopsy study, William Greenoughand colleagues expanded this hypothesis beyond the apparent spine immaturity, noting that spine density was increased in Layer 5 cortical pyramidal neurons (Irwin et al., 2001). Next, in a series of Golgi studies that systematically investigated dendritic spine pathology in adult Fmr1 KO mice, the same group reported that in both visual cortex and somatosensory cortices, Layer 5 pyramidal neurons display a significantly elevated spine density (Comery et al., 1997, Galvez and Greenough, 2005, McKinney et al., 2005). Another Golgi study recently reported an elevated spine density in Layer 5 pyramidal neurons of primary somatosensory cortex in KO mice at 1, 7 and 8 weeks of age (though, oddly, not at 2 weeks of age) (Su et al., 2011). Other groups have likewise found a higher than normal spine densityin Layer 2/3 pyramidal neurons from adult Fmr1 KO mice (Dolen et al., 2007, Hayashi et al., 2007, Liu et al., 2010), suggesting that alterations in spine numbers might be a common defect of all pyramidal neurons across different layers of the neocortex (Figure 1). Because the increase in spine density seen in adult FXS subjects and in mutant mice was unique to this neurodevelopmental disorder (as opposed to the presence of longer, immature-looking spines), this work led to the hypothesis that FXS might result from inadequate synaptic pruning due to “an absence of the [hypothetical and FMRP-dependent] stabilization protein at inactive synapses” (Irwin et al., 2000, Bagni and Greenough, 2005).
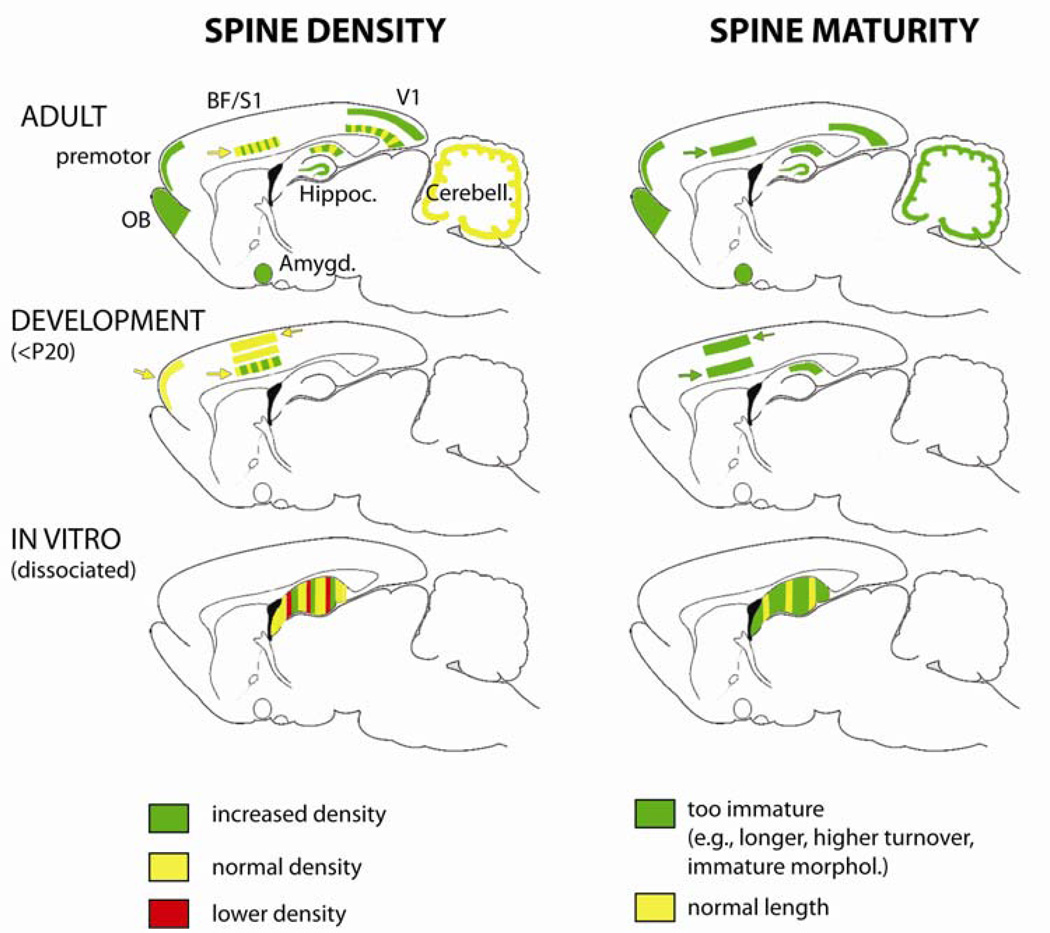
Figure 1. Regional distributions of reported spine defects in the brain of Fmr1 KO mice.
Data on dendritic spines in Fmr1 KO mice is displayed on sagittal cuts through the mouse brain (see Table 1). Data from the adult brain, developing brain (≤P20), and in vitro results from dissociated neurons in culture (hippocampus only) are shown in the top, middle and bottom rows, respectively. Colored stripes indicate the existence of published studies reporting different results on either spine density or maturity. The relative thickness of the colored stripes reflects approximately the relative number of studies supporting one finding or another. Studies that used imaging in living neurons (in vivo or in acute/organotypic brain slices) are indicated by arrowheads next to the stripes. Note that results of studies are much more consistent for the spine immaturity phenotype than for the spine density phenotype.
Abbreviations: BF: barrel field; OB: olfactory bulb; S1: somatosensory cortex; V1: visual cortex.
However, some of the more recent morphological analyses of spines inFmr1 KO mice have challenged this theory. These new studies differ from previous Golgi studies in two critical parameters: First, Fmr1 KO pyramidal neurons were now examined during early postnatal development (instead of adulthood, a critical difference considering that FXS presents in early childhood); and second, these surveys have relied more often on two-photon fluorescence microscopy (rather than using the Golgi method in fixed tissue), allowing investigators to image living neurons. The developmental studies have over whelmingly failed to identify differences in spine density in Layer 2/3, Layer 4, or Layer 5 pyramidal neurons between Fmr1 KO mice and wild type mice (Meredith et al., 2007, Ruan et al., 2009, Cruz-Martin et al., 2010, Harlow et al., 2010, Pan et al., 2010). Perhaps these contradictory results derive from differences in how the analyses were performed. For instance some investigators may choose to use for their statistics n= number of dendrites (for density) and n= number of spines (for spine length), whereas others use n= number of cells (or n= number of mice). It is also possible that, for unknown reasons, the Golgi impregnation method (or tissue fixation) could by itself bring about an elevated spine density in Fmr1 KO mice, but not wild type mice. Indeed, some have commented that different methodologies of labeling neurons (e.g., Golgi vs. intracellular neurobiotin, DiI, or Lucifer Yellow) might yield different quantitative results for spine density (Nimchinsky et al., 2001, Beltran-Campos et al., 2011). It has also been suggested that Golgi staining may preferentially label certain neuronal populations (e.g., cells with spine abnormalities (Nimchinsky et al., 2001) or cells with higher physiological levels of activity (Benshalom, 1992, Rao et al., 1993). Whether these suggestions are applicable to Fmr1 KO mice as compared to WT mice remains unknown. But in at least one study that compared spine density in fixed tissue vs. living neurons in cultured slices from Fmr1 KO mice, the authors reported a higher spine density in fixed tissue at postnatal day (P) 7 (though not at P14 or P28), but normal spine density in living neurons in organotypic slice cultures of an equivalent age (Nimchinsky et al., 2001). This again implies that the elevated spine density may be a bizarre consequence of fixation, but also that there may be differences in spine density between the first and subsequent postnatal weeks. Indeed, we have also found that in acute brain slices, spine density in Layer 2/3 cortical neurons is transiently elevated in mutant mice at P4-7, but not thereafter (Cruz-Martin & Portera-Cailliau, unpublished). More recently, two in vivo imaging studies have reported that spine density is normal in somatosensory cortex of Fmr1 KO mice at early postnatal stages (Cruz-Martin et al., 2010, Pan et al., 2010), as well as juvenile and adult stages (Pan et al., 2010).
In addition to the possible effects of age and fixation/staining method on spine density, one also wonders whether changes in spine density in Fmr1 KO mice may vary across different cortical layers, different brain regions, or different mouse strains. After a careful review of the literature, we concluded that the spine density profile in Fmr1 KO mice is independent of cortical layer (Table 1) or genetic background (Portera-Cailliau, 2011). In contrast, whether a particular spine density phenotype is apparent in the mouse model does depend on the brain region being examined (Figure 1). Given the well-established deficits in mGluR-dependent LTD in the hippocampus of Fmr1 KO mice (reviewed by (Ronesi and Huber, 2008) and the important role of this region in learning and memory, investigators have also looked for spine alterations in hippocampal pyramidal neurons. Spine density changes appear to be especially variable in the hippocampus, where studies of Fmr1 KO mice have found either normal (Pfeiffer and Huber, 2007, de Vrij et al., 2008, Levenga et al., 2011b, Su et al., 2011), higher (Antar et al., 2006, Grossman et al., 2006, Gross et al., 2010, Levenga et al., 2011a, Swanger, 2011), or even lower spine densities (Braun and Segal, 2000, Segal et al., 2003). Additional studies of spines in the hippocampus will hopefully resolve these discrepancies. Spine density increases have also been reported in granule cells of the olfactory bulb neurons (Scotto-Lomassese et al., 2011) and in the amygdala (Qin et al., 2011) of Fmr1 KO mice. The olfactory bulb study provided two important contributions regarding spine density. First, the authors demonstrated that the elevated spine density was cell-autonomous, suggesting that this phenotype may be triggered by loss of FMRP in single neurons and not by some large-scale network effect or by defects in presynaptic axons. Second, the authors showed that spine density in mutant neurons was already elevated at the earliest time-point examined (14 days in vitro), suggesting that the defect resulted from a developmental over production of spines rather than a failure to prune them (Scotto-Lomassese et al., 2011).
Table 1. Summary of abnormal dendritic spine phenotypes reported in Fmr1 KO mice.
The spine phenotypes reported in different brain regions of Fmr1 KO mice have been grouped according to three main results: increased density, normal density, and relative immaturity. Studies that used imaging in living neurons (in vivo or in acute/organotypic brain slices) are highlighted in gray.
| Region → | Cortex | Hippocampus | Cerebellum | Olfactory bulb | Amygdala |
|---|---|---|---|---|---|
| Phenotype ↓ | |||||
|
Increased spine density |
Layer 2/3: | Pyramidal cells in CA1: | Granule cells: | Pyramidal-like neurons: | |
| Dolen et al., 2007 (P30) | Levenga et al., 2011a (25 weeks, CA1) | Scotto-Lomassese et al., 2011 (2 months) | Qin et al., 2011 (~3 months) | ||
| Hayashi et al., 2007 (2 months) | |||||
| Liu et al., 2010 (12 weeks) | |||||
| Granule cells in dentate gyrus: | |||||
| Layer 5: | Grossman et al., 2010 (P15-60) | ||||
| Nimchinsky et al., 2001 (P7) | |||||
| ℘Su et al., 2011 (1, 7, 8 weeks) | Whole hippocampus dissociated: | ||||
| Comery et al., 1997 (16 weeks) | Antar et al., 2006 (P0+16div) | ||||
| McKinney et al., 2005 (P60-90) | Swanger et al., 2011(E17+18div) | ||||
| Galvez & Greenough, 2005 (P73) | |||||
|
Normal spine density |
Layer 2/3: | Pyramidal cells in CA1: | Purkinje neurons: | ||
| Cruz-Martin et al., 2010 (P7–P21) | Grossman et al., 2006 (P60–P90) | Koekkoek et al., 2005 (adult) | |||
| Meredith et al., 2007 (P14-23) | |||||
| Whole hippocampus dissociated: | |||||
| Layer 4: | Braun & Segal, 2000 (P0+7div) | ||||
| Harlow et al., 2010 (P7, P14) | Su et al., 2011 (P0+8 div) | ||||
| †Levenga et al., 2011b | |||||
| Layer 5: | (E16+14div) | ||||
| Nimchinsky et al., 2001 (P14, P28) | *Segal et al. 2003 (P1+14div) | ||||
| de Vrij et al., 2008 (E18+21div) | |||||
| Galvez & Greenough, 2005 (P25) | |||||
| Restivo et al., 2005 (12 weeks) | |||||
| Pan et al., 2010 (P30) | |||||
| Irwin et al., 2002 (60–90 days) | |||||
|
Relative immaturity of spines |
Layer 2/3: | Pyramidal cells in CA1: | Purkinje neurons: | Granule cells: | Pyramidal-like neurons: |
| Cruz-Martin et al., 2010 (P10-12; higher turnover; more filopodia / fewer mushroom spines) | Bilousova et al., 2009 (P7; more filopodia / fewer mushroom) | Koekkoek et al., 2005 (adult; longer) | Scotto-Lomassese et al., 2011 (2 months; longer) | Qin et al., 2011 (~3 months; longer) | |
| Grossman et al., 2006 (P60–P90; longer, more immature morphology) | |||||
| Meredith et al., 2007 (P14-23; longer) | ¥Levenga et al., 2011a (25 weeks; more filopodia / fewer mushroom) | ||||
| Liu et al., 2010 (13 weeks; longer) | |||||
| Layer 5: | Granule cells in dentate gyrus: | ||||
| Su et al., 2011 (1, 2, 7, 8 weeks; longer) | Grossman et al., 2010 (P15–P60; more immature morphology) | ||||
| Nimchinsky et al., 2001 (P7–P28) | Whole hippocampus dissociated: | ||||
| Pan et al., 2010 (P20, P30; high turnover) | |||||
| Comery et al., 1997 (16 weeks; longer) | Antar et al., 2006 (P0+16div; longer, more filopodia / fewer spine synapses) | ||||
| Restivo 2005 (12 weeks; more immature morphology) | de Vrij et al., 2008 (E18+21div; longer, more filopodia / fewer mature spines) | ||||
| Irwin et al., 2002 (60–90 days; more immature morphology) | Su et al., 2011 (P0+8div; longer) | ||||
| Galvez & Greenough, 2005 (P73; longer, more immature morphology) | ‡Levenga et al., 2011b (E16+14div; longer) | ||||
| Bilousova et al., 2009 (E15+14div; longer) | |||||
| McKinney et al., 2005 (P60–P90; longer, more immature morphology) | Swanger et al., 2011 (E17+18div; longer, more filopodia / fewer mushroom) | ||||
In this study, spine density in somatosensory cortex was elevated at 1, 7 and 8 weeks of age, but not at 2 weeks.
This is the only study that has reported a decrease in spine density in neocortical neurons (dissociated, E16+20div).
This study actually reported a lower than normal spine density in Fmr1 KO mice.
This study found that spines were not immature in CA3; also, they found no change in average spine length.
This study found that spine length was normal in neocortical neurons (dissociated, E16+20div).
More work is clearly needed to resolve the ongoing controversies regarding spine density in different brain regions of Fmr1 KO mice. However, one emerging theme is that the majority of studies to date, and in particular the developmental studies and those that used live imaging in the neocortex, report normal spine density for pyramidal neurons in Fmr1 KO mice (Table 1).
2. Spine maturity: size, shape and dynamics
The size and shape of dendritic spines are also critical parameters of neuronal function and connectivity. For instance, large spines are associated with bigger presynaptic terminals, and with larger post-synaptic currents (reviewed by Kasai et al., 2010). Likewise, newly formed spines tend to be smaller and therefore establish weaker synapses (Holtmaat et al., 2006). It follows that changes in spine size and turnover (that is, the rate of appearance and disappearance of spines) will also affect network function, and possibly to a greater degree than changes in spine density. For example, sensory deprivation produces dramatic changes in spine turnover, but has a negligible impact on spine density (reviewed by Holtmaat and Svoboda, 2009). Therefore, it is conceivable that loss of FMRP, a protein important for synaptic plasticity, would lead to alterations in spine size, shape or stability.
Size and shape
The original autopsy studies of FXS were the first to raise the issue of dendritic spine immaturity, because of the high prevalence of abnormally long, thin and tortuous spines observed in the dendrites of Layer 3 and Layer 5 pyramidal cells (Rudelli et al., 1985, Hinton et al., 1991). Because these spine morphologies were reminiscent of dendritic protrusions seen during normal cortical development (reviewed by (Portera Cailliau and Yuste, 2001, Yuste and Bonhoeffer, 2004), this led to the hypothesis that FXS could be caused by a failure in spine maturation, much as had been proposed earlier for other types of mental retardation (Purpura, 1974). A decade later, William Greenough conducted the only other human FXS study on spines, and for the first time used a quantitative approach to the analysis. His group reported not only a higher spine density in Layer 5 neurons, but also a higher proportion of longer spines (Irwin et al., 2001). In that study, the authors strived to categorize, for the first time in FXS, different classes of spines based on their morphology along a spine maturity continuum. They found that the proportion of spines exhibiting immature morphologies was greater in the brains of individuals with FXS.
Subsequent studies by the same lab focused on Fmr1 KO mice, and similarly reported an overabundance of immature spines in the neocortex (Comery et al., 1997, Irwin et al., 2002, Galvez and Greenough, 2005). Independent groups using a similar Golgi-staining approach in fixed tissue also found that pyramidal neurons in the neocortex of adult Fmr1 KO mice exhibit either unusually long spines (Meredith et al., 2007, Liu et al., 2010), or a preponderance of immature subtypes like dendritic filopodia (Restivo et al., 2005). But not all Golgi studies found changes in average spine length in the mutant mice (Hayashi et al., 2007), and neither did the two recent in vivo imaging studies (Cruz-Martin et al., 2010, Pan et al., 2010). Thus, although not all studies agree that spines are abnormally long in Fmr1 KO mice, most studies that evaluated the relative maturity of spines based on morphological criteria did find too many immature protrusions in the neocortex (Table 1; Figure 1). This immature spine phenotype has also been observed in pyramidal neurons and dentate gyrus granule cells of the hippocampus (Antar et al., 2006, Grossman et al., 2006, de Vrij et al., 2008, Bilousova et al., 2009, Grossman et al., 2010), Purkinje neurons of the cerebellum (Koekkoek et al., 2005), granule cells of the olfactory bulb (Scotto-Lomassese et al., 2011), and pyramidal-like neurons in the amygdala of Fmr1 KO mice (Qin et al., 2011). The immaturity of spines may also be subregion-specific, at least in the hippocampus, because it is present in CA1 but not CA3 (Levenga et al., 2011a), which is interesting in light of the different roles that these subregions play in learning and memory.
That said, morphology alone may not be the best way to differentiate between mature and immature spines. Time-lapse imaging studies have revealed that the shape and size of spines is rather dynamic, especially during early brain development. Immature spines undergo rapid changes in size and morphology on a minutes-to-hours scale (Bonhoeffer and Yuste, 2002). In adult mice, spines also display structural transformations, albeit on an hours-to-days scale (Holtmaat et al., 2005). Therefore, we have argued that a snapshot of a dendritic spine in fixed tissue is not the most accurate representation of its actual maturity, and that time-lapse imaging, which provides information on spine dynamics (motility and turnover), is the ideal approach to distinguish between different protrusion subtypes (Portera-Cailliau et al., 2003, Cruz-Martin et al., 2010, Portera-Cailliau, 2011). Dynamic imaging of spines in Fmr1 KO mice may reveal additional aspects of synaptic dysfunction in FXS.
Spine dynamics: Turnover and motility
To date, there have only been three independent studies published in which spine dynamics in Fmr1 KO mice were investigated using time-lapse imaging. In 2001, a two-photon imaging study of Layer 5 cortical neurons in organotypic slice cultures (P2 + 5 days in vitro) reported no changes in spine motility or turnover in the mutant mice (Nimchinsky et al., 2001). Last year, two different studies used in vivo two-photon microscopy in GFP-expressing mice to examine spine dynamics in the intact neocortex. In our study, we did not observe differences in spine motility between genotypes, but we did find an abnormally high turnover of spines in Layer 2/3 neurons in the barrel cortex of Fmr1 KO mice at P10-12 (Cruz-Martin et al., 2010). By P21-24, spine turnover in the mutant mice was normal, as measured at 10 min intervals over a 1 h period, so our results were consistent with a delay in spine stabilization during early postnatal development. The other study examined Layer 5 neurons in primary somatosensory cortex and found higher 2-d and 2-week spine turnover rates at P20 and P30 (Pan et al., 2010). The authors also noted that this phenotype was consistent across C57 and FVB genetic backgrounds. Although it remains to be seen whether Layer 2/3 neurons in KO mice exhibit elevated turnover rates at P30 over 2-d or 2-week intervals (or whether Layer 5 neurons are too unstable over 1 h intervals at P10-12), the results from these two independent groups are very similar. These data suggest that spine instability could be a problem that begins early in brain development and then persists throughout a lifetime in FXS. Incidentally, our finding of increased spine turnover in KO mice at P10-12 (Cruz-Martin et al., 2010) coincides with a critical period in barrel cortex development during which the absence of cortical FMRP also results in significant functional synaptic defects (Harlow et al., 2010). Thus, the increased spine turnover at this early age may be one of the earliest synaptic defects in this disorder.
A variety of spine abnormalities have been reported in Fmr1 KO mice (Figure 2). But, the most consistent abnormal spine phenotype, the one that matches what has been observed in autopsy material from humans with FXS, is a relative immaturity of dendritic spines, which is manifested by an overabundance of immature morphologies (filopodia-like protrusions) and a higher than normal spine turnover.
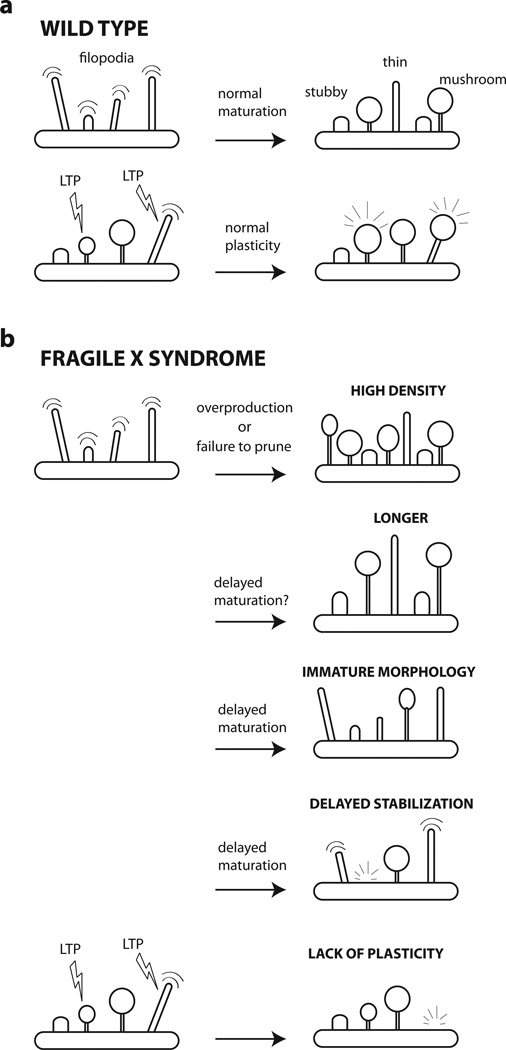
Figure 2. Possible mechanisms to explain spine defects in fragile X syndrome.
These cartoons depict the various dendritic spine defects that have been reported or proposed in patients with FXS or in Fmr1 KO mice, as well as the theories that may bring about those defects.
(a) Spine maturation and plasticity in wild type mice. During early brain development dendrites are studded with headless protrusions called filopodia. These are highly motile and transient protrusions that play a role in early synaptogenesis. In the adult, dendritic spines can be classified morphologically into three main types: thin, stuffy and mushroom. Thin spines tend to be smaller and have shorter lifetimes (days), whereas mushroom spines tend to be the largest and most stable (weeks to months). Spines are plastic structures such that changes in network activity (e.g., sensory inputs, learning new tasks) can modify the size, shape and turnover to influence synaptic strength (e.g., experience-dependent plasticity, LTP).
(b) Spine maturation and plasticity in fragile X syndrome. An over-production of spines (or a failure to prune them after development) can lead to a higher spine density as reported in some studies of Fmr1 KO mice. Alternatively, a delayed maturation of dendritic protrusions results in an overabundance of spines with immature morphologies or higher than normal turnover. In addition, spines in FXS may exhibit defects in activity-dependent plasticity.
Potential consequences of a higher spine density on the network might include seizures or an exaggerated response to sensory stimuli due to hyperconnectivity. Potential consequences of having immature spines (delayed stabilization and/or immature morphology) might include problems with learning and memory consolidation, due to a reduction in synapse number or to the formation of weaker, short-lasting synapses. Portential consequences of failure of spines to exhibit normal plasticity (e.g., experience-dependent plasticity) would also include problems with learning and memory, as well as sensory integration defects.
3. Future studies: Spine plasticity, synaptic ultrastructure, and molecular pathways
After more than two decades of research into the pathophysiology of fragile X syndrome, we are closer to understanding the molecular pathways regulated by FMRP and the synaptic defects associated with this disorder (Bassell and Warren, 2008). Alterations of dendritic spines, the major recipients of excitatory synapses in the brain, are an important clue to the pathophysiology of FXS. This review underscores the need for additional studies to resolve many outstanding controversies surrounding the various defects in spines that have been reported in Fmr1 KO mice. In particular, much could be learned from additional surveys of spine density size, shape, and turnover in the amygdala, hippocampus, and cerebellum throughout development. Whenever possible, such studies should be carried out with in vivo imaging in GFP-expressing mice, because this methodology preserves all brain connectivity and peripheral inputs. This approach has the added benefit that more homogeneous neuronal populations are labeled (compared to the Golgi method), which might reduce variability in spine analyses.
Perhaps the most exciting insights into spines in FXS will come from work that investigates the contribution of spines to circuit plasticity during learning or sensory processing. How spine plasticity contributes to circuit remodeling - for example, after changes in sensory experience - is one of the most important aspects of spine function (Holtmaat and Svoboda, 2009). In the context of a disease like FXS, characterized by deficits in learning and adaptive behavior, spine plasticity becomes an even more pertinent topic of investigation. Indeed, it has been proposed that FXS is caused by aberrant protein synthesis at the synapse (Bassell and Warren, 2008, Kelleher and Bear, 2008), which leads to alterations in activity-dependent circuit plasticity. Several studies have shown that Fmr1 KO mice exhibit defects in experience-dependent and other types of synaptic plasticity (such as LTP/LTD; reviewed by Pfeiffer and Huber, 2009), all of which are associated with changes in spine size and/or stabilization. We recently hypothesized that dendritic spines in FXS may be unable to modulate their size or stability in response to changes in neuronal activity, such that the LTP/LTD defects and the alterations of spine stability/maturation are actually different aspects of the same phenotype (Portera-Cailliau, 2011).
Thus far, spine plasticity has only been explored in a handful of studies of Fmr1 KO mice. One paper reported that while chronic stress increases spine density in WT mice, it failed to do so in the mutant mice (Qin et al., 2011). More strikingly, spines in adult Fmr1 KO mice were insensitive to modulation by sensory experience by whisker trimming for 2 weeks (Pan et al., 2010). In contrast, a different study showed that stimulating neuronal activity with bicuculline affected spines in dissociated hippocampal neurons from wild type and Fmr1 KO mice to a similar degree (Segal et al., 2003). Thus, future work ought to investigate which activity-dependent spine plasticity events are affected in Fmr1 KO mice. In addition, studies are needed to evaluate the effects of input deprivation on dendritic spines in various layers of visual or barrel cortex to confirm the initial study by Pan et al. (2010). It would also be interesting to investigate spine plasticity after sensory stimulation or after learning a behavioral task, as was recently done in motor cortex with in vivo imaging (Xu et al., 2009). But these studies need not be explored solely in vivo, as imaging spine size/turnover in acute slices during LTP/LTD protocols would also yield valuable results.
Another aspect of spines that has been understudied in Fmr1 KO mice is their appearance at the ultrastructural level, which would inform us on their ability to establish functional synapses. The original study of FXS autopsy material reported a reduction in the length of synapses and in the mean synaptic contact zone area (Rudelli et al., 1985). Indeed, one would predict that abnormally immature and unstable spines would form small, and therefore weak, synapses; future studies will need to confirm this prediction with electron microscopy in Fmr1 KO mice. One recent ultrastructural study in the olfactory bulb showed no differences in spine colocalization with PSD95, as well as ostensibly normal dendrodendritic synapsesongranule cells lacking Fmr1 (Scotto-Lomassese et al., 2011). In contrast, hippocampal neurons lacking FMRP had fewer spines that colocalized with synapsin, a presynaptic marker (Antar et al., 2006), indicating an inability of mutant neurons to establish functional synapses. Another interesting finding is that polyribosomes are scarcer in spines from Fmr1 KO mice (Weiler et al., 2004). This is intriguing because polyribosomes normally redistribute into spines with enlarged synapses following LTP (Ostroff et al., 2002), again supporting the argument that in the absence of FMRP, dendritic spines fail to undergo the classic signs of synaptic plasticity.
The molecular mechanisms that bring about this inability of spines in Fmr1 KO mice to play their part in modulating synaptic strength after changes in neuronal activity also merit investigation. Over the last two decades, a variety of signaling pathways have been identified that regulate spine density, turnover size or plasticity (Yuste, 2010, Portera-Cailliau, 2011). Honing in on those pathways that are dysregulated in FXS will be a difficult and time-consuming task, but one that will identify new therapeutic avenues for this disorder (Gross et al., 2011).
4. Spine Pathology: Cause or Consequence of Fragile X Syndrome
The neuropathological defect in fragile X syndrome is the overabundance of immature dendritic spines in cortical pyramidal neurons. In addition, a number of defects in synaptic plasticity have been uncovered with electrophysiology in Fmr1 KO mice (Pfeiffer and Huber, 2009). In theory, such dysfunctional circuits could lead to abnormal spines and vice versa, so it is still not clear which problem comes first. A better understanding of this cause-and-effect relationship will require additional experiments. Given the tight structure-function relationships at the synapse, we have previously argued that the two problems are intimately linked to one another (Portera-Cailliau, 2011). Therefore, as our understanding of the mechanisms that regulate the formation, morphology and lifetime of dendritic spines grows, so will our knowledge of the alterations in functional synaptic plasticity in Fmr1 KO mice. Interestingly, abnormal spines are a common feature of several neurodevelopmental disorders (as is apparent from this Special Issue). In addition, many of the defects in spines and in synaptic/circuit plasticity in these disorders occur during critical periods of early neocortical development. Therefore, further research into experience-dependent synaptic plasticity in sensory cortices will likely uncover candidate signaling pathways that could translate into therapeutic avenues for a variety of clinical syndromes.
The title of this review had what is now an obvious double-entendre. On one hand, dendritic spines are clearly abnormalin FXS. On the other hand, the scientific “trouble” we face is that the exact nature of the spine abnormality is not yet fully understood. The next decade, however, can no doubt find a solution to this puzzle through studies that combine electrophysiology, animal behavior and structural imaging, in order to better define the synaptic defects, both structural and functional, that are triggered by loss of FMRP.
Highlights.
Defects in the density, shape and turnover of spines occur in FXS
How these lead to circuit dysfunction and cognitive-behavioral symptoms is unknown
There are still several controversies regarding the exact spine defects in FXS
The real problem may be a defect in activity-dependent spine plasticity/maturation
Future research should explore further spine integrity and plasticity in FXS
Acknowledgments
This work was supported by FRAXA, the Dana Foundation, the NICHD/NIH (grant R01HD54453), the NIGMS/NIH (training grant GM08042), and the Medical Scientist Training Program at the David Geffen School of Medicine at UCLA.
Abbreviations
- div
days in vitro
- EM
electron microscopy
- FMRP
fragile X mental retardation protein
- FXS
fragile X syndrome
- GFP
green fluorescent protein
- KO
knockout
- L
layer
- LTD
long-term depression
- LTP
long-term potentiation
- mGluR
metabotropic glutamate receptor
- P
postnatal day
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP traficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nature Rev Neurosci. 2005;376:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Campos V, Prado-Alcala RA, Leon-Jacinto U, Aguilar-Vazquez A, Quirarte GL, Ramirez-Amaya V, Diaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Res. 2011;1369:119–130. doi: 10.1016/j.brainres.2010.10.105. [DOI] [PubMed] [Google Scholar]
- Benshalom G. Determining the neuronal connectivity of Golgi-impregnated neurons: ultrastructural assessment of functional aspects. Microsc Res Tech. 1992;23:324–333. doi: 10.1002/jemt.1070230407. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35:1019–1027. doi: 10.1016/s0896-6273(02)00906-6. [DOI] [PubMed] [Google Scholar]
- Braun K, Segal M. FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb Cortex. 2000;10:1045–1052. doi: 10.1093/cercor/10.10.1045. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martin A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, Bagni C. Fragile X mental retardation protein control of neuronal mRNA metabolism: Insights into mRNA stability. Mol Cell Neurosci. 2010;43:43–50. doi: 10.1016/j.mcn.2009.09.013. [DOI] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- Gross C, Berry-Kravis EM, Bassell GJ. Therapeutic Strategies in Fragile X Syndrome: Dysregulated mGluR Signaling and Beyond. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Lee KJ, Zeman MK, Jun CS, Azam HS, Arii T, Imoto K, Greenough WT, Rhyu IJ. Developmental characteristics of dendritic spines in the dentate gyrus of Fmr1 knockout mice. Brain Res. 2010;1355:221–227. doi: 10.1016/j.brainres.2010.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Elisseou NM, McKinney BC, Greenough WT. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Hagerman R, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, Patel B, Swain RA, Weiler IJ, Oostra BA, Greenough WT. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet. 2002;111:140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Bear MF. The autistic neuron: troubled translation? Cell. 2008;135:401–406. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Levenga J, de Vrij FM, Buijsen RA, Li T, Nieuwenhuizen IM, Pop A, Oostra BA, Willemsen R. Subregion-specific dendritic spine abnormalities in the hippocampus of Fmr1 KO mice. Neurobiol Learn Mem. 2011a;95:467–472. doi: 10.1016/j.nlm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, Song C, Buijsen RA, Pop AS, Gomezmancilla B, Nelson DL, Willemsen R, Gasparini F, Oostra BA. AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol Dis. 2011b;42:311–317. doi: 10.1016/j.nbd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol. 2010:1–13. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Grossman AW, Elisseou NM, Greenough WT. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am J Med Genet B Neuropsychiatr Genet. 2005;136:98–102. doi: 10.1002/ajmg.b.30183. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile × syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C. Which Comes First in Fragile X Syndrome, Dendritic Spine Dysgenesis or Defects in Circuit Plasticity? Neuroscientist. 2011 doi: 10.1177/1073858410395322. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera Cailliau C, Yuste R. [On the function of dendritic filopodia] Rev Neurol. 2001;33:1158–1166. [PubMed] [Google Scholar]
- Purpura DP. Dendritic spine "dysgenesis" and mental retardation. Science. 1974;186:1126–1128. doi: 10.1126/science.186.4169.1126. [DOI] [PubMed] [Google Scholar]
- Qin M, Xia Z, Huang T, Smith CB. Effects of chronic immobilization stress on anxietylike behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience. 2011;194:282–290. doi: 10.1016/j.neuroscience.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao BS, Desiraju T, Raju TR. Neuronal plasticity induced by self-stimulation rewarding experience in rats--a study on alteration in dendritic branching in pyramidal neurons of hippocampus and motor cortex. Brain Res. 1993;627:216–224. doi: 10.1016/0006-8993(93)90324-g. [DOI] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci U S A. 2005;102:11557–11562. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Metabotropic glutamate receptors and fragile × mental retardation protein: partners in translational regulation at the synapse. Sci Signal. 2008;1:pe6. doi: 10.1126/stke.15pe6. [DOI] [PubMed] [Google Scholar]
- Ruan YW, Lei Z, Fan Y, Zou B, Xu ZC. Diversity and fluctuation of spine morphology in CA1 pyramidal neurons after transient global ischemia. J Neurosci Res. 2009;87:61–68. doi: 10.1002/jnr.21816. [DOI] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol (Berl) 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Nissant A, Mota T, Neant-Fery M, Oostra BA, Greer CA, Lledo PM, Trembleau A, Caille I. Fragile X mental retardation protein regulates new neuron differentiation in the adult olfactory bulb. J Neurosci. 2011;31:2205–2215. doi: 10.1523/JNEUROSCI.5514-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Kreher U, Greenberger V, Braun K. Is fragile X mental retardation protein involved in activity-induced plasticity of dendritic spines? Brain Res. 2003;972:9–15. doi: 10.1016/s0006-8993(03)02410-7. [DOI] [PubMed] [Google Scholar]
- Su T, Fan HX, Jiang T, Sun WW, Den WY, Gao MM, Chen SQ, Zhao QH, Yi YH. Early continuous inhibition of group 1 mGlu signaling partially rescues dendritic spine abnormalities in the Fmr1 knockout mouse model for fragile X syndrome. Psychopharmacology (Berl) 2011;215:291–300. doi: 10.1007/s00213-010-2130-2. [DOI] [PubMed] [Google Scholar]
- Swanger SA. Automated 4D analysis of dendritic spine morphology: applications to stimulus-induced spine remodeling and pharmacoligcal rescue in a disease model. Molecular Brain. 2011;4:38–52. doi: 10.1186/1756-6606-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, Base CK, Greenough WT. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci U S A. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Dendritic Spines. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]