Abstract
This review describes the neuroendocrinological aspects of catamenial epilepsy, a menstrual cycle-related seizure disorder in women with epilepsy. Catamenial epilepsy is a multifaceted neuroendocrine condition in which seizures are clustered around specific points in the menstrual cycle, most often around perimenstrual or periovulatory period. Three types of catamenial seizures (perimenstrual, periovulatory and inadequate luteal) have been identified. The molecular pathophysiology of catamenial epilepsy remains unclear. Cyclical changes in the circulating levels of estrogens and progesterone (P) play a central role in the development of catamenial epilepsy. Endogenous neurosteroids such as allopregnanolone (AP) and allotetrahydrodeoxycorticosterone (THDOC) that modulate seizure susceptibility could play a critical role in catamenial epilepsy. In addition, plasticity in GABA-A receptor subunits could play a role in the enhanced seizure susceptibility in catamenial epilepsy. P-derived neurosteroids such as AP and THDOC potentiate synaptic GABA-A receptor function and also activate extrasynaptic GABA-A receptors in the hippocampus and thus may represent endogenous regulators of catamenial seizure susceptibility. Experimental studies have shown that neurosteroids confer greater seizure protection in animal models of catamenial epilepsy, especially without evident tolerance to their actions during chronic therapy. In the recently completed NIH-sponsored, placebo controlled Phase 3 clinical trial, P therapy proved to be beneficial only in women with perimenstrual catamenial epilepsy but not in non-catamenial subjects. Neurosteroid analogs with favorable profile may be useful in the treatment of catamenial epilepsy.
Keywords: Allopregnanolone, estradiol, neurosteroids, progesterone, catamenial epilepsy, GABA-A receptor
Introduction
Epilepsy is one of the most common chronic neurological disorders characterized by the unpredictable occurrence of seizures that differ in type, cause, and severity. However, seizures do not occur randomly in many women with epilepsy. Seizure clusters occur with a temporal periodicity following circadian or lunar periodicity. In women with epilepsy, seizure periodicity may conform to the menstrual cycle according to a “menstrual clock” provided by a common phase marker of the onset of menses (Gowers, 1881). Catamenial epilepsy, derived from the Greek word katomenios, meaning “monthly”, is characterized by seizures that cluster around specific points in the menstrual cycle (Fig.1). Catamenial epilepsy is a multifaceted neuroendocrine condition in which seizures are most often clustered around perimenstrual or periovulatory period. Epilepsy affects an estimated 1.3 million women in the United States (Kaplan et al., 2007; Pennell, 2008). Catamenial epilepsy affects from 10 – 70% of women with epilepsy (Herzog et al., 2004; Bazan et al., 2005; Gilad et al., 2008; Reddy, 2009). The large variation in prevalence of catamenial epilepsy is partly because of methodological differences such as the criteria used for defining seizure exacerbation in relation to menstrual cycle, patients’ self-reporting, diaries, and other records of seizures relating to menses. Overall, these studies support the prevailing notion that at least 1 in every 3 women with epilepsy show catamenial seizure exacerbation. Catamenial epilepsy is a form of intractable epilepsy because catamenial seizures are often quite resistant to available drug treatments. Presently, there is no effective prevention or cure for catamenial epilepsy. There is a large gap in our understanding of what changes occur in the brain in relation to the hormonal fluctuations associated with catamenial epilepsy and how these changes alter sensitivity to anticonvulsant drugs. Thus, a detailed understanding of the patterns and pathophysiology is essential for the development of rational approaches for the prevention or treatment of catamenial epilepsy.
Fig. 1. Temporal relationship between ovarian hormones and occurrence of catamenial seizures during the menstrual cycle.

The upper panel illustrates the strong relationship between seizure frequency and estradiol/P levels. The lower panel illustrates the three types of catamenial epilepsy. The vertical gray bars (left and right) represent the likely period for the perimenstrual (C1) type, while the vertical gray bar (middle) represents the likely period for the periovulatory (C2) type. The horizontal dark gray bar (bottom) represents the inadequate luteal (C3) type that likely occur starting early ovulatory to menstrual phases. In general, the female reproductive cycle is estimated to last 29 days. Day 1 is the onset of menstruation, and ovulation occurs 14 days before the onset of menstruation. The menstrual cycle is divided into four phases: (i) menstrual phase, days -3 to +3; (ii) follicular phase, days +4 to +9; (iii) ovulatory phase, days +10 to +16; and (iv) luteal phase, days +17 to -4. The early follicular phase is associated with low levels of estrogens and P. The synthesis and secretion of estrogens and P from the ovaries is controlled primarily by the hypothalamic GnRH and pituitary gonadotropins, FSH and LH. As ovulation approaches, the level of estrogen rises and triggers the release of a large surge of LH leading to ovulation. Following ovulation, the ruptured follicle luteinizes and forms a corpus luteum that secretes P and estrogen. Estradiol is secreted in the second half of the follicular phase and increases to a peak at midcycle, while P is elevated during the luteal phase and declines before menstruation begins. The neurosteroid AP is increased in parallel to its precursor, P (Reddy, 2009).
Three types of catamenial seizures, perimenstrual (C1), periovulatory (C2), and inadequate luteal-phase (C3), have been identified (Herzog et al., 1997) (Figure 1; Table 1). The perimenstrual type is the most common clinical type. Perimenstrual and periovulatory types are illustrated in Figure 1. The specific pattern of catamenial epilepsy can be identified simply by charting menses and seizures and obtaining a mid-luteal phase serum progesterone (P) level to distinguish between normal and inadequate luteal phase cycles (Herzog et al., 2008; Quigg et al., 2009). The diagnosis of catamenial epilepsy is mainly based on the assessment of menstruation and seizure records (Foldvary-Schaefer and Falcone, 2003; Herzog, 2006). The simple approach of evaluation of catamenial epilepsy, that is, whether the patient’s seizures tend to worsen at certain points of the menstrual cycle, is to record seizure diary in relation to menstrual cycle. Using the first day of menstrual bleeding as the first day of the cycle, the menstrual cycle is divided into four phases: (a) menstrual phase, days -3 to +3; (b) follicular phase, days +4 to +9; (c) ovulatory phase, days +10 to +16; and (d) luteal phase, days +17 to -4 (see Fig. 1). The number of seizures in each phase is counted for at least 2 cycles and a two-fold or greater increase in frequency during a particular phase of the menstrual cycle can be used as diagnostic criteria of catamenial epilepsy as demonstrated by a mathematical waveform (cosinor) analysis (Quigg et al., 2009).
Table 1.
Three patterns of catamenial epilepsy.
| Type | Characteristics |
|---|---|
| C1: Perimenstrual | Characterized by a greater average daily seizure frequency during the menstrual phase (day −3 to +3) compared with the midfollicular (day 4 to 9) and midluteal (day −12 to 14) phases in normal ovulatory cycles. Incidence: High |
| C2: Periovulatory | Characterized by a greater average daily seizure frequency during the ovulatory phase (day 10 to −13) compared with the midfollicular and midluteal phases in normal ovulatory cycles. Incidence: Medium |
| C3: Inadequate luteal-phase | Characterized by a greater seizure frequency during the ovulatory, luteal, and menstrual phases than during the midfolliclar phase in women with inadequate (anovulatory) luteal-phase cycles. Incidence: Low |
In the primary clinical type, perimenstrual catamenial epilepsy (C1), women with epilepsy experience an increase in seizure activity before, during or after the onset of menstruation (Reddy, 2009). Catamenial epilepsy is observed in women with ovulatory or anovulatory cycles. Women with ovulatory cycles could experience either the perimenstrual or periovulatory catamenial types or even both within a single cycle (Bauer et al., 1998; Bauer, 2001). The diagnosis of ovulatory or anovulatory cycles is often made by estimating the midluteal phase P levels. P levels lower than 5 ng/ml during days 20 through 22 of the cycle would certainly indicate inadequate luteal phase. Subjects can be examined by pelvic-abdominal ultrasound to measure size of mature graffian follicle as a sign of ovulation. About 16.5% of cycles in study subjects are found to be anovulatory (Herzog et al., 2004), and these women showed a third type, referred to as inadequate luteal-phase or anovulatory luteal seizures.
This review describes the neuroendocrinological aspects of catamenial epilepsy. It focuses on the role of hormones and GABA-A receptor-modulating neurosteroids in the pathophysiology of catamenial epilepsy. It also summarizes the promise of neurosteroid analogs as specific treatment for catamenial seizures in women with epilepsy.
Pathophysiology of catamenial epilepsy
The exact cause of catamenial epilepsy remains unclear. Catamenial epilepsy is among the oldest neurological disorders known with early reports in 1881 (Gowers, 1881), yet the molecular mechanisms involved in the pathophysiology of catamenial epilepsy are not well understood. Catamenial epilepsy is a multifaceted condition attributed to numerous causes. Epilepsy typically develops due to certain genetic defect or often after a presumed initiating injury. Catamenial epilepsy, in many cases, is assumed to be an acquired disorder and currently there is no clear evidence of genetic components. A variety of mechanisms such as fluctuations in antiepileptic drug (AED) levels, changes in water and electrolyte balance, and physiological variation in ovarian hormone secretion have been proposed as causes for catamenial epilepsy (see Reddy, 2009). Overall, cyclical changes in the circulating levels of estrogens and P are now widely accepted to play a central role in the development of this condition (Fig.1).
Catamenial seizures are more common among women with focal epilepsy, especially temporal lobe epilepsy, compared with those who have generalized epilepsy, but it is associated with every epilepsy syndrome (Marques-Assis, 1981; Morrel, 1999; Foldvary-Schaefer and Falcone, 2003). Catamenial seizures are seen in women with idiopathic, cryptogenic or symptomatic epilepsy and in subjects showing focal and generalized seizures (El-Khayat et al., 2008). Many women with catamenial epilepsy show cyclical increase in seizure exacerbations despite treatment with AEDs, and therefore, catamenial epilepsy can be considered as a form of intractable or pharmacoresistant epilepsy. Despite employing the best available drug treatment, many women do not respond or fail to exhibit resolution of catamenial seizures.
Changes in seizure activity in women can occur during changes in reproductive status (i.e. entering puberty, during pregnancy or after menopause). Although there is no overall consensus, puberty can affect the course of epilepsy. A significant increase in the incidence of generalized tonic-clonic seizures is observed in adolescents with epilepsy during puberty as compared with before puberty (Nijima and Wallace, 1989; Rosciszewka, 1987). Catamenial seizures can originate in women at puberty or in adolescent females with regular menstrual cycles. During puberty, the level of steroid hormones increases and menstrual period begins. Because steroid hormones influence seizure susceptibility, seizure types may change as females with epilepsy go through puberty. There is little information on the relationship between epilepsy and menopause. Natural reductions in steroid hormones around perimenopause and menopause are associated with alterations in frequency or severity of seizures in women with epilepsy (Abbasi et al., 1999; Harden et al., 1999; 2006).
Some AEDs are linked to the exacerbation of catamenial seizures. AED use is associated with changes in the serum levels of biologically active steroid hormones. AEDs can be divided into two groups, enzyme-inducing and non-enzyme-inducing AEDs (Table 2). AEDs such as phenytoin, carbamazepine and phenobarbital are potent inducers of liver cytochrome P450 isoforms (Rogawski and Loescher, 2004). The CYP3A4 and the other cytochrome P450 isoenzymes metabolize AEDs to a more water-soluble form, rendering them available for renal excretion. Because of common metabolic pathways, the AED-induced enzyme induction leads to enhanced metabolism of steroid hormones (Isojarvi et al., 2005) which may play a role in breakthrough seizures in women. Conversely, hepatic enzyme inhibitors like sodium valproate can increase the active steroid hormone levels. However, there is no direct clinical data available regarding the occurrence of catamenial seizures due to use of enzyme-inducing AEDs. In addition, the use of the enzyme-inducing AEDs phenobarbital, phenytoin and carbamazepine increases serum sex hormone-binding globulin concentrations in women with epilepsy, which may ultimately result in diminished concentrations of “free” or “biologically active” forms of steroid hormones. However, it remains to be determined to what extent such mechanisms contribute to catamenial epilepsy.
Table 2.
List of antiepileptic drugs (AEDs) that do and do not induce hepatic enzymes.
| Enzyme-inducing AEDs | Enzyme non-inducing AEDs |
|---|---|
| Carbamazepine (Tegretol) | Clonazepam (Rivotril) |
| Felbamate (Felbatol) | Ethosuximide (Zarontin) |
| Lamotrigine (Lamictal)* | Gabapentin (Neurontin) |
| Oxcarbazepin (Trileptal) | Levetiracetam (Keppra) |
| Phenobarbitone (Luminal) | Pregabalin (Lyrica) |
| Phenytoin (Dilantin) | Tiagabine (Gabitril) |
| Primidone (Mysoline) | Valproate (Depakote) |
| Topiramate (Topamax) | Vigabatrin (Sabril) |
| Zonisamide (Zonegran) |
weak enzyme inducer
Reproductive disorders that affect the normal ovarian cycle function are implicated in catamenial epilepsy. In women with epilepsy, both seizures and antiepileptic drugs can disturb the menstrual cycle (Morrell and Montouris, 2004). Seizures can profoundly affect steroid hormone secretion and regulation in women with epilepsy and are the leading cause of increased incidence of menstrual disturbances in epilepsy. For example, seizures can alter the release of hypothalamic and pituitary hormones such as LS/FSH secretion, while some antiepileptic drugs alter concentrations of sex steroid hormones. Sexual dysfunction is common in women with epilepsy. Other reproductive issues such as decreased fertility, libido, anovulatory cycles and early menopause are also evident in women with epilepsy. Such hormonal issues in the comorbidities of epilepsy are recently reviewed (Pack et al., 2011). From a neuroendocrine perspective, stress of chronic illness and psychosocial stigma may lead to hypothalamo-pituitary-adrenal hypersecretion which is generally accompanied by suppression of gonadal secretion. In women with epilepsy, menstrual disorders and infertility appear to be more common than in the general population and are often related to reproductive endocrine disorders such as polycystic ovary syndrome. The increased occurrence of this syndrome among women with epilepsy may be due to altered temporolimbic modulation of the hypothalamo-pituitary-ovarian axis.
There is no evidence that the use of oral contraceptives (OCs) increase the risk of seizures in women with epilepsy (Reddy, 2010). However, there are many factors to consider in the choice of AED therapy and hormonal contraception since some AEDs can reduce the efficacy of OCs due to pharmacokinetic interactions (Crawford, 2005; Harden and Leppik, 2006). The enzyme-inducing AEDs cause enhanced metabolism of either or both the estrogenic or progestogenic component of OCs, thereby reducing their efficacy in preventing pregnancy. Moreover, there is evidence that OCs can also decrease the concentrations of AEDs such as lamotrigine and thereby increase the risk of seizures (Sabers et al., 2003; Sabers and Gram, 2006).
Role of hormones and neurosteroids in the pathophysiology of catamenial epilepsy
Steroid hormones play a key role in the neuroendocrine control of neuronal excitability and seizure susceptibility. As illustrated in Figure 1, cyclical changes of ovarian hormone estrogens and P are now widely believed to be important in the pathogenesis of catamenial epilepsy. Generally, estrogens are found to be proconvulsant, while P has powerful antiseizure effect and reduces seizures, and thus they play a central role in the pathophysiology of catamenial epilepsy (Reddy, 2009). There is emerging evidence that endogenous neurosteroids influence seizure susceptibility in epilepsy (Reddy, 2011).
Estrogens
The role of estrogens in seizure susceptibility is complex. In general, estrogens have proconvulsant and epileptogenic properties in animals and humans. There are also studies that support protective effects of estrogens, and it may also be anticonvulsant under some circumstances. Estradiol has been widely investigated in animal epilepsy models. However, the effect of estrogens on seizure susceptibility is highly variable and depends on factors such as treatment duration, dosage, hormonal status and the seizure model (Veliskova, 2007). Early studies of estradiol administration to ovariectomized rats revealed proconvulsant effects (see Reddy, 2009). The effect of estrogens on hippocampus seizure susceptibility is controversial. While estradiol has been shown to be proconvulsant in several studies, there is also evidence that support lack of effect or protective effect of estrogens (Reibel et al., 2000; Veliskova et al., 2000; Veliskova and Velisek, 2007). In low doses, estradiol can produce neuroprotective effects (Velísek and Velísková, 2008).
Estradiol has been known to play a role in the exacerbation of seizures in women with epilepsy (Logothetis et al., 1959; Backstrom, 1976; Jacono and Robinson, 1987). Plasma estradiol levels are found to increase during both the follicular and luteal phase of the normal menstrual cycle (Fig.1). Backstrom (1976) was the first investigator to characterize the relationship between seizures and steroid hormones. In women with epilepsy, a positive correlation between seizure susceptibility and the estrogen-to-P ratio was observed, peaking in the premenstrual and preovulatory periods and declining during the midluteal phase. Logothetis and colleagues (1959) have demonstrated that intravenous infusions of estrogen were associated with rapid interictal epileptiform activity in women with epilepsy and seizures were exacerbated when estrogen was given premenstrually. Therefore, it is hypothesized that estrogens may facilitate some forms of catamenial seizures observed during these phases. The periovulatory catamenial exacerbation has been attributed to the midcycle surge of estrogen that is relatively unopposed by P until early luteal phase (Logothesis et al., 1959). An increase in the ratio of estrogen-to-P levels during perimenstrual period (described below) might at least partly contribute to the development of perimenstrual seizure exacerbation (Bonuccelli et al., 1989; Herzog et al., 1997). Nevertheless, the exact relationship between circulating estrogens and the perimenstrual or anovulatory catamenial seizures remains unclear.
Progesterone
P plays a key role in catamenial epilepsy. P has consistent anticonvulsant and antiepileptic properties in animals and humans. P has long been known to have antiseizure activity in a variety of animal models of epilepsy (Craig, 1966; Landgren et al., 1978). In recent years, numerous studies have confirmed the powerful anticonvulsant activity of P in diverse animal seizure models (see Reddy, 2009). There are two mechanisms by which P affects reproduction and seizure susceptibility: binding to P receptors (PRs) and being metabolized to the neurosteroid allopregnanolone (AP) (Fig.2). In P-responsive target cells, P binds to cytoplasmic PRs and the hormone-nuclear receptor complexes translocate to the cell nucleus where they activate or silence the transcription of downstream gene networks, thus affecting the physiological response of the target cell. There is strong evidence that the antiseizure effects of P are not related to interactions with classical PRs, because antiseizure activity of P was undiminished in PR knockout mice (Reddy et al., 2004). Further studies established that 5α-reduced metabolites of P, including 5α-reduced neurosteroids, are responsible for the seizure protection conferred by P. Thus, seizure susceptibility is very low during physiological conditions associated with high P.
Fig. 2. Biosynthesis of neurosteroids AP and THDOC in the brain.
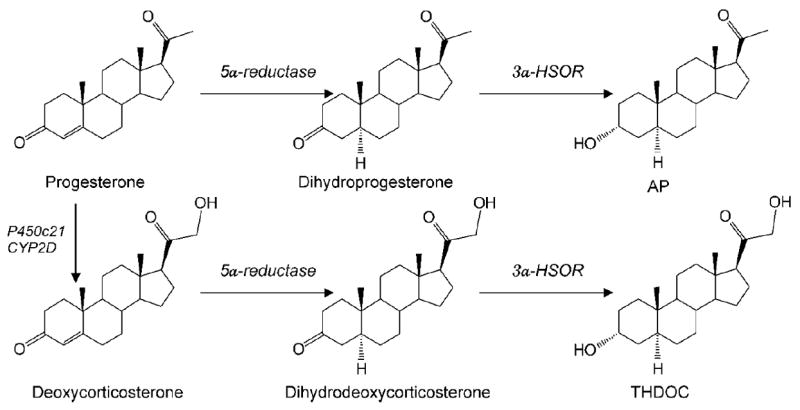
Enzymatic pathways for the production of two prototype neurosteroids AP and THDOC is illustrated from steroid precursors. P and deoxycorticosterone undergo two sequential A-ring reduction steps catalyzed by 5α-reductase and 3α-HSOR to form the 5α, 3α-reduced neurosteroids. The conversion of P or deoxycorticosterone into neurosteroids occurs in several regions within the brain. The 5α-reductase, 3α-hydroxysteroid oxidoreductase (3α-HSOR), cytochrome P450 21-hydroxylase (P450c21) and other enzymes are present in the brain.
Recent studies in our lab confirm the antiepileptogenic effects of P in the kindling model of epileptogenesis (Reddy et al., 2010; Reddy and Mohan, 2011). Epileptogenesis is a process whereby a normal brain becomes progressively epileptic due to precipitating risk factors. Preclinical and clinical evidence suggest that P interrupts epileptogenic events. It is hypothesized that 5α-reductase converts P to AP and related neurosteroids that retards epileptogenesis in the brain. Using finasteride, a 5α-reductase and neurosteroid synthesis inhibitor, we showed that neurosteroids such as AP may mediate the disease-modifying or antiepileptogenic effect of P (Reddy and Mohan, 2011), indicating the potential of P to protect against epileptogenic factors.
In women with epilepsy, natural cyclic variations in P during the menstrual cycle could influence catamenial seizure susceptibility (Fig.1). Seizures decrease in the mid-luteal phase when serum P levels are high and increase premenstrually when P levels fall and there is a decrease in the serum P-to-estrogen ratio (Backstrom, 1976; Bonucelli et al., 1989; Herzog et al., 2001). Changes in P levels have been directly correlated with catamenial seizures (Tuveri et al., 2008; El-Khayat et al., 2008). Despite some limitations, these findings provide evidence that disruption in ovarian cycle-related fluctuations in P can be correlated to catamenial seizure exacerbation. The emerging evidence clearly indicates that perimenstrual catamenial seizures are associated with a rapid decline in P around menstruation.
In clinical studies P has been found to reduce seizures (Backstrom et al., 1984; Herzog, 2009). Previous open-label studies suggest that the cyclic administration of adjunctive natural P supplement may lessen seizure frequency by over 50% in the majority of women with catamenially-exacerbated, intractable seizures (Herzog, 1995; 1999). Oral synthetic progestins, in contrast, have not shown significant efficacy. P is not widely prescribed because its benefits have yet to be conclusively demonstrated.
In a recently completed, NIH-sponsored Phase 3 trial, P’s efficacy was evaluated in women with epilepsy (Herzog et al., 2012). In this randomized, placebo-controlled, double-blind, multicenter clinical trial, Herzog and colleagues assessed the short term efficacy and safety of adjunctive cyclic natural P therapy in the treatment of intractable seizures in 462 women with partial epilepsy. There was no significant difference in the proportions of responders for all seizures combined between P and placebo in women with catamenial and noncatamenial epilepsy. This study provides Class III evidence that cyclic P is ineffective in women with intractable partial epilepsy. However, the prespecified exploratory findings suggest that the level of perimenstrual seizure exacerbation is a significant predictor of the responder rate with P therapy. Therefore, P therapy may provide a clinically significant benefit for many women with perimenstrual catamenial epilepsy.
Neurosteroids
Neurosteroids are steroids synthesized within the brain with unconventional, rapid effects on neuronal excitability (Table 3). Neurosteroids and their precursor steroid hormones play an important role in the neuronal excitability, seizure susceptibility, and pathophysiology of neurological conditions such as catamenial epilepsy. The term neurosteroid was coined in 1981 by the French endocrinologist Étienne-Émile Baulieu to refer to steroids that are synthesized de novo in the nervous system from cholesterol independent of the peripheral steroidogenic endocrine glands (Baulieu, 1981). It has been known since the 1940s, from the pioneering work of Hans Selye, that naturally occurring steroids such as the ovarian steroid P and the adrenal steroid deoxycorticosterone (DOC) can exert anesthetic and anticonvulsant actions (Selye, 1941). In the early 1980s, the synthetic steroid alphaxolone was found to enhance synaptic inhibition via an action on GABA-A receptors in the brain (Harrison and Simmonds, 1984). A major advance occurred when 5α-reduced metabolites of P and DOC were also found to enhance GABAA receptor function (Majewska et al., 1986). It was speculated that the anesthetic and anticonvulsant properties of P and DOC were due to their conversion to AP and THDOC, respectively (Reddy, 2011). The androgenic neurosteroid androstanediol (5α-androstan-3α,17β-diol) is synthesized from testosterone (Reddy and Jian, 2010). There is now compelling evidence that all of the enzymes required for the biosynthesis of the neurosteroids from cholesterol are present in the brain. Since neurosteroids are highly lipophilic and can readily cross the blood-brain barrier, neurosteroids synthesized in peripheral tissues accumulate in the brain. For the purpose of this revew, we limit our discussion mainly to AP, THDOC and structurally related neurosteroids (Fig.2).
Table 3.
Summary of steroid hormone modulation of seizure susceptibility.
| Hormone | Actions | Potential Mechanisms of action |
|---|---|---|
| Estrogens | Proconvulsant | Hippocampal dendritic spine density |
| Enhanced NMDA receptor function | ||
| Progesterone | Anticonvulsant | Neurosteroid AP precursor |
| Potentiation of GABA-A receptor function | ||
| Androgens | Pro- and anticonvulsant | Estrogen precursor (proconvulsant) |
| Androstanediol precursor (anticonvulsant) | ||
| Deoxycorticosterone | Anticonvulsant | Neurosteroid THDOC precursor |
| Potentiation of GABA-A receptor function | ||
| Cortisol | Proconvulsant | Corticosteroid receptors and plasticity |
| AP | Anticonvulsant | Potentiation of GABA-A receptor function |
| THDOC | Anticonvulsant | Potentiation of GABA-A receptor function |
| Androstanediol | Anticonvulsant | Potentiation of GABA-A receptor function |
| Pregnenolone sulfate | Proconvulsant | Inhibitiion of GABA-A receptor function |
| Potentiaton of NMDA receptor function | ||
| DHEA sulfate | Proconvulsant | Inhibitiion of GABA-A receptor function |
| Potentiaton of NMDA receptor function |
Recent evidence indicates that neurosteroids are present mainly in principal neurons in many brain regions that are relevant to focal epilepsies, including the hippocampus and neocortex (Agís-Balboa et al., 2006; Saalmann et al., 2007; Do Rego et al., 2009). The highly restricted distribution of neurosteroids to excitatory neurons suggests that they are mainly derived from local synthesis, although it is clear that peripheral neurosteroids do easily cross the blood–brain barrier. The biosynthesis of neurosteroids is controlled by the translocator protein (18kDa; TSPO), formerly called peripheral or mitochondrial benzodiazepine receptor (Rupprecht et al., 2009, 2010). Activation of TSPO by endogenous signals and ligands facilitates the intramitochondrial flux of cholesterol and thereby promotes neurosteroid synthesis. Neurosteroids are localized to the neurons that contain their target receptors, which is consistent with the concept that neurosteroids function in an autocrine fashion in which they reach their targets by lateral membrane diffusion. However, the factors that regulate local neurosteroid synthesis are unclear.
Neurosteroids rapidly alter neuronal excitability through direct interaction with GABA-A receptors (Harrison et al., 1984; 1987; Majewska et al., 1986; Gee et al., 1988; Hosie et al., 2007; 2009), which are the major receptors for the inhibitory neurotransmitter GABA. Activation of the GABA-A receptor by various ligands leads to an influx of chloride ions and to a hyperpolarization of the membrane that dampens the excitability. AP and other structurally-related neurosteroids act as positive allosteric modulators and direct activators of GABA-A receptors (Figure 3). At low concentrations, neurosteroids potentiate GABA-A receptor currents, whereas at higher concentrations, they directly activate the receptor (Harrison et al., 1987; Reddy and Rogawski, 2002). Unlike benzodiazepines or barbiturates, neurosteroid enhancement of GABA-A receptors occurs, in a hybrid fashion, through increases in both the channel open frequency and channel open duration (Twyman and Macdonald, 1992; Lambert et al., 2009; Ramakrishnan and Hess, 2010).
Fig. 3. Neurosteroid modulation of GABA-A receptors.

AP and related neurosteroids binds and potentiate the GABA-A receptor function leading protective effects against seizures. GABA-A receptors are believed to be pentameric with five protein subunits that form the chloride ion channel pore. The AP and THDOC binding site is thought to be at the “neurosteroid binding site”, which is distinct from sites for GABA, benzodiazepines and barbiturates. Postsynaptic GABA-A receptors, which are pentameric chloride channels composed of 2α2βγ subunits, mediate the phasic portion of GABAergic inhibition, while extrasynaptic GABA-A receptors, pentamers composed of 2α2βδ subunits, primarily contribute to tonic inhibition in the hippocampus. Neurosteroids activate both synaptic and extrasynaptic receptors and enhance the phasic and tonic inhibition. Therefore, they may promote maximal protection against seizure susceptibility.
The GABA-A receptor is a pentamer consisting of five subunits that form a chloride channel. Sixteen subunits (α1-6, β1-3, γ1-3, δ,ε,θ, and π subunits) have been identified so far. The GABA site is located at the interface between α and β subunits. Benzodiazepines bind at the interface between α and γ subunits and they interact with subunit combinations α1,2,3,5β2γ2. The effect of neurosteroids on GABA-A receptors occurs by binding to discrete sites on the receptor-channel complex that are located within the transmembrane domains of the α- and β-subunits (Hosie et al., 2006; 2007), which they access by lateral membrane diffusion (Chisari et al., 2009; 2010). The binding sites for neurosteroids are distinct from the recognition sites for GABA, benzodiazepines, and barbiturates (Hosie et al., 2009; Reddy and Jian, 2010). The GABA-A receptor mediates two types of GABAergic inhibition, now stratified into synaptic (phasic) or extrasynaptic (tonic) inhibition. Although GABA activates synaptic (γ2-containing) GABA-A receptors with high efficacy, GABA activation of the extrasynaptic (δ-containing) GABA-A receptors are limited to low-efficacy activity characterized by minimal desensitization and brief openings. Although neurosteroids act on all GABA-A-receptor isoforms, they have large effects on extrasynaptic δ-subunit containing GABA-A receptors that mediate tonic currents (Wohlfarth et al., 2002; Belelli et al., 2002). Consequently, GABA-A receptors that contain the δ-subunit are highly sensitive to neurosteroid potentiation (Mihalek et al., 1999; Spigelman et al., 2002). Tonic current causes a steady inhibition of neurons and reduces their excitability. Neurosteroids therefore could play a role in setting the level of excitability by potentiation of tonic inhibition (Stell et al., 2003).
AP-like neurosteroids are powerful anticonvulsants (Reddy, 2011). Neurosteroids protect against seizures induced by GABA-A receptor antagonists, including pentylenetetrazol and bicuculline, and are effective against pilocarpine-induced limbic seizures and seizures in kindled animals (Table 4). Neurosteroids are inactive or only weakly active against seizures elicited by maximal electroshock. In addition, neurosteroids are also highly effective in suppressing seizures due to withdrawal of GABA-A receptor modulators including neurosteroids and benzodiazepines, as well as other types of agents such as ethanol and cocaine (Devaud et al., 1996; Reddy and Rogawski, 2001). In contrast to benzodiazepines, where utility in the chronic treatment of epilepsy is limited by tolerance, anticonvulsant tolerance is not evident with neurosteroids (Kokate et al., 1998; Reddy and Rogawski, 2000a). Thus, neurosteroids have the potential to be used in the chronic treatment of epilepsy. Unlike benzodiazepines, neurosteroids are able to modulate all isoforms of GABA-A receptors, including those that contain benzodiazepine-insensitive α4 and α6 subunits or lack the obligatory γ2 subunit required for benzodiazepine-sensitivity. Thus, it is clear that neurosteroids can act on GABA-A receptors where the proposed benzodiazepine tolerance mechanisms have been invoked by chronic GABAergic drug therapy or other endogenous conditions. Surprisingly, while chronic neurosteroid exposure does not lead to anticonvulsant tolerance, neurosteroid exposure does lead to tolerance for benzodiazepines (Reddy and Rogawski, 2000a). Thus, it appears that the same plastic changes that underlie benzodiazepine tolerance are brought into play by chronic neurosteroid exposure. However, neurosteroids acting at distinct sites on GABA-A receptors and exhibiting effects on the full range of GABA-A receptor isoforms, do not exhibit anticonvulsant tolerance. In addition to anticonvulsant activity, there is emerging evidence that endogenous neurosteroids play a role in regulating epileptogenesis (Biagini et al., 2006; 2009; Reddy et al., 2010). Exogenous treatment with neurosteroids or P has also been reported to delay the occurrence of epileptogenesis. Indeed, P has been shown to retard epileptogenesis in kindling models, even at doses that do not affect seizure expression (Reddy et al., 2010). Overall, neurosteroids are more robust anticonvulsants than benzodiazepines in various animal models.
Table 4.
Antiseizure potency of three prototype neurosteroids in animal seizure models.
| Seizure Model | AP | THDOC | Androstanediol |
|---|---|---|---|
| Kindling models: | |||
| Amygdala kindling | 14 (8–23) | 15 (10–30) | ND |
| Hippocampus kindling | 3.5 | ND | 50 (36-64) |
| Electroshock models: | |||
| Maximal electroshock | 29 (19–44) | 48 (35–66) | ND |
| 6-Hz stimulation | 14 (10–19) | ND | ND |
| Chemoconvulsant models: | |||
| Pentylenetetrazol | 12 (10–15) | 19 (77–122) | 40 (27–60) |
| Bicuculline | 12 (10–15) | 12 (10–15) | 44 (24–81) |
| Picrotoxin | 10 (5–19) | 10 (5–19) | 39 (21–74) |
| N-methyl-D-aspartate | >40** | >40** | >200** |
| 4-Aminopyridine | >40** | >40** | >200** |
| Status epilepticus models: | |||
| Pilocarpine | 7 (4–13) | 7 (4–13) | 81 (45–133) |
| Kainic acid | >40** | >40** | >200** |
The potency of neurosteroids is expressed in terms of ED50, which is the dose in mg/kg producing seizure protection in 50% of animals. Values in parentheses are 95% confidence limits. ND, not determined.
Considered as inactive because of such high (sedative or anesthetic) doses.
Endogenous neurosteroids may play a role in catamenial seizure susceptibility in women with epilepsy. When neurosteroid levels fluctuate, loss of seizure control can occur. Neurosteroids have been implicated in perimenstrual seizure exacerbations in women with normal menstrual cycle. It is hypothesized that withdrawal of P-derived neurosteroids leads to enhanced excitability predisposing to seizures (Reddy, 2009). In addition, plasticity in GABA-A receptor subunits could play a role in the enhanced seizure susceptibility in perimenstrual catamenial epilepsy. Animal studies have shown that prolonged exposure to AP followed by withdrawal such as that occurs during menstruation causes a marked increase in expression of α4-subunit, a key subunit linked to enhanced neuronal excitability, seizure susceptibility and benzodiazepine resistance (Smith et al., 2007; Gangisetty and Reddy, 2010). These neuroendocrine changes can result in reduced inhibition resulting in enhanced excitability, which, among other effects, predisposes to seizures.
The overall potential mechanisms of catamenial seizure patterns are summarized in Table 5 (Reddy, 2009). Perimenstrual type (C1) occurs in women with ovulatory cycle possibly due to a sharp decline (“withdrawal”) in the serum level of P and, consequently, of the level of P-derived anticonvulsant neurosteroids in the brain around the perimenstrual period. The estradiol/neurosteroid ratio is highest during menstruation. Because neurosteroid potentiates GABA-A receptor-mediated inhibition, the rapid loss of neurosteroid-mediated inhibition, such as that occur before, during or after the onset of menses, could exacerbate seizures in many women with catamenial epilepsy. Periovulatory type (C2) occurs in women with ovulatory cycle possibly due to estradiol surge just before ovulation, and low neurosteroid levels do not offset the estradiol-induced excitation because the rise of anticonvulsant neurosteroid levels would not occur until after ovulation. The relatively low neurosteroid inhibition and marked estradiol excitation could lead to periovulatory seizures. Inadequate luteal-type (C3) occurs in women with anovulatory cycles possibly due to a loss of neurosteroid-mediated inhibition during luteal phase for a prolonged time and also due to elevated estrogen levels. P secretion that occurs normally during the luteal phase is markedly decreased during anovulatory cycle resulting in abnormally low levels of neurosteroid in the brain.
Table 5.
Summary of potential neuroendocrine mechanisms of catamenial epilepsy.
| Type | Hormonal changes | Neuronal mechanism | Seizure susceptibility |
|---|---|---|---|
| C1 Perimenstrual | Very low neurosteroids (withdrawal) | Decreased GABAergic inhibition | Increased |
| Low estradiol | |||
| C2 Periovulatory | High estradiol | Increased excitation | Increased |
| Low neurosteroids | Decreased GABAergic inhibition | ||
| C3 Inadequate luteal | Very low neurosteroids | Decrease in GABAergic inhibition | Increased |
| Moderate estradiol | Persistent excitation |
Animal models of catamenial epilepsy: implication for understanding the molecular mechanisms
Conventional seizure models, which are largely based on the utilization of acutely induced seizures in naive animals, are not suitable because they do not allow testing of specific therapies that are targeted to catamenial epilepsy. These models are clearly different from such models as kindling, pilocarpine or chronic kainic acid that induce severe damage and remodeling response in the brain and thereby result in secondary seizures. Generally, animal models of catamenial epilepsy could be designed specifically to simulate the menstrual cycle and ovarian hormone-related changes in seizure susceptibility. During the luteal phase of the menstrual cycle, circulating levels of P are increased for 10 to 12 days before declining (withdrawal) to low levels. Recently, three types of models have been described in animals that partially resemble catamenial seizure patterns. In the first category of models, attempts are made to mimic the luteal phase by inducing extended high levels of P and estrogens followed by rapid decline to simulate the menstruation in normal rodents. These include pseudopregnancy, chronic P, and P (neurosteroid) withdrawal models (Smith et al., 1998; Moran and Smith, 1998; Reddy et al., 2001). The second category of models is based on the naturally occurring estrous cycle or administration of exogenous hormones that simulate the specific stages of estrous cycle in ovariectomized rats (Frye et al., 1998; Frye and Bayon, 1998). These physiological models better mimic the normal ovarian cycle. In the third category of models, epilepsy animals are exposed to steroid hormones and neurosteroid withdrawal conditions, and the frequency and severity of spontaneous or evoked seizures are utilized as indices of catamenial-like seizure exacerbation (Reddy and Zeng, 2007; Reddy et al., 2012).
The pseudopregnancy model
We developed a rodent model of perimenstrual catamenial epilepsy (Reddy et al., 2001; Reddy and Rogawski, 2001). Rodents have a 4 to 5 day estrous cycle and studies of fluctuations in seizure susceptibility in cycling female rodents have not led to results that are relevant to the human menstrual cycle. In order to provide a model that more closely mimics the human situation, a condition of elevated P was created in rats by gonadotropin treatment. This resulted in prolonged high circulating levels of estrogen and P similar to those that occur in the luteal phase of the menstrual cycle. Then, to simulate the withdrawal of AP that occurs at the time of menstruation, the animals were treated with finasteride (a 5α-reductase and neurosteroid synthesis inhibitor) 11 days after the initiation of gonadotropin treatment. Withdrawal of neurosteroids had led to decreased seizure threshold and increased seizure activity (Reddy et al., 2001), suggesting that endogenous neurosteroids do modulate seizure susceptibility.
The neurosteroid withdrawal model of catamenial epilepsy was used to investigate therapies for perimenstrual catamenial epilepsy (Reddy and Rogawski, 2000b; 2001). A key result is that conventional AEDs, including benzodiazepines and valproate, are less potent in protecting against seizures during the period of enhanced seizure susceptibility following neurosteroid withdrawal. This pharmacoresistance appears to mimic the situation in women with catamenial epilepsy where breakthrough seizures occur despite treatment with antiepileptic drugs. In contrast to the results with conventional antiepileptic drugs, neurosteroids, including AP, THDOC and their 5β-isomers, were found to have enhanced activity in the catamenial epilepsy model (Reddy and Rogawski, 2001). This suggested a “neurosteroid replacement” approach to treat catamenial seizure exacerbations (Reddy and Rogawski, 2009). A neurosteroid could be administered in a “pulse” prior to menstruation and then withdrawn, or continuously administered throughout the month. The neurosteroid would be administered at low doses to avoid sedative side effects. Such low doses are expected to contribute little anticonvulsant activity during most of the menstrual cycle, but may prevent the occurrence of perimenstrual catamenial seizures.
The exogenous P model
There are models in which P was administered for extended periods using silastic implants or multiple daily injections (Costa et al., 1995; Smith et al., 1998; Moran and Smith, 1998). These models mimic the high P levels found during luteal phase of the menstrual cycle. Withdrawal of P containing implants or cessation of chronic P treatment induces an abrupt decline of P (neurosteroids) levels that could simulate the hormonal milieu of menstruation. Akin to finasteride-induced neurosteroid withdrawal, withdrawal of P has been associated with marked decrease in seizure threshold (Smith et al., 1998; Moran and Smith, 1998). The model has limitations because of intermittent, supraphysiological levels of neurosteroids achieved with exogenous P treatment.
The natural estrous cycle model
The estrous cycle models have been used to demonstrate that the structure of extrasynaptic GABA-A receptor undergoes drastic alterations due to changing levels of P during the ovarian cycle (Maguire et al., 2005; Reddy et al., 2011). During the late diestrous phase (associated with high P levels), expression of the δ-containing GABA-A receptors was elevated, which was associated with an increase in tonic inhibition and diminished seizure susceptibility in mice. During the estrous phase (associated with low P levels), tonic inhibition was reduced by 50% with corresponding increases in both seizure susceptibility and anxiety behavior in mice. These cyclic alterations in the δ-subunit are also observed following exogenous P treatment in ovariectomized female mice (Maguire and Mody, 2007). Unlike the phasic inhibition mediated by the γ-containing GABA-A receptors, the δ-containing GABA-A receptors are highly sensitive to neurosteroids (Mihalek et al., 1999; Stell et al., 2003). Susceptibility to epileptogenesis is lower at diestrous that estrous stage (Reddy et al., 2011). These findings are consistent with the possibility that the abundance of extrasynaptic, δ-containing GABA-A receptors is increased during diestrous, likely due to elevated neurosteroids, and thereby contribute to AP-sensitive GABAergic currents in the hippocampus, a key region for the pathophysiology of epilepsy (Fig.4). It is suggested that deficiencies in regulatory mechanisms controlling normal cycling of the δ-subunit-containing GABA-A receptors in the hippocampus could be a potential molecular mechanism for catamenial seizures. Thus, the δ-containing GABA-A receptor is an important target for developing specific treatments for catamenial epilepsy.
Fig. 4. A proposed model of ovarian-cycle related changes in the plasticity and function of synaptic and extrasynaptic GABA-A receptors in the hippocampus.
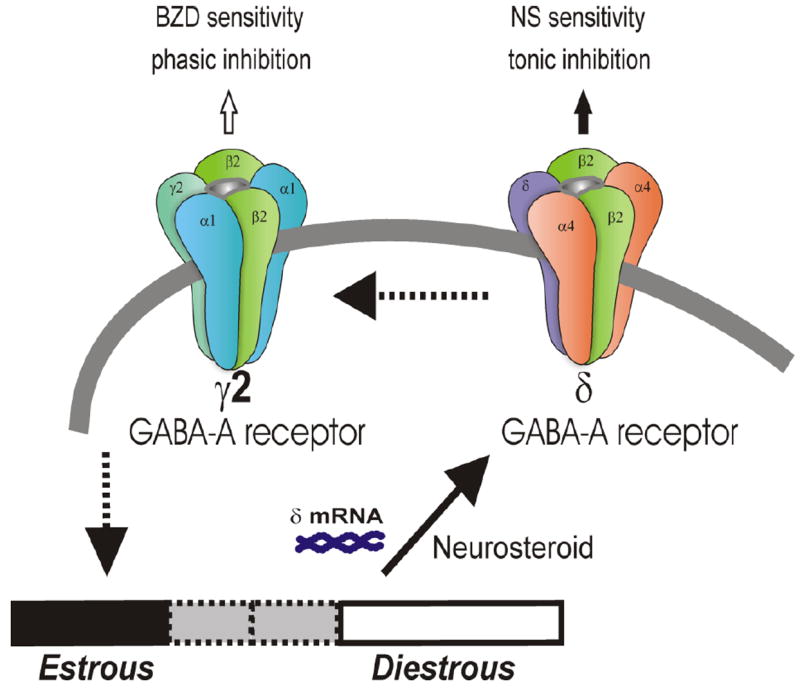
Postsynaptic GABA-A receptors, which are pentameric chloride channels composed of 2α2βγ subunits, mediate the phasic portion of GABAergic inhibition, while extrasynaptic GABA-A receptors, pentamers composed of 2α42βδ subunits, primarily contribute to tonic inhibition in the hippocampus. The abundance of δ-subunit-containing GABA-A receptors is elevated at diestrous (high P) than estrous (low P) stage. Such plasticity promotes greater tonic inhibition in the hippocampus and thereby provides resistance against seizure susceptibility (Reddy et al., 2011).
The spontaneous model in epilepsy animals
Previous models have utilized non-epileptic animals to study catamenial seizures. However, epilepsy animals are needed to model the catamenial seizure exacerbations. Currently available methods of epilepsy induction lead to severe reproductive dysfunction in female rats (Amado and Cavalheivo, 1998; Edwards et al., 1999). The pilocarpine model is widely used for inducing SE, followed by chronic spontaneous seizures after a latent period. The modified lithium-pilocarpine model of chronic epilepsy in rats may provide a stable epileptic condition and maintains reproductive function (Reddy et al., 2007). We devised the neurosteroid-withdrawal model in rats with spontaneous seizures based on the hypothesis that prolonged exposure followed by withdrawal of P, and therefore the neurosteroid AP, mimics the hormonal state associated with heightened vulnerability to seizures (Reddy and Zeng, 2007). In this new model, female rats with chronic epilepsy are subjected to neurosteroid withdrawal paradigm exhibited a significant (two-fold) increase in seizure frequency. These findings were confirmed in an independent study (Lawrence et al., 2010). An improvement in estrous cyclicity has also been reported in a rat model of epilepsy (Scharfman et al., 2009). In women with epilepsy, finasteride therapy had led to an increase in seizure frequency and severity (Herzog and Frye, 2003), indicating the role of neurosteroids in controlling seizure susceptibility.
The evoked model in kindled animals
Recently we developed and characterized a mouse model of catamenial epilepsy using the neurosteroid withdrawal paradigm (Gangisetty and Reddy, 2010; Reddy et al., 2012). A chronic seizure condition was created using the hippocampus kindling model in female mice. Elevated neurosteroid levels were induced by sequential gonadotropin treatment and withdrawal was induced by the neurosteroid synthesis inhibitor finasteride. Fully-kindled mice subjected to neurosteroid withdrawal showed increased generalized seizure frequency and intensity and enhanced seizure susceptibility. They also showed reduced benzodiazepine sensitivity and enhanced neurosteroid potency, similar to the clinical catamenial seizure phenotype. The increased susceptibility to seizures and alterations in antiseizure drug responses are associated with increased abundance of the α4- and δ-subunits of GABAA receptors in the hippocampus (Reddy et al., 2012). However, the signaling mechanisms underlying the upregulation of α4-subunit expression remain unclear. The role of PRs and the transcription factor early growth response factor-3 (Egr3) in regulation of the GABA-A receptor α4-subunit expression in the hippocampus was investigated in a mouse neurosteroid withdrawal paradigm (Gangisetty and Reddy, 2010). Neurosteroid withdrawal induced a threefold increase in α4-subunit expression in WT mice, but this upregulation was undiminished in PR knockout mice. The expression of the transcription factor early growth response factor-3 (Egr3), which controls α4-subunit transcription, was increased significantly following neurosteroid withdrawal in WT and PR knockout mice. Neurosteroid withdrawal-induced α4-subunit upregulation was completely suppressed by antisense Egr3 inhibition. These results support that neurosteroid withdrawal-induced upregulation of GABA-A receptor α4-subunit expression is mediated by the Egr3 via a PR-independent signaling pathway.
Hormonal and neurosteroid-based treatment of catamenial epilepsy
Presently there is no specific, FDA-approved drug treatment for catamenial epilepsy. The conventional AEDs are the mainstay for the management of catamenial seizures in women. Approximately one third of women with epilepsy use more than one AED appropriate to their seizure type. This is partly because catamenial seizures are often refractory to conventional AEDs. Many of these drugs are prescribed for treatment of catamenial epilepsy without direct studies of effectiveness, with their use based primarily on empirical evidence (Guille et al., 2008). Treatment of epilepsy in women must consider several important issues such as pharmacokinetics of AEDs, drug interactions, and contraceptive use (Crawford, 2005; Reddy, 2010). Some AEDs can reduce the levels of ovarian hormones due to pharmacokinetic interactions. Estrogens and progestogens are metabolized by CYP34A. AEDs such as phenytoin, phenobarbital, carbamazepine, felbamate, topiramate, oxcarbazepine, and primidone induce CYP3A4 leading to enhanced metabolism of either or both the estrogen and P, thereby influencing the dynamic equilibrium of ovarian hormones during the menstrual cycle (McAuley and Anderson, 2002). AEDs such as gabapentin, levetiracetam, tiagabine, zonisamide and pregabalin do not cause enzyme induction (Table 2), and hence do not cause pharmacokinetic interactions with steroid hormones. Oral contraceptives also pose risk because they can decrease the concentrations of AEDs such as lamotrigine (Sabers et al., 2003) and thereby increase the risk of seizures.
Table 6 lists an overview of various drugs investigated for the treatment of catamenial epilepsy. Many patients received these agents as supplements or adjunct drugs in a continuous or intermittent approach for inhibition of catamenial seizures (Zimmerman et al., 1973; Herzog, 1999). While these agents may be helpful for the treatment of catamenial seizures, each is based on small, unblinded studies or anecdotal reports except P, which was evaluated in Phase 3 trial.
Table 6.
List of hormonal, non-hormonal and neurosteroid-based agents tested in catamenial epilepsy.
| Drug | Mechanism | Efficacy | Limitations |
|---|---|---|---|
| Hormonal agents: | |||
| Medroxyprogesterone acetate | P analogue | Mixed | Reproductive dysfunction |
| Clomiphene | Estrogen receptor antagonist | Moderate | Reproductive dysfunction |
| Triptorelin | GnRH analogue | Moderate | Menopausal symptoms |
| Leuprolide | GnRH analogue | Moderate | Menopausal symptoms |
| Progesterone | Neurosteroid precursor | High | Hormonal side effects |
| Non-hormonal agents: | |||
| Acetazolamide | Carbonic anhydrase inhibitor | Moderate | Tolerance |
| Clobazam | GABA-A receptor modulator | Moderate | Sedation/tolerance |
| Lamotrigine | Sodium channel blocker | Moderate | Dizziness, pilot study |
| Neurosteroid-based agents: | |||
| Ganaxolone | GABA-A receptor modulator | High | Small pilot study |
Benzodiazepine therapy
Benzodiazepines such as clonazepam and clobazam are potent, positive allosteric modulators of GABAA receptor and broad-spectrum antiseizure agents. Clonazepam is highly useful in the therapy of absence and myclonic seizures. However, development of tolerance to their antiseizure effects usually limits their clinical utility (Haigh and Feely, 1988). Clobazam has been found to be an effective agent for the treatment of catamenial epilepsy (Feely et al., 1982; Feely and Gibson, 1984). Clobazam (20 to 30 mg/day) was administered intermittently (from 2 to 4 days before menses) probably to avoid the tolerance usually associated with long-term continual therapy. Moreover, tolerance to its antiseizure effects was not evidenced after 6 to 13 months of clobazam therapy (Feely and Gibson, 1984). The most common adverse effects of clobazam are sedation and depression. However, cross-tolerance to benzodiazepines has been described in animal model due to chronic exposure to neuroactive steroids (Reddy and Rogawski, 2000a). This could further impact the clinical utility of benzodiazepines in catamenial epilepsy therapy.
Hormonal therapy
A small number of hormonal treatment trials have been carried out in women with epilepsy (see Reddy, 2009). These trials can be divided into two categories: (i) hormonal suppression; and (ii) cyclic P supplementation. Suppression of ovarian hormonal secretion has been accomplished by treatment with intramuscular depomedroxyprogesterone. Ovarian secretion can also be suppressed with the use of parenteral depot gonadotropin-releasing hormone analog (Bauer et al., 1992). Because P has anticonvulsant effects and estrogen has proconvulsant effects, treatment with P or estrogen antagonists may prove to be useful adjunctive treatments in appropriate patients. Cyclic supplement studies have used both synthetic progestogens (progestins) and natural progestogen (P). Although oral synthetic progestin supplement has not been shown to benefit seizure control in small trials (Dana Haeri and Richens, 1983), natural P has shown a favorable seizure response (see below).
MPA therapy. Medroxyprogesterone acetate (MPA) is a widely investigated progestin-only contraceptive agent and is active by the parenteral and oral routes of administration. Zimmerman and colleagues (1973) used depot administration of MPA to treat a woman with catamenial seizures. Mattson et al (1984) found that MPA produces a 39% reduction in seizure frequency at a mean follow-up of 1 year. Suppression of seizures was evident when the patients were treated with parenteral MPA at dosages that were designed to eliminate menses. Therefore, it is conceivable that long-term MPA therapy is associated with undesirable reproductive disturbances. Although the mechanism of MPA action is not clearly understood, PRs appear to be important in its actions in catamenial epilepsy. Unlike P, MPA (17-acetyloxy-6-methyl-pregn-4-ene-3,20-dione) is not extensively metabolized to GABA-A receptor-modulating neurosteroids. This could partly explain the moderate efficacy of MPA relative to P, which is a prohormone for the synthesis of AP in the brain.
Natural P therapy. P therapy represents a potential hormonal approach for the treatment of catamenial epilepsy. The results of earlier clinical trials have shown that adjunctive P therapy produces significant benefits in reducing seizures in women with perimenstrual catamenial epilepsy (Herzog, 1999). It is recommended that the hormone be administered during the entire second half of the menstrual cycle and tapered gradually, as it is believed that abrupt discontinuation can result in rebound seizure exacerbation. However, there is no class III evidence on the efficacy of P therapy in women with epilepsy. The NIH-sponsored clinical study to assess P treatment of intractable seizures in women with partial epilepsy was recently completed (Herzog et al., 2012). This randomized, double-blind, placebo-controlled, phase III, multicenter, clinical trial compared the efficacy and safety of adjunctive cyclic natural P therapy versus placebo treatment of intractable seizures in 294 subjects randomized 2:1 to P or placebo, stratified by catamenial and non-catamenial status. It compared treatments on proportions of ≥50% responders and changes in seizure frequency from three baselines to three treated menstrual cycles. The results indicate that there was no significant difference in proportions of responders between P and placebo in the catamenial and non-catamenial patients. However, there was a significantly higher responder rate in women with perimenstrual seizure exacerbation (C1). Reductions in seizure frequency correlated with increasing C1 levels for P but not placebo, progressing from 26% to 71% for P vs. 25% to 26% for placebo. These findings suggest that P may provide a clinically important benefit for a subset of women with perimenstrual catamenial epilepsy. For C1 pattern, cyclic P supplement on days 14-25 followed by 3-day taper may provide beneficial outcome. The mechanisms underlying the lack of P effect on overall seizure frequency remains unclear. The dramatic response of P, which is a neurosteroid precursor, in women with perimenstrual catamenial epilepsy is attributable to the unique neurosteroid sensitivity of perimenstrual catamenial seizures (Reddy, 2009).
Neurosteroid therapy
Although P therapy is beneficial in perimenstrual catamenial epilepsy, it may be associated with hormonal side effects (Herzog, 2009; Reddy, 2009). Neurosteroids that are devoid of hormonal side effects represent a rational treatment strategy for perimenstrual catamenial epilepsy. However, natural neurosteroids such as AP have severe limitations because they have short half-life, are orally-inactive, and may produce hormonal effects due to their metabolism to hormonally-active compounds. Synthetic analogs of neurosteroids may overcome these obstacles and side effects associated with natural P therapy. Neurosteroid therapy possesses several advantages: (i) Neurosteroids can be effective for broad seizure types, even in diazepam-refractory seizures because they can activate most GABA-A receptor isoforms; (ii) Unlike benzodiazepines, neurosteroids lack tolerance upon repeated or chronic treatment which has been proven in clinical trials; (iii) They show a rapid onset and intermediate duration of action; (iv) Well established mechanism of action at GABA-A receptors; (v) Maximal efficacy is expected even in resistant seizures, due to their positive and direct (non-allosteric) actions in promoting GABAergic inhibition at high dosage, and (vi); They promote tonic inhibition that does not rely on interneurons that may be damaged in some patients with epilepsy.
Although neurosteroids seems to be the most direct approach to the treatment of catamenial epilepsy, there is only limited anecdotal data available to support their use (McAuley et al., 2001). Despite intense research on neurosteroids, there is no neurosteroid-based drug available for patients. Ganaxolone, the synthetic 3β-methyl derivative of AP, is the only neurosteroid that has been evaluated for the treatment of epilepsy in humans (Carter et al., 1997; Monaghan et al., 1999). Unlike AP and related natural neurosteroids that can undergo back conversion by 3α-HSOR isoenzymes to hormonally active intermediates, the 3β-methyl substituent of ganaxolone eliminates such metabolism and thereby avoids hormonal side effects. Ganaxolone has similar pharmacological properties to the natural neurosteroids such as AP (Reddy and Woodward, 2004). It has protective activity in diverse rodent seizure models (see Reddy and Rogawski, 2009; 2010; Reddy, 2009). In our recent study in female amygdala kindled mice, ganaxolone elicited strong suppression of behavioral and electrographic seizures (Reddy and Rogawski, 2010b). During prolonged daily treatment, tolerance does not develop with the anticonvulsant activity of neurosteroids (Kokate et al., 1998; Reddy and Rogawski, 2000a), a highly favorable profile compared to benzodiazepines.
Ganaxolone has been tested in various clinical trials to assess efficacy in the treatment of epilepsy (Reddy and Woodward, 2004; Rogawski et al., 2010). More than 900 subjects have received the drug at doses up to 1875 mg/day in adults and up to 54 mg/kg/day in children in phase 1 normal volunteer studies, epilepsy trials, and also clinical trials for migraine. Overall, the drug is safe and well tolerated. The most common side effect is reversible dose-dependent sedation. One epilepsy trial used the in-patient presurgical study design in adults with partial seizures (Laxer et al., 2000). A second study was an open-label, add-on trial in pediatric patients with a history of infantile spasms (Kerrigan et al., 2000). A third study was an open-label nonrandomized, dose-escalation, add-on trial in highly refractory pediatric and adolescent patients; 3 patients in this latter study were followed in an extension phase over 3.5 years (Pieribone et al., 2007). There is limited anecdotal information supporting the efficacy of ganaxolone in the treatment of catamenial seizure exacerbations (McAuley et al., 2001). Recently, a double-blind, randomized, placebo controlled study was completed in adults with partial seizures (Rogawski, et al., 2010). A separate trial was completed in children with infantile spasms. In this study, there was no clear statistically significant treatment effect although some subjects did appear to demonstrate a treatment-related reduction in spasm clusters. The adult trial included 147 subjects with partial onset seizures with or without secondary generalization who were refractory to conventional antiepileptic drugs. Ganaxolone treatment produced an 18% decrease in mean weekly seizure frequency, compared with a 2% increase for placebo over the 10-week treatment period. It is not end of the road to ganaxolone. Further clinical studies are needed to demonstrate the efficacy of ganaxolone in epilepsy and catamenial epilepsy.
Conclusions
Catamenial epilepsy is a specific form of pharmacoresistant epilepsy that impacts a substantial proportion (~70%) of an estimated 1.5 million women of child-bearing age with epilepsy in the United States. Catamenial epilepsy is a multifaceted neuroendocrine condition. Although ovarian hormones play a central role, the exact cause of catamenial epilepsy is unknown. Despite the increased information, there is a large gap in our understanding of catamenial epilepsy. Experimental studies to this point have indicated a clear role of estrogen, P and endogenous neurosteroids in the pathophysiology of catamenial epilepsy. Although there are several forms of catamenial epilepsy, neurosteroids have been implicated only in the seizure exacerbations that occur around the perimenstrual period. It is hypothesized that withdrawal of P-derived neurosteroids leads to enhanced brain excitability predisposing to seizures. In addition to neurosteroid fluctuations, there is emerging evidence that plasticity in GABA-A receptor subunits could play a role in the enhanced seizure susceptibility in catamenial epilepsy. Animal studies have shown that prolonged exposure to AP followed by withdrawal such as that occurs during menstruation causes a marked increase in expression of the extrasynaptic α4 and δ-subunits, which are linked to enhanced neuronal excitability, seizure susceptibility and benzodiazepine resistance. Overall, these neuroendocrine changes can result in reduced inhibition resulting in enhanced excitability, which, among other effects, predisposes to catamenial seizures. In the recent phase 3 trial, P therapy proved beneficial to many women with perimenstrual catamenial epilepsy. Treatment with synthetic neurosteroids may be beneficial to patients with catamenial seizures and avoid the hormonal side effects of P therapy. Alternatively, novel agents that increase the brain synthesis of neurosteroids, such as TSPO ligands, may find utility in the treatment of epilepsy.
It is important to recognize unique issues of women with epilepsy, including comorbidity and associated conditions such as bone health, reproductive, emotional and pregnancy issues. The Institute of Medicine released a report on epilepsy in 2012 with several recommendations on epilepsy care. It is important to tackle the significant issues identified in this landmark report, including women’s health issues.
Highlights.
-
►
This review describes the neuroendocrinological aspects of catamenial epilepsy.
-
►
Neurosteroids such as allopregnanolone play a critical role in catamenial epilepsy.
-
►
Alterations of GABA-A receptor plasticity and function are evident in catamenial models.
-
►
Synthetic neurosteroids may be useful for pharmacotherapy of catamenial epilepsy.
Acknowledgments
The original research described in this article was supported in part by the NIH grants NS051398, NS052158 and NS071597 (to D.S.R.) and the seed grant of TAMHSC Women’s Health in Neuroscience (WHIN) program. The author thanks Chase Carver for reading the manuscript.
ABBREVIATIONS
- AED
antiepileptic drug
- AP
allopregnanolone
- DOC
deoxycorticosterone
- Egr3
early growh response factor-3
- 3α-HSOR
3α-hydroxysteroid oxidoreductase
- MPA
medroxyprogesterone acetate
- OC
oral contraceptive
- P
progesterone
- PR
progesterone receptor
- SE
status epilepticus
- THDOC
allotetrahydrodeoxycorticosterone
- TSPO
translocator protein
Footnotes
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abassi F, Krumholz A, Kittner SJ, Langenberg P. Effects of menopause on seizures in women with epilepsy. Epilepsia. 1999;42:205–210. doi: 10.1111/j.1528-1157.1999.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado D, Cavalheiro EA. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998;32:266–274. doi: 10.1016/s0920-1211(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Zetterlund B, Blom S, Romano M. Effect of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand. 1984;69:240–248. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- Bauer J. Interactions between hormones and epilepsy in female patients. Epilepsia. 2001;42(Suppl 3):20–22. doi: 10.1046/j.1528-1157.2001.042suppl.3020.x. [DOI] [PubMed] [Google Scholar]
- Bauer J, Wildt L, Flugel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. J Neurol. 1992;239:284–286. doi: 10.1007/BF00810354. [DOI] [PubMed] [Google Scholar]
- Bauer J, Burr W, Elger CE. Seizure occurrence during ovulatory and anovulatory cycles in patients with temporal lobe epilepsy: a prospective study. Eur J Neurol. 1998;5:83–88. doi: 10.1046/j.1468-1331.1998.510083.x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Steroid hormones in the brain: several mechanisms? In: Fuxe F, Gustafsson JA, Wetterberg L, editors. Steroid Hormone Regulation of the Brain. Vol. 3. Oxford: Pergamon Press; 1981. p. 14. [Google Scholar]
- Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arg Neuro-Psiquiatria. 2005;63(3B):751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, Avoli M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp Neurol. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. Neurosteroids and epileptogenesis in the pilocarpine model: Evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(Suppl 1):53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–106. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABAA receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AM, Spence KT, Smith SS, ffrench-Mullen JM. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- Craig CR. Anticonvulsant activity of steroids: separability of anticonvulsant from hormonal effects. J Pharmacol Exp Ther. 1966;153:337–343. [Google Scholar]
- Crawford P. Best practice guidelines for the management of women with epilepsy. Epilepsia. 2005;46(Suppl 9):117–124. doi: 10.1111/j.1528-1167.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- El-Khayat HA, Soliman NA, Tomoum HY, Omran MA, El-Wakad AS, Shatla RH. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619–1626. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- Feely M, Gibson J. Intermittent clobazam for catamenial epilepsy: tolerance avoided. J Neurol Neursurg Psychiatry. 1984;47:1279–1282. doi: 10.1136/jnnp.47.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy: a model for evaluating anticonvulsants. Lancet. 1982;2:71–73. doi: 10.1016/s0140-6736(82)91691-9. [DOI] [PubMed] [Google Scholar]
- Foldvary-Schaefer N, Falcone T. Catamenial epilepsy: pathophysiology, diagnosis, and management. Neurology. 2003;61(Suppl 2):S2–15. doi: 10.1212/wnl.61.6_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3α,5α-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ, Bayon LE. Finasteride blocks the reduction in ictal activity produced by exogenous estrous cyclicity. J Neuroendocrinol. 1998;10:291–296. doi: 10.1046/j.1365-2826.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABAA receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Gilad R, Sadeh M, Rapoport A, Dabby R, Lampl Y. Lamotrigine and catamenial epilepsy. Seizure. 2008;17:531–534. doi: 10.1016/j.seizure.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Guille C, Spencer S, Cavus I, Epperson CN. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment. Epilepsy Behav. 2008;13:12–24. doi: 10.1016/j.yebeh.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers WR. Their causes, symptoms, and treatment. London: J & A Churchill; 1881. Epilepsy and other chronic convulsive diseases; p. 197. [Google Scholar]
- Haigh JRM, Feely M. Tolerance to the anticonvulsant effect of benzodiazepines. Trends Pharmacol Sci. 1988;9:361–366. doi: 10.1016/0165-6147(88)90255-6. [DOI] [PubMed] [Google Scholar]
- Harden CL, Leppik I. Optimizing therapy of seizures in women who use oral contraceptives. Neurology. 2006;67(Suppl 4):S56–S58. doi: 10.1212/wnl.67.12_suppl_4.s56. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pulver MC, Ravdin L, Jacobs AR. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–1407. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interactions with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1600–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–1918. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Menstrual disorders in women with epilepsy. Neurology. 2006;66(6 Suppl 3):S23–28. doi: 10.1212/wnl.66.66_suppl_3.s23. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Hormonal therapies: progesterone. Neurotherapeutics. 2009;6:383–391. doi: 10.1016/j.nurt.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM The NIH Progesterone Trial Study Group. Sensitivity and specificity of the association between catamenial seizure patterns and ovulation. Neurology. 2008;70:486–487. doi: 10.1212/01.wnl.0000278102.55701.d0. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Friedman MN, Freund S, Pascual-Leone A. Transcranial magnetic stimulation evidence of a potential role for progesterone in the modulation of premenstrual corticocortical inhibition in a woman with catamenial seizure exacerbation. Epilepsy Behav. 2001;2:367–369. doi: 10.1006/ebeh.2001.0232. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Smithson SD the Progesterone Trial Study Group. Progesterone versus placebo therapy for women with epilepsy: A randomized clinical trial. Neurology. 2012 doi: 10.1212/WNL.0b013e318259e1f9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AD, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABAA receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Isojarvi JI, Tauboll E, Herzog AG. Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS Drug. 2005;19:207–223. doi: 10.2165/00023210-200519030-00003. [DOI] [PubMed] [Google Scholar]
- Jacono JJ, Robinson J. The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle in epileptic women. Epilepsia. 1987;28:571–577. doi: 10.1111/j.1528-1157.1987.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Kaplan PW, Norwitz ER, Ben Menachem E, Pennell PB, Druzin M, Robinson JN, Gordon JC. Obstetric risks for women with epilepsy during pregnancy. Epilepsy Behav. 2007;11:283–291. doi: 10.1016/j.yebeh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RDJ, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34S:S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Landgren S, Bäckström T, Kalistratov G. The effect of progesterone on the spontaneous interictal spike evoked by the application of penicillin to the cat’s cerebral cortex. J Neurol Sci. 1978;36:119–133. doi: 10.1016/0022-510x(78)90166-1. [DOI] [PubMed] [Google Scholar]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia. 2000;41:187–1194. doi: 10.1111/j.1528-1157.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Logothetis J, Harner R, Morrel F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology. 1959;9:352–360. doi: 10.1212/wnl.9.5.352. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA-A receptors: Relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Marques-Assis L. Influence of menstruation on epilepsy. Arq Neuropsiquiatr. 1981;39:390–395. doi: 10.1590/s0004-282x1981000400004. [DOI] [PubMed] [Google Scholar]
- Mattson RH, Cramer JA, Caldwell BV, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology. 1984;34:1255–1258. doi: 10.1212/wnl.34.9.1255. [DOI] [PubMed] [Google Scholar]
- McAuley JW, Anderson GD. Treatment of epilepsy in women of reproductive age: pharmacokinetic considerations. Clin Pharmacokinet. 2002;41:559–79. doi: 10.2165/00003088-200241080-00002. [DOI] [PubMed] [Google Scholar]
- McAuley JW, Reeves Al, Flyak J, Monaghan EP, Data J. A pilot study of the neurosteroid ganaxolone in catamenial epilepsy: clinical experience in two patients. Epilepsia. 2001;42(Suppl 7, 85) 1.267. [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrock DW, Lee DA. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia. 1999;38:1026–1031. doi: 10.1111/j.1528-1157.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith S. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Epilepsy in women: the science of why it is special. Neurology. 1999;53(Suppl 1):S42–S48. [PubMed] [Google Scholar]
- Morrell MJ, Montouris GD. Reproductive disturbances in patients with epilepsy. Cleve Clin J Med. 2004;71(Suppl 2):S19–24. doi: 10.3949/ccjm.71.suppl_2.s19. [DOI] [PubMed] [Google Scholar]
- Niijima S, Wallace SJ. Effects of puberty on seizure frequency. Dev Med Child Neurol. 1989;31:174–180. doi: 10.1111/j.1469-8749.1989.tb03976.x. [DOI] [PubMed] [Google Scholar]
- Pack AM, Reddy DS, Duncan S, Herzog A. Neuroendocrinological aspects of epilepsy: important issues and trends in future research. Epilepsy Behav. 2011;22:94–102. doi: 10.1016/j.yebeh.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Pennell PB. Antiepileptic drugs during pregnancy: What is known and which AEDs seem to be safest? Epilepsia. 2008;49(suppl.9):43–55. doi: 10.1111/j.1528-1167.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Tsai J, Soufflet C, Rey E, Shaw K, Giller E, Dulac O. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia. 2007;48:1870–1874. doi: 10.1111/j.1528-1167.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG. NIH Progesterone Trial Study Group: Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology. 2009;73(3):223–227. doi: 10.1212/WNL.0b013e3181ae7adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L, Hess GP. Mechanism of potentiation of a dysfunctional epilepsy-linked mutated GABA(A) receptor by a neurosteroid (3alpha, 21-dihydroxy-5alpha-pregnan-20-one): transient kinetic investigations. Biochemistry. 2010;49:7892–7901. doi: 10.1021/bi901241g. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol. 2011;2:38. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310(1):230–9. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59:573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.192377. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334:1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000a;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000b;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Wu X. Ovarian cycle-related changes in expression and function of extrasynaptic GABA-A receptors in the hippocampus subfields. Soc Neurosc. 2011 Abstr 338/07 (C44) [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:303–310. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neuroactive steroids modulates GABAA receptor function and seizure susceptibility. J Neurosci. 2002;42:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010;89:254–260. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Woodward R. Ganaxolone: a prospective overview. Drugs Future. 2004;29:227–242. [Google Scholar]
- Reddy DS, Zeng YC. Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J. 2007;21(6):A1179–A11179. [Google Scholar]
- Reddy DS, Zeng Y-C, Dhanushkodi A, Hattiangady B, Shetty AK. Hippocampal interneuron damage, spontaneous recurrent seizures, mossy fiber sprouting, and neurosteroid efficacy in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2007;48(Suppl. 6):291–292. [Google Scholar]
- Reibel S, André V, Chassagnon S, André G, Marescaux C, Nehlig A, Depaulis A. Neuroprotective effects of chronic estradiol benzoate treatment on hippocampal cell loss induced by status epilepticus in the female rat. Neurosci Lett. 2000;281:79–82. doi: 10.1016/s0304-3940(00)00784-9. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Nohria V, Tsai J, Shaw K, Farfel G, Pieribone VA. Ganaxolone 2010 [Google Scholar]; Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 92(2-3):89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Rosciszewska D. In: Epilepsy and menstruation. Hopkins A, editor. Epilepsy London: Chapman & Hall; 1987. pp. 373–381. [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9(12):971–88. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325(5939):490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABA-A receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Sabers A, Gram L. Lamotrigine pharmacokinetics during anticonception and pregnancy. Letters in Drug Design & Discovery. 2006;3:321–322. [Google Scholar]
- Saberi M, Pourgholami MH. Estradiol alters afterdischarge threshold and acquisition of amygdala kindled seizures in male rats. Neurosci Lett. 2003;340:41–44. doi: 10.1016/s0304-3940(03)00074-0. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Malthankar-Phatak GH, Friedman D, Pearce P, McCloskey DP, Harden CL, Maclusky NJ. A rat model of epilepsy in women: a tool to study physiological interactions between endocrine systems and seizures. Endocrinology. 2009;150:4437–4442. doi: 10.1210/en.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (metrazol) J Lab Clin Med. 1942;27:1051–1053. [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABA-A receptor α4-subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA-A receptors: focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velísek L, Velísková J. New avenue of research: antiepileptic drug and estradiol neuroprotection in epilepsy. Recent Patents CNS Drug Discov. 2008;3:128–37. doi: 10.2174/157488908784534577. [DOI] [PubMed] [Google Scholar]
- Velíšková J. Estrogens and Epilepsy: Why are we so excited? Neuroscientist. 2007;13:77–88. doi: 10.1177/1073858406295827. [DOI] [PubMed] [Google Scholar]
- Velísková J, Velísek L. Beta-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide-Y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41(Suppl.6):S30–35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ-subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AW, Holder DT, Reiter EO, Dekaban AS. Medroxyprogesterone acetate in the treatment of seizures associated with menstruation. J Pediatr. 1973;83:959–963. doi: 10.1016/s0022-3476(73)80529-3. [DOI] [PubMed] [Google Scholar]


