INTRODUCTION
In assessing the pharmacokinetics (PK) of therapeutic agents, studies are commonly performed to evaluate the ‘washout’ of the drugs and metabolites after single or multiple doses. The author is often frustrated when viewing reports and publications where the lower concentrations of drugs are absent and reported as Below the Limit of Quantitation (BLQ). Similar circumstances occur in pharmacodynamic (PD) studies where biomarkers are suppressed to very low, but meaningful, concentrations. When seeking these missing values, a common response is referral to the 2001 FDA Guidance on Bioanalytical Method Validation (1) and the later 2007 AAPS Workshop Report (2). The former advises that use of measurements below the LLOQ is “not recommended”. Thus such values are not usually provided. The author herein provides arguments for provision and acceptance of such data in many circumstances. While this issue has been addressed previously in somewhat similar vein (3), it seems appropriate to revisit at the present time in view of the persistence of this analytical dilemma and the advancement of pharmacometric concepts and methods for dealing with low exposure data.
COMMON BIOANALYTICAL SITUATION
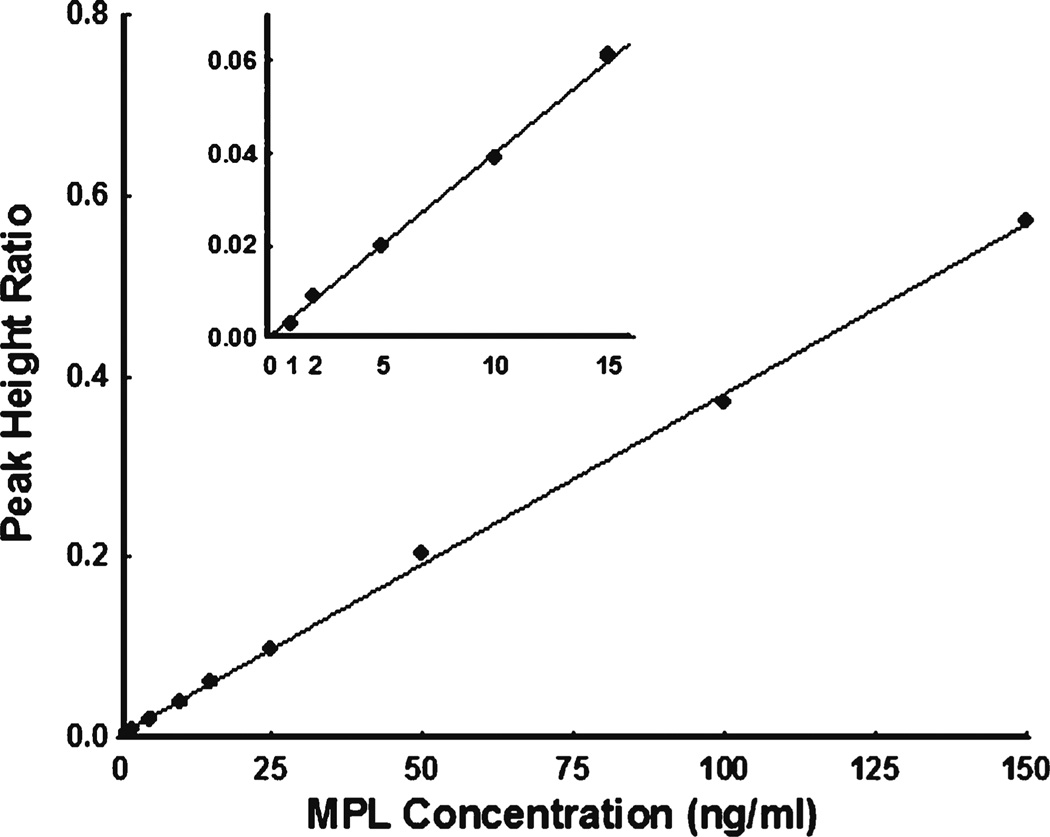
Chromatographic (HPLC, GC, or LC/MS/MS) assays typically produce linear standard curves. An example is shown in Fig. 1 for analysis of methylprednisolone standards in plasma. An adaptation of our general HPLC method for corticosteroids was utilized (4). As commonly done, 10 replicates of each standard concentration were assessed. For the lower standards, the Coefficients of Variation (CV%) were: 1 ng/ml–36.5%, 2 ng/ml–25.3%, 5 ng/ml–12.5%, 10 ng/ml–3.9%, and 15 ng/ml–17.2%. According to the FDA Guidance for determining the LLOQ, the lowest standard should be selected with an analyte response which is at least 5 times the blank response, has precision of 20% or less, and allows accuracy of 80–120%. Here 5 ng/ml would be designated as the LLOQ. The FDA Guidance does not require determination of the Limit of Detection (LOD). Here a drug concentration of 0.2 ng/ml produced the lowest detector response above baseline.
Fig. 1.
Standard curve for methylprednisolone analysis by HPLC: Peak Height Ratio versus drug concentration. The insert shows measurements near the LLOQ of the assay. The regression line was fitted using all data.
With these assay results, strict interpretation of the FDA Guidance would call for formal reporting of only those concentrations falling at or above the LLOQ (and at or below the Upper Limit of Quantitation (ULOQ) without dilution and reanalysis). A reasonable approach would be utilization of the concentrations down to 1 ng/ml with appropriate consideration of the greater potential variability and lesser accuracy of such values. As will be described, this can be handled in pharmacokinetic modeling by suitable weighting of the data and consideration of companion PK and/or PD profiles.
COMMON PHARMACOKINETIC SITUATIONS
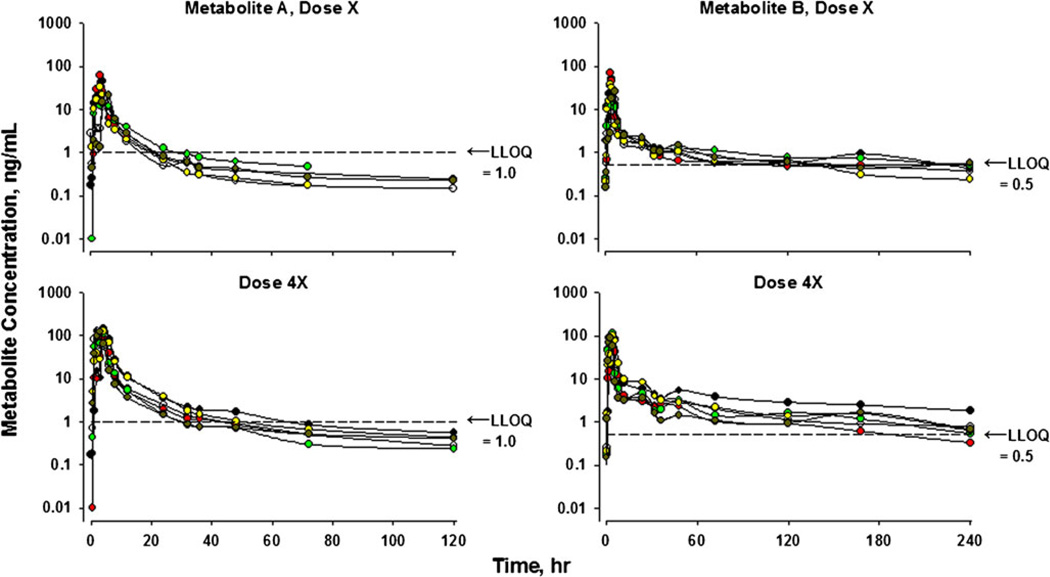
There are at least two common pharmacokinetic situations where an expert in drug disposition will recognize the likelihood of meaningful drug or metabolite concentrations with values reported below the LLOQ. A dual example of such situations is provided in Fig. 2. This graph provides disposition profiles of two drug metabolites in six human subjects where the parent drug was administered orally in parallel group studies at doses X and 4X (unpublished observations). As indicated, the LLOQ using a validated LC/MS/MS method was 1 ng/ml for Metabolite A and 0.5 ng/ml for Metabolite B.
Fig. 2.
Time course of plasma concentrations of two drug metabolites following an oral dose of the parent drug in parallel group studies using six healthy volunteers at each dose level. The top panels depict data at Dose X while the bottom panels reflect Dose 4X. Measurements were made using a validated LC/MS/MS assay with the indicated LLOQ for each compound.
With these PK profiles, strict interpretation of the FDA Guidance would call for recognition of only the early phase of the upper profile for Metabolite A. The later terminal phase which is more evident for the 4X dose would be neglected. Additional support for including the late phase for Dose X for Metabolite A is derived from the profiles of Metabolite B. The two compounds have very similar chemical structures with expectations of similar metabolic pathways and rates as well as tissue distribution properties based on structure-activity principles in PK. The values below the LLOQ are consistent with the upper curve shapes and directions of decline of all of the metabolites. Further, the remarkable reproducibility of the profiles between the dose levels (viz. dose-proportionality) and among the 6 subjects augments the credibility of the lower dose profiles. Providing early and late BLQ concentrations, rather than leaving gaps in a graph, also serves to indicate the directional changes of the adjacent data points. This type of dose-related dispositional behavior is commonplace. Like these profiles, numerous PK publications show plasma concentration versus time profiles for a range of drug doses with the data for the lower doses often truncated owing to assay limitations (one example cited (5)).
An analogous situation often occurs when following biomarker profiles in pharmacodynamics. For example, corticosteroids such as methylprednisolone suppress adrenal function where the cortisol concentrations can fall below the LLOQ of an HPLC assay. In one instance, this problem was handled by implementing a more sensitive radioimmunoassay method (6). The low concentrations are meaningful and should not be neglected in considering the effects of the administered drug. For the corticosteroids, the early use of HPLC has been supplanted by much more sensitive LC/MS/MS methods which can achieve a LLOQ of 5 pg/ml (7). However, it is not always possible to revisit a completed PK or PD study with a more sensitive analytical method or using larger sample volumes when seeking to complete the final data analysis.
UTILIZATION OF PHARMACOMETRICS
Other experts in pharmacokinetics have argued for retaining measurements of drug and metabolite concentrations between the LLOQ and limit of detection (LOD), but with due consideration of greater variability (9). All software programs for nonlinear least-squares fitting of PK data have various options for weighting experimental data. Reported measurements below the LLOQ can be assigned a lesser weight based on the actual or expected larger CV% of the analytical method. When applying noncompartmental methods for preliminary assessment of disposition profiles for single doses, what is best done is the fitting of polyexponential equations to the entire data set rather than calculating incremental AUC values for which a linear terminal slope is needed for extrapolation of AUC and AUMC values to time infinity (8).
Ultimately, the preferred approach for characterizing two or more drug disposition profiles is simultaneous fitting of all data to a generalized pharmacokinetic model to obtain a set of parameters which are universal for the drug and study. This systems (or population) approach allows the profiles from the higher doses to inform the fittings of the less reliable lower and later concentrations. If the data in Fig. 2 were handled in such a manner, this would effectively superimpose the dose-normalized plasma concentrations adding greater certainty to the observed data below the LLOQ. More complex models for simultaneous examination of drug and metabolite profiles would allow implementation of standard precursor-product relationships where metabolite profiles could not exhibit terminal slopes which decline more sharply than the parent drug (8).
Beal, in 2001, addressed several ways to fit a pharmacokinetic model with some data below the LLOQ using the population software, NONMEM (10). His assessments were limited to consideration of a one-compartment model seeking the clearance (CL) and volume of distribution (V) and assuming that some late measurements were reported as BLQ. Many investigators in pharmacometrics have accepted his recommendation of “Method M3” which retains later BLQ measurements, but handles them statistically as censored observations, a capability implemented in NONMEM. The Beal M3 method has been applied to more complex situations including pharmacodynamic data and found to be of value (11–14). Censoring of observations below the LLOQ has been found to result in biased parameter estimates. It appears timely to address these types of questions with consideration of real (an instrument signal), but less certain, measurements between the LLOQ and LOD.
The FDA Guidance (1) provides specific recommendations for ascertaining the LLOQ of an analytical procedure. However, there is no indication of need for the LOD and thus no guideline provided for its calculation. Nix and Wilson (15) have reflected on the clinical importance of such a measure and have reviewed published methods for determination of the LOD. With the absence of consideration of the LOD in the FDA Guidance, it is usually not assessed in many assay validation efforts and not reported in most publications. Further, some studies simply use the lowest analytical standard as the LLOQ (and the highest as the ULOQ) without pursuing the analytical limits of the system.
Further insight into the validity of drug and metabolite concentrations below the LLOQ can be often found in assessing the pharmacodynamics. It is well appreciated that the PK of the active agent serves as the driving force for most pharmacologic and physiologic changes, albeit in ways that range from simple and immediate Hill-type nonlinear relationships (direct effects) to complex delayed responses (indirect and transduction models)(16). Occasionally it is possible to infer the entire biophase kinetics of an active agent from the time-course of observed responses (17). Models frequently can reflect the properties of a “black box” using the input/output characteristics of the system. All mechanistic equations and models in pharmacodynamics must contain a function for the active drug and/or metabolite concentrations driving the system. Thus, just as a higher dose disposition profile can affirm the expected behavior of lower doses, the dynamics of a drug can inform the validity of drug concentration measurements falling below the LLOQ. This might be the case, for example, when simple (without rebound) drug effects appear to outlast drug concentrations available only above the LLOQ. A systems PK/PD approach can supplement the statistical weakness in part of the analytical methodology.
CONCLUSIONS
Increasingly selective and sensitive assays for therapeutic agents and biomarkers have provided advantages in studying drug disposition and action. Direct measurements of low, meaningful concentrations of therapeutic agents should remain an analytical goal. Drug and metabolite concentrations falling between the LLOQ and LOD as generated from chromatographic procedures with linear standard curves can offer considerable value in PK and PD. This does not pertain to ligand-based assays where linearity is unlikely (1,2). Although deviations from FDA Guidances are unusual, such deviations from recommendations should be permitted if suitably justified.1 Companion PK and PD information from a particular study or from the literature can augment the utility of instrument responses to low analyte concentrations. In turn, the pharmacometric handling of low concentration data with less certainty in the measurements requires caution by implementation of suitable statistical weighting and insightful modeling and computational approaches.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURES
The author appreciates the technical assistance of Ms. Nancy Pyszczynski. This work was supported by NIH Grant GM 24211.
Footnotes
My conversation with a senior FDA pharmacometrician on this issue produced the following response, “From a pharmacometrics point of view one should use good scientific judgment to decide whether to include BLOQ “observations” or not. We are open to all reasonable approaches.” (personal communication)
REFERENCES
- 1.Guidance for Industry, Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) 2001 May
- 2.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, et al. Workshop/Conference Report – Quantitative bioanalytical methods validation and implementation: Best practices for chromatographic and ligand binding assays. AAPS J. 2007;9:E30–E42. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 3.Humbert H, Cabiac MD, Barradas J, Gerbeau C. Evaluation of pharmacokinetic studies: is it useful to take into account concentrations below the limit of quantification? Pharm Res. 1996;13:839–845. doi: 10.1023/a:1016088609005. [DOI] [PubMed] [Google Scholar]
- 4.Jusko WJ, Pyszczynski NA, Bushway MS, D’Ambrosio R, Mis SM. Fifteen Years of operation of a high-performance liquid chromatographic assay for prednisolone, cortisol and prednisone in plasma. J Chrom Biomed Appl. 1994;658:47–54. doi: 10.1016/0378-4347(94)00218-5. [DOI] [PubMed] [Google Scholar]
- 5.Parasrampuria DA, de Boer P, Desai-Krieger D, Chow AT, Jones CR. Single-dose pharmacokinetics and pharmacodynamics of RWJ 67657, a specific p38 mitogen-activated protein kinase inhibitor: a first-in-human study. J Clin Pharmacol. 2003;43:406–413. doi: 10.1177/0091270002250615. [DOI] [PubMed] [Google Scholar]
- 6.Kong A-N, Ludwig EA, Slaughter RL, DiStefano PM, DeMasi J, Middleton E, et al. Pharmacokinetics and pharmacodynamic modeling of direct suppression effects of methylprednisolone on serum cortisol and blood histamine in human subjects. Clin Pharmacol Ther. 1989;46:616–628. doi: 10.1038/clpt.1989.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu J, Qu Y, Straubinger RM. Ultra-sensitive quantification of corticosteroids in plasma samples using selective solid-phase extraction and reversed-phase capillary high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2007;79:3786–3794. doi: 10.1021/ac062184r. [DOI] [PubMed] [Google Scholar]
- 8.Jusko WJ. Guidelines for collection and analysis of pharmacokinetic data. In: Burton ME, Shaw LM, Schentag JJ, Evans WE, editors. Applied Pharmacokinetics & Pharmacodynamics. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 9–29. [Google Scholar]
- 9.Holford N, Jelliffe R, et al. Pharm PK Discussion – BLQ Values. http://www.boomer.org/pkin/PK07/PK2007116.html.
- 10.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokin Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 11.Duval V, Karlsson MO. Impact of omission or replacement of data below the limit of quantification on parameter estimates in a two-compartment model. Pharm Res. 2002;19:1835–1840. doi: 10.1023/a:1021441407898. [DOI] [PubMed] [Google Scholar]
- 12.Ahn JE, Karlsson MO, Dunn A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35:401–421. doi: 10.1007/s10928-008-9094-4. [DOI] [PubMed] [Google Scholar]
- 13.Byon W, Fletcher CV, Brundage RC. Impact of censoring data below an arbitrary quantification limit on structural model misspecification. J Pharmacokinet Pharmacodyn. 2008;35:101–116. doi: 10.1007/s10928-007-9078-9. [DOI] [PubMed] [Google Scholar]
- 14.Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11:371–380. doi: 10.1208/s12248-009-9112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nix ABJ, Wilson DW. Assay detection limits: concept, definition, and estimation. Eur J Clin Pharmacol. 1990;39:203–206. doi: 10.1007/BF00315096. [DOI] [PubMed] [Google Scholar]
- 16.Mager DE, Wyska E, Jusko WJ. Diversity of mechanism-based pharmacodynamic models. Drug Met Disp. 2003;31:510–518. doi: 10.1124/dmd.31.5.510. [DOI] [PubMed] [Google Scholar]
- 17.Gabrielsson J, Jusko WJ, Alari L. Modeling of dose-response-time data: four examples of generating kinetic functions from response profiles. Biopharm Drug Disp. 2000;21:41–52. doi: 10.1002/1099-081x(200003)21:2<41::aid-bdd217>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]