Abstract
Our earlier studies showed that ahyRI (AI-1) and LuxS-based (AI-2) quorum sensing (QS) systems were positive and negative regulators of virulence, respectively, in a diarrheal isolate SSU of A. hydrophila. Recently, we demonstrated that deletion of the QseBC two-component signal transduction system (AI-3 QS in enterohemorrhagic E. coli) also led to an attenuation of A. hydrophila in a septicemic mouse model of infection, and that interplay exists between AI-1, AI-2, and the second messenger cyclic-di-guanosine monophosphate (c-di-GMP) in modulating bacterial virulence. To further explore a network connection between all of the three QS systems in A. hydrophila SSU and their cross-talk with c-di-GMP, we overproduced a protein with a GGDEF domain, which increases c-di-GMP levels in bacteria, and studied phenotypes and transcriptional profiling of genes involved in biofilm formation and motility of the wild-type (WT) A. hydrophila and its ΔqseB mutant. Over-expression of the GGDEF domain-encoding gene (aha0701h) resulted in a significantly reduced motility of the WT A. hydrophila similar to that of the ΔqseB mutant. While enhanced protease production was noted in WT A. hydrophila that had increased c-di-GMP, no enzymatic activity was detected in the ΔqseB mutant overexpressing the aha0701h gene. Likewise, denser biofilm formation was noted for WT bacteria when c-di-GMP was overproduced compared to its respective control; however, overproduction of c-di-GMP in the ΔqseB mutant led to reduced biofilm formation, a finding similar to that noted for the parental A. hydrophila strain. These effects on bacterial motility and biofilm formation in the ΔqseB mutant or the mutant with increased c-di-GMP were correlated with altered levels of fleN and vpsT genes. While we noted transcript levels of qseB and qseC genes to be increased in the ahyRI mutant, down-regulation of the ahyR and ahyI genes was observed in the ΔqseB mutant, which correlated with decreased protease activity. Finally, an enhanced virulence of WT A. hydrophila with increased c-di-GMP was noted in a mouse model when compared to findings in the parental strain with vector alone. Overall, we conclude that cross talk between AI-1- and QseBC-systems exist in A. hydrophila SSU, and c-di-GMP modulation on QseBC-system is dependent on the expression of the AI-1 system.
Keywords: Aeromonas hydrophila, 2-component QseBC system, c-di-GMP, quorum sensing systems, motility, biofilm formation, gene transcription, mouse model of infection
INTRODUCTION
QseBC has recently been described as a novel, two-component-based quorum sensing (QS) system that responds to eukaryotic hormone-like signals, such as autoinducer-3 (AI-3), epinephrine, and norepinephrine [1]. In the presence of signal molecules, the inner membrane-localized QseC first undergoes autophosphorylation and then transfers this phosphate to intracellular response regulator QseB. Phosphorylated QseB binds and activates the transcription of the flhDC master regulator of the flagella regulon in enterohemorrhagic E. coli (EHEC), and it also binds to its own promoter [2]. QseC activates transcription of the genes encoding Shiga toxin, and, in addition to activating expression of the LEE (locus of enterocyte effacement)-encoded type 3 secretion system (T3SS), the majority of the genes encoding effectors and translocated through this T3SS, are also regulated by QseC [3]. It has been demonstrated that QseBC is an important virulence regulator contributing to intracellular colonization and systemic infection caused by EHEC [4], uropathogenic E. coli (UPEC) [5], and Salmonella enterica Serovar Typhimurium [6].
Recently, we demonstrated that two modified autoinducer systems exist in a diarrheal isolate SSU of A. hydrophila. One of them is an N-acylhomoserine lactone (AHL)-based QS system, designated as AI-1 [7], while the other is an S-ribosylhomocysteinase (LuxS)-based AI-2 system [8]. These QS systems had opposing effects on modulating biofilm formation in an in vitro model and virulence in a septicemic mouse model of infection [7, 8]. The regulatory network of AI-1 and AI-2 QS systems in A. hydrophila SSU also includes second-messenger c-di-GMP-dependent modulation of virulence genes [9].
More recently, we reported identification of a functional QseBC system in A. hydrophila SSU. We provided evidence that deletion of the qseB gene from A. hydrophila decreased swimming and swarming motility, increased biofilm formation, and reduced the protease and hemolytic activity associated with the cytotoxic enterotoxin (Act). Moreover, the mutant was attenuated in a septicemic mouse model of infection [10]. Since we have already demonstrated an interplay between AI-1 and AI-2 QS systems in A. hydrophila SSU through c-di-GMP [9], we were interested in studying the potential interaction of these two QS systems with the QseBC system found in A. hydrophila.
Here we present data showing an impact of the QseBC system on motility, biofilm formation, and the QS-dependent virulence regulatory network in A. hydrophila SSU as a function of the c-di-GMP level. We further show that transcript levels of the genes involved in AI-1- and QseBC systems are co-regulated in A. hydrophila SSU.
RESULTS
2.1. Identification of qseB and qseC gene transcripts in A. hydrophila SSU
As with the EHEC QseBC two-component system [11], the transcriptional stop codon of the qseB gene overlaps with the transcriptional start codon of the qseC gene in A. hydrophila [10]. However, we identified by reverse transcriptase (RT)-polymerase chain reaction (PCR) two independent transcripts, one each for the qseB and qseC gene (data not shown), in contrast to qseBC co-transcription which was described for EHEC [11] and Edwarsiella tarda [12]. Additionally, the qseC transcript was detected at a much reduced level, when compared to the results with the qseB transcript in A. hydrophila, possibly because qseC is a GC-rich gene (67.1%). At the same time, a 4-bp overlap at the ATGA motif, which was found for the qseB and qseC gene open reading frames (ORFs), obviously was necessary to enhance the expression of the GC-rich qseC gene [13].
2.2. Overproduction of GGDEF domain protein regulates motility and biofilm formation in the ΔqseB mutant A. hydrophila of SSU
Recently, we successfully used the overproduction of AHA0701h protein with a GGDEF domain, which increases c-di-GMP levels in bacteria, for gene profiling and phenotypic alterations in WT A. hydrophila and its luxS and ahyRI mutants [9]. The gene-encoding AHA0701h is located downstream of the luxS gene in the genome of A. hydrophila [8]. The GGDEF domain is typically found in proteins as part of modular diguanylate cyclase (DGC) enzymes, which catalyze the synthesis of the signaling molecule c-di-GMP [14]. For the present study, the gene encoding AHA0701h was cloned into the pBAD/Myc-HisB vector and transformed into the ΔqseB mutant, with the latter harboring only the vector serving as a negative control. The phenotypic alterations of these strains were studied after arabinose induction (section 4.1) and compared to the similarly transformed WT A. hydrophila strain.
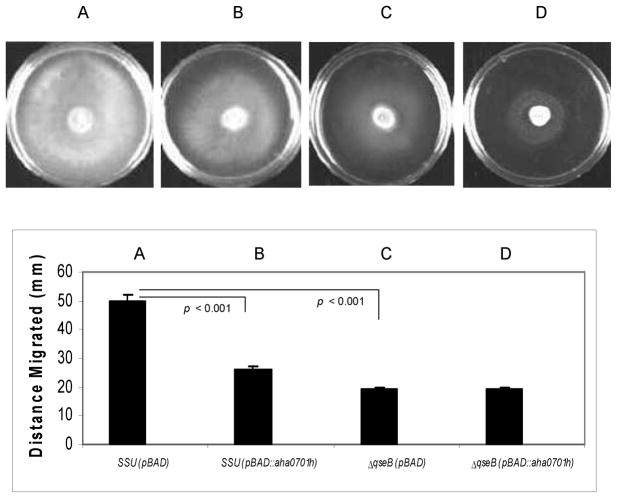
QS functions to control population size in a biofilm and is also involved in the biosynthesis of flagella [15], which modulates swimming or swarming motility [10]. Recently, we demonstrated that over-expression of the aha0701h gene in the ΔqseB mutant resulted in no swimming motility [10]. Here, we show that overproduction of c-di-GMP in WT A. hydrophila significantly reduced swarming motility compared to the parental bacteria with vector alone (Fig. 1). This decrease in swarming motility of WT A. hydrophila with overexpression of the aha0701h gene was similar to that found with the ΔqseB mutant (Fig. 1). However, while the swimming motility of the ΔqseB mutant was further decreased when c-di-GMP was overproduced [10], no further reduction in the swarming motility of the ΔqseB mutant was observed when c-di-GMP was overproduced (Fig. 1).
Fig. 1.
Swarming motility of the WT A. hydrophila SSU and its ΔqseB mutant with either the empty pBAD/Myc-HisB vector or the pBAD::aha0701h plasmid under arabinose induction. The WT A. hydrophila with vector alone (A) migrated in the swarming Difco nutrient agar plate with 0.5% Eiken agar, whereas the WT bacteria with overproduction of c-di-GMP (B) showed a decrease in motility (p < 0.001).The ggdef-overexpression in the ΔqseB mutant (D) did not alter its migration compared to the ΔqseB mutant with vector alone (C), but the swarming motility of the latter was decreased statistically when compared to WT A. hydrophila harboring the pBAD/Myc-HisB vector alone (A) (p < 0.001). Three independent experiments were performed, and the arithmetic means ± standard deviations were plotted.
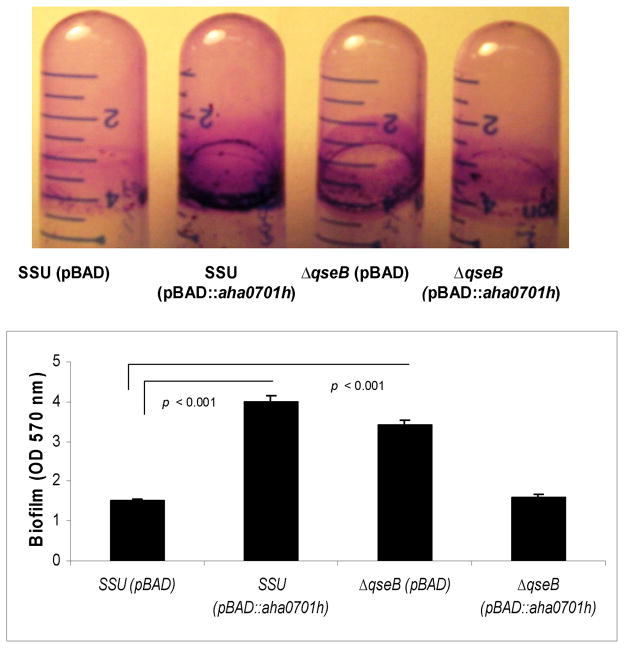
Biofilm formation is critical not only for environmental survival but also to cause successful infection in the host by numerous pathogenic bacteria. To measure solid surface-associated biofilm formation, we performed a crystal violet (CV) staining assay and also examined biofilm formation on thermanox cover slips by scanning electron microscopy (SEM). The biofilm formation in polystyrene tubes was observed after 24 h of growth of WT A. hydrophila and its ΔqseB mutant as well as of the ΔqseB mutant with overproduction of GGDEF domain protein after induction of the latter strain with 0.2% arabinose in the Luria-Bertani (LB) medium. Recently, we demonstrated that the ΔqseB mutant formed significantly increased solid-surface-associated biofilms in polystyrene tubes, with a more than 2-fold increase in CV staining when compared to that of the WT A. hydrophila harboring the pBAD/Myc-HisB vector [10]. While an increase in c-di-GMP levels in WT A. hydrophila led to a significant increase in biofilm formation (Fig. 2), similar to that with the ΔqseB mutant, overproduction of c-di-GMP in the ΔqseB mutant resulted in decreased biofilm formation; the latter finding was similar to that noted for the WT A. hydrophila with vector alone (Fig. 2).
Fig 2.
Measurement of biofilm mass by CV staining formed on polystyrene plastic by the WT A. hydrophila SSU and its ΔqseB mutant. Biofilms were quantified after 24 h of incubation at 37°C. The results were reproduced in three independent experiments, and the error bars represent standard deviations. Overproduction of AHA0701h in the WT A. hydrophila statistically increased biofilm formation compared to the biofilm formed by the WT bacteria with empty vector (p < 0.001). The ΔqseB mutant with pBAD/Myc-HisB vector alone formed biofilms comparable to that seen with the WT A. hydrophila with GGDEF domain protein overproduction. When AHA0701h was overproduced in the ΔqseB mutant, the biofilm mass was similar to that seen in the WT A. hydrophila with the pBAD/Myc-HisB vector alone.
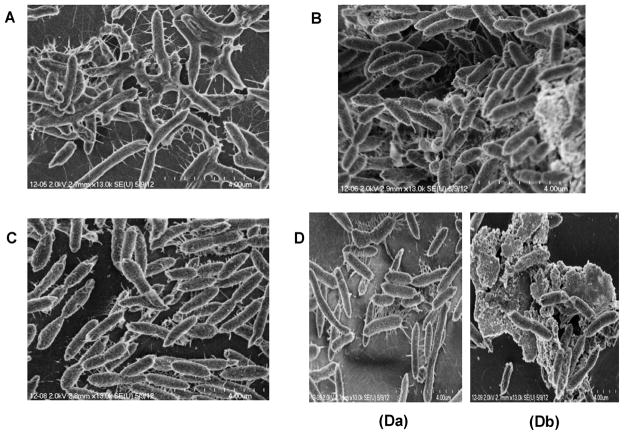
We then used SEM to investigate in detail the surface attachment and architecture of bacterial cell aggregation in biofilms formed by the WT A. hydrophila and its qseB mutant, both harboring the pBAD/Myc-HisB vector alone, as well as when c-di-GMP overproduced, after 48 h of cultivation on thermanox cover slips. As shown in Fig. 3A, biofilms of WT A. hydrophila with pBAD/Myc-HisB vector alone demonstrated compact and three-dimensional cell aggregation with exopolysaccharide (EPS) that covered and extended over the cells similar to that found in WT A. hydrophila without the plasmid vector [8, 10]. On the contrary, SEM of WT A. hyrophila, when c-di-GMP was overproduced, showed biofilms with greater number of cells forming highly compact three-dimensional structure and EPS appeared as pellets of condensed material around aggregated sessile cells (Fig. 3B). Aggregated cells of the qseB mutant harboring the pBAD/Myc-HisB vector alone formed a dense biofilm with intercellular filament connections and a very little EPS (Fig. 3C) that appeared similar to the biofilms in the qseB mutant [10]. Overproduction of c-di-GMP in the qseB mutant resulted in irregular shape of the biofilms with sparser cell aggregation (Fig. 3Da). Importantly, several biofilm regions of the qseB mutant with c-di-GMP overproduction included extensive EPS surrounding the mutant cells (Fig. 3Db).
Fig. 3.
Representative SEM images of biofilm formation by WT A. hydrophila SSU and its ΔqseB mutant after 48 h of cultivation at on37°C thermanox cover slips: A. WT A. hydrophila with the pBAD/Myc-HisB vector alone; B. WT A. hydrophila when c-di-GMP was overproduced; C. ΔqseB mutant with the pBAD//Myc-HisB vector alone; D (a, b) ΔqseB mutant with overproduction of c-di-GMP.
2.3. Overproduction of the GGDEF domain protein further ablates protease activity of the ΔqseB mutant A. hydrophila of SSU
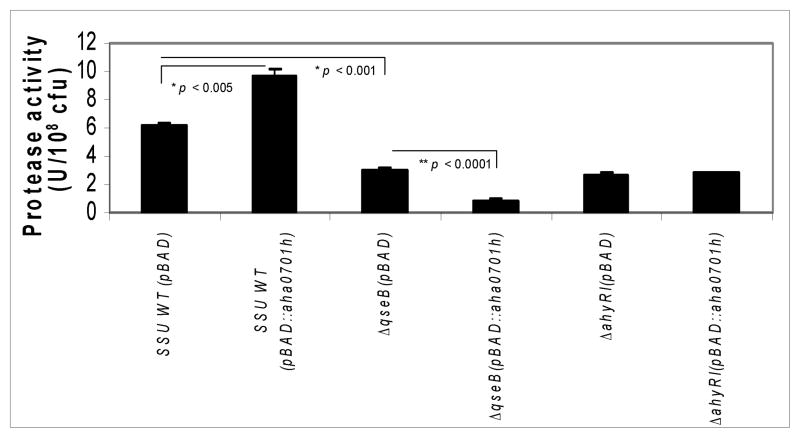
We examined the protease activity in the culture supernatants of WT A. hydrophila and its qseB and ahyRI mutants with and without the overproduction of c-di-GMP. While as we showed earlier, deletion of the ahyRI and qseB genes resulted in decreased protease production [7, 10], increase of c-di-GMP levels in the ΔqseB mutant further ablated protease production, with no further change in protease activity in the ahyRI mutant with increased levels of c-di-GMP.
On the contrary, the protease level was increased in the WT A. hydrophila when c-di-GMP was overproduced, compared to the parental strain with the pBAD/Myc-HisB empty vector (Fig. 4). These results were different from our earlier published data [10] in which we showed decreased protease level when c-di-GMP was overproduced from WT A. hydrophila compared to the parental strain with vector alone. We believe these differences could be attributed to bacterial growth conditions used. For example, in our earlier study [10], the bacteria were directly grown from −80°C stocks in the liquid culture for measuring enzymatic and other biological activities. We followed this method based on a reported study of Waters et al. [16] which indicated that levels of c-di-GMP were much higher in Vibrio cholerae when the bacteria were directly grown from −80°C stocks compared to when the bacteria were first grown in a liquid culture overnight and then subcultures were made. This was possibly related to decrease in c-di-GMP levels over time in the stationary phase of the bacterial growth.
Fig. 4.
Protease activity in the culture supernatants of WT A. hydrophila SSU and its ΔqseB and ahyRI mutants with pBAD/Myc-HisB vector alone, compared to the WT and mutants with overproduction of GGDEF domain protein. Overproduction of c-di-GMP in the WT A. hydrophila resulted in statistically significant increases of protease activity compared to that seen in the WT bacteria with the pBAD/Myc-HisB vector (p < 0.005). No protease activity was measured when AHA0701h-encoding gene was over-expressed in the ΔqseB mutant. No statistically significant differences in protease activity were found when the GGDEF domain protein was overproduced in the ahyRI mutant as compared to the mutant with the empty pBAD/Myc-HisB vector. The data were normalized to 1 × 108 cfu to account for any minor differences in the growth rates between the WT bacteria and mutant strains. All of the experiments were performed in triplicate and the data presented as arithmetic means ± standard deviations. * denotes statistically significant increase (WT SSU versus SSU with overproduction of c-di-GMP) and decrease (WT SSU versus ΔqseB mutant), respectively. ** denotes statistically significant decrease between the ΔqseB mutant with and without the overproduction of c-di-GMP (p < 0.0001).
However, in the current paper, we grew the cultures overnight in the liquid medium from −80°C stocks and then the subcultures were made. As a result, we noticed increase in protease activity in the WT A. hydrophila with increased c-di-GMP when compared to the parental bacteria with vector alone. We also noted that this effect on c-di-GMP levels in bacteria in terms of how we grew them modulated only some biological effects and only in some mutants of A. hydrophila.
2.4. Addition of aspartate does not restore the altered phenotypes (motility and biofilm formation) in the luxS mutant of A. hydrophila SSU
Mutations in the qseB and luxS genes of A. hydrophila resulted in decreased motility [8, 10]. The luxS mutation seems to alter cellular metabolism leading to decreased AI-3 production, possibly by reducing tyrosine levels in E. coli, while AI-3 signaling had little effect on bacterial metabolism [17]. To examine whether the decreased motility noted in the ΔqseB mutant(Fig. 1) could be interrelated with the LuxS-dependent metabolism of A. hydrophila, we assessed the restoration of QS-dependent phenotypes by complementing the defects in the luxS mutant at the level of oxaloacetate-homocysteine pathway. The addition of aspartate to the growth medium could change the nitrogen and carbon levels in the luxS mutant [17]. However, the addition of L-aspartate to the luxS mutant was not able to restore either the luxS mutant’s motility or biofilm formation (data not shown).
2.5. Alteration in the transcription of genes that encode three QS systems in the qseB, luxS, and ahyRI mutants of A. hydrophila SSU
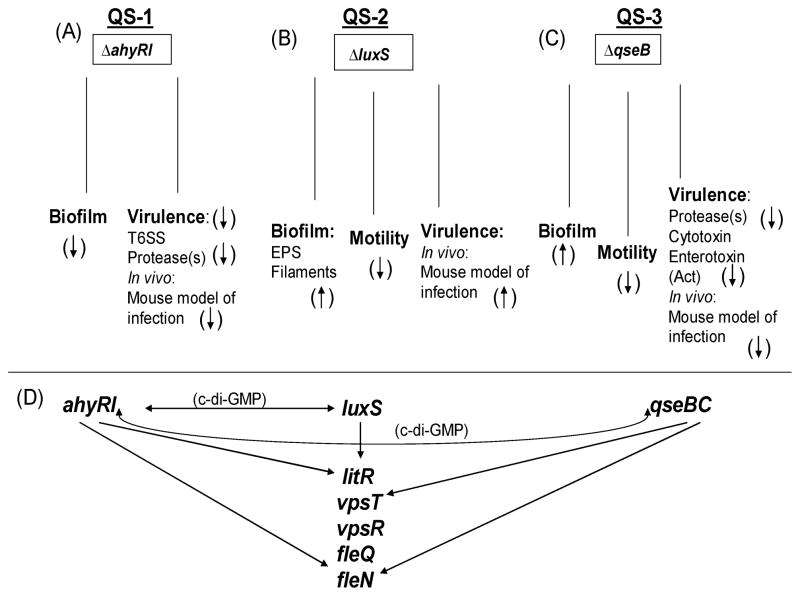
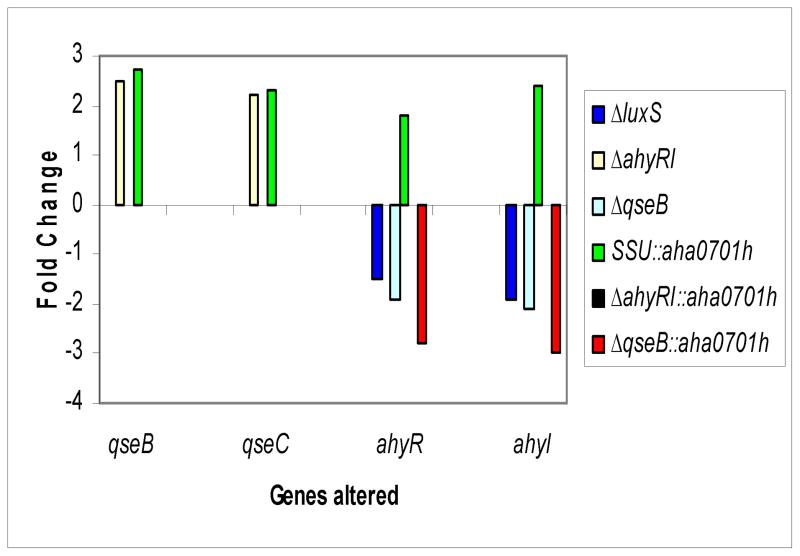
The existence in A. hydrophila of three QS systems (Fig. 5) prompted us to study the possibility of interconnections between them. Indeed, we found transcriptional interplay between LuxS-dependent and AhyRI-based QS systems [9]. We did not find alterations in the transcript levels of qseB and qseC genes in the luxS mutant of A. hydrophila when compared to that in the WT bacteria (Supplemental data, Table I). However, the transcription levels of qseB and qseC genes were increased (by over 2 fold) in the ahyRI mutant (Fig. 6 [yellow bars], Supplemental data, Table I). The mutation in the qseB gene did not alter the luxS transcript level (Supplemental data, Table 1), which was in contrast to the ahyRI deletion that caused the luxS gene transcript to be up-regulated [9]. The ahyR and ahyI genes were down-regulated (by 2 fold) in the ΔqseB mutant compared to that in the WT A. hydrophila (Fig. 6 [cyan bars], Supplemental data, Table I).
Fig. 5.
Integrated schematic illustrating QS-dependent regulatory network in A. hydrophila SSU. Three modified autoinducer (AI) systems, which are known to date in other bacteria, exist in A. hydrophila. (A) While the ahyRI-based (AI-1) QS system positively regulates bacterial virulence [7], the LuxS-based QS system (AI-2) negatively regulates A. hydrophila virulence [8] (B). (C) QseBC has both positive and negative regulation on various virulence factors/mechanisms of A. hydrophila [10] that play an important role in fine tuning the expression of virulence genes (Current study). (D) Cross talk between AI-1- and QseBC- systems was identified (Current study) in addition to interplay between AI-1 and AI-2 systems [10]. An alteration in the transcription of major genes involved in biofilm formation and motility of the WT A. hydrophila is dependent on the existence of AI-1, AI-2, and QseBC systems, and they are co-regulated via di-GMP. (↑) denotes an increase and (↓) shows a decrease in biofilm formation, motility, and virulence in ΔahyRI, ΔluxS, and ΔqseB mutants of A. hydrophila.
Fig. 6.
Comparison by RT-PCR of the expression of qseB, qseC, ahyR, and ahyI genes in different genetic backgrounds of A. hydrophila SSU. The transcript levels of the qseB and qseC genes were increased in the ahyRI mutant compared to those of the WT A. hydrophila (yellow bars). The qseB and qseC gene transcript levels were increased when c-di-GMP was overproduced in the WT A. hydrophila compared to those of the parental strain with pBAD/Myc-HisB vector (green bars). The level of expression of the ahyR and ahyI genes was down-regulated in the ΔluxS mutant. The transcript levels ahyR of the and ahyI genes were down-regulated in the ΔqseB mutant (cyan bars). The increased levels of c-di-GMP further down-regulated the expression levels of ahyR and ahyI genes in the ΔqseB mutant (red bars). Finally, the expression ahyR of the and ahyI genes was increased in the WT A. hydrophila with increased c-di-GMP levels (green bars). The data used to generate fold changes (arithmatic means ± standard deviations) with statistical analysis are shown in Supplemental data, Table I.
2.6. Alteration in the transcription of genes involved in biofilm formation and motility in the ΔqseB mutant A. hydrophila of SSU
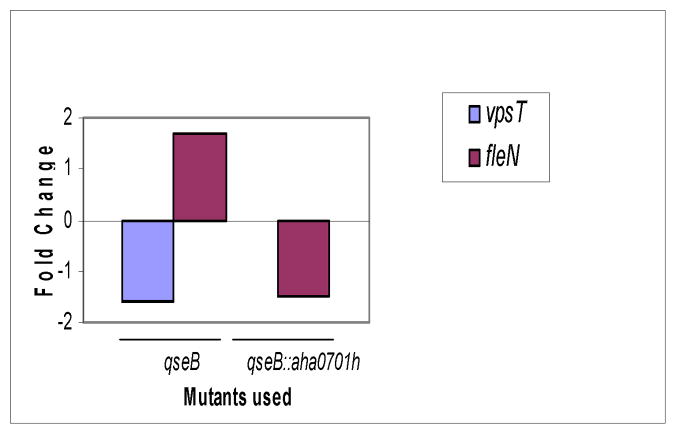
In our recent study, we examined the transcription of five major genes involved in biofilm formation and motility of the WT A. hydrophila and its ΔahyRI and luxS mutants [9]. We analyzed transcript levels of the same genes in the ΔqseB mutant, and, in contrast to findings in the luxS and ahyRI mutants, the transcript levels of only vpsT and fleN genes (Fig. 7, Supplemental data, Table 1 were altered in the ΔqseB mutant. As noted, the transcript level of fleN was increased and that of vpsT decreased in the ΔqseB mutant compared to that of the WT bacteria.
Fig. 7.
Alteration in the transcription of genes involved in biofilm formation and motility in the ΔqseB mutant A. of hydrophila SSU. The analysis of transcripts by RT-PCR demonstrated increases in the levels of the fleN gene and decreases in the levels of vpsT gene, compared to that of WT A. hydrophila. The GGDEF domain protein overproduction in the ΔqseB mutant returned the transcript level of vpsT the gene to the level found in the WT bacteria with the pBAD/Myc-HisB vector alone, while the expression of the fleN gene was down-regulated. The data used to generate fold changes (arithmatic means ± standard deviations) with statistical analysis are shown in the Supplemental data, Table I.
2.7. Effect of overproduction of the GGDEF domain protein on qseB and qseC gene expression in WT A. hydrophila SSU and its ΔahyRI mutant
The qseB and qseC gene transcript levels were increased (approximately 2–3 fold) when c-di-GMP was overproduced in the WT A. hydrophila compared to those of the parental bacteria with pBAD/Myc-HisB vector alone (Fig. 6 [green bars], Supplemental data, Table I). The level of expression of the qseB and qseC genes in the ahyRI mutant, when c-di-GMP was overproduced was comparable to the level seen in WT bacteria which harbored only the pBAD/Myc-HisB vector (Supplemental data, Table I). This was in contrast to increased expression levels of these genes (qseB and qseC) in the ahyRI mutant with the pBAD/Myc-HisB vector alone (Fig. 6 [yellow bars], Supplemental data, Table I). Thus, increased production of c-di-GMP in the ΔahyRI mutant normalized the transcriptional response of the qseB and qseC genes and made their levels comparable to those seen in the WT A. hydrophila.
2.8. Effect of overproduction of GGDEF domain protein on QS and QS-dependent gene transcripts in the ΔqseB mutant A. hydrophila of SSU
We found that, when compared to the WT A. hydrophila with the pBAD/Myc-HisB vector alone, ahyR and ahyI gene transcripts were even more down-regulated by approximately 3 fold [red bars] when c-di-GMP was overproduced in the ΔqseB mutant, compared to its already decreased level of expression [cyan bars] caused by qseB deletion (Fig. 6, Supplemental data, Table I). This finding was similar to the decreased levels of expression of these genes (ahyR and ahyI) in the luxS mutant, again when compared to the WT bacteria with the pBAD/Myc-HisB vector alone (Fig. 6 [blue bars], Supplemental data, Table I). When c-di-GMP was overproduced in the ΔqseB mutant, the expression of the fleN gene was down-regulated, and the transcription level of vpsT was restored to the level found in the WT bacteria (Fig. 7, Supplemental data, Table I). Thus, overall our results showed that the increased production of c-di-GMP in the ΔqseB mutant resulted in differential transcriptional responses of studied genes (motility and biofilm formation) when compared to that seen in the WT and the ahyRI mutant of A. hydrophila.
2.9. Effect of overproduction of the GGDEF domain protein on WT A. hydrophila SSU virulence in an animal model
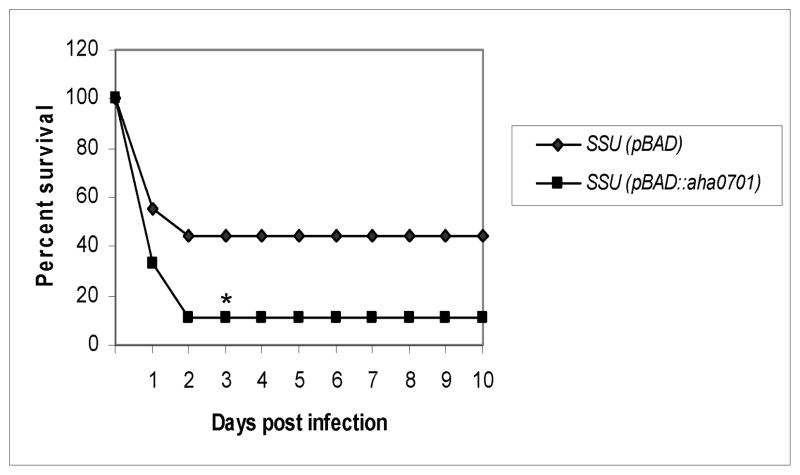
To study the effect of overproduction of c-di-GMP in WT A. hydrophila, we injected mice via the intraperitoneal (i.p.) route with either the WT A. hydrophila harboring the pBAD/Myc-HisB vector alone or the parental bacteria having increased c-di-GMP levels. As noted in Fig. 8, 56% of the animals infected with the WT A. hydrophila (pBAD/Myc-HisB) died within 2 days at a dose of 1 × 107 colony forming units (cfu), and when GGDEF domain protein was overproduced in the WT bacteria, an 89% mortality rate was noted at the same dose. These data indicated that increased production of c-di-GMP enhanced the virulence of the WT A. hydrophila.
Fig. 8.
The virulence of the WT A. hydrophila SSU and the parental bacteria with increased production of c-di-GMP in a mouse model. Swiss-Webster mice (n=9/group) were infected at doses of 1 × 107 cfu of the above-mentioned strains by the i.p. route. The animals were observed for mortality over a period of 10 days. The data were statistically analyzed by using a Kaplan-Meier survival estimate. * represents statistical significance at a p value of < 0.05.
DISCUSSION
In this study, we described the impact of the QseBC system on QS and that of c-di-GMP network regulation in A. hydrophila SSU. Our earlier report demonstrated that the AI-1 and AI-2 QS systems were interconnected, at least at the transcriptional level in A. hydrophila [9], and both of them were involved in the regulation of biofilm formation, albeit in an opposite way. Interestingly, however, the LuxS-dependent AI-2 QS system regulated bacterial motility [8], while the AhyRI-based AI-1 QS system did not regulate either the swimming or swarming motility of A. hydrophila [7].
The deletion of the qseB gene in A. hydrophila SSU resulted in decreased swarming and swimming motility, and an increase in c-di-GMP levels in the ΔqseB mutant further ablated the swimming motility [10]. Importantly, our current results showed contrasting data in terms of the swimming and the swarming motility associated with the ΔqseB mutant when c-di-GMP was overproduced (Fig. 1). While increased c-di-GMP levels led to a further decrease in the swimming motility [10], no further ablation of the swarming motility of the ΔqseB mutant was noted. Clarke et al. reported that QseBC regulated flagella and motility through the flagellar master regulator FlhDC in E. coli [4, 11]. They also demonstrated that in order to control motility, QseB directly bound to the flhDC promoter, both at the low- and high-affinity binding sites [4]. However, the flhDC genes do not exist in the A. hydrophila genome [18]. Recently, QseB was demonstrated as a negative regulator of bacterial motility in UPEC [5] and S. enterica serovar Typhimurium [19]. It was shown the qseB gene deletion did not influence the flagellar gene expression; however, the expression of the qseB gene was upregulated in the absence of QseC and that resulted in the downregulation of flagella, pili and curli genes in UPEC [5]. In contrast, the ΔqseB and ΔqseC mutants displayed significantly impaired motility, and this defect was rescued by complementation of the respective mutants with either the intact qseB or the qseC gene in E. tarda [12]. We showed that the deletion of the qseB gene in A. hydrophila SSU resulted in a dramatic decrease in the qseC transcript; at least we could not detect this transcript by RT-PCR. However, detectable levels of qseB and qseC gene transcripts were seen in the WT A. hydrophila strain.
Interestingly, over-expression of the aha0701h gene encoding the GGDEF domain protein in WT A. hydrophila SSU resulted in decreasing both swimming [9] and swarming motility (Fig. 1). These results demonstrated that high levels of c-di-GMP regulate the polar and lateral flagella of WT bacteria; however, this effect is restricted to polar flagellar regulation when the qseB gene was deleted, as polar and lateral flagella are involved in swimming and swarming motility, respectively [20]. Since we also showed that an increase in c-di-GMP levels in WT A. hydrophila led to enhanced transcripts for qseB and qseC genes (Fig. 6), this could lead to down-regulation of the flagellar genes and, hence, bacterial motility, as shown for UPEC [5]. In the future, it will be intriguing to delete the qseC gene and show the effect of this deletion on the expression of the qseB gene in WT A. hydrophila.
Biofilm formation represents a virulence mechanism in both Gram-positive [21, 22] and Gram-negative bacteria [23, 24]. Further, c-di-GMP has been shown to induce biofilm formation in V. cholerae [25, 26]. Although increased c-di-GMP levels resulted in enhanced biofilm formation in V. vulnificus, the degree of induction and the final level of biofilm formed were strain specific [27]. Our recent results showed that the overproduction of AHA0701h protein resulted in a dramatic increase in biofilm formation, as measured by CV staining and SEM, in WT A. hydrophila SSU (Figs. 2 and 3B) compared to that in bacteria harboring only the vector (Figs. 2 and 3A). However, increased production of c-di-GMP in the ahyRI mutant did not cause any quantitative alteration in biofilm formation (data not shown). Surprisingly, overproduction of c-di-GMP in the ΔqseB mutant decreased biofilm formation when compared to that in the ΔqseB mutant with vector alone (Figs. 2, 3C, 3Da and 3Db), and it was similar to that noted for the WT A. hydrophila SSU as measured by CV staining (Fig. 2). However, the distribution of qseB mutant cells with overproduction of GGDEF domain protein that formed a biofilm on the thermanox cover slip, was different, when compared to that of WT bacteria with increased c-di-GMP levels (Fig. 3B), and could be described as cells attached to islands including extensive EPS surrounding the mutant cells uniformly distributed all over the cover-slip surface (Fig. 3D). The differential biofilm-forming capacities of the strains could be due to differences in the expression or activity of downstream effector proteins, such as the proteins that bind c-di-GMP and transduce the signal to systems that regulate adhesion factor(s) [28].
Although many bacteria produce metalloproteases that have a zinc (II) ion in the catalytic site, other types of proteases, such as serine proteases, are also produced by pathogenic bacteria [29]. The role of proteases in the virulence of A. hydrophila has been well established [30]. The contribution of the AI-2 system, but not of the AI-1 system, to protease production in V. vulnificus was suggested [31]. However, this regulation was temperature dependent [32]. It appears that V. vulnificus produces protease via the QS system only in the tissues of the limbs, as the temperature there is lower than that in the bloodstream [29]. However, in human serum, V. vulnificus protease production increased at 37°C without an increase in the expression of the luxS gene [33]. Thus, V. vulnificus protease production in the host serum may be regulated by a system other than the AI-2-dependent QS system [29]. In our study, we found that protease activity in WT A. hydrophila SSU was modulated by the QseBC and AI-1 systems in a c-di-GMP-dependent manner (Fig. 4). In addition, our observation in a mouse model of the increased virulence of WT A. hydrophila with GGDEF domain protein over-production, compared to that of its parental strain with vector alone, demonstrated the role of c-di-GMP in the regulation of A. hydrophila virulence.
Based on our observation of LuxS-dependent and AhyRI-based QS system interplay (Fig. 5) [9], we studied the possible cross talk between three QS systems which exist in A. hydrophila SSU. A study on the QseBC system of E. coli showed that a mutation in the qseC gene did not have any effect on the transcription of other genes and phenotype regulation of the AI-2 QS mechanism [34]. However, the luxS gene mutation led to decreased levels of AI-3 in E. coli, which was dependent on the metabolic defect present in the ΔluxS mutant [17]. No phenotypic alterations were noted when L-aspartate was used as an alternative source of the homocysteine pathway during the growth of the A. hydrophila SSU ΔluxS mutant. In agreement with this, we did not find an alteration in the transcript levels of the qseB and qseC genes in the ΔluxS mutant of A. hydrophila (Supplemental data, Table I). However, the transcript levels of qseB and qseC genes were increased in the ahyRI mutant (Fig. 6, Supplemental data, Table I).
Mutation in the qseB gene did not alter the luxS transcript level, while the ahyR and ahyI genes were down-regulated in the ΔqseB mutant (Fig. 6). Of note is that only one common phenotype in addition to in vivo virulence attenuation, when the qseB and ahyRI genes were deleted, was the reduction in protease activity (Fig. 4). The observation of decreased ahyRI transcript levels in the ΔqseB mutant is in agreement with earlier studies [7, 35, 36]. Overall, mutations in either the qseB gene or the ahyRI genes caused an alteration in gene transcripts of the remaining functional QS system of A. hydrophila; these data lead us to suggest that the QseBC system interplays with the AI-1 QS system.
Recently, we observed that mutations in either the luxS gene or the ahyRI genes caused an alteration in the expression of the major genes involved in biofilm formation and motility of the WT A. hydrophila. [9]. In contrast to the luxS and ahyRI mutants, the transcription levels of vpsT and fleN genes only were altered in the ΔqseB mutant (Figs. 5 and 7). VpsT is a transcriptional activator involved in biofilm formation in Vibrio spp. [37]. Because we already demonstrated that vpsT gene expression is dependent on the existence of ahyRI, luxS and c-di-GMP levels in A. hydrophila SSU [9], we were interested in studying the effects of AHA0701h (GGDEF) over-production in the ΔqseB mutant.
Consequently, we showed that over-expression of one of the genes encoding a protein with a GGDEF domain in the ΔqseB mutant A. hydrophila of SSU resulted in no swimming motility, had no effect on swarming motility, exhibited an altered biofilm phenotype, and demonstrated no detectable protease activity. Interestingly, the qseB and qseC gene transcript levels were increased when c-di-GMP was overproduced in the WT A. hydrophila (Fig. 6). However, the level of expression of the qseB and qseC genes returned to that of the WT bacteria when c-di-GMP was overproduced in the ahyRI mutant (Supplemental data, Table I), while the ΔahyRI mutant showed an increase in these transcripts (Fig. 6).
We demonstrated that the QseBC system, in addition to c-di-GMP-dependent AI-1 system regulation, is involved in the modulation of expression of two major regulators of biofilm and motility, such as VpsT and FleN. An over-expression of the GGDEF domain protein-encoding gene in the ΔqseB mutant down-regulated the fleN gene transcript level (Fig. 7) in a manner similar to that seen in the ahyRI mutant [9]. However, the effect of overproduction of c-di-GMP on the expression of the vpsT gene was different in those two (qseB and ahyRI) mutants. For example, in contrast to the down-regulation of the vpsT gene expression by high levels of c-di-GMP in the ahyRI mutant [9], overproduction of the GGDEF domain protein in the ΔqseB mutant resulted in normalization of the vpsT gene expression to the level of WT A. hydrophila SSU (Fig. 7, Supplemental data, Table I).
Interestingly, interruption/mutation of either the QseBC or the AhyRI QS system abolished positive regulation of c-di-GMP overproduction on protease activity and, consequently, the mortality rate in mice, which was noted in animals infected with the WT A. hydrophila (pBAD::aha0701h). In contrast, Wang et al. [38] showed that c-di-GMP negatively regulated the production of HA/protease in V. cholerae. The authors proposed a model showing an interplay between c-di-GMP, HapR and RpoS in this regulation. Based on our observations on the gene transcript levels for qseB, qseC, ahyR, and ahyI in the qseB, ahyRI and luxS mutants, we believe that QseB regulates protease production indirectly through the AI-1 QS system in A. hydrophila. In addition, an interplay which alters transcript levels of vpsT and vpsR genes in the qseB, ahyRI and luxS mutants after c-di-GMP overproduction suggested that a downstream regulatory system, e.g., O-antigen lipopolysaccharide biosynthesis or flagellin glycosylation [39], could be involved in QS-dependent regulatory network and impact the virulence of A. hydrophila. Moreover, some of these genes could be regulated by local c-di-GMP, independent of the global alteration in c-di-GMP levels [40].
Overall, our data indicated an impact of the QseBC system on the c-di-GMP-dependent, quorum-sensing virulence regulatory network in a clinical isolate SSU of A. hydrophila, and demonstrated the interplay between three existing QS systems.
4. MATERIALS AND METHODS
4.1. Bacterial strains and plasmids
The sources of A. hydrophila strains, as well as the plasmids used in this study, are listed in Table 1. The antibiotics ampicillin (Ap), kanamycin (Km), streptomycin (Sp), and spectinomycin (Sm) were used at concentrations of 50–500, 50–100, 50–100 and 50–100 μg/ml, respectively, in Luria-Bertani (LB) medium or agar plates. Rifampicin (Rif) was utilized at a concentration of 100 μg/ml for bacterial growth and 300 μg/ml during transformation experiments. All of the antibiotics used were obtained from Sigma (St. Louis, MO). Aspartate at a concentration of 0.5 mM was added to the growth medium for the ΔluxS mutant cultivation. The Advantage cDNA PCR Kit was purchased from Clontech (Palo Alto, CA). The digested plasmid DNA or DNA fragments from agarose gels were purified by using a QIApreps Miniprep Kit (Qiagen, Inc., Valencia, CA). The medium was supplemented with L-arabinose (0.2%) when the ggdef gene was expressed from the pBAD/Myc-HisB::aha0701h plasmid (Table 1) under the control of an arabinose-inducible araC promoter in the pBAD vector.
Table 1.
Strains and plasmids used in this study
| Strain or Plasmid | Relevant characteristic (s) | Source or reference |
|---|---|---|
| A. hydrophila SSU | CDCa | |
| SSU-Rifr | Rifr strain of A. hydrophila SSU | Laboratory stock |
| ΔqseB | qseB gene deletion mutant of A. hydrophila SSU-R strain Rifr Kmr | [10] |
| ΔahyRI | ahyRI gene deletion mutant of A. hydrophila SSU-R strain Rifr Smr Spr | [7] |
| ΔluxS | luxS mutant of A. hydrophila SSU-R strain Rifr Kmr | [8] |
| SSU (pBAD::aha0701h) | WT A. hydrophila SSU with GGDEF domain protein overproduced | [9] |
| ΔahyRI (pBAD::aha0701h) | ahyRI gene deletion mutant of A. hydrophila with GGDEF domain protein overproduced | [9] |
| ΔqseB (pBAD::aha0701h) | qseB gene deletion mutant of A. hydrophila SSU with GGDEF domain protein overproduced | This study |
| Plasmids | ||
| pBAD/Myc-HisB | vector, ara BAD promoter Apr | Invitrogen |
| pBAD:: aha0701h | GGDEF domain encoded gene of A. hydrophila cloned into pBAD/Myc-HisB ara Kmr Apr | [9] |
Abbreviations: Rif, rifampin; Km, kanamycin, Sm, streptomycin, Sp, spectinomycin,
Centers for Disease Control and Prevention
4.2. Motility assay
LB medium with 0.3% Difco Bacto-agar (Difco Laboratories, Detroit, MI) was used to characterize the swimming motility, while Difco nutrient broth with 0.5% Eiken agar (Eiken Chemical Co., Ltd., Tokyo, Japan) was employed for measuring the swarming motility of WT A. hydrophila SSU and its ΔqseB mutant strain, as described in our previous study [10]. The overnight cultures grown in the presence of the antibiotics used were adjusted to the same optical density, and equal numbers of cfu (106) were stabbed onto 0.35% LB agar plates. Plates were incubated at 37°C overnight, and the motility was assayed by examining the migration of bacteria through the agar from the center towards the periphery of the plate.
4.3. Crystal violet (CV) biofilm assay
The WT A. hydrophila SSU and its ΔqseB mutant were grown in 3 ml of LB broth contained in polystyrene tubes at 37°C for 24 h with shaking. Biofilm formation was quantified according to the procedure described elsewhere [10]. The biofilm formation results were normalized to 1 × 109 cfu to account for any minor differences in the growth rate of the various bacterial strains used. The experiment was repeated independently three times.
4.4. In vitro growth of the biofilm
Briefly, 6-well, sterile polystyrene microtiter plates were filled with 2.5 ml of LB broth supplemented with the appropriate antibiotics. Next, a sterile, 13-mm-diameter thermanox plastic cover slip was laid in each of the wells. Medium was inoculated with 106 cfu of WT A. hydrophila or its mutants, and the plates were incubated at 37°C. After 12, 24 or 48 h of incubation with gentle shaking (65 rpm), those bacterial cells that were not sufficiently adherent, were removed along with the planktonic cells, by 3 washes of water. Glass or Thermanox plastic cover slips containing the attached biofilms were removed, rinsed with water, and viewed under light microscopy.
4.5 Scanning electron microscopy (SEM) of biofilms
SEM on biofilm formation of A. hydrophila SSU and its mutants was performed using 13-mm-diameter thermanox plastic cover slips. After 48 h of incubation, unattached cells were removed by washing with distillate water, the cover slips were fixed, and samples were examined in a Hitachi S4700 field emission scanning electron microscope (Hitachi High Technologies America) according to the procedure described in our previous studies [7, 8].
4.6. Primers and PCR assays
The primers used for various experiments are indicated in Table 2 and were synthesized by Sigma-Aldrich Biotechnology LP (The Woodlands, TX). The PCR assays were performed with 100 ng of genomic DNA (gDNA) and 50 ng of plasmid DNA.
Table 2.
Oligonucleotides used and RT-PCR products
| Primers | Oligonucleotide sequences | Amplification of the genes | RT-PCR product (bp) |
|---|---|---|---|
|
| |||
| qseBF2 | TGCTCAAGAGCGAGGAGTTTG | qseB | 551 |
| qseBR2 | CTTCTTGCGCAGGTGGTGAAT | ||
|
| |||
| qseCF2 | ATGGAGGAGCTGTTCGATGCC | qseC | 793 |
| qseCR2 | AGCAGCATCTTCTGCAGGGAGT | ||
|
| |||
| vpsTF1 | TCAGAGATACTCCTTGGCCCA | vpsT (csgAB) | 645 |
| vpsTR1 | CGCTTCATGATCACCCCATA | ||
|
| |||
| fleNF1 | TGGTCTGCGCAAAATGCGT | fleN | 856 |
| fleNR1 | TTATTCACGGGAACCTTCCTG | ||
|
| |||
| luxSF2 | ACCTCCAAGTGGGATGCGTAT | luxS | 971 |
| luxSR2 | CGGGCCATCGAAAAAATGT | ||
|
| |||
| ahyRF1 | TATTGCATCAGCTTGGGGAA | ahyR | 781 |
| ahyRR1 | TGAAACAAGACCAACTGCTTG | ||
4.7. Reverse transcription–polymerase chain reaction (RT-PCR)
Standard conditions for the isolation of total RNA from cells grown overnight in LB medium, cDNA generation, reverse-transcription PCR procedure, and an estimation of gene transcript levels were performed as described [9]. Briefly, RT-PCR was performed by using SuperScript™ III Platinum (Invitrogen, Carlsbad, CA). Equal amounts of DNase-treated total RNA (500 ng) were used to generate cDNA with random hexamer primers according to the manufacturer’s protocol. Sequences of primers used in RT-PCR are shown in Table 2. The relative levels of the cDNAs of RT-PCR were determined by densitometric analyses with AlphaEasyFC software (AlphaInnotech, San Leandro, CA) by using 16S rRNA genes as references. Each RT-PCR reaction which was reported in our earlier paper [9] was rerun as control in the present study. The results were very reproducible and demonstrated exactly the same fold increases compared to a similar supplemental data table presented in our earlier study [9].
4.8. Measurement of the protease activity
Protease activity was measured in culture filtrates of overnight-grown WT A. hydrophila SSU and its ΔqseB and ahyRI mutants as described earlier [41]. The cultures were grown overnight in a liquid medium from −80°C stocks and then the subcultures were made. The protease activity was calculated per ml of the culture filtrate per 108 cfu. The hide azure powder substrate (Calbiochem, La Jolla, CA) was used for measuring protease activity because of the sensitivity and rapidity of the assay. The substrate incubated with Dulbecco’s phosphate-buffered saline (DPBS) alone served as a negative control.
4.9. Animal experiments
Groups (n=9) of Swiss Webster female mice (Taconic Farms, CA) were infected via the intraperitoneal (i.p.) route with the WT A. hydrophila (pBAD/Myc-HisB) and the WT A. hydrophila in which the GGDEF domain protein was overproduced, in accordance with an approved Institutional Animal Care and Use Committee protocol. The animals were infected at doses of 1 × 107 cfu of the above-mentioned strains. Deaths were recorded for 10 days post-infection. The data were statistically analyzed by using Kaplan-Meier survival estimates. * represents statistical significance at p < 0.05.
4.10. Statistical analysis
All of the experiments were performed in triplicate, and, wherever appropriate, the data were analyzed by using the Student’s t test, with a p value of ≤ 0.05 considered significant. The data were presented as an arithmetic mean ± standard deviation.
Supplementary Material
Highlights.
Connection of 3 QS systems through c-di-GMP was established in A. hydrophila.
Increase of c-di-GMP in WT A. hydrophila regulated bacterial virulence.
c-di-GMP levels in the qseB mutant modulated A. hydrophila virulence phenotypes.
c-di-GMP-dependent transcription of fleN and vpsT was noted in the qseB mutant.
Interplay between QseBC and AhyRI systems at the transcript level was established.
Acknowledgments
This work was supported by the grants from NIH/NIAID (AI041611) and the Environmental Protection Agency. We thank Ms. Mardelle Susman for editing the manuscript and Ms. Michelle Kirtley for providing assistance in animal experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria-host communication: the language of hormones. Proc Natl Acad Sci U S A. 2003;100:8951–6. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A. 2006;103:10420–5. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes DT, Clarke MB, Yamamoto K, Rasko DA, Sperandio V. The QseC adrenergic signaling cascade in Enterohemorrhagic E. coli (EHEC) PLoS Pathog. 2009;5(8):e1000553. doi: 10.1371/journal.ppat.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke MB, Sperandio V. Transcriptional regulation of flhDC by QseBC and sigma (FliA) in enterohaemorrhagic Escherichia coli. Mol Microbiol. 2005a;57:1734–49. doi: 10.1111/j.1365-2958.2005.04792.x. [DOI] [PubMed] [Google Scholar]
- 5.Kostakioti M, Hadjifrangiskou M, Pinkner JS, Hultgren SJ. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol Microbiol. 2009;173:1020–31. doi: 10.1111/j.1365-2958.2009.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira CG, Weinshenker D, Sperandio V. QseC mediates Salmonella enterica serovar Typhimurium virulence in vitro and in vivo. Infect Immun. 2010;78:914–26. doi: 10.1128/IAI.01038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khajanchi BK, Sha J, Kozlova EV, Erova TE, Suarez G, Sierra JC, Popov VL, Horneman AJ, Chopra AK. N-acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology. 2009;155:3518–31. doi: 10.1099/mic.0.031575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozlova EV, Popov VL, Sha J, Foltz SM, Erova TE, Agar SL, Horneman AJ, Chopra AK. Mutation in the S-ribosylhomocysteinase (luxS) gene involved in quorum sensing affects biofilm formation and virulence in a clinical isolate of Aeromonas hydrophila. Microb Pathog. 2008;45:343–54. doi: 10.1016/j.micpath.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Kozlova EV, Khajanchi BK, Sha J, Chopra AK. Quorum sensing and c-di-GMP-dependent alterations in gene transcripts and virulence-associated phenotypes in a clinical isolate of Aeromonas hydrophila. Microb Pathog. 2011;50:213–23. doi: 10.1016/j.micpath.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khajanchi BK, Kozlova EV, Sha J, Popov VL, Chopra AK. The two-component QseBC signaling system regulates in vitro and in vivo virulence of Aeromonas hydrophila. Microbiology. 2012;158:259–71. doi: 10.1099/mic.0.051805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke MB, Sperandio V. Transcriptional autoregulation by quorum sensing Escherichia coli regulators B and C (QseBC) in enterohaemorrhagic E. coli (EHEC) Mol Microbiol. 2005b;58:441–55. doi: 10.1111/j.1365-2958.2005.04819.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wang Q, Yang M, Xiao J, Liu Q, Wu H, Zhang Y. QseBC controls flagellar motility, fimbrial hemagglutination and intracellular virulence in fish pathogen Edwardsiella tarda. Fish Shellfish Immunol. 2011;30:944–53. doi: 10.1016/j.fsi.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Ishida M, Oshima T. Effective structure of a leader open reading frame for enhancing the expression of GC-rich genes. J Biochem. 2002;132:63–70. doi: 10.1093/oxfordjournals.jbchem.a003199. [DOI] [PubMed] [Google Scholar]
- 14.Navarro MV, De N, Bae N, Wang Q, Sondermann H. Structural analysis of the GGDEF-EAL domain-containing c-di-GMP receptor FimX. Structure. 2009;17:1104–16. doi: 10.1016/j.str.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yildiz FH, Visick KL. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 2009;17:109–18. doi: 10.1016/j.tim.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters CM, Lu W, Rabinowitz JD, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J Bacteriol. 2008;190:2527–36. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters M, Sircili MP, Sperandio V. AI-3 synthesis is not dependent on luxS in Escherichia coli. J Bacteriol. 2006;188:5668–81. doi: 10.1128/JB.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshadri R, Joseph SW, Chopra AK, Sha J, Shaw J, Graf J, Haft D, Wu M, Ren Q, Rosovitz M, Madupu R, Tallon L, Kim M, Jin S, Vuong H, Stine OC, Ali A, Horneman AJ, Heidelberg JF. Genome sequence of Aeromonas hydrophila ATCC 7966T: jack of all trades. J Bacteriol. 2006;188:8272–82. doi: 10.1128/JB.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bearson BL, Bearson SM, Lee IS, Brunelle BW. The Salmonella enterica serovar Typhimurium QseB response regulator negatively regulates bacterial motility and swine colonization in the absence of the QseC sensor kinase. Microb Pathog. 2010;48:214–9. doi: 10.1016/j.micpath.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Kirov SM, Tassell BS, Semmler ABT, O’Donovan LA, Rabaan AA, Shaw JG. Lateral Flagella and Swarming Motility in Aeromonas Species. J Bacteriol. 2002;184:547–55. doi: 10.1128/JB.184.2.547-555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oggioni MR, Trappetti C, Kadioglu A, Cassone M, Iannelli F, Ricci S, Andrew PW, Pozzi G. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol Microbiol. 2006;61:1196–210. doi: 10.1111/j.1365-2958.2006.05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen FC, Ahmed NA, Naemi A, Scheie AA. LuxS-mediated signalling in Streptococcus anginosus and its role in biofilm formation. Antonie Van Leeuwenhoek. 2006;90:109–21. doi: 10.1007/s10482-006-9065-y. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5:647–56. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 24.de Kievit TR. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2009;11:279–88. doi: 10.1111/j.1462-2920.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 25.Lim B, Beyhan S, Meir J, Yildiz FH. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol Microbiol. 2006;60:331–48. doi: 10.1111/j.1365-2958.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- 26.Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol. 2004;53:857–69. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDougald D, Lin WH, Rice SA, Kjelleberg S. The role of quorum sensing and the effect of environmental conditions on biofilm formation by strains of Vibrio vulnificus. Biofouling. 2006;22:133–44. doi: 10.1080/08927010600691879. [DOI] [PubMed] [Google Scholar]
- 28.Nakhamchik A, Wilde C, Rowe-Magnus DA. Cyclic-di-GMP regulates extracellular polysaccharide production, biofilm formation, and rugose colony development by Vibrio vulnificus. Appl Environ Microbiol. 2008;74:4199–209. doi: 10.1128/AEM.00176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinoda S, Miyoshi S. Proteases produced by vibrios. Biocontrol Sci. 2011;6:1–11. doi: 10.4265/bio.16.1. [DOI] [PubMed] [Google Scholar]
- 30.Cascón A, Yugueros J, Temprano A, Sánchez M, Hernanz C, Luengo JM, Naharro G. A major secreted elastase is essential for pathogenicity of Aeromonas hydrophila. Infect Immun. 2000;68:3233–41. doi: 10.1128/iai.68.6.3233-3241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SY, Lee SE, Kim YR, Kim CM, Ryu PY, Choy HE, Chung SS, Rhee JH. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol Microbiol. 2003;48:1647–64. doi: 10.1046/j.1365-2958.2003.03536.x. [DOI] [PubMed] [Google Scholar]
- 32.Miyoshi S, Sultan SZ, Yasino Y, Sinoda S. Growth phase-dependent production of a toxic metalloprotease by Vibrio vulnificus. Toxin Rev. 2006;25:19–30. [Google Scholar]
- 33.Kawase T, Miyoshi S, Sultan Z, Shinoda S. Regulation system for protease production in Vibrio vulnificus. FEMS Microbiol Lett. 2004;240:55–9. doi: 10.1016/j.femsle.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Sperandio V, Torres AG, Kaper JB. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol. 2002;43:809–21. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 35.Bi ZX, Liu YJ, Lu CP. Contribution of AhyR to virulence of Aeromonas hydrophila. J-1 Res Vet Sci. 2007;83:150–6. doi: 10.1016/j.rvsc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Swift S, Lynch MJ, Fish L, Kirke DF, Tomas JM, Stewart GS, Williams P. Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila. Infect Immun. 1999;67:5192–9. doi: 10.1128/iai.67.10.5192-5199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casper-Lindley C, Yildiz FH. VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J Bacteriol. 2004;186:1574–8. doi: 10.1128/JB.186.5.1574-1578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Wu JH, Ayala JC, Benitez JA, Silva AJ. Interplay among cyclic diguanylate, HapR, and the general stress response regulator (RpoS) in the regulation of Vibrio cholerae hemagglutinin/protease. J Bacteriol. 2011;193:6529–38. doi: 10.1128/JB.05166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabei SM, Hitchen PG, Day-Williams MJ, Merino S, Vart R, Pang PC, Horsburgh GJ, Viches S, Wilhelms M, Tomás JM, Dell A, Shaw JG. An Aeromonas caviae genomic island is required for both O-antigen lipopolysaccharide biosynthesis and flagellin glycosylation. J Bacteriol. 2009;191:2851–63. doi: 10.1128/JB.01406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuchma SL, Ballok AE, Merritt JH, Hammond JH, Lu W, Rabinowitz JD, O’Toole GA. Cyclic-di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa: the pilY1 gene and its impact on surface-associated behaviors. J Bacteriol. 2010;192:2950–64. doi: 10.1128/JB.01642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erova TE, Pillai L, Fadl AA, Sha J, Wang S, Galindo CL, Chopra AK. DNA adenine methyltransferase influences the virulence of Aeromonas hydrophila. Infect Immun. 2006;74:410–24. doi: 10.1128/IAI.74.1.410-424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.