Abstract
In addition to its cardinal symptoms of bradykinesia, muscle rigidity, resting tremor and postural disturbances, Parkinson’s disease (PD) also affects orolingual motor function. Orolingual motor deficits can contribute to dysphagia, which increases morbidity and mortality in this population. Previous preclinical studies describing orolingual motor deficits in animal models of PD have focused on unilateral nigrostriatal dopamine (DA) depletion. In this study we compared the effects of unilateral vs bilateral 6-hydroxydopamine (6-OHDA)-induced DA depletion in rats trained to lick water from an isometric force-sensing disc. Rats received either unilateral or bilateral 6-OHDA into the medial forebrain bundle and were tested for four weeks post-lesion. Dependent variables included task engagement (the number of licks per session), tongue force (mean and maximum), and tongue motility (the number of licks per second). While both lesion groups exhibited decreased tongue force output, tongue motility deficits were present in only the group that received unilateral nigrostriatal DA depletion. Task engagement was not significantly diminished by 6-OHDA. Analysis of striatal DA tissue content revealed that DA depletion was ~97% in the unilateral group and ~90% in the bilateral group. These results suggest that while nigrostriatal DA depletion affects tongue force output, deficits in tongue motility may instead result from a functional imbalance in neural pathways affecting this midline structure.
Keywords: Parkinson’s disease, orolingual, oromotor, isometric, operant, behavioral, nigrostriatal, dysarthria, dysphagia, force
Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disease with characteristic symptoms that include bradykinesia, muscle rigidity, resting tremor and postural disturbances. These symptoms result primarily from degeneration of dopamine (DA) neurons in the substantia nigra, and depletion of DA in the nigrostriatal pathway [2,11]. Preclinical studies have taken advantage of this well-characterized lesion by targeting the nigrostriatal pathway with neurotoxic agents such as 6-hydroxydopamine (6-OHDA) and MPTP (reviewed in [8]). These models, which typically involve unilateral DA depletion, generally recapitulate the cardinal symptoms of PD.
In addition to its cardinal symptoms, PD can also impair orolingual and pharyngeal motor function [1,18,27,40]. These impairments can increase morbidity and mortality [25], primarily by disrupting the oral phase of swallowing [44]. In contrast to limb deficits (and with the exception of levodopa-induced oral dyskinesias [15]), preclinical attention to the primary effects of nigrostriatal DA depletion on orolingual motor function is a relatively recent development [13,36,41,47]. These studies have reported that unilateral nigrostriatal DA depletion impairs tongue motility and protrusion force. The effects of bilateral nigrostriatal DA depletion on orolingual motor function have not been reported. This is an important consideration, not only because the tongue is a midline structure, but because onset in PD is typically unilateral, progressing bilaterally in later stages [23].
The purpose of this study was to compare the effects of bilateral and unilateral nigrostriatal DA depletion models on orolingual motor function in rats. Rats’ tongue force and tongue motility were measured as they licked water from an isometric disc as described previously by our lab [43,51] and others [36,41]. Orolingual motor function was measured prior to and for four weeks after treatment with 6-OHDA. We hypothesized that rats with bilateral striatal DA depletion would exhibit greater deficits in tongue force and tongue motility than rats with unilateral lesions.
Materials and Method
Animals
Male Sprague Dawley rats were obtained from Harlan. Rats were 3-months-old at the time of testing, were housed individually and were maintained on a 12 h light/dark cycle. After acclimation to the facility, access to water was gradually restricted with food made available ad libitum. The water restriction schedule allowed for slow weight gain and provided rats with necessary motivation to perform the water licking task. Procedures adhered to the Guide for the Care and Use of Laboratory Animals [31], and the experiment was approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Behavioral Testing
Licking behavior was recorded using a lick force chamber as described previously [20,41,43]. Briefly, thirsty rats were placed in individual customized Gerbrands rodent operant chambers, each with a front panel containing a 6 cm2 hole at floor level. Affixed to the square hole is a 6 cm3 transparent enclosure that, on its lower horizontal surface, contains a 12 mm-diameter hole through which the rat can extent its tongue downward to reach the operandum. The operandum is an 18 mm-diameter aluminum disc rigidly attached to the shaft of a Model 31 load cell (0–250 g range, Sensotec, Columbus, OH). The disc is centered 2 mm below the hole in the plastic enclosure. A computer-controlled peristaltic pump (Series E at 14 rpm; Manostat Corp., New York, NY), fitted with a solid-state relay (Digikey, Thief River Falls, MN), delivers water to the center of the disc through a 0.5 mm-diameter hole. The force transducer was capable of resolving force measurements to 0.2-g equivalent weights. A PC recorded the transducer’s force-time output sampled at 100 samples/s. Rats were exposed to the water-licking task during 6 minute sessions until they licked reliably. The force requirement was 1 g to register a response and 12 licks were required to produce 0.05 ml of water. Each session started with a free 0.05 ml delivery of water. Rats were tested daily (5 days/week) until they achieved a stable baseline. Testing resumed within a week after surgeries and continued for four weeks post-surgery. Brains were then removed for tissue harvest.
6-OHDA Infusion
After achieving a stable baseline, rats were anesthetized with isoflurane (5% induction; 2% maintenance) and placed in a stereotaxic frame atop an isothermal heating pad. Rats in the unilateral group (n=8) received 9.0 μg 6-OHDA (mixed in 0.9% NaCl with 0.02% ascorbate) into the right medial forebrain bundle (MFB; stereotaxic coordinates with respect to bregma: 6 mm posterior, 1.7 mm lateral, 7.0 mm ventral to the dural surface [34]). Rats in the bilateral lesion group (n=5) received 3.0 μg 6-OHDA into the right and left MFB. The sham group (n=6) received bilateral injections of saline (0.9% NaCl with 0.02% ascorbate) into the MFB. Infusion rate was 0.25 μL/min. Needles were left in place after infusions for 5 min and then slowly withdrawn. Animals were administered 0.05 mg/kg buprenorphine and 5 mg/kg ketoprofen post-surgery and were placed on a warm surface to prevent hypothermia until recovery.
Quantitative Analysis
Orolingual motor function were assessed in terms of four dependent variables: 1) number of licks per session, 2) the rhythm of licking in licks/second, 3) the mean peak lick force (g), and 4) the maximum lick force (g). The number of licks was a count of the number of tongue contacts that equaled or exceeded 1 g. The lick rhythm was determined as follows: computation of the power spectra was performed by MatLab’s Signal Processing Toolbox (The Math Works, Inc, Natick, MA). For this analysis, each 6-min session was divided into 35 series of 1024 samples from the lick-force transducer. With the Hanning data window selected, MatLab produced 35 corresponding power spectra. The power spectra were truncated to 25 Hz (based on prior work indicating little of behavioral interest beyond 25 Hz) and averaged together to yield a single power spectrum. A peak-find program written in Free Pascal was used to identify the peak in the averaged power spectrum, and the frequency at this peak was taken as the lick rhythm for a particular session. This method resolved lick rhythm to the nearest 0.1 Hz. The peak lick force was the mean of the peak forces exerted during a session, and the maximum lick force was the maximum force produced during a session. For statistical analyses, pre-lesion data for each variable were expressed as the mean of the values for the final three days prior to surgery. Post-lesion data were expressed as a percentage of the pre-lesion values for each week post-lesion, beginning with week 2 (due to the extent of response suppression during the first week post-lesion primarily in the bilateral group). Data for each measure were analyzed using a two-way analysis of variance (ANOVA) with group (sham vs unilateral vs bilateral) as the between-subjects variable and post-lesion time as the within-subjects repeated measure.
Analysis of Dopamine Content
On the 5th week post-lesion, brains were removed and placed in a chilled brain mold for sectioning as described previously [6,7,29,30]. Bilateral striatal sections were removed, weighed and frozen at −80 °C until processing. Striatal tissue was processed for analysis by high pressure liquid chromatography coupled with an electrochemical detector (HPLC-EC). Tissue samples were sonicated in 450 μL burnt, filtered citrate acetate mobile phase with 50 μL DHBA (0.1mM) added to each sample. After sonication, tubes were placed in a cold centrifuge for 10 min at 12000 rpm at 4 °C. Supernatant was then extracted and placed into Amicon Ultra 0.5 mL centrifugal filters and spun at 12,000 rpm for 1 h at 4 °C. HPLC-EC analysis was performed on collected eluent using an isocratic HPLC coupled to a dual-channel Coulochem III electrochemical detector (ESA Inc., Chelmsford, MA, USA; Model 5011A, E1 +0.35 mV and E2 −0.25 mV using a 5011 dual analytical cell). The citrate acetate mobile phase was comprised octane sulfonic acid (0.07375 g/L), ethylenediaminetetraacetic acid (0.05 g/L), citric acid (14 g/L), sodium acetate trihydrate (13.8 g/L), triethylamine (0.01%), and methanol (4%). The mobile phase was made from filtered water using a Milli-Q purification system (Millipore) and was filtered through a 0.2 μm nylon membrane filter (Whatman). We used a 3 μm CAPCELL PAK reversed phase C-18 column (Shiseido). Dopamine content was expressed in ng/g tissue wet weight.
Results
Orolingual Motor Function
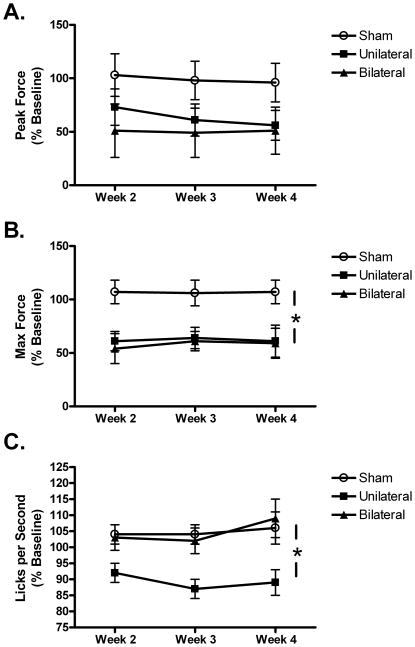
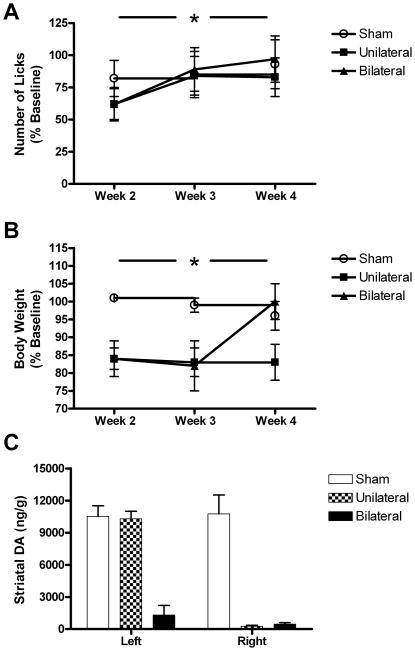
Pre-lesion values for body weight and the orolingual motor variables are provided in Table 1. None of the groups differed significantly with regard to body weight, tongue force, tongue motility or number of licks during these baseline measurements. Nigrostriatal DA depletion significantly attenuated tongue force in both lesion groups (Fig. 1). Although the effect for peak force did not differ significantly, maximum lick force was significantly decreased, F=5.236, p=0.01 (Fig. 2A & B). This effect was similar for the two 6-OHDA groups. Tongue motility was also decreased following 6-OHDA, but, unlike tongue force, only in the unilateral group, F=6.952, p<0.01 (Fig. 2C). The number of licks per second did not differ between the bilateral and sham group. Despite a slight decrease in week two post-lesion, animals in each group remained engaged in the task as the number of licks per session did not differ significantly between groups (Fig. 3A). The measure did increase between weeks two and four post-lesion, leading to an overall effect for week, F=9.172, p=0.001.
Table 1.
Baseline group means and standard errors of means for body weight and orolingual motor measures prior to lesions.
| Body Weight (g) | Peak Force (g) | Maximum Force (g) | Frequency (licks/second) | Number of Licks (licks/session) | |
|---|---|---|---|---|---|
| Group | |||||
| Sham | 460 ± 33 | 8.6 ± 2.6 | 21.6 ± 2.0 | 5.68 ± 0.54 | 592 ± 58 |
| Unilateral | 488 ± 9.0 | 8.8 ± 1.4 | 23.1 ± 3.4 | 5.69 ± 0.23 | 542 ± 43 |
| Bilateral | 451 ± 27 | 7.5 ± 0.6 | 20.0 ± 2.9 | 5.55 ± 0.29 | 482 ± 106 |
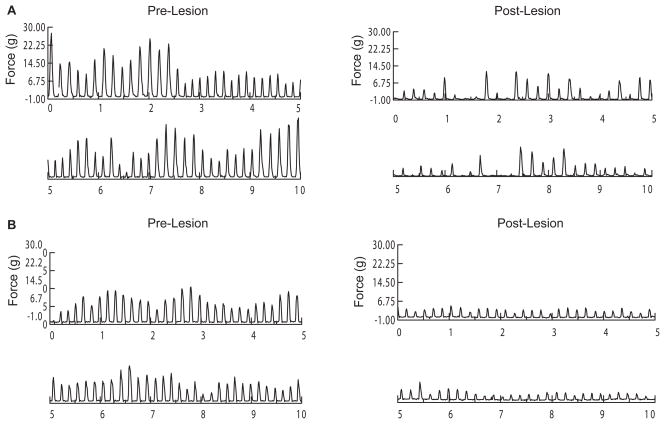
Figure 1.
Raw force-time waveforms from a representative unilaterally-lesioned rat (A) and bilaterally-lesioned rat (B). Each panel includes 10 seconds of responding during a 6-min session prior to (left panels) or 4 weeks post-lesion (right panel). The post-lesion decline in tongue force is apparent in both rats. Although the force deficit appears greater in this bilaterally-lesioned rat, the deficit was of similar magnitude between the two lesion groups. Despite this, the number of licks/second was affected only in the unilaterally-lesioned rat (from 5.8 licks/s to 4.8 licks/s).
Figure 2.
Tongue force and tongue motility as a function of lesion group. Although the decline in peak force (A) was not significant, maximum tongue force (B) was significantly attenuated in both the unilateral and bilateral groups. (C) Tongue motility (licks/s) was decreased in the unilateral but not the bilateral group. *p<0.01.
Figure 3.
Task engagement, body weight and striatal DA content as a function of lesion group. (A) The number of licks emitted during the session did not differ between the sham and the lesioned groups. An initial decrease in the measure during the second week normalized by the third week, leading to a significant overall effect of time. (B) Body weight was decreased similarly in the unilateral and bilateral groups during the weeks 2 and 3 post-lesion. At week 4, however, body weights recovered in the bilaterally-lesioned group. (C) Striatal DA content was decreased (compared to the contralateral striatum) by 97% in the unilateral group, and ~90% in the bilateral group (compared to the sham group). *p<0.05.
Body Weight and Dopamine Content
Body weight loss was equivalent for the unilateral and bilateral groups at weeks 2 and 3 (Fig. 3B). At week 4, however, body weights in the bilateral group recovered to pre-lesion values. This led to significant main effects for group, F=5.669, p=0.01, and a group-by-time interaction, F=4.093, p<0.01. It should be noted that there was some attrition in the bilateral group due to either aphagia or to incomplete bilateral lesions. Striatal DA depletion in the unilateral 6-OHDA group averaged 97% (Fig. 3C). Compared to the sham group, DA depletion in the bilateral 6-OHDA group was ~90%.
Discussion
In this study we found that nigrostriatal DA depletion impairs orolingual motor function in rats. While this finding is consistent with previous work, we extended these findings to report that bilateral DA depletion does not merely worsen the impairments observed in unilaterally-lesioned rats, but rather affects orolingual motor function in qualitatively different ways. Specifically, although both lesion groups exhibited decreased tongue force, tongue motility deficits were limited to the unilateral group. These differential effects may be related to the greater bilateral neural control of the tongue compared to the limbs.
Our finding of decreased tongue force following 6-OHDA is consistent with previous findings using this model and similar methods [13,36,41]. It is interesting to note, however, that in preclinical studies measuring maximal force output, nigrostriatal DA depletion tends to affect tongue force production to a greater extent than forelimb force production in young adult animals [6,13,28,36]. We recently reported that at unilateral striatal DA depletion levels between 40–60%, rats exhibit substantial forelimb force control deficits, but not decreased force production [6]. Interestingly, despite a lack of main effect for force in that study, middle-aged rats, who pressed with higher forelimb forces pre-lesion, did exhibit decreased force output post-lesion. One difference between these studies was the amount of DA depletion sustained. It is may be that equating depletion levels would yield similar results (it is also likely that the >75% depletion levels that are required to produce tongue force deficits would render animals incapable of performing an operant forelimb force task). However, while decreased force output has been measured in individuals with PD [17,33], it is not a central feature of the disease. Rather, it may be that the force-disrupting effects of nigrostriatal DA depletion are due to impaired sensorimotor [39] or effort-related [38] processing through the basal ganglia pathway. Unlike the forelimb force studies cited above, rats’ natural tongue forces while licking (between 7–9 grams under these conditions) were substantially greater than the 1-gram force required to register a lick and count toward reinforcement in this task (indeed, their diminished post-lesion force output still exceeded the minimal criterion). Similarly, the middle-aged rats in our recent forelimb study also exceeded minimal force requirements, and their diminished post-lesion forelimb forces were also still effective in producing reinforcement. It is likely that nigrostriatal DA depletion shifted the rats’ motor output toward more externally-driven cues (in this case, the task-specific force requirements). This interpretation is consistent with a previous clinical study that reported disrupted tongue force control in the absence of tongue force weakness in dysarthric PD patients [4]. Nevertheless, tasks requiring increased forelimb [42] or tongue forces [20] for reinforcement may produce results that are different than the ones we report here.
Our finding that tongue motility was affected in the unilateral but not the bilateral group was surprising, given the bradykinesia that is prominent in PD. This novel finding suggests that an imbalance in motor circuitry, rather than DA depletion-induced bradykinesia per se, accounts for the slowed licking movements that we and others have found in rats following unilateral 6-OHDA. There are many factors that contribute to tongue motility, and measuring licking speed in rodents is greatly influenced by experimental conditions [16,45]. During consummatory licking, activation of hypoglossal motor neurons that innervate tongue muscles must be coordinated with central pattern generators in the hypoglossal nucleus, cerebellum and medullary reticular formation that coordinate swallowing and respiration [10,46,48]. Although single neurons in the striatum [49] and substantia nigra reticulata [21] exhibit increased activity during licking behavior, and the striatum plays a role in tongue protrusion [35], the basal ganglia likely do not code high speed consummatory licking behavior as it does other movement sequences. Rather, tongue motility in this task may be a function of central pattern generators in the brainstem and cerebellum.
It is tempting to speculate that the lack of effect of bilateral nigrostriatal DA depletion on tongue motility indicates greater orolingual impairments during earlier PD stages, when unilateral symptoms are prevalent. Indeed, up to 70% of early-stage idiopathic PD patients have been found to exhibit impairments in the oral phase of swallowing, contributing to dysphagia [44]. In sporadic PD, however, progressively widespread extranigral and cortical pathology accompany nigrostriatal DA depletion [9]. Furthermore, as an age-related disease, PD is also superimposed on normal age-related neural and muscular changes that affect orolingual motor function [3,5,19,22,32,43,50]. These findings are consistent with preclinical studies of orolingual motor function during licking tasks, when rats exhibit robust age-related slowing of tongue motility beginning in middle age [14,43,51]. Finally, we did not quantify norepinephrine in our HPLC analysis. This is important because norepinephrine is diminished in PD [24] and is involved with orolingual motor-related central pattern generators [3]. Our previous studies have found no changes in NE when 6-OHDA was delivered to the medial forebrain bundle however [30], so we suspect that NE levels were not affected in this study. Repeating the current study in older animals, including the locus ceruleus as a target for 6-OHDA, or using a different model of PD (e.g., overexpressing alpha-synuclein in the substantia nigra) may produce effects that better reflect the progression of orolingual motor deficits in PD.
Overall, our results reveal a dissociation between tongue force and tongue motility as measures of orolingual motor function, at least during licking in rodents. The orolingual motor deficits we observed were not related to aphagia or loss of body weight, as body weights were affected similarly for the two groups initially, and normalized in the bilateral group by the fourth week post-lesion. Previous studies of bilaterally-lesioned rats report severe aphagia with nigrostriatal lesions greater than 95% [37]. The fact that our depletions (~90%) did not reach this threshold, along with our post-lesion efforts to supplement this group with palatable food and extra water, probably contributed to the lack of severe aphagia and recovery of body weight. Regarding translation of our findings to PD, tongue force and motility contribute to both speech articulation and swallowing in humans. Recent reports of impaired ultrasonic vocalizations in rats following nigrostriatal DA depletion (e.g., [12,26]) likely better model the pharyngeal and laryngeal contributions to human speech deficits than do our licking measures. Rather, due to tight coordination of tongue movements, swallowing and respiration, our measures likely reflect important orolingual motor processes involved with normal and impaired ingestion. Our results suggest that when modeling tongue force and motility in PD, factors other than nigrostriatal DA, such as age of the animals and extranigral pathology, are important considerations.
Research Highlights.
Unilateral and bilateral nigrostriatal dopamine depletion affects orolingual motor function in rats.
Both unilateral and bilateral nigrostriatal dopamine depletion decrease tongue force during licking.
Tongue motility is decreased following unilateral but not bilateral nigrostriatal dopamine depletion.
These results reveal a dissociation between these two movement modalities in the 6-hydroxydopamine rat model of Parkinson’s disease.
Acknowledgments
This work was supported by NIH grants AG023549, AG026491, the Kansas Training Program in Neurological and Rehabilitation Sciences (T32 HD57850), a Lied Endowed Basic Science Research grant, and by the Kansas Intellectual and Developmental Disabilities Research Center (HD02528).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbs JH, Hartman DE, Vishwanat B. Orofacial motor control impairment in Parkinson’s disease. Neurology. 1987;37:394–398. doi: 10.1212/wnl.37.3.394. [DOI] [PubMed] [Google Scholar]
- 2.Agid Y, Javoy-Agid F, Ruberg M. Biochemistry of neurotransmitters in Parkinson’s disease. Movement disorders. 1987;2:166–230. [Google Scholar]
- 3.Aldes LD, Champman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- 4.Barlow SM, Abbs JH. Force transducers for the evaluation of labial, lingual, and mandibular motor impairments. J Speech Hear Res. 1983;26:616–621. doi: 10.1044/jshr.2604.616. [DOI] [PubMed] [Google Scholar]
- 5.Baum BJ, Bodner L. Aging and oral motor function: evidence for altered performance among older persons. J Dent Res. 1983;62:2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- 6.Bethel-Brown CS, Morris JK, Stanford JA. Young and middle-aged rats exhibit isometric forelimb force control deficits in a model of early-stage Parkinson’s disease. Behav Brain Res. 2011;225:97–103. doi: 10.1016/j.bbr.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bethel-Brown CS, Zhang H, Fowler SC, Chertoff ME, Watson GS, Stanford JA. Within-session analysis of amphetamine-elicited rotation behavior reveals differences between young and middle-aged F344/BN rats with partial unilateral striatal dopamine depletion. Pharmacol Biochem Behav. 2010;96:423–8. doi: 10.1016/j.pbb.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol. 2012;2012:845618. doi: 10.1155/2012/845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, Del Tredici K, Rub U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 10.Brozek F, Zhuravin IA, Megirian D, Bures J. Localization of the central rhythm generator involved in spontaneous consummatory licking in rats: functional ablation and electrical brain stimulation studies. Proc Natl Acad Sci USA. 1996;93:3325–3329. doi: 10.1073/pnas.93.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calne DB, Langston JW. Aetiology of Parkinson’s disease. Lancet. 1983;2:1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- 12.Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behav Brain Res. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciucci MR, Russell JA, Schaser AJ, Doll EJ, Vinney LM, Connor NP. Tongue force and timing deficits in a rat model of Parkinson disease. Behav Brain Res. 2011;222:315–320. doi: 10.1016/j.bbr.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52:732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–91. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler SC, Pinkston J, Stanford JA, Hughes JC. Lick-force-rhythm test. In: Kompoliti K, Verhagen Metman L, editors. Encyclopedia of Movement Disorders. 2. Oxford: Academic Press; 2010. pp. 137–141. [Google Scholar]
- 17.Franz EA, Miller J. Effects of response readiness on reation time and force output in people with Parkinson’s disease. Brain. 2002;125:1733–50. doi: 10.1093/brain/awf192. [DOI] [PubMed] [Google Scholar]
- 18.Fuh JL, Lee RC, Wang SJ, Lin CH, Wang PN, Chiang JH, Liu HC. Swallowing difficulty in Parkinson’s disease. Clin Neurol Neurosurg. 1997;99:106–112. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 19.Gould TJ, Bowenkamp KE, Larson G, Zahniser NR, Bickford PC. Effects of dietary restriction on motor learning and cerebellar noradrenergic dysfunction in aged F344 rats. Brain Res. 1995;684:150–158. doi: 10.1016/0006-8993(95)00407-h. [DOI] [PubMed] [Google Scholar]
- 20.Guggenmos DJ, Barbay S, Bethel-Brown C, Nudo RJ, Stanford JA. Effects of tongue force training on orolingual motor cortical representation. Behav Brain Res. 2009;201:229–232. doi: 10.1016/j.bbr.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulley JM, Kosobud EK, Rebec GV. Behavior-related modulation of substantia nigra pars reticulata neurons in rats performing a conditioned reinforcement task. Neuroscience. 2002;111:337–349. doi: 10.1016/s0306-4522(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 22.Hilber P, Caston J. Motor skills and motor learning in Lurcher mutant mice during aging. Neuroscience. 2001;102:615–623. doi: 10.1016/s0306-4522(00)00509-1. [DOI] [PubMed] [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Jellinger KA. Pathology of Parkinson’s disease: changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- 25.Johnston BT, Li Q, Castell JA, Castell DO. Swallowing and esophageal function in Parkinson’s disease. Am J Gastroenterol. 1995;90:1741–6. [PubMed] [Google Scholar]
- 26.Kane JR, Ciucci MR, Jacobs AN, Tews N, Russell JA, Ahrens AM, Ma ST, Britt JM, Cormack LK, Schallert T. Assessing the role of dopamine in limb and cranial-oromotor control in a rat model of Parkinson’s disease. J Commun Disord. 2011;44:529–537. doi: 10.1016/j.jcomdis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia. 1996;11:14–22. doi: 10.1007/BF00385794. [DOI] [PubMed] [Google Scholar]
- 28.Liao RM, Fowler SC, Kallman MJ. Quantifying operant behavior deficits in rats with bilateral 6-hydroxydopamine lesions of the ventrolateral striatum. Chin J Physiol. 1997;40:71–8. [PubMed] [Google Scholar]
- 29.Morris JK, Bomhoff GL, Stanford JA, Geiger PC. Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1082–R1090. doi: 10.1152/ajpregu.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris JK, Seim NB, Bomhoff GL, Geiger PC, Stanford JA. Effects of unilateral nigrostriatal dopamine depletion on peripheral glucose tolerance and insulin signaling in middle aged rats. Neurosci Lett. 2011;504:219–222. doi: 10.1016/j.neulet.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 32.Ota F, Connor NP, Konopacki R. Alterations in contractile properties of tongue muscles in old rats. Ann Otol Rhinol Laryngol. 2005;114:799–803. doi: 10.1177/000348940511401010. [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Stelmach GE. Force development during target-directed isometric force production in Parkinson’s disease. Neurosci Lett. 2007;412:173–8. doi: 10.1016/j.neulet.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- 35.Pisa M. Motor functions of the striatum in the rat: Critical role of the lateral region in tongue and forelimb reaching. Neuroscience. 1988;24:453–63. doi: 10.1016/0306-4522(88)90341-7. [DOI] [PubMed] [Google Scholar]
- 36.Plowman EK, Kleim JA. Behavioral and neurophysiological correlates of striatal dopamine depletion: a rodent model of Parkinson’s disease. J Commun Disord. 2011;44:549–556. doi: 10.1016/j.jcomdis.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai K, Gash DM. Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat. Brain Res. 1994;633:144–150. doi: 10.1016/0006-8993(94)91533-4. [DOI] [PubMed] [Google Scholar]
- 38.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schallert T, Hall S. ‘Disengage’ sensorimotor deficit following apparent recovery from unilateral dopamine depletion. Behav Brain Res. 1988;30:15–24. doi: 10.1016/0166-4328(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 40.Schneider JS, Diamond SG, Markham CH. Deficits in orofacial sensorimotor function in Parkinson’s disease. Ann Neurol. 1986;19:275–82. doi: 10.1002/ana.410190309. [DOI] [PubMed] [Google Scholar]
- 41.Skitek EB, Fowler SC, Tessel RE. Effects of unilateral striatal dopamine depletion on tongue force and rhythm during licking in rats. Behav Neurosci. 1999;113:567–573. doi: 10.1037//0735-7044.113.3.567. [DOI] [PubMed] [Google Scholar]
- 42.Stanford JA, Vorontsova E, Fowler SC. The relationship between isometric force requirement and forelimb tremor in the rat. Physiol Behav. 2000;69:285–93. doi: 10.1016/s0031-9384(99)00248-6. [DOI] [PubMed] [Google Scholar]
- 43.Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered orolingual motor function: Relationships with nigrostriatal neurochemical measures. Neurobiol Aging. 2003;24:259–266. doi: 10.1016/s0197-4580(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 44.Volonte MA, Porta M, Comi G. Clinical assessment of dysphagia in early phases of Parkinson’s disease. Neurol Sci. 2002;23:S121–S122. doi: 10.1007/s100720200099. [DOI] [PubMed] [Google Scholar]
- 45.Weijnen JAWM. Licking behavior in the rat: Measurement and situational control of licking frequency. Neurosci Biobehav Rev. 1998;22:751–760. doi: 10.1016/s0149-7634(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 46.Welsh JP, Lang EJ, Sugihara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–7. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 47.Whishaw IQ, Coles BL, Pellis SM, Miklyaeva EI. Impairments and compensation in mouth and limb use in free feeding after unilateral dopamine depletions in a rat analog of human Parkinson’s disease. Behav Brain Res. 1997;84:167–77. doi: 10.1016/s0166-4328(96)00148-9. [DOI] [PubMed] [Google Scholar]
- 48.Wiesenfeld Z, Halpern BP, Tapper DN. Licking behavior: evidence of hypoglossal oscillator. Science. 1977;196:1122–1124. doi: 10.1126/science.558653. [DOI] [PubMed] [Google Scholar]
- 49.Yanase Y, Amano N, Satoda T, Masuda Y, Kawagish S, Toshino K. Neostriatal stimulation activates tongue-protruder muscle, but not tongue-retractor or facial muscles: an electrical and chemical microstimulation study in rats. Brain Res. 2001;893:282–286. doi: 10.1016/s0006-8993(00)03299-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhang JH, Sampogna S, Morales FR, Chase MH. Age-related alterations in immunoreactivity of the midsized neurofilament subunit in the brainstem reticular formation of the cat. Brain Res. 1997;769:196–200. doi: 10.1016/s0006-8993(97)00853-6. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Bethel CS, Smittkamp SE, Stanford JA. Age-related changes in orolingual motor function in F344 vs. F344/BN rats. Physiol Behav. 2008;93:461–466. doi: 10.1016/j.physbeh.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]