Abstract
In bowel ischemia, impaired mucosal integrity may allow intestinal pancreatic enzyme products to become systemic and precipitate irreversible shock and death. This can be attenuated by pancreatic enzyme inhibition in the small bowel lumen. It is unresolved, however, whether ischemically-mediated mucosal disruption is the key event allowing pancreatic enzyme products systemic access, and whether intestinal digestive enzyme activity in concert with increased mucosal permeability leads to shock in the absence of ischemia. To test this possibility, the small intestinal lumen of non-ischemic rats was perfused for two hours with either digestive enzymes, a mucin disruption strategy (i.e., mucolytics) designed to increase mucosal permeability, or both, and animals were observed for shock. Digestive enzymes perfused included trypsin, chymotrypsin, elastase, amylase and lipase. Control (n=6) and experimental animals perfused with pancreatic enzymes only (n=6) or single enzymes (n=3 for each of the five enzyme groups) maintained stable hemodynamics. After mucin disruption using a combination of enteral N-acetylcysteine, atropine, and increased flow rates, rats (n=6) developed mild hypotension (p<0.001 compared to groups perfused with pancreatic enzymes only after 90 minutes) and increased intestinal permeability to intralumenally perfused FITC-dextrans-20kD (p<0.05) compared to control and enzyme-only groups, but there were no deaths. All animals perfused with both digestive enzymes and subjected to mucin disruption (n=6) developed hypotension and increased intestinal permeability (p<0.001 after 90 minutes). Pancreatic enzymes were measured in the intestinal wall of both groups subjected to mucin disruption, but not in the enzyme-only or control groups. Depletion of plasma protease inhibitors was found only in animals perfused with pancreatic enzymes plus mucin disruption, implicating increased permeability and intralumenal pancreatic enzyme egress in this group. These experiments demonstrate that increased bowel permeability via mucin disruption in the presence of pancreatic enzymes can induce shock and increase systemic protease activation in the absence of ischemia, implicating bowel mucin disruption as a key event in early ischemia. Digestive enzymes and their products, if allowed to penetrate the gut wall may trigger multiorgan failure and death.
Keywords: Autodigestion, small intestine permeability, pancreatic enzymes, inflammatory mediators
INTRODUCTION
Multisystem organ failure and shock is a common cause of mortality in critically ill patients, with rates ranging from 30-100% [1, 2]. The pathophysiology is incompletely understood but loss of barrier integrity in the small bowel is increasingly implicated as a key step. Pancreatic digestive enzymes in the lumen of the small intestine may play a major role in this pathology [3].
The bowel mucosal barrier plays a central role protecting the organism from the external environment, including proteolytic degradation, while at the same time allowing systemic access for necessary nutrients and foodstuffs [4]. The selectively permeable mucin-containing mucous layer is the ‘first line’ of defense against inadvertent autodigestion and external pathogens, and as such is largely impermeable to digestive enzymes [5, 6]. Underlying the mucin layer, enterocytes are adherent via tight-junctions that act as differentially permeable filters and further limit the systemic absorption of macromolecules. In shock this barrier may become compromised, allowing egress of inflammatory bowel mediators into the systemic circulation.
Blockade of pancreatic enzymes in the intestinal lumen, but not in the systemic circulation, abolishes morbidity and mortality normally seen in experimental models of hemorrhagic, septic and splanchnic artery occlusion (SAO) shock [7]. In contrast, systemic intravenous (i.v.) enzymatic inhibition in shock has only limited efficacy, presumably because of the limited ability of inhibitors to reach the bowel in sufficiently high concentrations necessary to block these enzymes [8]. The mechanisms by which bowel enzymatic inhibition prevents shock are unknown but may be in part due to their ability to decrease the destruction of gut epithelium in the event the protective mucin barrier layer is breached [6].
In shock, bowel permeability increases with increasing ischemia; however the particular role of the mucin layer is unknown. We postulate that a competent mucin barrier is necessary in order to maintain bowel integrity in the presence of pancreatic enzymes, and that its disruption leads to proteolytic destruction of the underlying mucus barrier and the subsequent transport of cytotoxic enteral contents systemically.
To test this hypothesis, we determined whether the presence of pancreatic enzymes in the bowel of healthy non-ischemic animals with and without an intact mucin layer is by itself sufficient to provoke cardiovascular collapse.
MATERIALS AND METHODS
The experiments were reviewed and approved by the University of California, San Diego Animal Use and Care Committee and were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals. Unless noted otherwise, all reagents were purchased from Sigma-Aldrich Chemical Corp (St Louis, MO).
Animal preparation
Male Wistar rats (250-350 g, Charles River Laboratories, Wilmington, MA) were housed in a controlled environment and maintained on a standard pellet diet for at least three days before the experiments. After induction of general anesthesia (sodium pentobarbital 50 mg/kg, i.m., Abbott Laboratories, North Chicago, IL), femoral venous and arterial catheters (PE-50 tubing, Clay Adams, Parsippany, NJ) were introduced for vascular access and continuous blood pressure and pulse measurements, respectively (MACLAB, GE Medical Systems, Milwaukee, WI). Supplemental anesthetic (5 mg/kg, i.v.) was given as necessary as indicated by tail pinch reflex testing. Body temperature was maintained by a feedback-controlled flow-heating pad with heat cover and was measured using rectal monitoring. The small intestine was carefully exteriorized via a small abdominal midline incision. The proximal duodenum and terminal ileum were both ligated and small enterotomies were made for insertion of catheters. The lumen of the proximal duodenum was cannulated with PE-190 tubing (Clay Adams, Parsippany, NJ) and the intestinal contents were gently flushed with 50 mL of 0.9% normal saline (NS) (Baxter Healthcare, Deerfield, IL). PE-240 tubing was then inserted into the terminal ileum and the cannulae were connected to a peristaltic pump (MasterFlex, Cole Parmer Instrument Co., Chicago, IL) with a reservoir containing a priming volume of 50 ml at constant pressure (10–15 mmHg) and temperature (37°C) to make a complete circuit.
Hemodynamic effects of enteral pancreatic enzyme infusion
After a 15 minute stabilization period, 100 mL NS containing a combination of trypsin, chymotrypsin, amylase, lipase, and elastase in supraphysiological concentrations (n=6), NS alone (n=6) as a control, or single enzymes (trypsin, chymotrypsin, amylase, lipase, or elastase) in NS (n=3 for each group), was circulated through the small intestinal lumen at 4 mL/minutes for 120 minutes. Concentrations used were as follows: trypsin 30 mg/ml; chymotrypsin 15 mg/ml; elastase 750 μg/ml; amylase 25 mg/ml; lipase 3.5 mg/ml. These concentrations were chosen as estimates of approximately 5 times basal intestinal pancreatic enzyme concentrations, in order to reliably test our hypothesis [9, 10]. Hemodynamics were recorded continuously during the procedure.
Hemodynamic effects of an enteral mucin disruption strategy (MDS)
We developed a strategy to disrupt the mucin layer coating the small intestine. Based on previously published studies, N-acetyl cysteine (NAC) (20mg/ml) and atropine (0.2 mg/ml) in NS were circulated at 8 mL/minutes in the small intestinal lumen for 120 minutes (n=6) and hemodynamics were continuously recorded.
The rationale for this intervention was as follows. NAC is a mucolytic that has been used to deplete the mucin layer protecting the bowel [11]. Atropine has been reported to stabilize goblet cells and decrease mucin secretion [12]. Finally, to minimize adherent mucin we increased the mechanical shear by maintaining a pump flow rate of 8 ml/minutes, greater than predicted yield shear stress for intestinal mucin (on the order of 10 Pa, assuming a tube length of 120 cm, 2 mm mean diameter, and viscosity of 6 cP) [13, 14]).
Hemodynamic effects of a combined enteral mucin disruption strategy (MDS) and pancreatic enzymes
In these experiments a combination of the MDS and pancreatic enzymes were perfused simultaneously through the small bowel lumen for 120 minutes (n=6). Hemodynamics were measured as above.
To verify results from these experiments in physiologically more relevant bowel enzyme concentrations, rat pancreas from donor animals were collected, homogenized and incubated for 2.5 hours and enterally perfused as a substitute for the purified pancreatic enzymes, in combination with MDS (n=2) [15].
Bowel permeability measurements
In all groups FITC-dextrans20 (1 mg/ml) was added to the lumenal fluid at the pump reservoir 20 minutes before the end of the infusion period (100 minutes into the procedure) to measure intestinal permeability. 20 kD weight FITC-dextrans was used as its molecular weight approximates that of active pancreatic proteases. Intestinal fluid and blood aliquots were collected at the conclusion of the infusion period, the fractions were isolated by centrifugation at 1000G and the ratio of the plasma:intestinal fluorescence (excitation: 480nm, emission: 520nm) was used as an index of bowel permeability. Samples were measured in triplicate in 96-well plates (Molecular Devices, Sunnyvale, CA). Results are reported in relative fluorescence units (RFUs).
Intestinal histology and immunohistochemistry
To assess mucin integrity, at the conclusion of the experiments, animals were euthanized with i.v. sodium pentobarbital (120 mg/kg). 2 cm intestinal wall (mid-jejunum) sections were collected and flash frozen for in-situ enzyme zymography and histology. Longitudinal sections (7μm thickness) were cut across the intestine with a cryostat at −23°C, air dried for 30 minutes and fixed in cold purified acetone for 10 minutes. Tissue sections were stained using Alcian Blue 8GX for 30 minutes and subsequently washed with H2O. Sections were counterstained with Nuclear fast red (ScyTek Laboratories, Logan, UT), dehydrated with increasing alcohol concentrations, cleared with xylene and mounted.
To assess pancreatic enzyme penetration of the mucosa, chymotrypsin as a representative pancreatic enzyme was measured via in-situ enzyme zymography in the usual fashion [9]. Briefly, samples were fixed in cold purified acetone for 10 minutes, washed with 1X Phosphate Buffered Saline (PBS), and incubated with 5% normal goat serum blocking solution. Tissue sectors were blocked with Avidin and Biotin solutions for 15 minutes each. After four washings, slides were incubated with primary antibody against chymotrypsin (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), then washed and incubated with secondary antibody (1:1000, Santa Cruz Biotechnology) for 90 minutes at room temperature. Slides were washed and incubated with Texas Red-Avidin D conjugated antibody (1:1000, Vector Labs, Burlingame, CA) for 30 minutes at room temperature. Slides were washed, dehydrated with alcohol and cleared with xylene. Tissue sectors were counterstained with DAPI and mounted for fluorescence microscopy.
Plasma protease determination
We measured non-specific plasma protease activity using a fluorescent casein substrate (EnzChek, Sigma) to obtain an index of possible systemic (pancreatic) protease transport. Because it was assumed that little, if any, active (unbound) protease would be recovered, we titrated exogenous trypsin into experimental plasma samples to create calibration curves for each sample. In this way a decrease in circulating protease inhibitor buffer capacity was measured as an index of the amount of activated protease in plasma. Samples (100 μl buffer with added trypsin in 25 μl plasma, 125 μl casein substrate) were measured at steady-state (excitation: 589 nm, emission: 617 nm, 45 minutes), and results were normalized by reagent without trypsin and blank values subtracted. Results are presented as a calibration curve to added trypsin. The inflection points on each curve correspond to the concentration of added trypsin required to overcome endogenous plasma protease inhibitors.
Western blotting
Western blot analysis was done in the standard fashion. Equal aliquots of diluted plasma (1:20) were fractionated using 10% SDS-PAGE and transferred to nitrocellulose (NC) membranes (BioRad, Hercules, CA). Trypsin and elastase were assayed by incubating the NC membrane with rabbit anti-porcine primary antibody (Pierce, Rockford, IL), diluted 1:1000 in 5% non-fat milk blocking reagent (Cell Signaling Technology, Danvers, MA). Peroxidase conjugated goat anti-rabbit secondary was added (1:5000) (Santa Cruz Biotechnology, Santa Cruz, CA), and chemiluminescence was measured (Pierce, Rockford, IL).
Statistical analysis
Measurements are expressed as the means ± SD. Differences in mean values were tested by analysis of variance (ANOVA) with Bonferroni’s correction. A Fisher’s exact test was used for the calculation of significance between binary outcomes (SPSS v18, Chicago, IL). P<0.05 was considered to be significant.
RESULTS
Hemodynamics
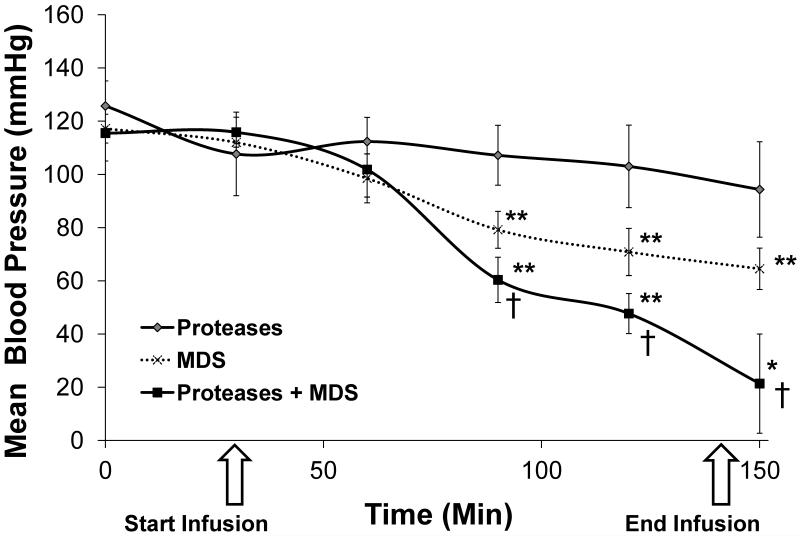
Mean arterial pressure (MAP) did not change significantly among animals enterally perfused with the pancreatic enzymes, either separately or in combination during the perfusion period. MAP of the MDS-treated group was, however, significantly different from those perfused with enzymes alone, starting at 90 minutes of perfusion (p<0.001). Small intestinal perfusion of pancreatic enzymes with MDS resulted in significantly decreased MAP compared to pancreatic enzymes and MDS-only groups by 90 minutes (p<0.01) (Figure 1). Animals in the enzymes with MDS group died or displayed blood pressure readings incompatible with continued survival by the end of the 120-minute perfusion period; no animals in the other groups died (p<0.001).
Figure 1. Effect of intralumenal small bowel pancreatic enzyme administration and/or mucin depletion on mean arterial blood pressure (MAP).
Rats perfused with digestive enzymes in the presence of a depleted mucin layer (n=6) display significantly lower blood pressures compared to those perfused with digestive enzymes (n=6) or MDS alone (n=6) (*p<0.05, **p<0.01 for Proteases plus MDS and MDS groups compared to Proteases alone, †p<0.05 for Proteases plus MDS compared to MDS alone).
Histology
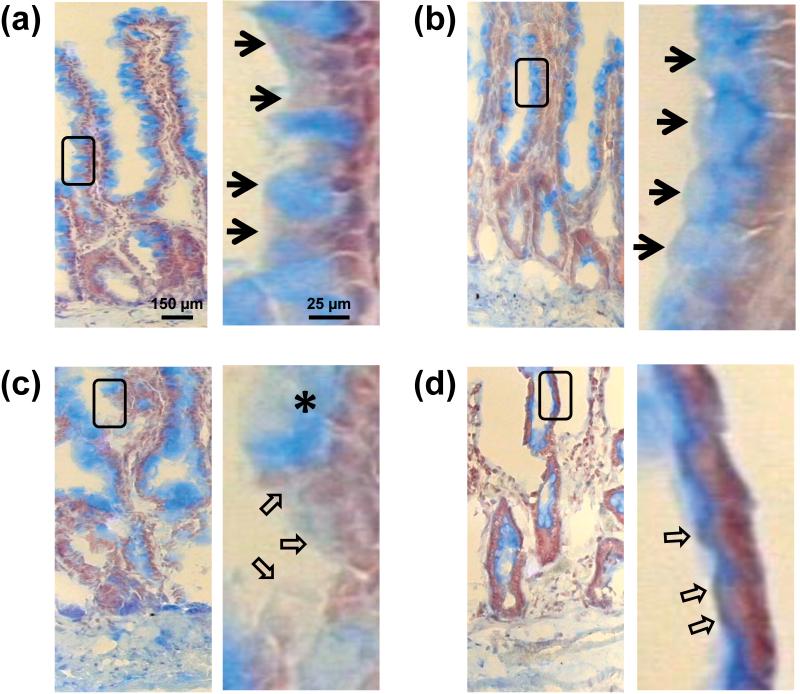
Alcian blue staining of control bowel mucosa for mucin revealed distinct staining of goblet cells on the apical intestinal villi (Figure 2a) and an intact mucin layer (Figure 2a inset). Addition of pancreatic digestive enzymes did not appreciably alter the morphology (Figure 2b). The MDS resulted in diffusion of lumenal mucin compared to controls (Figure 2c), implicating disruption of the mucin layer [16]. However, the overall morphology of the villus structures remained intact. Perfusion of the small intestine with digestive enzymes plus MDS resulted in near obliteration of the mucosal wall, with destruction of the villi and compromise of the basement membrane (Figure 2d).
Figure 2. Disruption of bowel mucin.
Representative frozen sections of rat small bowel mucosa displaying mucin (stained by Alcian blue) and Nuclear fast red counterstain of (a) Controls (b) Proteases alone (c) MDS alone and (d) Proteases plus MDS groups. Note the destruction of the mucosal wall in (d). Bordered insets are shown to the right of each figure for visualization at higher magnification. Arrowheads denote mucin border, the asterisk denotes (probably) degraded mucin; the hollow arrows indicate areas of denuded epithelium.
Bowel permeability measurements
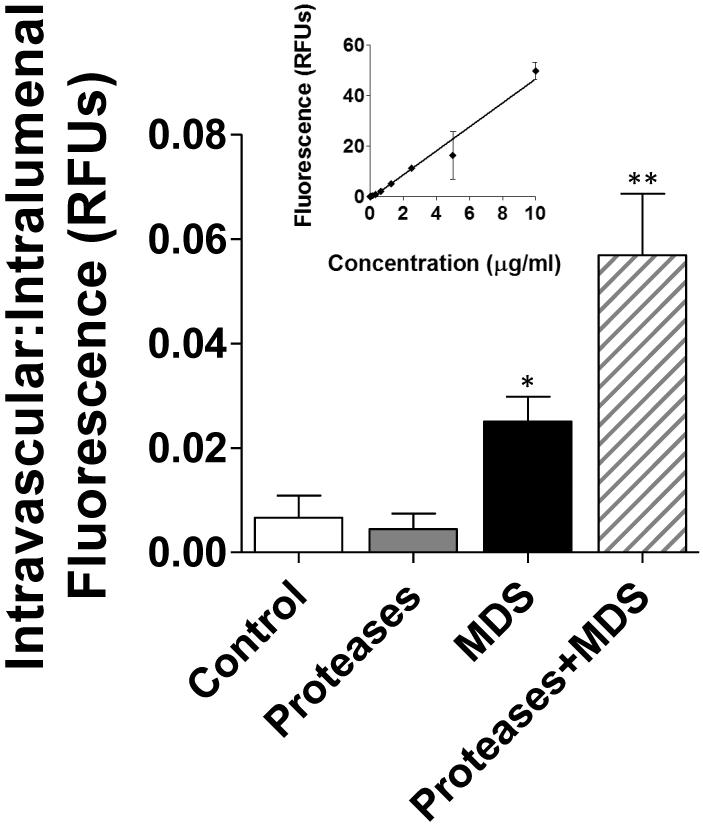
Small intestinal wall permeability to FITC-dextrans20 (n=5) was significantly increased in the digestive enzyme plus MDS group (p<0.001 compared to all groups, including MDS only) after 120 minutes of bowel perfusion. Permeability was also significantly increased in the MDS group compared to control and digestive enzymes-only groups (p<0.05) (Figure 3).
Figure 3. Intestinal permeability.
Comparison of intestinal permeability between animals perfused with digestive enzymes + MDS (n=6) and those perfused with either digestive enzymes (n=6) or MDS alone (n=6). Permeability was measured as the ratio between the concentrations of fluorescent substrate (FITC-dextrans–20kD) in the lumen compared to that seen intravascularly as measured in relative fluorescent units (RFUs). **p<0.01 between Proteases plus MDS group and all other groups. *p<0.05 for MDS vs. Proteases only and Controls. Inset is calibration curve for FITC-dextrans-20kD.
Immunohistochemistry
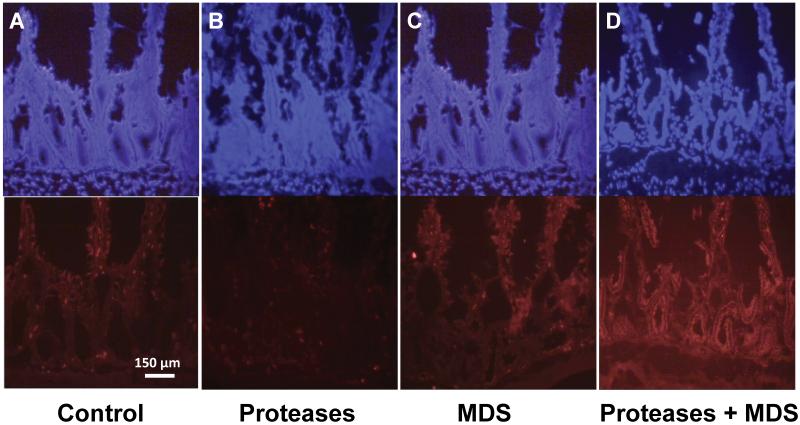
With increases in permeability there were concomitant increases in pancreatic enzyme (as shown by chymotrypsin antibody labeling, Figure 4) involvement in the mucosal, sub-mucosal and serosal layers. Antibody labeling of control and enzyme-only groups displayed little chymotrypsin immunofluorescence (Figures 4a, 4b). Increases in bowel permeability induced by the MDS increased chymotrypsin immunofluorescence in the intestinal villi (Figure 4c), suggesting the presence of residual pancreatic enzyme in the bowel, even after washout of intestinal contents [17]. Bowel immunofluorescence was greatly increased with the addition of pancreatic enzymes to the MDS (Figure 4d) and resulted in extensive fluorescence deep into the mucosal and serosal layers.
Figure 4. Permeability to proteases in the bowel wall.
Representative frozen sections of small bowel mucosa with DAPI nuclear stain (top) and antibody to chymotrypsin (bottom) of (a) Control (b) Proteases alone (c) MDS and (d) Proteases plus MDS groups. Note chymotrypsin infiltration of the mucosa and basement membrane in (d).
Intravascular protease measurements
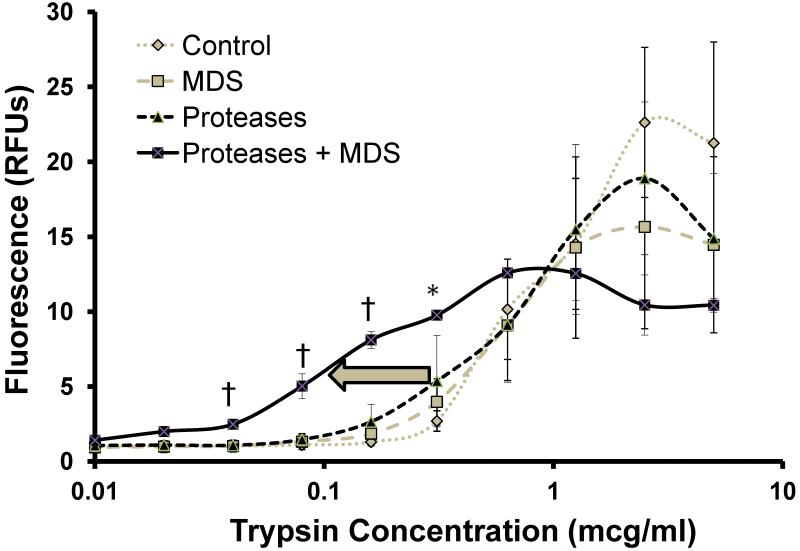
Measured plasma protease activity was negligible at low concentrations of added trypsin in all groups (Figure 5) suggesting little free plasma enzyme. However, the pancreatic enzyme plus MDS group displayed significantly increased activity at almost an order of magnitude lower added trypsin compared to all other groups (p<0.01), implicating increased binding of endogenous plasma protease inhibitors by enzymes of gut origin in this group.
Figure 5. Protease buffer capacity of plasma.
Proteolytic activity in plasma after 120 minutes of bowel perfusion with Proteases, MDS, Proteases plus MDS or NS control as measured by fluorescence of a non-specific fluorescently-labeled casein substrate after addition of increasing trypsin concentrations to samples. Results are shown in relative fluorescence units (RFUs). Initial levels in all groups are close to zero, indicating little to no active plasma proteolytic activity at baseline. However, the Proteases + MDS group (n=6) displays significantly decreased anti-protease buffer capacity compared to all other groups (n=6 for each group) (see arrow, essentially shifting the calibration curve to the left), indirectly reflecting the amount of active protease that achieved the systemic circulation in this group. †p<0.001 between Protease + MDS group and all other groups, *p<0.05 between Proteases plus MDS versus Control plasma only.
Digestive protease levels in plasma
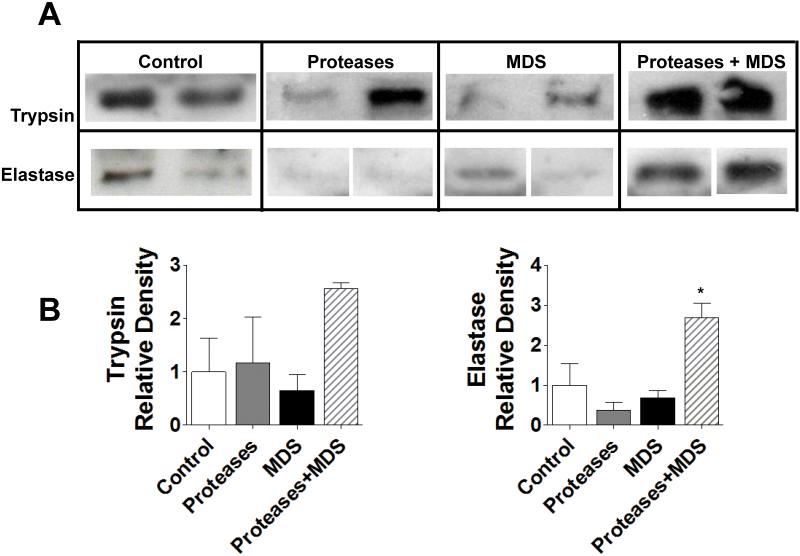
Plasma levels of trypsin and elastase were increased in the pancreatic enzyme plus MDS group compared all other groups as measured by Western blotting, indicating that these pancreatic enzymes may have achieved the systemic circulation in this model (Figure 6).
Figure 6. Digestive protease levels in plasma.
Western blot of digestive enzymes in plasma, displaying relative amounts of trypsin and elastase in rat plasma after bowel perfusion with Proteases, MDS, or Proteases + MDS. *p<0.05 between Proteases plus MDS and all other groups. 2 animals/measurement.
DISCUSSION
The present study shows in a non-ischemic bowel the ability of enteral pancreatic enzymes to breach the mucosal barrier in the presence of an impaired mucus layer. In the absence of ischemia, enhanced enteral pancreatic enzyme concentrations in the presence of a disrupted mucin-containing mucus layer were sufficient to penetrate the bowel mucosa, significantly increase bowel permeability, reach the systemic circulation, and result in significant hemodynamic compromise within a few hours. Mucin disruption via MDS without supplemented pancreatic enzymes had only minimal effects on measured protease levels in the systemic circulation and significant, but lesser effects on blood pressure, while the addition of exogenous pancreatic enzymes to a non-ischemic and otherwise healthy bowel had no detectable histological, immunological or systemic effects. These results are in agreement with studies that demonstrate local permeability increases after mucin disruption in the presence of pancreatic enzymes [18, 19].
Bowel dysfunction, increased mucosal permeability and the subsequent egress of bowel inflammatory mediators into the systemic circulation are important early events in shock [20]. Elevated permeability in the intestine may occur before central blood pressure falls (e.g. in shock after endotoxin administration) and an ischemic state arises in the systemic circulation. Mucosal permeability can increase via depletion of the protective mucin layer, disruption of tight junctions, and destruction of enterocytes themselves [21], establishing a conduit from the external environment to the systemic circulation, with subsequent entry and generation of circulating inflammatory mediators, including possibly pancreatic digestive enzymes, bacterial toxins, and partially digested food.
This study implicates disruption of the mucin in the mucosal barrier and degradation of the mucosal barrier in conjunction with a constitutive presence of high concentrations of digestive enzymes as being mechanisms that lead to central blood pressure reduction. To test our hypothesis in the acute setting, higher than normal exogenous pancreatic enzyme concentrations were circulated in the small bowel. Using these concentrations in the absence of the MDS also confirmed that permeability in an intact bowel cannot be compromised simply by an increase in lumenal pancreatic enzyme concentrations. Substitution of homogenized pancreas for purified enzymes for more “physiologic” protease concentrations in conjunction with MDS led to similar cardiovascular collapse and death of the animals, but within a four-hour time period, suggesting that this mechanism may indeed be operant during clinical shock and contribute to the hemodynamic compromise seen in this condition.
The splanchnic circulation is only mildly autoregulated and as such, is vulnerable to decreases in systemic perfusion [22-24]. Previous work has shown that inhibition of gut lumenal pancreatic digestive enzymes is an effective treatment against different forms of circulatory shock with reduced intestinal perfusion, including hemorrhage [21], splanchnic arterial occlusion [3], and endotoxemia [25], suggesting a common mechanism for these forms of experimental shock. Bowel integrity appears to be preserved in all of these models [7]. Ischemia increases bowel permeability via many possible mechanisms including decreased mucin production via ATP-dependent processes, decreased enterocyte tight junctions, and direct necrosis of enterocytes themselves [26]. Because there is most probably a continuum of ischemia-related bowel damage and there are numerous simultaneous events occurring during ischemia, this study used a non-ischemic model in an attempt to parse the specific role of enteral mucin.
A limitation in the current study is the MDS employed. It is recognized that there is no universally satisfactory method available to achieve adequate mucin disruption without potentially confounding results. In order to minimize this possibility, we used a combination of an established mucolytic and mucopenic agent to degrade the mucin layer, and in concentrations lower (approximately 10%) than previously reported [18, 19]. Atropine was added to enhance mucin degradation and was without systemic effect on heart rate at the selected concentration [12]. In addition, we estimated the yield shear stress for wall mucin and modified flow rates accordingly. The histological evidence indicates that the interventions applied were effective in disrupting the mucin, but there is the possibility that this strategy may have had other effects (i.e., antioxidant properties of NAC, etc). Therefore, the small but evident increases in permeability and changes in hemodynamics in the MDS-only groups may have been in part secondary to direct effects of this strategy as opposed to endogenous pancreatic enzyme synergy. The detection of appreciable chymotrypsin as a representative pancreatic enzyme in the bowel mucosa of the MDS-only group provides evidence for the latter.
The current results demonstrate that ischemia per se may not be necessary to produce experimental bowel-derived circulatory shock. In tandem with previous work demonstrating protection by gut pancreatic enzyme inhibition in experimental shock [3, 7, 17, 21, 27], these results further implicate digestive enzymes as a major mechanism in the pathogenesis of shock and the mucin layer as their principal deterrent. We propose that in shock, ischemia to the bowel results in an impairment in the proteolytically impermeable mucin layer with subsequent mucosal destruction by bowel pancreatic enzymes and deleterious systemic sequelae.
Acknowledgement
This work was supported by NIH grant GM085072 and the Foundation for Anesthesia Education and Research and the American Society of Critical Care Anesthesiologists.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rocker G, Cook D, Sjokvist P, Weaver B, Finfer S, McDonald E, Marshall J, Kirby A, Levy M, Dodek P, et al. Clinician predictions of intensive care unit mortality. Crit Care Med. 2004;32:1149–1154. doi: 10.1097/01.ccm.0000126402.51524.52. [DOI] [PubMed] [Google Scholar]
- 2.Gustot T. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Current opinion in critical care. 2011;17:153–159. doi: 10.1097/MCC.0b013e328344b446. [DOI] [PubMed] [Google Scholar]
- 3.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci U S A. 2000;97:1772–1777. doi: 10.1073/pnas.97.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pusztai A. Transport of proteins through the membranes of the adult gastro-intestinal tract- a potential for drug delivery? Adv Drug Delivery Reviews. 1989;3:215–228. [Google Scholar]
- 5.Godl K, Johansson ME, Lidell ME, Morgelin M, Karlsson H, Olson FJ, Gum JR, Jr., Kim YS, Hansson GC. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 6.Chang M, Kistler EB, Schmid-Schonbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37:297–305. doi: 10.1097/SHK.0b013e318240b59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205–209. doi: 10.1097/00024382-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Kistler EB, Lefer AM, Hugli TE, Schmid-Schonbein GW. Plasma activation during splanchnic arterial occlusion shock. Shock. 2000;14:30–34. doi: 10.1097/00024382-200014010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Rosario HS, Waldo SW, Becker SA, Schmid-Schonbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol. 2004;164:1707–1716. doi: 10.1016/S0002-9440(10)63729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldo SW, Rosario HS, Penn AH, Schmid-Schonbein GW. Pancreatic digestive enzymes are potent generators of mediators for leukocyte activation and mortality. Shock. 2003;20:138–143. doi: 10.1097/01.shk.0000073866.47824.ae. [DOI] [PubMed] [Google Scholar]
- 11.Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116P–117P. [PubMed] [Google Scholar]
- 12.Bounous G, McArdle AH, Hodges DM, Hampson LG, Gurd FN. Biosynthesis of intestinal mucin in shock: relationship to tryptic hemorrhagic enteritis and permeability to curare. Ann Surg. 1966;164:13–22. doi: 10.1097/00000658-196607000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berlin SC, Goske MJ, Obuchowski N, Alexander F, Zepp RC, Goldblum JR, Godec K. Small bowel obstruction in rats: diagnostic accuracy of sonography versus radiography. J Ultrasound Med. 1998;17:497–504. doi: 10.7863/jum.1998.17.8.497. [DOI] [PubMed] [Google Scholar]
- 14.Celli JP, Turner BS, Afdhal NH, Ewoldt RH, McKinley GH, Bansil R, Erramilli S. Rheology of gastric mucin exhibits a pH-dependent sol-gel transition. Biomacromolecules. 2007;8:1580–1586. doi: 10.1021/bm0609691. [DOI] [PubMed] [Google Scholar]
- 15.Kistler EB, Hugli TE, Schmid-Schonbein GW. The pancreas as a source of cardiovascular cell activating factors. Microcirculation. 2000;7:183–192. [PubMed] [Google Scholar]
- 16.Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respir Care. 2007;52:859–865. [PubMed] [Google Scholar]
- 17.Doucet JJ, Hoyt DB, Coimbra R, Schmid-Schonbein GW, Junger WG, Paul LW, Loomis WH, Hugli TE. Inhibition of enteral enzymes by enteroclysis with nafamostat mesilate reduces neutrophil activation and transfusion requirements after hemorrhagic shock. J Trauma. 2004;56:501–510. doi: 10.1097/01.ta.0000114536.98447.f7. discussion 510-501. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe SM, Qin X, Lu Q, Feketeova E, Palange DC, Dong W, Sheth SU, Lee MA, Reino D, Xu DZ, Deitch EA. Loss of the intestinal mucus layer in the normal rat causes gut injury but not toxic mesenteric lymph nor lung injury. Shock. 2010;34:475–481. doi: 10.1097/SHK.0b013e3181dc3ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin X, Sheth SU, Sharpe SM, Dong W, Lu Q, Xu D, Deitch EA. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock. 2011;35:275–281. doi: 10.1097/SHK.0b013e3181f6aaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheth SU, Lu Q, Twelker K, Sharpe SM, Qin X, Reino DC, Lee MA, Xu DZ, Deitch EA. Intestinal mucus layer preservation in female rats attenuates gut injury after trauma-hemorrhagic shock. The Journal of trauma. 2010;68:279–288. doi: 10.1097/TA.0b013e3181caa6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 22.Chang RW, Chang JB, Longo WE. Update in management of mesenteric ischemia. World J Gastroenterol. 2006;12:3243–3247. doi: 10.3748/wjg.v12.i20.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leaphart CL, Tepas JJ., 3rd The gut is a motor of organ system dysfunction. Surgery. 2007;141:563–569. doi: 10.1016/j.surg.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Malinoski DJ, Hadjizacharia P, Salim A, Kim H, Dolich MO, Cinat M, Barrios C, Lekawa ME, Hoyt DB. Elevated serum pancreatic enzyme levels after hemorrhagic shock predict organ failure and death. J Trauma. 2009;67:445–449. doi: 10.1097/TA.0b013e3181b5dc11. [DOI] [PubMed] [Google Scholar]
- 25.Fitzal F, DeLano FA, Young C, Rosario HS, Schmid-Schonbein GW. Pancreatic protease inhibition during shock attenuates cell activation and peripheral inflammation. J Vasc Res. 2002;39:320–329. doi: 10.1159/000065544. [DOI] [PubMed] [Google Scholar]
- 26.Hansson GC. Role of mucus layers in gut infection and inflammation. Current opinion in microbiology. 2012;15:57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HD, Malinoski DJ, Borazjani B, Patel MS, Chen J, Slone J, Nguyen XM, Steward E, Schmid-Schonbein GW, Hoyt DB. Inhibition of intraluminal pancreatic enzymes with nafamostat mesilate improves clinical outcomes after hemorrhagic shock in swine. The Journal of trauma. 2010;68:1078–1083. doi: 10.1097/TA.0b013e3181da78b1. [DOI] [PubMed] [Google Scholar]