Abstract
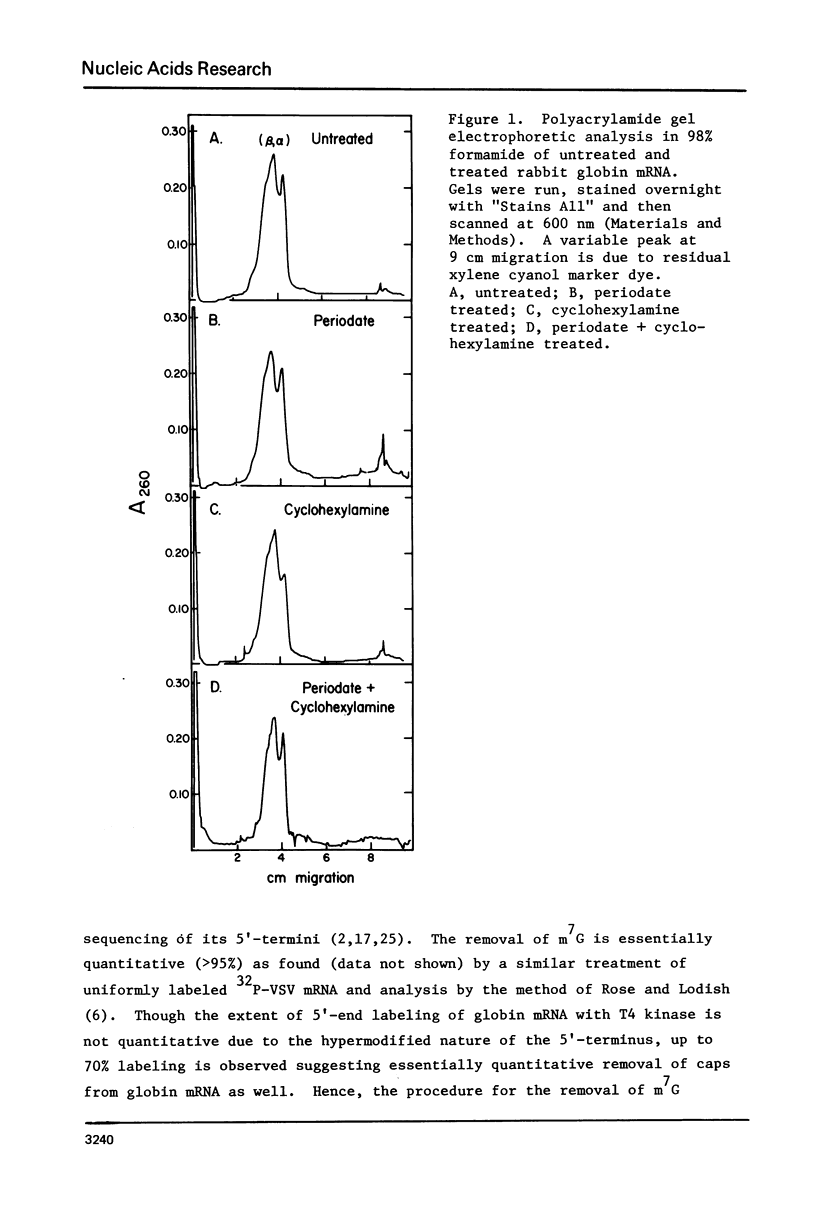
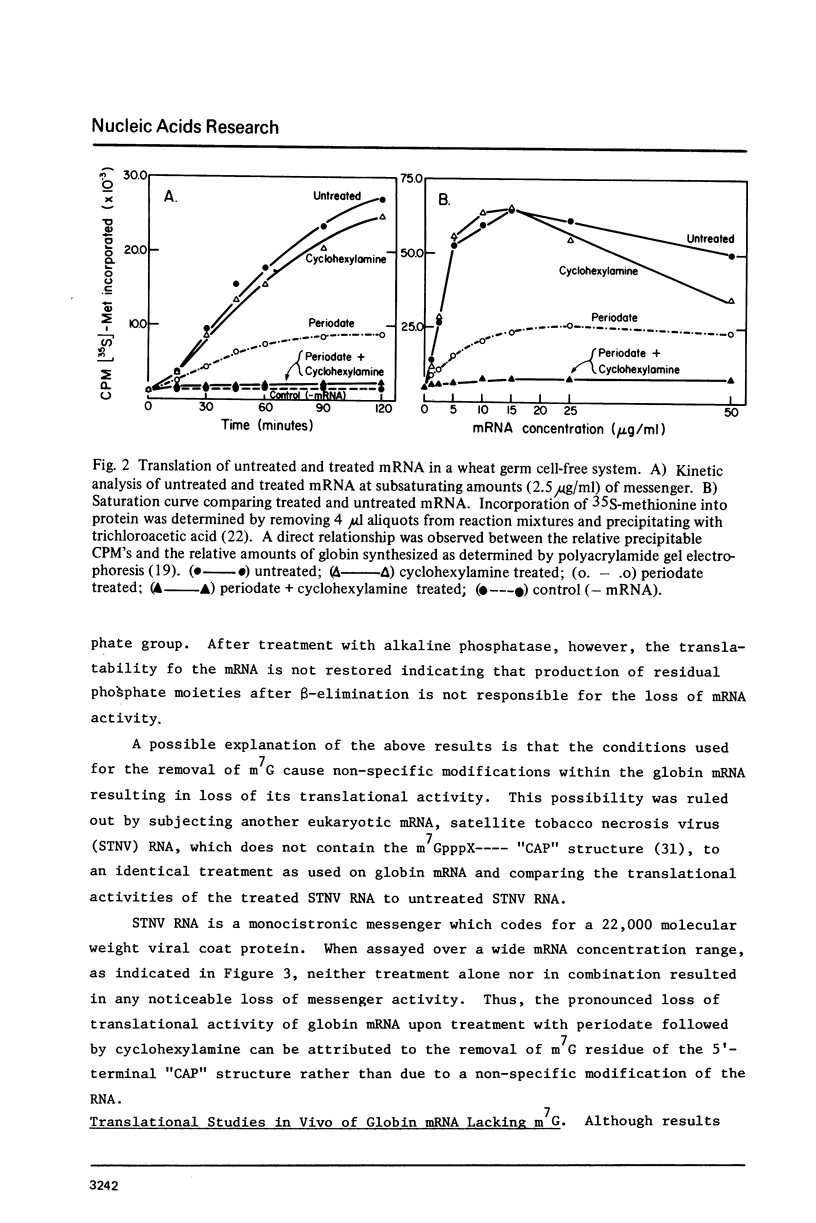
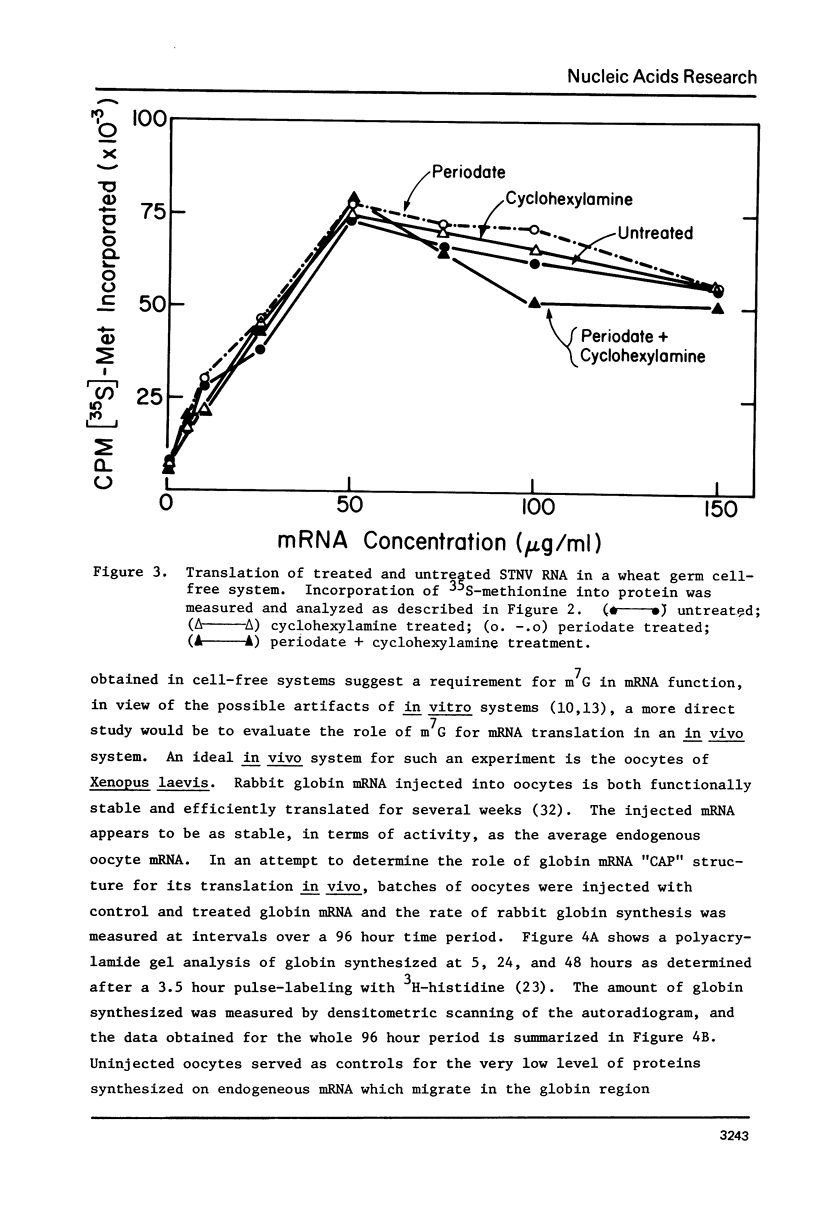
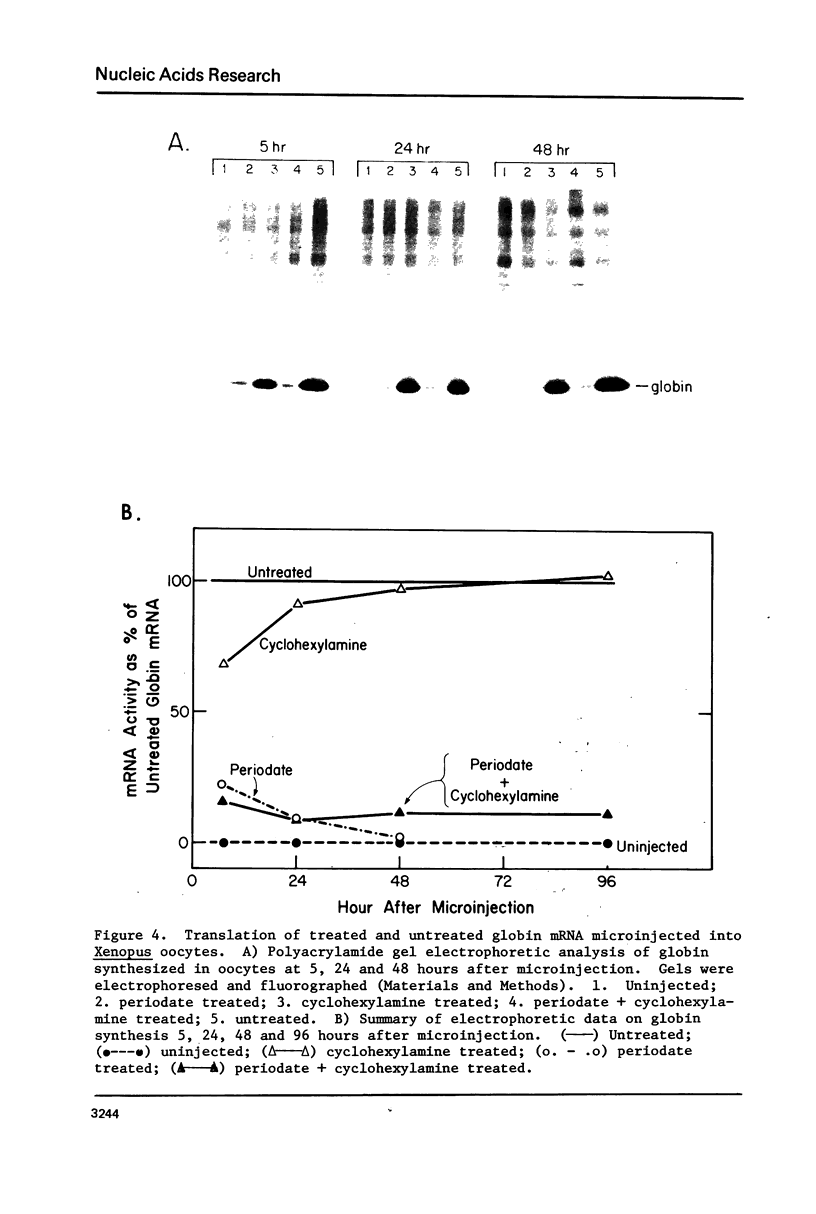
The 7-methylguanosine (m7G) residue present in the m7G5' ppp5'X-"CAP" structure of rabbit globin mRNA was removed quantitatively by periodate oxidation followed by beta-elimination in the presence of cyclohexylamine. The RNA thus treated was intact and exhibited no signs of degradation as examined by polyacrylamide gel electrophoresis in formamide. Assay for protein synthesis using a wheat germ cell-free system showed that the globin mRNA lacking m7G had lost most of its messenger activity. Identical treatment, of satellite tobacco necrosis virus (STNV) RNA, which does not contain the 5'-terminal "CAP" structure, resulted in no loss of its mRNA activity. Since the importance of the m7G residue in eukaryotic mRNA has not yet been shown essential for translation in vivo, both untreated and treated globin mRNAs were injected into frog oocytes and their translation into globin was measured at intervals over a ninety-six hour period. Globin mRNA either treated with periodate alone or lacking in m7g altogether were both found to have lost more than 90% of their activity in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham K. A., Pihl A. Translation of enzymically decapped messenger RNA. Eur J Biochem. 1977 Aug 1;77(3):589–593. doi: 10.1111/j.1432-1033.1977.tb11703.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Lane C. D. Translation of Xenopus liver messenger RNA in Xenopus oocytes: vitellogenin synthesis and conversion to yolk platelet proteins. Cell. 1976 Jun;8(2):283–297. doi: 10.1016/0092-8674(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lingrel J. B., Marbaix G. Message stability in injected frog oocytes: long life of mammalian alpha and beta globin messages. J Mol Biol. 1973 Nov 5;80(3):539–551. doi: 10.1016/0022-2836(73)90421-x. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C., Kim C. H., Sarma R. H. A relation between inhibition of protein synthesis and conformation of 5'-phosphorylated 7-methylguanosine derivatives. J Mol Biol. 1977 Jan 15;109(2):173–183. doi: 10.1016/s0022-2836(77)80027-2. [DOI] [PubMed] [Google Scholar]
- Kemper B. Inactivation of parathyroid hormone mRNA by treatment with periodate and aniline. Nature. 1976 Jul 22;262(5566):321–323. doi: 10.1038/262321a0. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Sarma R. H. Spatial configuration of mRNA 5'-terminus. Nature. 1977 Nov 17;270(5634):223–227. doi: 10.1038/270223a0. [DOI] [PubMed] [Google Scholar]
- Kohl R. J., Hall T. C. Loss of infectivity of brome mosaic virus RNA after chemical modification of the 3' or 5' terminus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2682–2686. doi: 10.1073/pnas.74.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Lockard R. E., Alzner-deWeerd B., RajBhandary U. L., Bol J. F. Nucleotide sequence of 5' terminus of alfalfa mosaic virus RNA 4 leading into coat protein cistron. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5504–5508. doi: 10.1073/pnas.74.12.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. D., Marbaix G., Gurdon J. B. Rabbit haemoglobin synthesis in frog cells: the translation of reticulocyte 9 s RNA in frog oocytes. J Mol Biol. 1971 Oct 14;61(1):73–91. doi: 10.1016/0022-2836(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Gilbert C. W., Smith R. E., Sasavage N. L., Clark J. M., Jr Translation of satellite tobacco necrosis virus ribonucleic acid by an in vitro system from wheat germ. Biochemistry. 1976 Nov 2;15(22):4943–4950. doi: 10.1021/bi00667a030. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhard R. E., Rajbhandary U. L. Nucleotide sequences at the 5'termini of rabbit alpha and beta globin mRNA. Cell. 1976 Dec;9(4 Pt 2):747–760. doi: 10.1016/0092-8674(76)90138-0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Rose J. K. Relative importance of 7-methylguanosine in ribosome binding and translation of vesicular stomatitis virus mRNA in wheat germ and reticulocyte cell-free systems. J Biol Chem. 1977 Feb 25;252(4):1181–1188. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Moss B., Ingram V. M. Hemoglobin synthesis during amphibian metamorphosis. I. Chemical studies on the hemoglobins from the larval and adult stages of Rana catesbeiana. J Mol Biol. 1968 Mar 28;32(3):481–492. doi: 10.1016/0022-2836(68)90336-7. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Morgan M., Banerjee A. K., Shatkin A. J. Influence of 5'-terminal m7G and 2'--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry. 1976 Dec 28;15(26):5761–5768. doi: 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Moss B., Cooper J. A., Maxwell E. S. Influence of 5'-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J Biol Chem. 1978 Mar 10;253(5):1710–1715. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Wu B. C., Waxman J., Busch H. Rigid structural requirement of the 5' terminus of mRNA for translational activity. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1186–1193. doi: 10.1016/0006-291x(75)90484-2. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F. Translation in vitro of vesicular stomatitis virus mRNA lacking 5'-terminal 7-methylguanosine. Nature. 1976 Jul 1;262(5563):32–37. doi: 10.1038/262032a0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Kodama Y., Hashimoto J., Miura K. I. Importance of 5'-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2734–2738. doi: 10.1073/pnas.74.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Miwa M., Kato K., Noguchi M., Matsushima T., Sugimura T. A novel phosphodiesterase from cultured tobacco cells. Biochemistry. 1976 May 18;15(10):2185–2190. doi: 10.1021/bi00655a024. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Maroney P. A., Baglioni C. Inhibition of protein synthesis by Cl-. J Biol Chem. 1977 Jun 10;252(11):4007–4010. [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Nuss D. L., Baglioni C. 5'-Terminal 7-methylguanosine and mRNA function: influence of potassium concentration on translation in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3254–3258. doi: 10.1073/pnas.74.8.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Moss B. 5'-Terminal capping of RNA by guanylyltransferase from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3758–3761. doi: 10.1073/pnas.74.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan-Kowalczewska M., Bretner M., Sierakowska H., Szczesna E., Filipowicz W., Shatkin A. J. Removal of 5'-terminal m7G from eukaryotic mRNAs by potato nucleotide pyrophosphatase and its effect on translation. Nucleic Acids Res. 1977 Sep;4(9):3065–3081. doi: 10.1093/nar/4.9.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi-Willner T., Lane C. Subcellular compartmentation of albumin and globin made in oocytes under the direction of injected messenger RNA. Cell. 1977 Jul;11(3):683–693. doi: 10.1016/0092-8674(77)90085-x. [DOI] [PubMed] [Google Scholar]




