Abstract
Animal and cellular work has shown that central cannabinoid-1 receptors modulate neural oscillations in the gamma range (40 Hz), which may be important for normal perceptual and cognitive processes. In order to assess the effect of cannabinoids on broadband-frequency neural oscillations in humans, the current study examined the effect of chronic cannabis use on auditory steady-state responses (ASSRs) utilizing electroencephalography (EEG). Passive ASSRs were assessed using varying rates of binaural stimulation (auditory click-trains; 10–50 Hz in increments of 5 Hz; 80 dB SPL) in carefully screened cannabis users and controls. Chronic cannabis users (n=22; 12 h abstinence before study; positive 11-nor-9-carboxy-delta-9-tetrahydrocannabinol urine levels) and cannabis naïve controls (n=24) were evaluated. Time X frequency analyses on EEG data were performed using Fourier-based mean trial power (MTP) and phase-locking (inter-trial coherence; ITC). Transient ERPs to stimulus onset (auditory N100 components) were also evaluated. As predicted, a decrease in spectral power (MTP) at 40 Hz was observed in the cannabis group (p<0.018). No effects on phase-locking (ITC) or the N100 were observed. Further, within the cannabis group, lower 40 Hz power correlated with an earlier age of onset of cannabis use (p<0.04). These data suggest that chronic exposure to exogenous cannabinoids can alter the ability to generate neural oscillations, particularly in the gamma range. This is consistent with preclinical animal and cellular data, which may have implications for understanding the short- and long-term psychopharmacological effects of cannabis.
Keywords: cannabis, cannabinoid, neural oscillations, neural synchrony, EEG, auditory steady-state response
INTRODUCTION
Cannabis remains one of the most widely used psychoactive substances in the world (United Nations Office on Drugs and Crime, 2009). The principal psychoactive constituent in cannabis, Δ9-tetrahydrocannabinol (THC; Gaoni and Mechoulam, 1971), affects' the brain via the action of central cannabinoid-1 receptors (CB1R; Devane et al, 1988; Pertwee et al, 2010). The CB1R is one of the most abundant G-protein-coupled receptors in the central nervous system, with high densities in areas such as the cerebral cortex, basal ganglia, hippocampus, and cerebellum (Egertova and Elphick, 2000; Eggan and Lewis, 2007; Glass et al, 1997; Herkenham et al, 1990; Pertwee, 1997, 1999; Tsou et al, 1998). CB1Rs are primarily located presynaptically, and their activation (by either endogenous or exogenous cannabinoids) inhibits the release of other neurotransmitters such as gamma-amino butyric acid (GABA) and glutamate by decreasing Ca2+ influx via the inhibition of adenylate cyclase and N-type Ca2+ channels (Freund et al, 2003). In the cerebral cortex and hippocampus, this neuromodulation principally occurs in networks of cholecystokinin-containing GABAergic interneurons (Ali and Todorova, 2010; Bacci et al, 2004; Bodor et al, 2005; Eggan and Lewis, 2007; Eggan et al, 2010; Foldy et al, 2006; Hill et al, 2007; Katona et al, 2000). Thus, it appears that CB1Rs may function as a molecular ‘brake,' regulating the timing and release of GABA and other neurotransmitters (Farkas et al, 2010).
Concerning the precise role that cannabinoids have in GABA function, it has been hypothesized that ‘cannabis' neurobehavioral effects may involve alterations in neural synchronization' (Skosnik et al, 2006a). Indeed, in vitro and in vivo animal studies have shown that CB1Rs modulate gamma- (30–80 Hz) and theta-band (4–7 Hz) synchronized oscillations in networks of GABAergic interneurons in the cerebral cortex and hippocampus (Hajos et al, 2000, 2008; Katona et al, 1999; Morgan et al, 2008; Reich et al, 2005; Robbe et al, 2006). This may be particularly germane to the psychopharmacological effects of cannabis, as neural oscillations are thought to be involved in several domains of cognitive and perceptual function. For example, cellular studies and computational models have suggested that gamma-band rhythmic synchronization across neuronal assembles has an important role in the integration and binding of perceptual features, associative learning, and conscious awareness (Melloni et al, 2007; Singer, 1999; Uhlhaas et al, 2009; Whittington et al, 2000).
Although several experiments have examined the effect of cannabinoid manipulations on synchronized oscillations using cellular and animal preparations (Hajos et al, 2000, 2008; Katona et al, 1999; Morgan et al, 2008; Reich et al, 2005; Robbe et al, 2006), there has been a paucity of studies assessing CB1R effects on frequency-specific neural oscillations in humans. One non-invasive technique that can be used to study neural oscillations in humans is electroencephalography (EEG). EEG is one of the few available neuroimaging methodologies that can directly measure neural events (postsynaptic potentials) with high temporal precision in humans (Luck et al, 2011). Neural network oscillations can be probed and assessed by entrainment of the EEG to rhythmic sensory stimulation (eg, auditory click trains at specific frequencies). Because the EEG waveform entrains to the frequency and phase of the presented stimulus, it serves as an indicator of the functional state of the neural circuits supporting synchronized oscillations. In the auditory modality, the output of such stimulation is termed the auditory steady-state response (ASSR). Evidence suggests that the ASSR has a preferred gamma-band resonance (Galambos et al, 1981; Picton et al, 2003), is generated by the brainstem, thalamus, cerebellum, and auditory cortex (Hari et al, 1989a, 1989b; Makela and Hari, 1987; Pantev et al, 1996; Pastor et al, 2002, 2006; Steinmann and Gutschalk, 2011), and is mediated by the GABAergic system (Vohs et al, 2010). As aptly described by Spencer et al (2008), ‘Although it is not thought that the ASSR itself reflects any process related to the formation of cell assemblies, its 40-Hz resonance suggests that the underlying neural circuits preferentially oscillate at this frequency and thus might rely on some of the same circuit and intrinsic neuron properties as non-driven (sensory evoked and cognitive-related) oscillations (Spencer et al, 2008).' Thus, the auditory steady-state paradigm may represent a valid probe with which to test the ability of neural networks to oscillate at frequencies important for normal perceptual and cognitive processes. Indeed, the ASSR has been used successfully to demonstrate neural oscillation deficits in psychotic illnesses such as schizophrenia and bipolar disorder (Krishnan et al, 2009; Kwon et al, 1999; Light et al, 2006; O'Donnell et al, 2004; Rass et al, 2010; Spencer et al, 2008).
In the only previous study of the effects of cannabis use on neural oscillations using the ASSR paradigm, it was demonstrated that 20 and 40 Hz harmonic EEG spectral power were decreased during beta-band auditory stimulation (Skosnik et al, 2006a). However, this initial study only assessed a sample of self-reported cannabis users, with no objective confirmation of recent cannabis use (eg, detection of urinary THC metabolites). Further, only three frequencies of stimulation were utilized (20, 30, and 40 Hz), and the fast Fourier transform measure of power did not examine the temporal dynamics of the ASSR, or differentiate effects on power vs phase locking. Therefore, the current study examined the effect of chronic cannabinoids on broadband-frequency neural oscillations in confirmed cannabis users utilizing the ASSR paradigm. On the basis of previous animal and cellular work, it was hypothesized that the cannabis group would exhibit ASSR deficits (decreases in mean trial power (MTP) and inter-trial coherence (ITC)), specifically in the gamma band.
MATERIALS AND METHODS
Subjects
This study was approved by the Indiana University Bloomington Human Subjects Committee. Current cannabis users (n=22) and healthy drug-naive controls (n=24) were recruited from the local university community, paid for their participation, and written informed consent was obtained from each.
The inclusion criteria were as follows: (1) For the cannabis group: current cannabis consumption (smoked joints) at the rate of at least once per week during the last month, a positive urine toxicology screen for THC metabolites (THC-COOH), no other illicit substance use during the past 3 months (including a negative urine toxicology screen for other illicit drugs), and no DSM-IV diagnosis of Axis I or II disorders, including no current or past history of illicit substance abuse or dependence (other than cannabis); (2) For the control group: no history of illicit substance use, a negative urine toxicology screen for all drugs tested, and no history of psychiatric illness (Axis I or II); (3) For all participants: ages 18–35, completion of high-school education, no family history of schizophrenia or bipolar disorder, no history of cardiovascular disease, hearing problems, neurological disease, learning disability, or head injury resulting in loss of consciousness. In addition, participants were excluded if they reported consumption of more than two alcoholic drinks per day (one per day for females). The cannabis group drug-use inclusion criteria (cannabis use at least once per week; 12 h abstinence) were chosen to minimize acute cannabis effects. Human studies indicate that 80–90% of the total amount of THC is excreted within 5 days, so a minimum use of once per week enabled detection of THC metabolites (Hunt and Jones, 1980).
Clinical Interviews, Questionnaires, and Drug Use Assessment
The structured clinical interview for DSM-IV axis I and II disorders (SCID I and SCID II) were administered to assess current and past history of psychopathology. The SCID I module E and a locally developed drug-use questionnaire were used to ascertain current and past diagnoses for alcohol and substance abuse and dependence. Levels of cannabis consumption (estimated number of joints) were determined via the interview and questionnaire for lifetime, the past 6 months, 3 months, 1 month, and then for the week before the test session as has been described previously (Fridberg et al, 2010; Skosnik et al, 2006a, 2008). Participants were instructed to consider each day of the week and indicate, for an average week, how much they consumed per drug-use occasion for each length of time assessed. Age of first use, number of joints smoked in the last month, and time since last use are reported in Table 1.
Table 1. Demographic and Substance Use Data (mean±SD).
| Controls (n=24) | Users (n=22) | P | |
|---|---|---|---|
| Age (years) | 21.6 (3.0) | 21.1 (2.6) | 0.59 |
| Education (years) | 15.0 (1.6) | 14.4 (1.4) | 0.20 |
| Gender (no. of females)a | 12 | 7 | 0.21 |
| WAIS (piccom) Scores | 12.9 (2.92) | 12.3 (2.42) | 0.43 |
| WAIS (digit) scores | 11.7 (2.53) | 11.4 (2.40) | 0.68 |
| WAIS (sim) scores | 12.4 (2.57) | 12.5 (2.41) | 0.91 |
| WAIS (dspan) scores | 10.8 (2.44) | 11.7 (3.03) | 0.28 |
| Average number of alcoholic drinks/week | 0.96 (2.5) | 5.3 (4.5) | 0.001a |
| Age of first cannabis use | — | 15.3 (1.4) | — |
| Frequency of cannabis use (joints in last month) | — | 54.0 (36.6) | — |
| Hours since last cannabis use (h) | — | 41.4 (34.0) | — |
Note: WAIS version was the WAIS-III. Subscales included were picture completion (piccom); digit symbol (digit); similarities (sim); digit span (dspan).
Results of χ2 test.
Urine screens (Q10-1, Proxam Diagnostics, Sunnyvale, CA, USA) were administered immediately preceding EEG testing in order to corroborate self-reports from the drug questionnaire and clinical interviews. The Q10-1 kit screens for cannabis (THC-COOH; 50 ng/ml sensitivity), opiates, amphetamines, cocaine, MDMA (ecstasy), tricyclic antidepressants, phencyclidine, benzodiazepines, methamphetamines, and barbiturates.
In addition to assessment of psychopathology and substance use, subscales of the Wechsler Adult Intelligence Scale III (WAIS-III; picture completion, digit symbol, similarities, and digit span) were used to assess possible deficits in general neuropsychological function.
Auditory Steady-state Responses
During the assessment of ASSRs, participants were seated comfortably in a sound-attenuated room with eyes open while passively listening to click trains presented through Etymotic insert earphones (Etymotic Research, Elk Grove Village, IL, USA). Auditory stimuli consisted of click trains (square waves) presented at nine different frequencies in each of the ten blocks (10, 15, 20, 25, 30, 35, 40, 45, and 50 Hz; 80 dB SPL). Each block contained 100 trials of the frequency of interest, which were presented for 500 ms each (interstimulus interval of 1000 ms). The order of frequency blocks was randomized across subjects.
EEG Recording
The EEG was recorded continuously (band pass 0.1–100 Hz; sampling rate 1000 Hz) from the scalp using a 32 channel electrode cap with a nose reference, along with additional electrodes to record the vertical electrooculogram (VEOG; Neuroscan SynAmps, Compumedics Neuroscan, Charlotte, NC, USA). Electrode impedances were maintained below 10 kΩ. The recorded EEG was segmented into epochs consisting of the 500 ms during stimulus presentation, along with a 500 ms baseline and a 500 ms offset period. Any trial with a voltage >±100 μV was excluded from analysis. Ocular movement correction was applied using Gratton's algorithm (Gratton et al, 1983).
EEG Signal Analysis
MTP and ITC were determined using a time X frequency spectrogram with the Signal Processing and EEGLab toolbox in MATLAB (Delorme and Makeig, 2004; Rass et al, 2010; Skosnik et al, 2006b). For MTP, a baseline-normalized event-related spectral perturbation (ERSP) was obtained by applying a fast Fourier Transform using a time-sliding window on single-trial data. This results in a time X frequency transform consisting of a complex number for every timepoint, frequency, and trial. The 500 ms interval before stimulus onset was used as the baseline for computing the ERSP, and the sliding window had a duration of 128 ms. After sufficient padding a frequency resolution of 0.98 Hz was obtained and the time resolution was 3.8 ms. A Hanning window (100%) was applied on the data before the fast Fourier transform. No other taper functions were used. Thus, MTP represents the average of spectral power from individual trials after subtracting the mean from the baseline period (500 ms before stimulus onset).
For ITC (which represents phase synchronization of EEG activity across trials at particular temporal intervals and frequencies), the complex output of the ERSP was divided by its complex norm (absolute value), which was then averaged across trials. The complex norm of this averaged value results in the ITC value for different time and frequency points. ITC values range from 0 (absence of synchronization) to 1 (perfect synchronization, or phase reproducibility across trials at a given frequency).
MTP and ITC values (frequency bins ±5 Hz from the frequency of stimulation in each condition) were averaged using sequential 100 ms windows between onset and offset of the stimuli (500 ms) as has been reported previously (Rass et al, 2010; Skosnik et al, 2006b). This resulted in 5 MTP and ITC values for every subject and every channel.
For analysis of the transient auditory-evoked response (N100), epochs were low-pass filtered at 15 Hz (24 dB/octave) before averaging, and were baseline-corrected (300 ms prestimulus baseline) after averaging. Peak amplitude and latency values after stimulus onset were used as the dependent measures, and were obtained for each electrode within the time window of interest using an automated algorithm (Vision Analyzer, Brain Products GmbH, Gilching, Germany). The N100 was defined as the most negative voltage between 90 and 150 ms after stimulus onset as reported previously (Skosnik et al, 2008). The signal amplitude showed local maxima in the frontal region (FCz), and all subsequent statistical analyses were conducted on data from this site. All N100 EEG processing was performed using commercially available software (Vision Analyzer, Brain Products GmbH).
Statistical Analysis
All analyses were conducted in the software package PASW Statistics 18.0. For the primary EEG outcome measures (MTP and ITC), a repeated measures ANOVA was utilized to examine the between-subjects factor of group (2) and the within-subjects factor of time (5) at electrode FCz (where MTP and ITC were maximal). Separate ANOVAs were performed for each frequency condition. Greenhouse–Geisser corrections for non-sphericity were used, where appropriate. The addition of gender, age, and level of alcohol use as covariates did not alter the results for the MTP or ITC analyses. For the transient N100 ERP, amplitude and latency were assessed within each frequency condition using a one-way ANOVA (at electrode FCz). In order to examine possible relationships between the ASSR and cannabis use variables (age of first use, number of joints in the last month, and time since last use), Pearson correlation coefficients were utilized. For the correlational analyses, a single value was calculated for MTP and ITC at the frequency of interest for the entire interval after stimulus onset (average MTP and ITC between 0–500 ms). All EEG data were normally distributed, as assessed with Shapiro–Wilk tests for normality. A criterion of p<0.05 was used throughout to determine statistical significance, and all tests were two-tailed.
RESULTS
Table 1 provides basic demographic information as well as cannabis/alcohol use rates. A one-way ANOVA revealed that there were no significant differences between the groups in age, years of education, or WAIS-III subscale scores. A χ2 test showed that the gender distribution within each group was not significantly different (Table 1). Because of the stringent exclusion criteria (see below), alcohol use rates for both groups were extremely low. However, there was a significant difference in the average number of alcoholic drinks consumed per week between the two groups (Table 1).
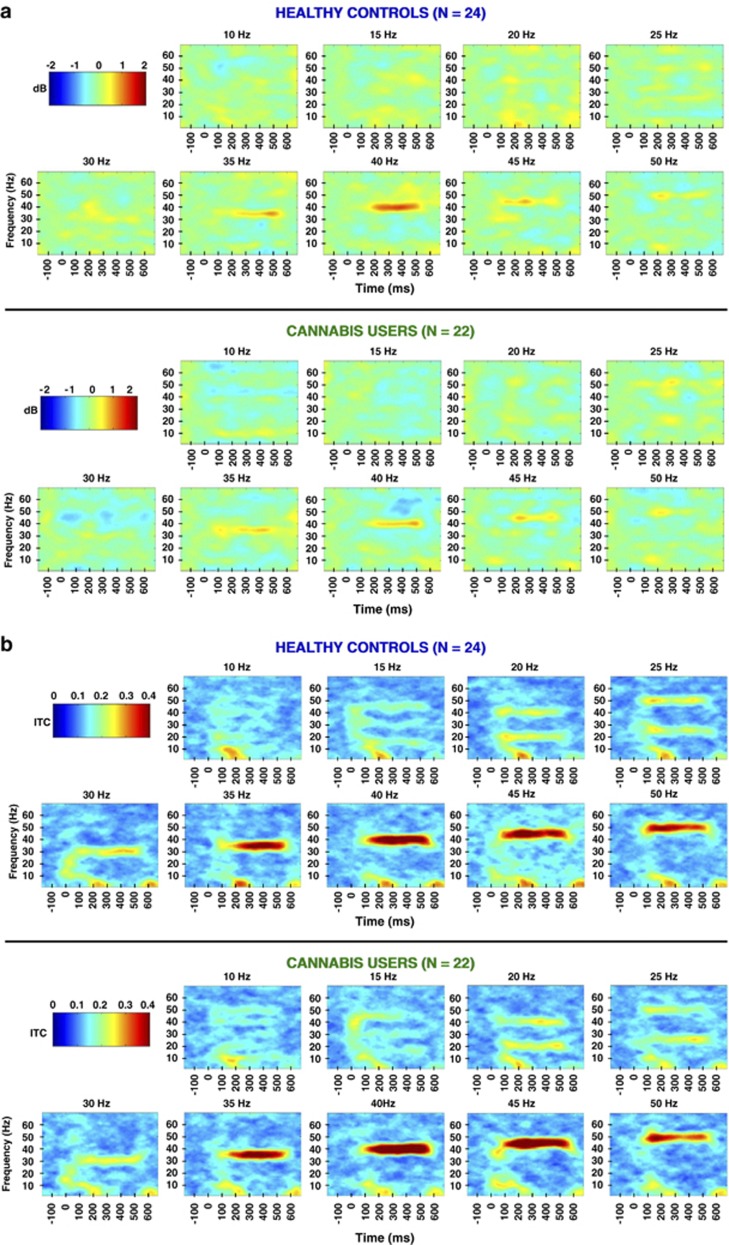
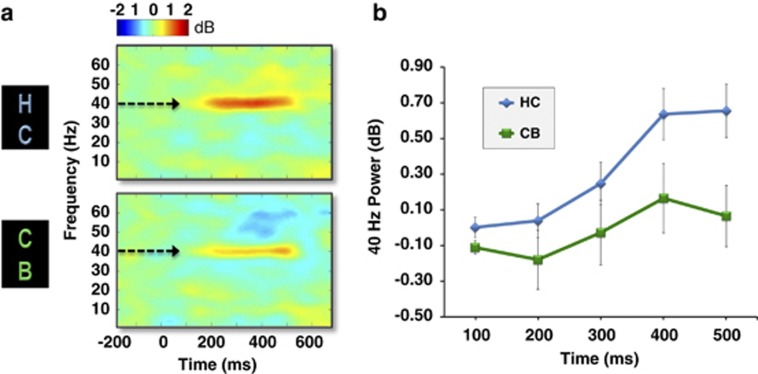
In the ASSR EEG protocol, subjects in both groups entrained to all nine stimulus frequencies, which can be seen in the grand averaged time X frequency plots of MTP and ITC across all frequencies taken from electrode FCz (Figure 1a and b). Note the preferred resonance of the ASSR at 40 Hz, particularly in MTP (Figure 1a), which has been described previously (Galambos et al, 1981; Picton et al, 2003). For MTP, a repeated measures ANOVA revealed a main effect of time for the 35, 40, and 50 Hz frequency conditions, an indication of change in spectral power across the stimulation time window, with the strongest response at 40 Hz (Table 2, top). Moreover, a main effect of group was observed in the 40 Hz (F(1,44)=6.01, p<0.018) and 15 Hz (F(1,44)=4.19, p<0.047) frequency conditions, indicating that the cannabis group had significantly decreased spectral power in the gamma and low beta bands. No group X time interactions were observed for MTP. Time X frequency plots of MTP comparing cannabis users vs controls in the 40 Hz condition can be seen in Figure 2.
Figure 1.
(a) Grand-averaged time X frequency plots demonstrating spectral power across all stimulation frequencies (FCz). Note the preferred resonance of mean trial power (MTP) at 40 Hz stimulation. (b) Grand-averaged time X frequency plots for inter-trial coherence (ITC) across all stimulation frequencies (FCz).
Table 2. ANOVA Table Showing Results for All Time X Frequency Analyses (MTP and ITC) Across All Frequency Conditions.
| ASSR condition (Hz) | Group | Time | Group X time |
|---|---|---|---|
| Spectral power (MTP) | |||
| 10 | F(1,44)=0.645, p=0.426 | F(2.99,131.56)=1.46, p=0.23 | F(2.99,131.56)=0.37, p=0.78 |
| 15 | F(1,44)=4.19, p<0.047* | F(2.57,112.85)=0.224, p=0.851 | F(2.57,112.85)=1.33, p=0.27 |
| 20 | F(1,44)=0.771, p=0.385 | F(2.04,89.73 )=2.13, p=0.12 | F(2.04,89.73 )=1.01, p=0.37 |
| 25 | F(1,44)=0.034, p=0.854 | F(3.46,152.1 )=0.6, p=0.64 | F(3.46,152.1 )=0.1, p=0.72 |
| 30 | F(1,44)=0.96, p=0.332 | F(2.0,87.6)=0.53, p=0.59 | F(2.0,87.6 )=1.0, p=0.39 |
| 35 | F(1,44)=0.210, p=0.649 | F(3.44,151.3 )=3.92, p=0.007* | F(3.44,151.3)=0.5, p=0.71 |
| 40 | F(1,44)=6.01, p<0.018* | F(3.15,138.6)=7.8, p<0.000* | F(3.15,138.6)=1.47, p=0.22 |
| 45 | F(1,44)=1.43, p=0.24 | F(2.53,111.46)=2.58, p<0.07 | F(2.53,111.46)=0.64, p=0.56 |
| 50 | F(1,44)=0.14, p=0.91 | F(3.32,146.2)=4.0, p<0.007* | F(3.32,146.2)=0.48, p=0.49 |
| Intertrial coherence (ITC) | |||
| 10 | F(1,44)=0.04, p=0.85 | F(2.49,109.32)=22.08, p=0.000* | F(2.49,109.32)=0.75, p=0.50 |
| 15 | F(1,44)=0.85, p=0.36 | F(3.13,137.78)=17.38, p<0.000* | F(3.13,137.78)=2.3, p=0.08 |
| 20 | F(1,44)=0.21, p=0.65 | F(3.23,142.17)=20.1, p<0.000* | F(3.23,142.17)=1.69, p=0.17 |
| 25 | F(1,44)=0.045, p=0.83 | F(3.20, 140.61)=5.22, p<0.002* | F(3.20, 140.61)=1.54, p=0.21 |
| 30 | F(1,44)=0.02, p=0.89 | F(2.58,113.38)=12.6, p<0.000* | F(2.58,113.38)=0.34, p=0.76 |
| 35 | F(1,44)=0.36, p=0.55 | F(2.60,114.24)=84.57, p<0.000* | F(2.60,114.24)=0.76, p=0.50 |
| 40 | F(1,44)=1.71, p=0.20 | F(2.17, 95.43)=102.61, p<0.000* | F(2.17, 95.43)=1.34, p=0.27 |
| 45 | F(1,44)=1.58, p=0.22 | F(2.40,105.46)=83.84, p<0.000* | F(2.40,105.46)=2.20, p=0.11 |
| 50 | F(1,44)=0.62, p=0.44 | F(2.39,105.36)=57.23, p<0.000* | F(2.39,105.36)=1.52, p=0.22 |
Significant effects are denoted with an asterisk* and bold font.
Figure 2.
(a) Grand-averaged time X frequency plots of MTP during gamma-band (40 Hz) auditory stimulation at electrode FCz for healthy controls (HC; top; n=24) and cannabis users (CB; bottom; n=22). Greater 40 Hz power was seen in control subjects compared with cannabis users. (b) Average MTP values in 100 ms intervals during the 500 ms window after onset of the 40 Hz click trains for control subjects (blue line) and cannabis users (green line) at FCz (error bars indicate ±SE).
For ITC, a main effect of time was observed for all frequency conditions (Table 2, bottom). No group or group X time interactions were observed for ITC.
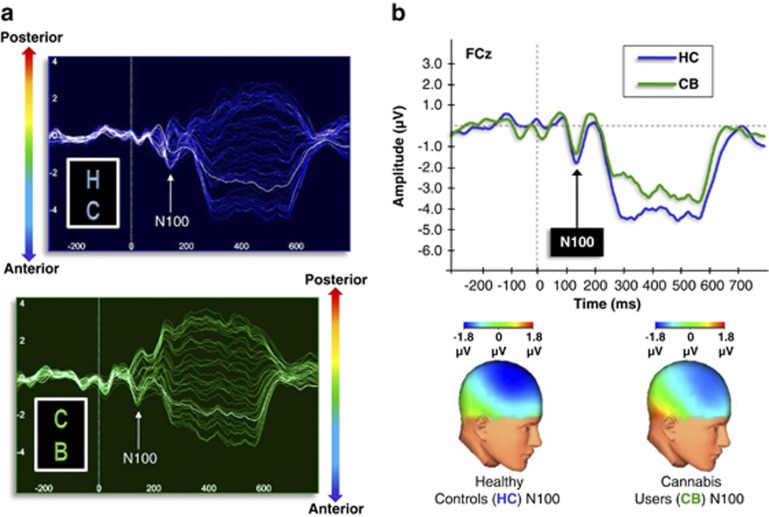
For transient ERPs to stimulus onset, no group differences were observed in the N100 component in any frequency condition, indicating that the group differences in MTP were not due to altered early stimulus processing and registration. For illustrative purposes, ERPs to stimulus onset in the 40 Hz condition can be seen in Figure 3.
Figure 3.
(a) Butterfly plots illustrating the grand-averaged ERPs across all electrodes during 40 Hz stimulation for healthy controls (HC) and cannabis users (CB). Waveforms from each source electrode are oriented generally from posterior (top) to anterior (bottom). (b) Grand-averaged ERPs (FCz) and topographic maps (at 100 ms) for healthy controls and cannabis users demonstrating the N100 to 40 Hz stimulation (top). No group differences were observed in the N100 component in any frequency condition, indicating normal early stimulus processing and registration.
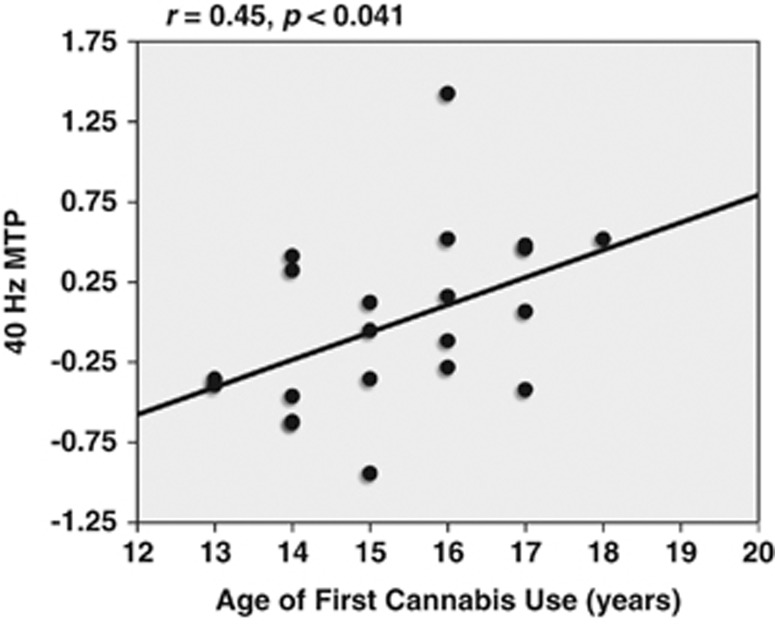
On the basis of a priori hypotheses, and the fact that the cannabis group exhibited decreased MTP in the gamma band (40 Hz), correlational analyses were carried out to examine the relationship between cannabis use variables and 40 Hz MTP. Cannabis use variables analyzed were age of first cannabis use, number of joints in the last month, and time since last use. A significant correlation was observed between 40 Hz spectral power and age of first cannabis use (r=0.45, p<0.041; Figure 4). In other words, individuals with the earliest age of cannabis use onset exhibited the lowest gamma-band (40 Hz) MTP.
Figure 4.
Correlation between 40 Hz spectral power (average MTP between 0–500 ms during 40 Hz stimulation) and age of first cannabis use within the cannabis group (n=22).
DISCUSSION
The present study evaluated neural oscillatory activity by way of EEG in chronic cannabis users. The primary finding was a decrease in spectral power (MTP) during gamma- (40 Hz) and low beta-band (15 Hz) auditory steady-state stimulation in the cannabis group. No group differences were observed in ITC. Across all frequency conditions, no differences were observed between the groups in the transient N100 ERP component, indicating intact early auditory processing and sensory registration. Lastly, within the cannabis group, earlier onset of cannabis use was associated with lower levels of 40 Hz oscillatory power.
The fact that cannabis users did not exhibit differences in the transient N100 ERP is not surprising, as previous work on this component has been mixed. The N100 is thought to be related to basic perceptual processing, and in the auditory domain, is likely generated by auditory and frontal cortices (Naatanen and Picton, 1987). Skosnik et al (2008) demonstrated that heavy cannabis users had decreased N100 amplitudes for discrete 1000 Hz tones during an associative learning task (Skosnik et al, 2008). However, a subsequent study utilizing the same auditory stimuli with a different sample of cannabis users failed to replicate this finding (Edwards et al, 2008). Whether heavy cannabis use can disrupt the transient N100 ERP therefore remains equivocal.
The current study showed that exogenous cannabinoid exposure decreased MTP, particularly in the gamma band. Although power is thought to reflect the amplitude of neural oscillations, ITC represents the variance of phase across single trials, and is mathematically independent of power and amplitude (Roach and Mathalon, 2008; Spencer et al, 2008). These results therefore suggest that for 40 Hz stimuli, cannabis preferentially affected amplitude, while the variance of phase across single trials was not disrupted.
The observed association between cannabis use and altered gamma band activity is in agreement with previous cellular work examining the effects of exogenous cannabinoids on neural oscillations. Hajos et al (2000) showed that the administration of the highly potent cannabinoid agonist CP 55,940 robustly reduced the power of 40-Hz oscillations elicited in hippocampal slices by kainate in vitro (Hajos et al, 2000). In an in vivo study using rat entorhinal cortical neurons, it was found that while the CB1R agonist arachidonylcyclopropylamide had no effect on neural oscillations, the CB1R antagonist LY320135 increased gamma-band power in the deep medial entorhinal cortex (Morgan et al, 2008). Interestingly, LY320135 suppressed gamma power in more superficial layers of the entorhinal cortex, illustrating the complex role of CB1Rs in cortical oscillations.
Although the results gleaned from slice preparations are highly informative, they are somewhat distal from the type of EEG data described in the current study. More analogous to the surface-based EEG data described here, several groups have examined the effects of cannabinoids on neural oscillations in vivo using animal-based local field potentials (LFPs). For example, it has been shown that both THC and CP 55,940 disrupt hippocampal theta and gamma oscillations in head-restrained and freely moving rats, effects that were blocked by the CB1R antagonist SR141716A (Robbe et al, 2006). Importantly, the alterations were shown to be functionally relevant, as the degree of theta power disruption was correlated with performance on a hippocampal-dependent memory task. In a study exploring hippocampal and cortical LFPs, Hajos et al (2008) demonstrated that rats engaged in a sensory gating paradigm exhibited decreases in gamma- and theta-band spectral power after the administration of CP 55,940. CP 55,940 similarly affected prefrontal cortical recordings during free movement (attenuation of gamma and theta-band power). Importantly, these results were both CB1R-specific, as the disruption in neural oscillations was reversed by the CB1R antagonist AM-251. Taken together, these results suggest that theta and gamma oscillations in networks of GABAergic interneurons are regulated by the endocannabinoid system, which can be disturbed by the exogenous application of CB1R agonists.
In terms of human studies, the present findings are consistent with the results of a number of experiments examining the effects of both chronic and acute cannabinoids on neural oscillations. For example, two studies have shown that chronic cannabis users exhibit disrupted neural oscillatory activity using different EEG paradigms. Edwards et al (2009) implemented a human analogue of the sensory gating paradigm described above by Hajos et al (2008), and found decreased gamma-band power during the auditory click stimuli, which was negatively correlated with levels of cannabis use (ie, those with the lowest gamma power had the greatest levels of cannabis exposure) (Edwards et al, 2009). Further, Skosnik et al (2006a) found evidence of decreased EEG spectral power using several frequencies of stimulation in the ASSR paradigm (Skosnik et al, 2006a). Regarding the acute effects of cannabinoids in humans, Morrison et al (2011) recently demonstrated that intravenous THC administration decreased theta power and inter-electrode coherence during performance on an n-back task of working memory (Morrison et al, 2011). Two previous studies showed similar results with inhaled THC, including decreased resting state theta power and disruptions in working memory performance (Bocker et al, 2010; Ilan et al, 2004, 2005). To date, no human studies have shown altered gamma-band activity in the context of acute cannabinoid administration.
The current finding that 40 Hz power was associated with a younger age of onset of cannabis use suggests that long-term exposure to cannabis (and not recency of use or residual cannabinoids) contributed to the observed findings. This is noteworthy, given the known role of cannabinoids in neurodevelopment. Both cellular and animal studies have shown that the endogenous cannabinoid system has a key role in neurogenesis, neural specification, neural maturation, neuronal migration, axonal elongation, and glia formation (Harkany et al, 2007, 2008a, 2008b). Hence, earlier cannabis exposure during adolescence may alter neurodevelopmental trajectories, which could permanently disrupt the ability of neural circuits to generate synchronized oscillations. Interestingly, cannabis use is now thought to represent a risk factor and component cause in the development of schizophrenia (Moore et al, 2007; Sewell et al, 2010). As schizophrenia patients and their first-degree relatives also demonstrate decreased 40 Hz ASSRs (Shin et al, 2011), it is reasonable to speculate that these alterations are mediated in part by disruptions in cannabinoid-GABA interactions. Future research is needed to explicitly test this postulate.
There are several limitations to the current experiment. First, the cross-sectional design of the study precludes the ability to ascertain precise cause and effect relationships. Hence, it remains unclear whether the observed results were due to the residual effects of THC, cannabis withdrawal, long-term cannabis exposure (eg, CB1R downregulation), or premorbid neurodevelopmental and/or personality differences predisposing individuals to use cannabis. Second, the cannabis plant contains nearly 70 phytocannabinoids, so it is unclear which specific constituent has a role in neural oscillations. Each of these limitations could be resolved in future studies by examining the ASSR in the context of acute THC administration in humans. A third limitation is that while cannabis use status was confirmed by urinary THC-COOH, no quantitative assays of THC or THC-COOH were undertaken, which would have been a more valid means to determine the magnitude of recent cannabis exposure. Fourth, as previous studies have shown that CB1Rs are also involved in theta-band oscillations, future work should examine the effect of cannabis on ASSRs in the 4–7 Hz range. Finally, the functional significance of the ASSR remains unclear, as it is unknown whether the neural oscillations evoked during auditory steady-state stimulation are related to spectral power and phase locking measured during cognitive tasks. These limitations notwithstanding, the current data suggest that chronic exposure to cannabis may alter the ability to generate neural oscillations in the gamma range, which may have implications for understanding the short- and long-term psychopharmacological effects of exogenous cannabinoids.
Acknowledgments
This research project was funded in part by grants from NIDA (1 R03 DA019630-01A1) and a NARSAD Young Investigator Award to PDS. We would like to thank Giri P Krishnan for his assistance with EEG data analysis, and Peter Finn, Jennifer Vohs, Charlotta Campbell, Ken Mackie, and the late Michael Walker for their input and help throughout the project.
Deepak Cyril D'Souza has in the past 3 years or currently receives research grant support administered through Yale University School of Medicine from Astra Zeneca, Abbott Laboratories, Eli Lilly, Organon, Pfizer, and Sanofi; he is a consultant for Bristol Meyers Squibb. Other authors of this manuscript declare no conflict of interest.
References
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci. 2010;31:1196–1207. doi: 10.1111/j.1460-9568.2010.07165.x. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bocker KB, Hunault CC, Gerritsen J, Kruidenier M, Mensinga TT, Kenemans JL. Cannabinoid modulations of resting state EEG theta power and working memory are correlated in humans. J Cogn Neurosci. 2010;22:1906–1916. doi: 10.1162/jocn.2009.21355. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD, Steinmetz AB, O'Donnell BF, Hetrick WP. Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations. Behav Neurosci. 2009;123:894–904. doi: 10.1037/a0016328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD, Steinmetz AB, Vollmer JM, O'Donnell BF, Hetrick WP. Assessment of forebrain-dependent trace eyeblink conditioning in chronic cannabis users. Neurosci Lett. 2008;439:264–268. doi: 10.1016/j.neulet.2008.04.102. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Kallo I, Deli L, Vida B, Hrabovszky E, Fekete C, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Vollmer JM, O'Donnell BF, Skosnik PD. Cannabis users differ from non-users on measures of personality and schizotypy. Psychiatry Res. 2010;186:46–52. doi: 10.1016/j.psychres.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA. 1981;78:2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hari R, Hamalainen M, Joutsiniemi SL. Neuromagnetic steady-state responses to auditory stimuli. J Acoust Soc Am. 1989a;86:1033–1039. doi: 10.1121/1.398093. [DOI] [PubMed] [Google Scholar]
- Hari R, Joutsiniemi SL, Hamalainen M, Vilkman V. Neuromagnetic responses of human auditory cortex to interruptions in a steady rhythm. Neurosci Lett. 1989b;99:164–168. doi: 10.1016/0304-3940(89)90283-8. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, Barabas K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. 2008a;286 (1–2 Suppl 1:S84–S90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Harkany T, Mackie K, Doherty P. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008b;18:338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, et al. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology. 2004;176:214–222. doi: 10.1007/s00213-004-1868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, et al. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O'Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Mathalon DH, O'Donnell BF, Hamalainen MS, Spencer KM, Javitt DC, et al. A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol Psychiatry. 2011;70:28–34. doi: 10.1016/j.biopsych.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela JP, Hari R. Evidence for cortical origin of the 40 Hz auditory evoked response in man. Electroencephalogr Clin Neurophysiol. 1987;66:539–546. doi: 10.1016/0013-4694(87)90101-5. [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL. Modulation of network oscillatory activity and GABAergic synaptic transmission by CB1 cannabinoid receptors in the rat medial entorhinal cortex. Neural Plast. 2008;2008:808564. doi: 10.1155/2008/808564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PD, Nottage J, Stone JM, Bhattacharyya S, Tunstall N, Brenneisen R, et al. Disruption of frontal theta coherence by Delta(9)-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011;36:827–836. doi: 10.1038/npp.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C. Tonotopic organization of the sources of human auditory steady-state responses. Hear Res. 1996;101:62–74. doi: 10.1016/s0378-5955(96)00133-5. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22:10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Thut G, Pascual-Leone A. Modulation of steady-state auditory evoked potentials by cerebellar rTMS. Exp Brain Res. 2006;175:702–709. doi: 10.1007/s00221-006-0588-2. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, et al. Auditory steady state response in bipolar disorder: relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 2010;12:793–803. doi: 10.1111/j.1399-5618.2010.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Karson MA, Karnup SV, Jones LM, Alger BE. Regulation of IPSP theta rhythm by muscarinic receptors and endocannabinoids in hippocampus. J Neurophysiol. 2005;94:4290–4299. doi: 10.1152/jn.00480.2005. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Sewell RA, Skosnik PD, Garcia-Sosa I, Ranganathan M, D'Souza DC. [Behavioral, cognitive and psychophysiological effects of cannabinoids: relevance to psychosis and schizophrenia] Rev Bras Psiquiatr. 2010;32 (Suppl 1:S15–S30. [PubMed] [Google Scholar]
- Shin YW, O'Donnell BF, Youn S, Kwon JS. Gamma oscillation in schizophrenia. Psychiatry Investig. 2011;8:288–296. doi: 10.4306/pi.2011.8.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W.1999Neuronal synchrony: a versatile code for the definition of relations Neuron 2449–65.111–125. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Edwards CR, O'Donnell BF, Steffen A, Steinmetz JE, Hetrick WP. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2008;33:1432–1440. doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Aydt EE, Kuhlenshmidt HA, O'Donnell BF. Psychophysiological evidence of altered neural synchronization in cannabis use: relationship to schizotypy. Am J Psychiatry. 2006a;163:1798–1805. doi: 10.1176/ajp.2006.163.10.1798. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Vohs JL, O'Donnell BF. The effect of cannabis use and gender on the visual steady state evoked potential. Clin Neurophysiol. 2006b;117:144–156. doi: 10.1016/j.clinph.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann I, Gutschalk A. Potential fMRI correlates of 40-Hz phase locking in primary auditory cortex, thalamus and midbrain. Neuroimage. 2011;54:495–504. doi: 10.1016/j.neuroimage.2010.07.064. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, et al. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, Krishnan GP, O'Donnell BF, Berg S, Morzorati SL. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13:487–497. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Faulkner HJ, Doheny HC, Traub RD. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000;86:171–190. doi: 10.1016/s0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]