Abstract
Growing evidence suggests schizophrenia may arise from abnormalities in early brain development. The prefrontal cortex (PFC) stands out as one of the main regions affected in schizophrenia. Latent inhibition, an interesting cognitive marker for schizophrenia, has been found in some studies to be reduced in acute patients. It is generally widely accepted that there is a dopaminergic dysfunctioning in schizophrenia. Moreover, several authors have reported that the psychostimulant, D-amphetamine (D-AMP), exacerbates symptoms in patients with schizophrenia. We explored in rats the effects in adulthood of neonatal transient inactivation of the PFC on behavioral and neurochemical anomalies associated with schizophrenia. Following tetrodotoxin (TTX) inactivation of the left PFC at postnatal day 8, latent inhibition-related dopaminergic responses and dopaminergic reactivity to D-AMP were monitored using in vivo voltammetry in the left core part of the nucleus accumbens in adult freely moving rats. Dopaminergic responses and behavioral responses were followed in parallel. Prefrontal neonatal inactivation resulted in disrupted behavioral responses of latent inhibition and latent inhibition-related dopaminergic responses in the core subregion. After D-AMP challenge, the highest dose (1.5 mg/kg i.p.) induced a greater dopamine increase in the core in rats microinjected with TTX, and a parallel increase in locomotor activity, suggesting that following prefrontal neonatal TTX inactivation animals display a greater behavioral and dopaminergic reactivity to D-AMP. Transitory inactivation of the PFC early in the postnatal developmental period leads to behavioral and neurochemical changes in adulthood that are meaningful for schizophrenia modeling. The data obtained may help our understanding of the pathophysiology of this disabling disorder.
Keywords: neonatal functional inactivation, prefrontal cortex, latent inhibition, D-amphetamine, nucleus accumbens, in vivo voltammetry
INTRODUCTION
The prefrontal cortex (PFC) stands out as one of the main regions affected in schizophrenia. In the past two decades, several studies have described cytoarchitectural and neuronal morphometric abnormalities consistent with defects in early neurodevelopment at the level of the PFC, particularly in the left hemisphere (Kalus et al, 2000; Garey, 2010; Yang et al, 2011). In schizophrenia, miswiring of neuronal connections due at least in some cases to neurodevelopmental failures would result in disconnectivity between different integrative brain regions, especially the PFC, temporal lobe, and striatal regions (Lawrie et al, 2002; Stephan et al, 2009). This disconnectivity may cause the psychic disintegration characteristic of the disease.
It is generally widely accepted that there is striatal dopaminergic dysfunctioning in schizophrenia (eg, Carlsson et al, 2001). Consistent with this proposal, a larger striatal dopamine release following administration of D-amphetamine (D-AMP) associated with the emergence or increase of positive symptomatology was observed in unmedicated patients with schizophrenia, in comparison with healthy controls (see Lyon et al, 2011 for review). It is also interesting to note that an improvement in cognitive function has been reported in medicated patients with schizophrenia after D-AMP administration (Barch and Carter, 2005). Latent inhibition, an interesting cognitive marker for schizophrenia, has also been found to be reduced in acute patients with schizophrenia (Baruch et al, 1988; Gray et al, 1995; Rascle et al, 2001; but see Swerdlow et al, 1996), whereas in chronic patients it has been found to be either enhanced (Rascle et al, 2001) or normal (see Kumari and Ettinger, 2010; Swerdlow, 2010 for reviews). Latent inhibition, as first described by Lubow and Moore (1959), is a behavioral phenomenon observed in many animal species. It was originally defined as retarded acquisition of the conditioned response (CR) when the conditional stimulus (CS) is first pre-exposed on its own. However, more recent theoretical explanations also lend support to the view that latent inhibition is a performance deficit characterized by a reduction or even disappearance of the CR, when the CS is presented without reinforcement, before conditioning (see De la Casa and Pineño, 2010 for review). Recent evidence from in vivo animal studies backs the existence of a differential involvement of dopaminergic neurons innervating the core and dorsomedial shell subregions of the nucleus accumbens in this phenomenon (Jeanblanc et al, 2002; Louilot et al, 2010). Moreover, as shown recently, unilateral neonatal functional blockade in the left hemisphere of two temporal regions linked to the PFC, the entorhinal cortex, or the ventral subiculum of the hippocampus (Jay and Witter, 1991; Hoover and Vertes, 2007) have been found to be sufficient to induce a disruption of the behavioral manifestation of latent inhibition and the latent inhibition-related dopaminergic variations in adult animals, with a differential reversal of these responses in the left core part of the nucleus accumbens (Peterschmitt et al, 2007; Meyer and Louilot, 2011).
The present study was designed to investigate, in adult rats, the consequences of neonatal functional inactivation of the left PFC for two schizophrenia-related markers: latent inhibition expression and reactivity to D-AMP. To that end, and given the aforementioned data, we performed a functional neonatal blockade of the left PFC by locally microinjecting tetrodotoxin (TTX), a well-known Na+ channel blocker, at postnatal day 8 (PND8). This point in time in the postnatal neurodevelopmental period in rats is comparable to the middle of the second trimester of gestation in humans (Clancy et al, 2001), considered a time window of high vulnerability for developing schizophrenia (Weinberger and Lipska, 1995; Fatemi and Folsom, 2009). TTX interrupts neuronal activity whereas, during development, impulse electrical activity is crucial for shaping connections, once developing fibers reach the target structure (Stryker and Harris, 1986; Katz and Shatz, 1996; Drakew et al, 1999; Hutchins and Kalil, 2008; see also Spitzer, 2006). D-AMP behavioral reactivity was assessed by measuring locomotor activity, which is considered as a good marker for animal modeling for schizophrenia (Lipska and Weinberger, 2000). The core part of the nucleus accumbens was chosen for dopamine measurements as it has been reported that it sends direct efferent connections to the motor outputs (eg, Zahm, 1999). Latent inhibition-related dopaminergic responses and D-AMP-induced dopamine release were monitored in the left core alongside behavioral responses using in vivo voltammetry in freely moving adult animals (11 weeks).
MATERIALS AND METHODS
Animals and Experimental Design
Animals were housed at 22±2 °C and fed ad libitum. Sprague–Dawley female rats (Janvier, Le Genest, France), obtained at 14 days gestation, were housed individually on a 12-h light/dark cycle (lights on at 0800 hours). The size of the litters was limited at birth (PND0) in 12 animals. The supernumerary neonates were given a lethal injection of pentobarbital. On PND8, half of the litter, chosen at random received a PBS microinjection (control groups) and the other half received a TTX microinjection (experimental groups). On PND56 male animals were individually housed on a 12-h reversed light/dark cycle (lights off at 1100 hours). On PND70, the grown-up male rats were surgically implanted with a microsystem designed for monitoring behavior and voltammetric responses in parallel. All the behavioral procedures commenced at least 1 week after implantation of the microsystem in the adult animals. All the experiments were performed during the dark phase of the light/dark cycle. A total of 128 male rats were used in the present study. All the experimental procedures were conducted in accordance with the European Community guidelines for the care and use of experimental animals (Council Directive 86/609/EEC) and authorized by the French Ministry of Agriculture (Authorization 67-244).
Surgery
Neonate surgery
On PND8, rat pups (weight 18.8±0.2 g) were anaesthetized by gas anesthesia by vaporizing isoflurane (Forene, ABBOTT, Rungis, France; for further details see Peterschmitt et al, 2007; Meyer et al, 2009; Meyer and Louilot, 2011). A stainless steel guide cannula (30 gauge, 12.5 mm length, Small Parts, Miami) was lowered into the PFC at coordinates 1.5 mm relative to bregma (AP), 0.4 mm lateral to the midline (L), and 3.9 mm below the cortical surface (H). PBS (NaCl 8 g/l, KCl 0.2 g/l, MgCl2, 6H2O 0.1 g/l, KH2PO4 0.2 g/l, Na2HPO4, 2H2O 1.15 g/l, pH: 7.4) or TTX (Sigma, St Quentin-Fallavier, France) dissolved in PBS were infused in a volume of 0.3 μl for a period of 135 s using an infusion pump (Razel, Stamford, CT). The cannula was left in the PFC for 4 min after the end of the microinjection to allow PBS and TTX to diffuse in the targeted structure. The amount of TTX microinjected in the PFC (100 μM × 0.3 μl), approximately 10 ng, is similar to that reported previously in the literature (Zhuravin and Bures, 1991; Peterschmitt et al, 2007; Meyer et al, 2009; Meyer and Louilot, 2011). It has been reported that the effects resulting from the TTX blockade last 24–48 h (Rothfeld et al, 1986). Zhuravin and Bures (1991) reported that microinjection of 10 ng TTX in a total volume of 1 μl at a rate of 1 μl/min has no functional consequences beyond 1 mm/3 h after the microinfusion. Pilot experiments we performed showed that TTX microinjected in neonates in the cortex 1 mm above the infralimbic/prelimbic region of the PFC does not change latent inhibition expression in adult animals. Moreover, according to Malpeli (1999), if the radius of spread varies as the cube root of the volume, the efficient spread for a volume of 0.3 μl TTX is only 0.67 mm, corresponding to an efficient spread of 1 mm for a volume of 1 μl TTX, according to Zhuravin and Bures (1991). Further more, in the Zhuravin and Bures experiment the microinjection speed was 1 μl/min, whereas in our study the infusion speed was 0.3 μl/135 s. This slow injection speed could result in a more limited functional effective spread as has been observed with a slow infusion speed (1 μl/3 min) of lidocaïne, which is more diffusible than TTX (Albert and Madryga, 1980; Zhuravin and Bures, 1991).
The microinjection site in the PFC was identified by postmortem using Evans blue (Sigma), a vital dye added to the solutions of PBS and TTX, and reported as remaining visible in the cerebral tissue several weeks after injection (Martin and Ghez, 1999; Peterschmitt et al, 2007; Meyer et al, 2009; Brooks et al, 2011; Meyer and Louilot, 2011). Each rat pup was identified by means of small three-digit ear tags (Ref 52-4716, Harvard Apparatus, Les Ulis, France). After surgery all of the pups microinjected with either PBS or TTX were placed under a warming lamp and then returned to their mother.
Adult surgery
On PND70, following anesthesia by chloral hydrate (400 mg/kg i.p.) and after being placed in a stereotaxic apparatus (incisor bar set at 3.3 mm below the interaural line; Unimécamique, Epinay/Seine, France), the fully grown male rats (weight 400±25 g) were implanted in the left core part of the nucleus accumbens at coordinates 10.2 mm (AP), 1.8 mm (L), and 6.9 mm (H; Paxinos and Watson, 2009), with a specially designed microsystem (Unimécanique; Louilot et al, 1987) allowing to monitor behavioral and dopaminergic responses in parallel. After surgery animals were given 7–8 days to recover.
Behavior Analysis
Latent inhibition
Procedure: A total of 70 animals were used for the latent inhibition experiment. Latent inhibition was measured in a three-stage latent inhibition paradigm involving a conditioned olfactory aversion procedure, with banana odor (amyl acetate, Prolabo, Strasbourg, France) as the conditional olfactory stimulus (CS), and LiCl-induced aversion as the unconditional stimulus, as previously reported (Jeanblanc et al, 2002; Peterschmitt et al, 2007; Louilot et al, 2010; Meyer and Louilot, 2011). All pre-exposed and non-pre-exposed animals were microinjected at PND8 with either PBS or TTX in the PFC as indicated before (see neonate surgery). After being implanted with the microsystem, the adult rats were randomly split up into two pre-exposed control groups (pre-exposed PBS-NaCl; pre-exposed TTX-NaCl), two pre-exposed-conditioned groups (pre-exposed PBS-LiCl; pre-exposed TTX-LiCl), two non-pre-exposed control groups (non-pre-exposed PBS-NaCl; non-pre-exposed TTX-NaCl), and two non-pre-exposed-conditioned groups (non-pre-exposed PBS-LiCl; non-pre-exposed TTX-LiCl).
Data analysis: The animals' position in the experimental cage (24 cm wide × 27 cm long × 44 cm high) was followed using a small infrared camera (Ref. 51.8050, CA-H34C, Selectronic, Lille, France) inserted into the ceiling of the cage and linked up to a video monitor and videotape. The banana odor was fed into the cage through a hole in the wall adjacent to the cage door. More precisely, the olfactory stimulus (amyl acetate) was applied to a ball of cotton wool placed in the bottom of a vial, which could be quickly inserted in the hole. Attraction or aversion to the olfactory stimulus was evaluated by the amount of time spent near the olfactive source. The cage floor was divided empirically into two virtual zones. One, containing the hole, was a semicircle accounting for 35% of the total surface area. The rest of the floor made up the second zone. The behavior was analyzed over 10-min periods. It was assumed that an animal moving about the cage at random should spend 35% (210 s) of the 10-min period in the zone containing the hole. Results are expressed as mean±SEM of the time spent near the hole.
D-AMP-induced locomotor activity
Procedure: A total of 58 animals were used for the D-AMP experiment. D-AMP-sulfate (Sigma) was dissolved in 0.9% saline, with D-AMP solutions freshly prepared before administration. Adult rats from the same litter were spread across the two controls groups (PBS-NaCl; TTX-NaCl), the two groups injected with D-AMP 0.75 mg/kg (PBS-D-AMP 0.75 mg/kg; TTX-D-AMP 0.75 mg/kg) and the two groups injected with D-AMP 1.5 mg/kg (PBS-D-AMP 1.5 mg/kg; TTX-D-AMP 1.5 mg/kg). Animals were habituated to the experimental cage (24 cm wide × 27 cm long × 44 cm high) for 1 h, after which they received, randomly, either an i.p. injection of NaCl (0.9%) or an i.p. injection of one of the two doses (0.75 mg/kg or 1.5 mg/kg) of D-AMP chosen from the studies that showed that D-AMP administered in this range induces a marked increase in locomotor activity (Sharp et al, 1987; Louilot and Choulli, 1997), a good index for animal modeling for schizophrenia (Lipska and Weinberger, 2000). The animals then remained for 90 min in the experimental cage during which their locomotor activity was assessed.
Data Analysis: The behavior of the animals in the experimental cage was monitored using a small infrared camera placed in the top of the cage and connected to a video monitor and videotape. The floor of the cage was divided into four equal virtual quadrants, so that locomotor activity could be measured by directly observing each animal via a video recording and counting the number of times it crossed from one quadrant to another in each 10-min period. Data are expressed as mean±SEM.
Voltammetric Analysis of the Dopaminergic Signal
The electrochemical procedures were those previously described (Gonzalez-Mora et al, 1991; Peterschmitt et al, 2007; Meyer et al, 2009; Meyer and Louilot, 2011). Differential normal pulse voltammetry (see O'Neill et al, 1998 for review), combined with the computerized waveform analysis of the catechol peak, was used to obtain the selective detection of the extracellular dopamine levels in the core part of the nucleus accumbens. It is unlikely that any of the change we observed in the voltammetric dopaminergic signal is due to the oxidation of noradrenaline, particularly because noradrenergic innervation has been found to be largely absent from the core part of the nucleus accumbens (Berridge et al, 1997). DNP voltammograms were recorded every minute. The average amplitude of the last 10 peaks (last 10 min) of dopamine signals obtained during the control period (variation of voltammetric signal <10%) was calculated for each animal and set at 100%. Voltammetric variations in the dopamine signal in the core subregion of the nucleus accumbens, recorded min by min, are expressed as percentages (mean±SEM) of the mean values before exposure to the olfactory stimulus or to the i.p. injection (NaCl 0.9% or D-AMP). Only variations obtained every 2 min are shown on the graphs.
Statistics
Statistical analysis was performed using a multifactorial ANOVA analysis with repeated measurements on the time factor. Only between-subject ANOVAs are shown unless otherwise indicated. For both the latent inhibition and D-AMP experiments, possible litter effects were first tested. For the latent inhibition experiment, between-subject grouping factors were the conditioning factor with two levels (NaCl, LiCl), microinjection factor with two levels (PBS, TTX), and pre-exposure factor with two levels (pre-exposed, non-pre-exposed). The dependent variables were the time spent near the hole for the behavioral study and the dopaminergic variations for the voltammetric study. For the D-AMP experiment, between-subject grouping factors were D-AMP doses with three levels (NaCl; 0.75 mg/kg; 1.5 mg/kg) and microinjection with two levels (PBS, TTX). The dependent variables were the number of crossings for the behavioral study and the dopaminergic variations for the voltammetric study. For both experiments (latent inhibition, D-AMP response), contrast analysis of the ANOVA was used to test specific hypotheses (see Rosenthal and Rosnow, 1985; Rosenthal et al, 2000). Statistical significance was set at P<0.05 for all analyses.
Histology
At the end of each experiment, the rats underwent electrocoagulation (see Meyer et al, 2009 for details), before receiving a lethal i.p. injection of pentobarbital and then being intracardially perfused, first with NaCl 0.9% and then with a 4% paraformaldehyde solution. Their brains were quickly removed from their skull. The postnatal microinjection site in the left PFC was visualized with the vital dye Evans blue and neutral red staining of the PFC sections, whereas the voltammetric recording site in the left core part of the nucleus accumbens was checked by staining the brain sections with thionin blue. Paxinos and Watson's atlas (Paxinos and Watson, 1998; 2009) was used as a reference.
RESULTS
Histology
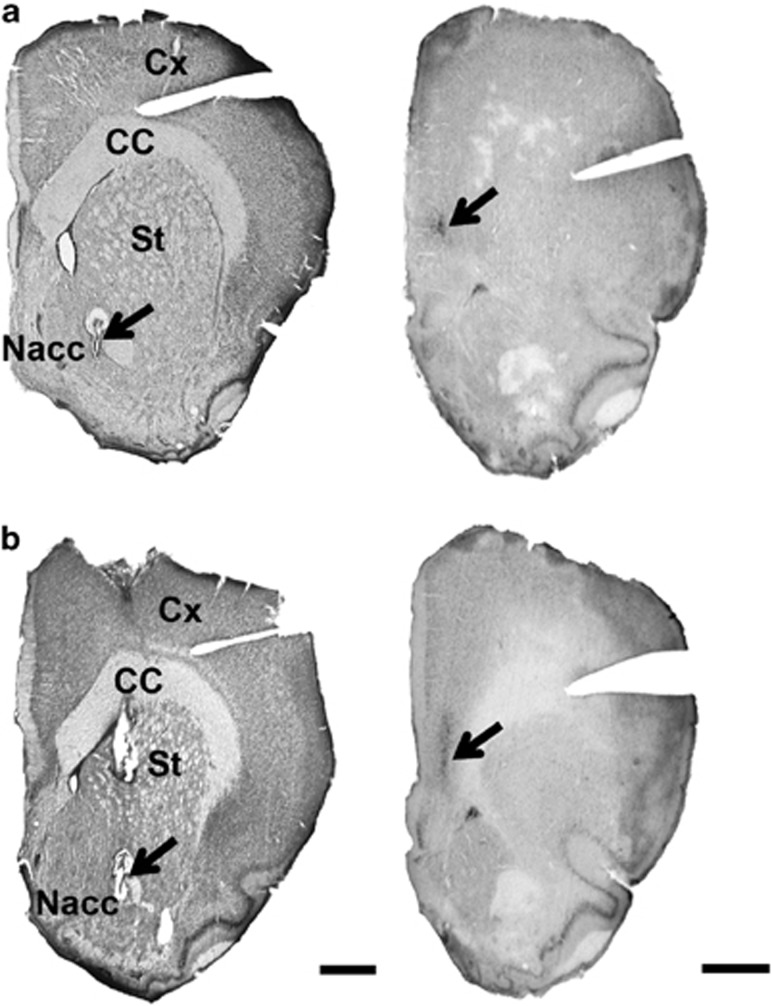
The qualitative macroscopic observations of brain sections at the level of the left PFC and left core part of the nucleus accumbens displayed no gliosis or anatomical changes between the animals microinjected with PBS or TTX in the PFC at PND8 (Figure 1).
Figure 1.
Microphotographs illustrating typical recording sites in the core part of the left nucleus accumbens (left panels) and typical injection sites in the left prefrontal cortex (right panels) of animals microinjected at postnatal day 8 with PBS (a) or tetrodotoxin (TTX) (b). Small arrows (left panels) indicate the location of tracks made by the recording electrodes following electrocoagulation at the end of the experiment. The microinjection sites in the left prefrontal cortex were identified by means of the vital dye Evans blue (arrows, right panels). The locations of both, recording sites and microinjection sites, were checked histologically by thionin blue and neutral red staining of sections, respectively. Scale bar=1 mm. CC, corpus callosum; Cx, cortex; Nacc, nucleus accumbens; St, striatum.
Latent Inhibition
Behavioral study (retention session)
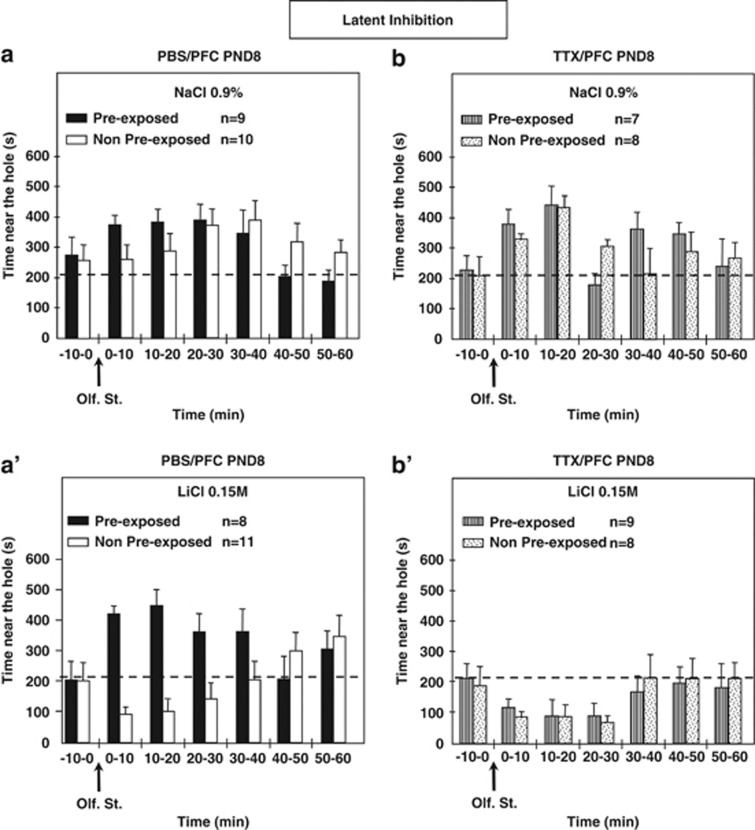
To summarize, pre-exposed PBS animals microinjected at PND8 in the left PFC displayed no significant conditioning effect, unlike other groups. The conditioning effects obtained for pre-exposed TTX animals and non-pre-exposed TTX animals were similar. Time spent near the hole differed statistically for pre-exposed PBS-conditioned animals and pre-exposed TTX-conditioned animals (Figure 2).
Figure 2.
Time spent near the hole during the test session in pre-exposed and non-pre-exposed adult animals microinjected in the left prefrontal cortex with either PBS (a and a′) or tetrodotoxin (TTX) (b and b′) at postnatal day 8. The arrows indicate presentation of the olfactory stimulus (banana odor). During the conditioning session, banana odor was associated in control animals with an i.p. injection of NaCl (0.9%) and in conditioned animals with an i.p. injection of the same volume of LiCl (0.15 M). The dashed line represents 35% of one 10-min period (210 s), corresponding to neutral distribution of the rats in the cage. n Represents the number of animals per group. Results were analyzed with factorial ANOVA.
The ANOVAs for the full hour following animals' exposure to the banana odor showed no significant litter effects (F[10,59]=1.53, NS) but revealed significant effects of conditioning (NaCl/LiCl; F[1,62]=18.07; P<0.0001), and microinjection (PBS/TTX; F[1,62]=6.96; P<0.05), as well as a significant interaction of microinjection × conditioning (F[1,62]=6.71; P<0.05), whereas no significant effects were found for pre-exposure or the other interactions (microinjection × pre-exposure, pre-exposure × conditioning, microinjection × pre-exposure × conditioning). Furthermore, contrast analysis of ANOVA for the time spent near the olfactory source during the full hour following stimulus presentation showed a significant conditioning effect for the non-pre-exposed-PBS groups (NPE-PBS-NaCl/NPE-PBS-LiCl; F[1,62]=7.09; P<0.01), non-pre-exposed-TTX groups (NPE-TTX-NaCl/NPE-TTX-LiCl; F[1,62]=9.46; P<0.005), and pre-exposed-TTX animals (PE-TTX-NaCl/PE-TTX-LiCl; F[1,62]=12.24; P<0.001), whereas in pre-exposed PBS groups (PE-PBS-NaCl/PE-PBS-LiCl) no significant differences were obtained. A clear significant effect of the neonatal microinjection was obtained for the pre-exposed-conditioned animals (PE-PBS-LiCl/PE-TTX-LiCl; F[1,62]=17.48; P<0.0001).
Dopaminergic variations recorded in the core part of the nucleus accumbens (retention session)
Only animals with implantation sites clearly located in the core part of the nucleus accumbens (Figure 1, left; Supplementary Figure S1) were considered for the voltammetric analysis (Figure 3).
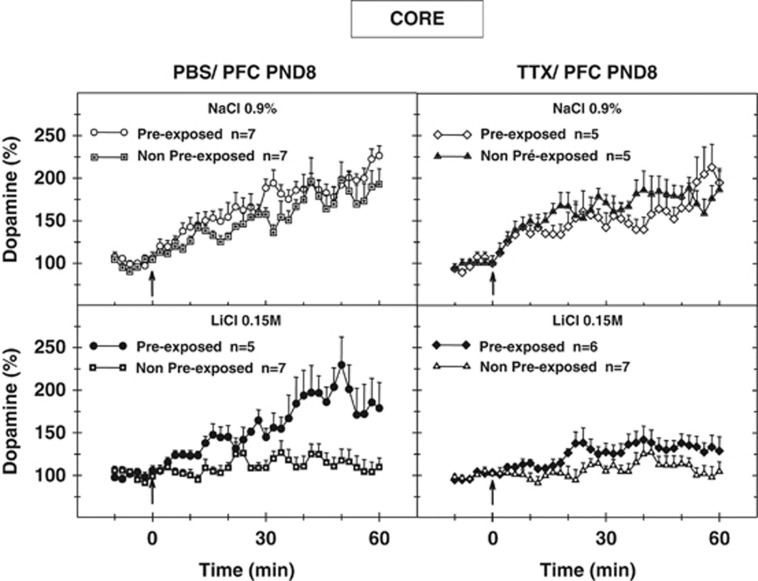
Figure 3.
Dopaminergic variations recorded in the core part of the left nucleus accumbens during the test session in adult pre-exposed and non-pre-exposed animals after transient neonatal inactivation of the left prefrontal cortex by tetrodotoxin (TTX) at postnatal day 8 (PND8). Extracellular dopaminergic variations were assessed using differential normal pulse voltammetry and computer-assisted numerical analysis in freely moving adult rats. Voltammograms were recorded every minute. Only mean values and SEM corresponding to two scans are presented. Where no SEM is given, the size is less than the radius of the symbol. The arrows indicate presentation of the olfactory stimulus (banana odor). n Is the number of rats per group. Results were analyzed with factorial ANOVA.
In summary, concerning the dopaminergic variations recorded in the core subregion of the nucleus accumbens no significant conditioning effect was observed for the pre-exposed PBS animals (conditioned vs controls), unlike the other groups. Moreover, increases in the dopamine signal in the pre-exposed PBS-conditioned animals were significantly different from the dopaminergic variations recorded in the non-pre-exposed PBS-conditioned animals, whereas in the case of the pre-exposed TTX-conditioned animals, the dopaminergic responses were moderate and not statistically different from those observed in the non-pre-exposed TTX-conditioned animals.
The overall ANOVA carried out for the dopaminergic variations in the core part of the nucleus accumbens with respect to the full hour following exposure to the banana smell showed significant differences for the conditioning factor (NaCl/LiCl; F[1,41]=20.88; P<0.00005), as well as the pre-exposure factor (NPE/PE; F[1,41]=8.09; P<0,01), but not the microinjection factor (PBS/TTX). The various interactions (conditioning × microinjection, microinjection × pre-exposure, pre-exposure × conditioning or conditioning × microinjection × pre-exposure) were not found to be statistically different. Furthermore, contrast analysis of ANOVA performed on the dopaminergic variations for the whole hour following exposure to the olfactory stimulus revealed a significant effect of conditioning (NaCl/LiCl) for the non-pre-exposed-PBS groups (NPE-PBS-NaCl/NPE-PBS-LiCl; F[1,41]=7.36; P<0.01), non-pre-exposed TTX groups (NPE-TTX-NaCl/NPE-TTX-LiCl; F[1,41]=11.46; P<0.005), and pre-exposed TTX groups (PE-TTX-NaCl/PE-TTX-LiCl; F[1,41]=6.67; P<0.05), whereas no significant difference was observed for the conditioning factor for the pre-exposed PBS animals (PE-PBS-NaCl/PE-PBS-LiCl). This contrast analysis also revealed a significant effect of pre-exposure for the PBS-conditioned animals (PE-PBS-LiCl/NPE-PBS-LiCl; F[1, 41]=9.08; P<0.005), but no statistical differences for the TTX-conditioned animals (PE-TTX-LiCl/NPE-TTTX-LiCl; F[1,41]=1.45; P=0.24, NS).
D-AMP Challenge
D-AMP-induced locomotor activity
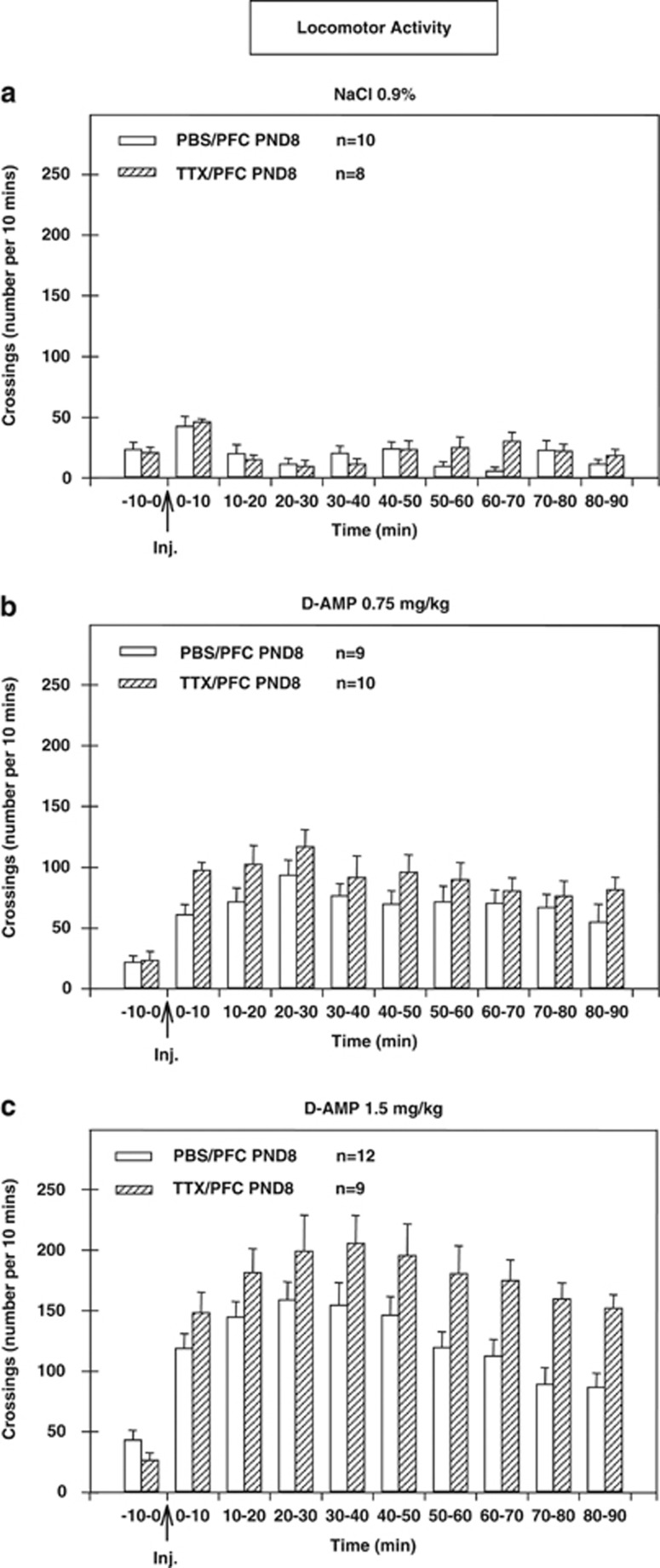
To summarize, after D-AMP challenge a difference in locomotor activity was observed depending on the dose (NaCl 0.9%, D-AMP 0.75 mg/kg, D-AMP 1.5 mg/kg; Figure 4). Moreover, a significant effect of the neonatal microinjection (PBS or TTX) on locomotor activity was obtained for the highest D-AMP dose (1.5 mg/kg; Figure 4c).
Figure 4.
Locomotor activity of animals microinjected at postnatal day 8 (PND8) with PBS (white bars) or tetrodotoxin (TTX; hatched bars) following a NaCl (0.9%) injection (a) or D-AMP challenge (0.75 mg/kg; 1.5 mg/kg, respectively b, c). The arrows indicate the moment of injection (NaCl, D-AMP 0.75 mg/kg, or D-AMP 1.5 mg/kg). n Is the number of animals per group. Results were analyzed with factorial ANOVA.
The overall ANOVAs carried out for the locomotor activity of the 58 animals during the 90 min postinjection showed no significant litter effects (F[8,49]=1.17, NS) but revealed significant effects of the dose (NaCl 0.9%/D-AMP 0.75 mg/kg, D-AMP 1.5 mg/kg; F [1,52]=64.55; P<10−6) and microinjection (PBS/TTX; F[1,52]=7.42; P<0.01), but not the dose × microinjection interaction. Contrast analysis of ANOVA for locomotor activity during the 90 min following the D-AMP challenge showed a significant microinjection effect only for the highest D-AMP dose 1.5 mg/kg (F[1,52]=10.76; P<0.01), but not for the lowest D-AMP dose 0.75 mg/kg.
Within-subjects analysis revealed a significant time effect (F[8,416]=8.12; P<10−5) and a significant time × dose effect (F[16,416]=5.78; P<10−5), but no significant effects for the time × microinjection or time × dose × microinjection interactions.
D-amp-induced dopaminergic changes recorded in the core part of the nucleus accumbens
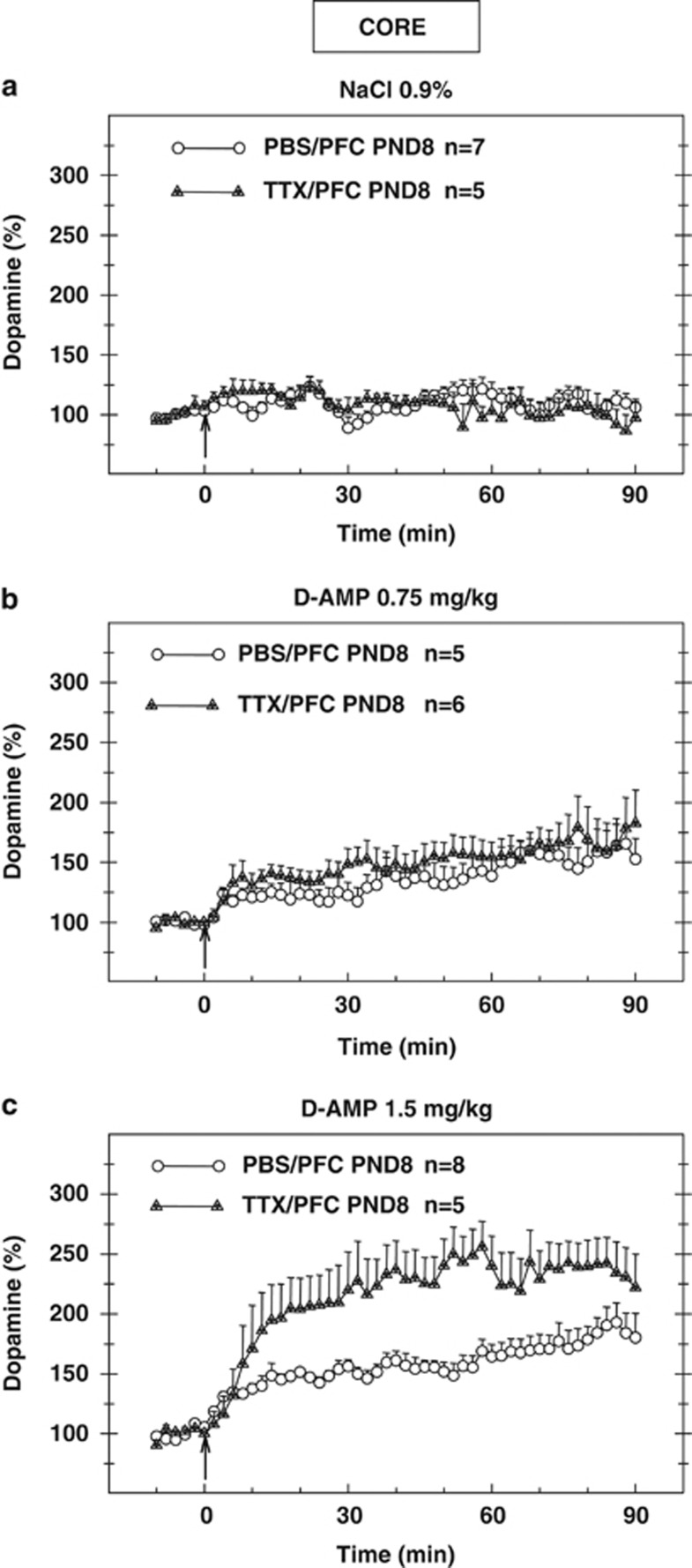
Only animals with implantation sites clearly located in the core part of the nucleus accumbens (Figure 1, left; Supplementary Figure S1) were considered for the voltammetric analysis (Figure 5). To summarize, after D-AMP challenge extracellular dopamine levels depend on the injected dose (NaCl 0.9%, D-AMP 0.75 mg/kg, D-AMP 1.5 mg/kg). A significant effect of the neonatal microinjection (PBS or TTX) on the dopaminergic levels was observed only for the highest D-AMP dose (1.5 mg/kg; Figure 5c).
Figure 5.
Dopaminergic levels recorded in the core part of the left nucleus accumbens following a NaCl (0.9%) injection (a) a D-AMP challenge (0.75 mg/kg, 1.5 mg/kg, respectively b, c) after neonatal inactivation of the left prefrontal cortex by tetrodotoxin (TTX) at postnatal day 8. Extracellular dopaminergic variations were assessed using differential normal pulse voltammetry and computer-assisted numerical analysis in freely moving adult rats. Voltammograms were recorded every minute. Only mean values and SEM corresponding to two scans are presented. Where no SEM is given, the size is less than the radius of the symbol. The arrows indicate the time of injection of either NaCl (0.9%) or D-AMP (0.75 mg/kg, 1.5 mg/kg). n is the number of rats per group. Results were analyzed with factorial ANOVA.
The overall ANOVA for the dopaminergic variations recorded in the core part of the nucleus accumbens in the 90 min postinjection revealed a significant effect of the dose (NaCl, 0.9%/D-AMP 0.75 mg/kg/D-AMP 1.5 mg/kg; F[2,30]=21.32; P<10−5) or neonatal microinjection (PBS/TTX; F[1,30]=5.28; P<0.05) but no significant effect of the interaction (dose × microinjection), although a trend was observed (F[2,30]=3.28; P=0.052). Contrast analysis of ANOVA revealed a significant effect of the neonatal microinjection (F[1,30]=11.67; P<0.05) for the highest dose of D-AMP (1.5 mg/kg), but no effect was obtained for the lowest dose (0.75 mg/kg).
Within-subjects analysis revealed a significant time effect (F[44,1320]=9.71; P<10−5) and significant effects for the time × dose interaction (F[88,1320]=5.12; P<10−5), the time × microinjection interaction (F[44,1320]=1.59; P<0.01), and the time × dose × microinjection interaction (F[88,1320]=1.71; P<10−4).
DISCUSSION
To the best of our knowledge, the present study is the first to report the consequences, in adult animals, of a neonatal transitory inactivation of the left PFC for two schizophrenia-related markers; latent inhibition expression and reactivity to D-AMP challenge. The results suggest that neonatal TTX inactivation of the left PFC performed in rats at PND8 leads to disruptions in adulthood of both latent inhibition-related behavioral and dopaminergic responses in the core part of the nucleus accumbens, as well as heightened behavioral and dopaminergic reactivity to D-AMP at the highest dose.
No macroscopic anatomical changes were observed in adulthood in either the PFC or nucleus accumbens of TTX animals. It is conceivable, therefore, that transitory blockade of the PFC early in development involves cellular/molecular mechanisms resulting in impaired communication between the PFC and structures that either receive direct projections from the PFC, such as the nucleus accumbens (Wright and Groenewegen, 1995; Heidbreder and Groenewegen, 2003), or which innervate the PFC, such as the ventral subiculum (Jay and Witter, 1991) or those presenting reciprocal connections with the PFC, such as the entorhinal cortex (Heidbreder and Groenewegen, 2003; Hoover and Vertes, 2007). The first 2 weeks after birth are a critical time window for the development of the PFC insofar as intrinsic and extrinsic connections of the PFC are growing significantly (van Eden et al, 1990). Electrical activity has an essential role in the early development of the nervous system (for review see Spitzer, 2006) and is involved in a number of cellular processes, such as axons' myelination (Demerens et al, 1996), rearrangement of synaptic connections in target structures (Stryker and Harris, 1986; Katz and Shatz, 1996; Hutchins and Kalil, 2008), and maturation of dendritic spines (Drakew et al, 1999). Thus, failure in one or more of these mechanisms, following TTX neonatal inactivation, could result in malfunctioning of the PFC and consequently in changes in control in the dopamine release in the core part of the nucleus accumbens. This suggestion is consistent with recent data showing that a neonatal PFC lesion enhances the sensitivity of the mesoaccumbal dopamine neurons (Bennay et al, 2004) and leads to deficient myelination in some projection areas of the PFC, including the nucleus accumbens, hippocampus, and amygdala (Schneider and Koch, 2005; Klein et al, 2008).
In the latent inhibition paradigm, neonatal TTX functional blockade of the left PFC at PND8 induced no significant behavioral and dopaminergic differences between non-pre-exposed PBS and non-pre-exposed TTX adult animals. For both postnatal microinjections, attraction or aversion toward the olfactory stimulus (banana odor) was observed for control and conditioned animals. These behavioral results were compatible with those previously obtained with the same aversive conditioning protocol in microinjection-naïve non-pre-exposed animals (Jeanblanc et al, 2002; Louilot et al, 2010), suggesting that early TTX inactivation of the PFC leaves the olfactory perception intact in microinjected animals. With respect to the pre-exposed PBS animals, latent inhibition-related behavioral and dopaminergic responses for pre-exposed PBS-control and pre-exposed PBS-conditioned rats are comparable to those reported previously in adult rats having had no postnatal microinjection in the PFC (Jeanblanc et al, 2002; Louilot et al, 2010). Thus, the neonatal microinjection and vital dye Evans blue do not appear to have any major effect.
Pre-exposed TTX-conditioned animals expressed no latent inhibition responses, but on the contrary displayed typical conditioned aversive responses toward the banana odor. These data could be interpreted to mean the prelimbic/infralimbic subregion of the PFC is involved in the latent inhibition phenomenon, which would be consistent with recent studies carried out with focal lesions in adults (George et al, 2010; Nelson et al, 2010), rather than previous works performed with more dorsal lesioning sites in the anteromedian PFC (Lacroix et al, 2000; Schiller and Weiner, 2004). However, lesions in the prelimbic/infralimbic subregion of the PFC in adults resulted in enhanced latent inhibition (George et al, 2010; Nelson et al, 2010), whereas what we observed was that latent inhibition disappeared after postnatal TTX microinjection in the same PFC subregion, suggesting that different mechanisms are involved after intervention in adults and during the postnatal developmental period. The disappearance of the behavioral expression of latent inhibition obtained after neonatal TTX blockade of the PFC is similar to that observed after an identical blockade of the entorhinal cortex or ventral subiculum (Peterschmitt et al, 2007; Meyer et al, 2009; Meyer and Louilot, 2011). The explanation given for former results was a malfunctioning of a recognition memory system that prevented proper learning and memorization of the characteristics related to CS (banana odor) during pre-exposure to the CS (see Meyer et al, 2009; Louilot et al, 2010). The PFC could be part of the recognition memory system thought to be involved in the latent inhibition phenomenon and defective after early neonatal inactivation. However, another interpretation is that the results obtained in pre-exposed TTX-conditioned animals are related to neurodevelopmental disturbances in PFC targets, secondary to the neonatal inactivation, such as myelination defects in PFC projection structures, particularly the hippocampal regions, and not to a functional impairment of the PFC per se. Such myelination defects have been observed after neonatal ibotenic lesion of the PFC (Schneider and Koch, 2005; Klein et al, 2008) and warrant investigation following TTX inactivation.
Dopaminergic variations recorded in pre-exposed TTX-conditioned animals are similar to those obtained in non-pre-exposed TTX-conditioned rats and are characteristic of aversion. A first explanation could be that the disrupted latent inhibition-related dopaminergic responses result mainly from impaired control exerted by the PFC over the extracellular dopamine release in the nucleus accumbens, in interaction with regulating influences from the basolateral nucleus of amygdala. Indeed, dopaminergic transmission in the core part of the nucleus accumbens depends on the functional integrity of the PFC, as shown in anesthetized animals (Louilot et al, 1989), and projections from the infralimbic/prelimbic PFC subregion toward the core subregion have been described (Wright and Groenewegen, 1995; Heidbreder and Groenewegen, 2003), as well as a close apposition between the PFC afferents and dopaminergic endings in the nucleus accumbens (Sesack and Pickel, 1992). In other respects, a convergence of afferents from the PFC and basolateral nucleus of amygdala has also been reported at the level of the nucleus accumbens (Wright and Groenewegen, 1995). Moreover, the basolateral nucleus has been shown to be involved in controlling aversively conditioned dopaminergic responses in the core subregion (Louilot and Besson, 2000). The specific mechanisms involved in the accumbal dopaminergic changes in latent inhibition following the TTX neonatal PFC blockade have still to be clarified, however, given that PFC also appears to be able to regulate the activity of dopaminergic neurons innervating the nucleus accumbens by PFC efferents reaching the ventral mesencephalon (Sesack and Pickel, 1992; Taber et al, 1995; Karreman and Moghaddam, 1996; Pennartz et al, 1994). The involvement of more complex or indirect regulating pathways can also not be ruled out (see Pennartz et al, 1994). Whatever the exact regulatory pathways involved in the observed core dopaminergic variations might be, the relationships between these variations and the behavioral responses could be interpreted with reference to Weiner's switching model of latent inhibition (2003; 2010), according to which the core subregion of the nucleus accumbens is involved in a switching mechanism between responding according to the CS-reinforcement associations acquired during conditioning and responding to the CS-no event associations acquired during pre-exposure, with the switching mechanism inhibited by the shell subregion of the nucleus accumbens. In the switching model, the latent inhibition response is coupled to a rapid increase in dopamine released in the core part of the nucleus accumbens, whereas the CR is associated with a lack of initial increase in dopamine levels (Weiner, 2003, 2010). The present dopaminergic variations recorded in the core in pre-exposed PBS-conditioned animals showing a preference for the CS and in the non-pre-exposed PBS-conditioned, non-pre-exposed TTX-conditioned, and pre-exposed TTX-conditioned animals displaying an aversion toward the CS appear to be consistent with this model.
Regarding the D-AMP challenge, our data show first and foremost that spontaneous locomotor activity, measured before injection of D-AMP, is not altered in TTX-microinjected animals compared with PBS animals. With respect to the PBS and TTX animals, the D-AMP i.p. injection induced a dose-dependent increase in locomotor activity. The highest D-AMP dose (1.5 mg/kg) triggered a larger increase in the locomotor response and longer-lasting effect compared with what was observed with the lowest dose (0.75 mg/kg). Data obtained for PBS-microinjected animals are consistent with previous findings showing that D-AMP induced an increase in locomotor activity in a dose-dependent manner, with a gradual return to baseline values from 60 min after the injection (Sharp et al, 1987; Louilot and Choulli, 1997). Our data showing more locomotor activity with the highest D-AMP dose (1.5 mg/kg) in animals subjected to postnatal TTX inactivation, compared with PBS animals, appear to be original. To the best of our knowledge, the behavioral response to D-AMP in animals undergoing TTX neonatal functional inactivation of the PFC has never before been investigated. Our data are consistent with those obtained by Flores et al (1996) after excitotoxic PFC postnatal lesion, which showed greater D-AMP-induced locomotor activity in postpubertal animals, but not with those obtained by Lipska et al (1998) in the same conditions. The reasons for the discrepancies were not obvious but may be related to the lesion's site or size.
Concerning dopaminergic responses recorded in the core, for PBS and TTX animals we observed a dose-dependent dopaminergic increase following the D-AMP challenge. As with the TTX-animals, a larger increase in dopamine release compared with PBS animals was obtained with the highest D-AMP dose (1.5 mg/kg). With the lower D-AMP dose (0.75 mg/kg) or saline injection, no statistical differences were obtained for the PBS- and TTX-microinjected rats. D-AMP dose-dependent dopaminergic increases observed for PBS-microinjected animals are consistent with results obtained with in vivo microdialysis measurements in the nucleus accumbens (Sharp et al, 1987). Furthermore, the extent of the present D-AMP-induced dopaminergic responses is consistent with that observed with the voltammetric approach (Ramsson et al, 2011). It is also interesting to observe that for the 1.5 mg/kg D-AMP dose, the difference in dopamine levels appears more marked during the 50–60 min postinjection period, whereas the difference in locomotor responses appears at its greatest during the 70–80 min period. At very least, this temporal disconnection can be interpreted as a long-lasting impact of released dopamine on postsynaptic sites in TTX-microinjected animals. In other respects, it is generally accepted that D-AMP increases dopamine levels in the brain by blockade and reversal of the plasmalemmal dopamine transporter (see Sulzer et al, 2005). It has also been reported that at a high dose D-AMP can induce a dopaminergic release from storage pools and thus redistribute dopamine from vesicles to the cytoplasm (Sulzer et al, 2005). With respect to the TTX animals, it is tempting to attribute the heightened behavioral and dopaminergic hyperactivity to the highest dose of D-AMP (1.5 mg/kg) in the core part of the nucleus accumbens to a much higher release from presynaptic vesicles pools. The greater storage of dopamine in dopaminergic terminals in the nucleus accumbens following early neonatal inactivation of the PFC, as well as the putative mechanisms involved, warrant further investigation. However, it is interesting to note that an increased vesicular storage of dopamine has been proposed as an explanation for the greater reactivity to D-AMP observed in patients with schizophrenia (Lyon et al, 2011).
To conclude, the present data suggest that subtle and transient functional inactivation of the infralimbic/prelimbic subregion of the PFC during the postnatal developmental period is sufficient to induce schizophrenia-related behavioral and dopaminergic abnormalities in adulthood. This further suggests that early functional impairment of PFC induced by TTX is a valid approach for modeling the pathophysiology of schizophrenia in animals. To refine this modeling, it would be important to identify the putative disruptions of latent inhibition and D-AMP responses before and after puberty, which is thought to be a second period of vulnerability to developing schizophrenia (Keshavan, 1999; Keshavan and Hogarty, 1999; Paus et al, 2008).
Acknowledgments
This research was supported by INSERM & Région Alsace, and Fondation de France (FM), and Electricité de France (AL). We thank S Eybrard and M Loureiro for their technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Albert DJ, Madryga FJ. An examination of the functionally effective spread of 4 microliters of slowly infused lidocaine. Behav Neural Biol. 1980;29:378–384. doi: 10.1016/s0163-1047(80)90335-0. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. J Nerv Ment Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- Bennay M, Gernert M, Schwabe K, Enkel T, Koch M. Neonatal medial prefrontal cortex lesion enhances the sensitivity of the mesoaccumbal dopamine system. Eur J Neurosci. 2004;19:3277–3290. doi: 10.1111/j.0953-816X.2004.03442.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Stratford TL, Foote SL, Kelley AE. Distribution of dopamine beta hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse. 1997;27:230–241. doi: 10.1002/(SICI)1098-2396(199711)27:3<230::AID-SYN8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Brooks JM, Sarter M, Bruno JP. Transient inactivation of the neonatal ventral hippocampus permanently disrupts the mesolimbic regulation of prefrontal cholinergic transmission: implications for schizophrenia. Neuropsychopharmacology. 2011;36:2477–2487. doi: 10.1038/npp.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–290. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- De la Casa LG, Pineño O.2010Inter-stage context and time as determinants of latent inhibitionIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia. Cambridge University Press: New York; 40–61. [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakew A, Frotscher M, Heimrich B. Blockade of neuronal activity alters spine maturation of dentate granule cells but not their dendritic arborization. Neuroscience. 1999;94:767–774. doi: 10.1016/s0306-4522(99)00378-4. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Wood GK, Liang JJ, Quirion R, Srivastava LK. Enhanced amphetamine sensitivity and increased expression of dopamine D2 receptors in postpubertal rats after neonatal excitotoxic lesions of the medial prefrontal cortex. J Neurosci. 1996;16:7366–7375. doi: 10.1523/JNEUROSCI.16-22-07366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J Anat. 2010;217:324–333. doi: 10.1111/j.1469-7580.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DN, Duffaud AM, Pothuizen HH, Haddon JE, Killcross S. Lesions to the ventral, but not the dorsal, medial prefrontal cortex enhance latent inhibition. Eur J Neurosci. 2010;31:1474–1482. doi: 10.1111/j.1460-9568.2010.07178.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mora JL, Guadalupe T, Fumero B, Mas M. Mathematical resolution of mixed in vivo voltammetry signals. Models, equipment, assessment by simultaneous microdialysis sampling. J Neurosci Methods. 1991;39:231–244. doi: 10.1016/0165-0270(91)90102-6. [DOI] [PubMed] [Google Scholar]
- Gray NS, Pilowsky LS, Gray JA, Kerwin RW. Latent inhibition in drug naive schizophrenics: relationship to duration of illness and dopamine D2 binding using SPET. Schizophr Res. 1995;17:95–107. doi: 10.1016/0920-9964(95)00034-j. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hutchins BI, Kalil K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. J Neurosci. 2008;28:143–153. doi: 10.1523/JNEUROSCI.4548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Hoeltzel A, Louilot A. Dissociation in the involvement of dopaminergic neurons innervating the core and shell subregions of the nucleus accumbens in latent inhibition and affective perception. Neuroscience. 2002;111:351–353. doi: 10.1016/s0306-4522(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Kalus P, Müller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Keshavan MS. Development, disease and degeneration in schizophrenia a unitary pathophysiological model. J Psychiatr Res. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Dev Psychopathol. 1999;11:525–545. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Klein S, Koch M, Schwabe K. Neuroanatomical changes in the adult rat brain after neonatal lesion of the medial prefrontal cortex. Exp Neurol. 2008;209:199–212. doi: 10.1016/j.expneurol.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Kumari V, Ettinger U.2010Latent inhibition in schizophrenia and schizotypy: a review of the empirical literatureIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia.Cambridge University Press: New York. pp419–447.
- Lacroix L, Spinelli S, White W, Feldon J. The effects of ibotenic acid lesions of the medial and lateral prefrontal cortex on latent inhibition, prepulse inhibition and amphetamine-induced hyperlocomotion. Neuroscience. 2000;97:459–468. doi: 10.1016/s0306-4522(00)00013-0. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lipska BK, al-Amin HA, Weinberger DR. Excitotoxic lesions of the rat medial prefrontal cortex. Effects on abnormal behaviors associated with neonatal hippocampal damage. Neuropsychopharmacology. 1998;19:451–464. doi: 10.1016/S0893-133X(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Louilot A, Besson C. Specificity of amygdalostriatal interactions in the involvement of mesencephalic dopaminergic neurons in affective perception. Neuroscience. 2000;96:73–82. doi: 10.1016/s0306-4522(99)00530-8. [DOI] [PubMed] [Google Scholar]
- Louilot A, Choulli MK. Asymmetrical increases in dopamine turn-over in the nucleus accumbens and lack of changes in locomotor responses following unilateral dopaminergic depletions in the entorhinal cortex. Brain Res. 1997;778:150–157. doi: 10.1016/s0006-8993(97)01050-0. [DOI] [PubMed] [Google Scholar]
- Louilot A, Jeanblanc J, Peterschmitt Y, Meyer F.2010Parahippocampal region-dopaminergic neuron relationships in latent inhibitionIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia. Cambridge University Press: New York; 319–341. [Google Scholar]
- Louilot A, Le Moal M, Simon H. Opposite influences of dopaminergic pathways to the prefrontal cortex or the septum on the dopaminergic transmission in the nucleus accumbens. An in vivo voltammetric study. Neuroscience. 1989;29:45–56. doi: 10.1016/0306-4522(89)90331-x. [DOI] [PubMed] [Google Scholar]
- Louilot A, Serrano A, D'Angio M. A novel carbon fiber implantationassembly for cerebral voltammetric measurements in freely moving rats. Physiol Behav. 1987;41:227–231. doi: 10.1016/0031-9384(87)90357-x. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: the effect of non-reinforced pre-exposure to the conditioned stimulus. J Comp Physiol Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–117. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpeli JG. Reversible inactivation of subcortical sites by drug injection. J Neurosci Methods. 1999;86:119–128. doi: 10.1016/s0165-0270(98)00161-7. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Meyer F, Louilot A. Latent inhibition-related dopaminergic responses in the nucleus accumbens are disrupted following neonatal transient inactivation of the ventral subiculum. Neuropsychopharmacology. 2011;36:1421–1432. doi: 10.1038/npp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Peterschmitt Y, Louilot A. Postnatal functional inactivation of the entorhinal cortex or ventral subiculum has different consequences for latent inhibition-related striatal dopaminergic responses in adult rats. Eur J Neurosci. 2009;29:2035–2048. doi: 10.1111/j.1460-9568.2009.06755.x. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Thur KE, Marsden CA, Cassaday HJ. Catecholaminergic depletion within the prelimbic medial prefrontal cortex enhances latent inhibition. Neuroscience. 2010;170:99–106. doi: 10.1016/j.neuroscience.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill RD, Lowry JP, Mas M. Monitoring brain chemistry in vivo: voltammetric techniques, sensors, and behavioral applications. Crit Rev Neurobiol. 1998;12:69–127. doi: 10.1615/critrevneurobiol.v12.i1-2.40. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence. Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C.1998The Rat Brain in Stereotaxic Coordinates,4th edn. Academic Press: San Diego [Google Scholar]
- Paxinos G, Watson C.2009The Rat Brain in Stereotaxic Coordinates(compact 6th edn). Academic Press: New York [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peterschmitt Y, Meyer F, Louilot A. Neonatal functional blockade of the entorhinal cortex results in disruption of accumbal dopaminergic responses observed in latent inhibition paradigm in adult rats. Eur J Neurosci. 2007;8:2504–2513. doi: 10.1111/j.1460-9568.2007.05503.x. [DOI] [PubMed] [Google Scholar]
- Ramsson ES, Covey DP, Daberkow DP, Litherland MT, Juliano SA, Garris PA. Amphetamine augments action potential-dependent dopaminergic signaling in the striatum in vivo. J Neurochem. 2011;117:937–948. doi: 10.1111/j.1471-4159.2011.07258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascle C, Mazas O, Vaiva G, Tournant M, Raybois O, Goudemand M, et al. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51:149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL. Contrast Analysis: Focused Comparaisons in the Analysis of Variance. Cambridge University Press: Cambridge; 1985. [Google Scholar]
- Rosenthal R, Rosnow RL, Rubin DB. Contrasts and Effect Sizes in Behavioral Research. Cambridge University Press: New York; 2000. [Google Scholar]
- Rothfeld JM, Harlan RE, Shivers BD, Pfaff DW. Reversible disruption of lordosis via midbrain infusions of procaine and tetrodotoxin. Pharmacol Biochem Behav. 1986;25:857–863. doi: 10.1016/0091-3057(86)90398-9. [DOI] [PubMed] [Google Scholar]
- Schiller D, Weiner I. Lesions to the basolateral amygdala and the orbitofrontal cortex but not to the medial prefrontal cortex produce an abnormally persistent latent inhibition in rats. Neuroscience. 2004;128:15–25. doi: 10.1016/j.neuroscience.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Behavioral and morphological alterations following neonatal excitotoxic lesions of the medial prefrontal cortex in rats. Exp Neurol. 2005;195:185–198. doi: 10.1016/j.expneurol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterström T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR.2010A cautionary note about latent inhibition in schizophrenia: are we ignoring relevant informationIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia. Cambridge University Press: New York; 448–456. [Google Scholar]
- Swerdlow NR, Braff DL, Hartston H, Perry W, Geyer MA. Latent inhibition in schizophrenia. Schizophr Res. 1996;20:91–103. doi: 10.1016/0920-9964(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Kros JM, Uylings HB. The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Prog Brain Res. 1990;85:169–183. doi: 10.1016/s0079-6123(08)62680-1. [DOI] [PubMed] [Google Scholar]
- Weiner I. The ‘two-headed' latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology. 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- Weiner I.2010What the brain teaches us about latent inhibition (LI): the neural substrates of the expression and prevention of LIIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia. Cambridge University Press. New York; 372–415. [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fung SJ, Rothwell A, Tianmei S, Weickert CS. Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biol Psychiatry. 2011;69:63–70. doi: 10.1016/j.biopsych.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zhuravin IA, Bures J. Extent of the tetrodotoxin induced blockade examined by pupillary paralysis elicited by intracerebral injection of the drug. Exp Brain Res. 1991;83:687–690. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.