Abstract
Alcoholic patients who have undergone multiple detoxifications/relapses show altered processing of emotional signals. We performed functional magnetic resonance imaging during performance of implicit and explicit versions of a task in which subjects were presented with morphs of fearful facial emotional expressions. Participants were abstaining, multiply detoxified (MDTx; n=12) or singly detoxified patients (SDTx; n=17), and social drinker controls (n=31). Alcoholic patients were less able than controls to recognize fearful expressions, and showed lower activation in prefrontal areas, including orbitofrontal cortex and insula, which mediate emotional processing. The decrease in activation was greater in MDTx patients who also showed decreased connectivity between insula and prefrontal areas, and between amygdala and globus pallidus. In the explicit condition, the strength of connectivity between insula and areas involved in regulation of emotion (inferior frontal cortex and frontal pole) was negatively correlated with both the number of detoxifications and dependency (measured by the severity of alcohol dependency (SADQ) and control over drinking score (Impaired Control questionnaire, ICQ)). In contrast, increased connectivity was found between insula and the colliculus neuronal cluster, and between amygdala and stria terminalis bed nucleus. In the implicit condition, number of detoxifications and ICQ score correlated positively with connectivity between amygdala and prefrontal cortical areas involved in attentional and executive processes. Repeated episodes of detoxification from alcohol are associated with altered function both in fear perception pathways and in cortical modulation of emotions. Such changes may confer increased sensitivity to emotional stress and impaired social competence, contributing to relapse.
Keywords: fMRI, fearful facial expression, detoxification, amygdala, insula, prefrontal cortex
INTRODUCTION
A primary aim of physicians treating alcoholic patients is to wean them from alcohol use, usually by a process of inpatient or outpatient detoxification, followed by pharmacological or behavioral support to maintain abstinence. Nevertheless, relapse is common, necessitating repeating the process, so that individual patients may undergo many detoxification episodes before achieving abstinence. Repeated detoxification results in increased sensitivity to withdrawal seizures (Adinoff, 1994; Ballenger and Post, 1978), and perhaps other effects, including both cognitive and emotional deficits (Stephens and Duka, 2008; Stephens et al, 2005). Such emotional impairments may contribute to deficits in social interaction that lead to social isolation, in turn supporting further drinking behavior.
In the present report we use a laboratory task, recognition of fearful expressions in the faces of others, to characterize the altered processing of such emotions in the brains of alcoholic patients. We studied fearful expressions because we had previously established that inaccurate fear recognition is related to the number of previous detoxifications (Townshend and Duka, 2003), and centered our approach on the amygdala and its connections. The role of amygdala in processing of fear (LeDoux, 2000), including the recognition of fearful facial expressions, is well established (Derntl et al, 2009). Repeated episodes of alcohol withdrawal in animals alter amygdala physiology, including impaired synaptic plasticity (Stephens and Duka, 2008; Stephens et al, 2005), associated with impaired fear conditioning (Stephens et al, 2001). As impaired fear conditioning is also seen in alcoholic patients and binge drinkers (Stephens and Duka, 2008; Stephens et al, 2005), amygdala physiology may be altered in these human populations too.
We therefore asked whether multiply detoxified (MDTx) patients would show changes in BOLD signals in amygdala pathways during a task in which they viewed fearful facial expressions. We included sublenticular extended amygdala (SLEA), which is involved in human fear circuitry (Liberzon et al, 2003), in our analyses. Impaired interpretation of the emotional reaction generated by amygdala dysfunction may also be attributable to dysfunction of insular cortex, which mediates the experience of emotion (Craig, 2009).
The amygdala interacts with prefrontal cortical structures, including orbitofrontal cortex (OFC) and medial prefrontal cortex (Peters et al, 2009). Withdrawal-induced dysfunction of cortical areas (Duka et al, 2011) might therefore elicit similar outcomes by failing to modulate the amygdala function. We thus tested the hypothesis that patients with MDTx may show increased amygdala activity accompanied by decreased activity of cortical structures involved in the experience and modulation of emotion. Amygdala and insula also interact with the other brain areas underlying emotional processing, and functional neuronal connectivity during the emotional challenge in these circuits could also be affected by withdrawal-induced impairments in neuroplasticity.
Two conditions, an implicit perception of emotion and an explicit labeling of the emotion in the facial expression were used to challenge both subcortical and cortical structures and their interaction (Hariri et al, 2000; Lange et al, 2003). The implicit condition implies a passive view of threat stimuli, whereas the explicit condition implies a requirement for recognition and regulation of emotion. The latter condition is thus more likely to recruit cortical areas, whereas the implicit task is likely to reflect only subcortical processes.
MATERIALS AND METHODS
Study Population
Seventy-six volunteers out of 83 originally recruited, physically healthy, and with no diagnosis of a psychiatric or neurological disease were eligible for inclusion; 38 alcohol-dependent subjects diagnosed by independent clinicians according to DSM-IV (APA, 1994) or ICD-10 (WHO, 1993) formed the patient group; thirty-eight mild-to-moderate social alcohol drinkers served as controls. All patients had been abstinent for a minimum of 2 weeks at the time of the study and had been medically supported during withdrawal with standard detoxification treatments, including administration of chlordiazepoxide and thiamine. All patients had ceased benzodiazepine treatment at least 72 h before testing. From the 76 participants, 60 provided eligible fMRI data (see below) and data from only those participants are presented.
Among the patient group, there was variable experience of medically supervised detoxifications (MSDs) allowing us to divide patients into two groups using information from the medical records (events clearly described in the medical records as periods of abstinence under medical supervision). The two groups consisted of those patients with two or fewer (including current) MSDs (single detoxification group; SDTx, n=17) and those patients with more than two MSDs (multiple detoxification group; MDTx, n=12).
Additional information on patient population and on inclusion exclusion criteria and restrictions during the study are provided in Supplementary Materials and Methods.
Design and Procedure
The experiment lasted ∼2.5. h. First, demographic details were collected and participants completed questionnaires including the Severity of Alcohol Dependence (SADQ) and the Impaired Control scale (ICQ) Questionnaires (see Supplementary Materials and Methods for details). They then underwent safety screening for MRI scanning before entering the scanner to complete the facial expression recognition task. Postscan, a match-to-sample visual search task taken from the CANTAB battery was carried out to confirm that participants had good visuospatial abilities and motor responses.
Facial Expression Recognition Task
Participants were presented with faces depicting expression of fear in 50 : 50 and 100 : 0 fear-neutral morphs (see Supplementary Figure S1). During the implicit task, participants were asked to indicate the gender of each face by pressing one of two buttons with their right index finger. During the explicit task, participants were asked to decide which of two emotions (fear or neutral) was displayed in each face by pressing one of two buttons with their right index finger.
Match to Sample Visual Search
An abstract pattern was presented in the center of the screen. After 1000 ms, a varying number (1, 2, 4, or 8) of similar patterns was presented in a circle of boxes around the edge of the screen. The participant had to indicate which of these matched the sample stimulus. See Supplementary Materials for details.
Functional MRI Data
Details of data acquisition, preprocessing and modeling are given in Supplementary Materials. To evaluate contrasts, we employed a model that included separate regressors for each of the three conditions of interest (ie, neutral, intermediate fear (50%), and maximal fear (100%)), and an additional regressor, which encoded button presses (see Supplementary Materials).
Group-Level Inference
At the group level, we performed a random effect analysis. Rescaled images of parameter estimates for each of the three affective conditions for both implicit and explicit task runs from the first-level analysis were entered into the second level of analysis of variance (group(3) × task(2) × emotion(3) full factorial). Statistical Parametric Maps (SPMs) of the t-statistic were constructed using a generalized Greenhouse–Geisser correction. For F-tests, SPMs were thresholded at p<0.05. Unless otherwise stated, all results presented are whole-brain corrected for multiple comparisons on the basis of cluster extent. Otherwise, small-volume correction was used where regional effects were predicted a priori. These SVC results are indicated in the statistical tables.
Connectivity Analysis
For regions showing significant group differences connectivity analyses were employed to determine whether the changes in activation were related to altered inter-regional communication (dysconnectivity). Amygdala and insula were included as previous evidence suggests their roles in emotional processing. As we expected significant between-group differences in hemodynamic response and thus could not confidently employ approaches dependent on deconvolution of the HRF (eg, DCM or PPI), we employed a simple seed-based functional connectivity analysis, based on clusters identified in our whole-brain analysis. The model also included white matter and CSF nuisance regressors drawn from spheres deep in white matter (10 mm radius at (27 −8 32)) and ventricular space (5 mm radius at (−17 −33 19)). All functional connectivity analysis models also included the task conditions as nuisance regressors to minimize the potentially confounding contribution of task-related recruitment to assessment of connectivity strength. These connectivity maps were entered into a Group (3) × Task (2) full factorial ANOVA.
Regression Analysis
Additionally, we employed a whole-brain regression model to identify any relationships between clinically relevant measures (ie, the SADQ, ICQ, and the number of supervised detoxifications) and the strength of connectivity. The ICQ subscale depicts the inability to control alcohol drinking and is therefore relevant for relapse. The SADQ score is cumulative score of four subscales, which addresses mostly physical dependency (see Supplementary Materials and Methods for detail on SADQ questionnaire). Finally, BOLD signal was extracted from all brain regions identified in the whole-brain analysis using independently derived ROIs. These regions were derived from the Automated Anatomical Labelling Toolbox (Tzourio-Mazoyer et al, 2002) atlas available for MARSBAR unless otherwise stated. The beta coefficients were entered into a three-factor ANOVA (ie, group (3) by task (2) by emotion (3)) for each hemisphere separately to assess the relationship between task-related BOLD response during task performance and clinical measures.
Statistical Analysis
Participants
Participants were excluded from fMRI and all other subsequent analyses if they showed excessive movement (>4.4 mm. in any plane; n=8), or made more than 10 errors or had more than 20 missing responses (>2 SDs from the group mean) in either the implicit or the explicit task for the 100% fear face stimuli (n=7; no group trends), or failed to complete the questionnaires (n=1). Sixty participants remained who were eligible for inclusion in the fMRI analysis.
Questionnaires and behavioral data
Questionnaires, fMRI facial expression task behavioral data and match to sample visual search data were analyzed using one-way ANOVA with group (control, SDTx, and MDTx) as the between subjects factor.
Significant main effects were interpreted using post-hoc comparisons. All analyses were performed using SPSS 16.0 (SPSS, Chicago, IL).
RESULTS
Population Characteristics
The groups were well matched for age, gender, and full scale IQ (for group characteristics and statistics see Table 1).
Table 1. Population Characteristics for the Three Groups.
| Characteristics | Controls (n=31) | SDTx (n=17) | MDTx (n=12) | Statistics |
|---|---|---|---|---|
| Age (years), mean (SD) | 40.2 (8.7) | 37.6 (9.6) | 44.4 (9.5) | F(2,59)=1.79, NS |
| Men, no | 16 | 11 | 7 | χ2(2)=.78, NS |
| IQa, mean (SD) | 106.7 (7.3) | 101.9 (7.1) | 106.3 (6.3) | F(2,59)=2.62, NS |
| Detoxb, mean (SD) | NA | 1.3 (0.5) | 4.3 (1.5)c | t(12.6)=−6.65, p<.001 |
| Age of starting heavy drinking, mean (SD) | NA | 23.9 (10.3) | 25.5 (7.9) | t(27)=-.477, NS |
| SADQb, mean (SD) | NA | 33.1 (14.3) | 46.8 (6.4)c | t(23.7)=−3.48, p<.001 |
| ICQ, mean (SD) | 11.2 (2.6) | 12.7 (1.4) | t(27)=−1.56, NS | |
| Units/dayb, mean (SD) | 2.1 (2.1) | 39.1 (39.8) | 48.1 (34.8) | F(2,59)=18.59, p<.001 |
| Smokers, nob | 6 | 14 | 9c | χ2(2)=26.00, p<.001 |
| Cigarettes per day, mean (SD) | 9.0 (5.7) | 24.6 (18.1) | 23.6 (8.2) | F(2,28)=2.90, NS |
| Years smoked, mean (SD) | 20.8 (12.9) | 20.4 (11.9) | 20.8 (9.0) | F(2,20)<.01, NS |
| Fagerstrom score (FTQ), mean (SD) | 3.8 (2.4) | 6.5 (2.8) | 8.0 (2.4) | F(2,21)=3.43, NS |
| BDIb mean (SD) | 6.4 (7.6) | 18.8 (11.3) | 13.7 (11.6) | F(2,59)=9.60, p<.001 |
| State anxiety (STAI)b, mean (SD) | 30.2 (7.6) | 38.8 (9.9) | 39.7 (14.2) | F(2,59)=6.35, p<.01 |
| Trait anxiety (STAI)b, mean (SD) | 36.6 (9.8) | 50.6 (10.3) | 46.3 (12.1) | F(2,59)=10.82, p<.001 |
Abbreviations: BDI, Beck Depression Inventory; ICQ, impaired control scale questionnaire; MDTx, multiply detoxified; NART, National Adult Reading Test; SADQ, Severity of Alcohol Dependence Questionnaire; SDTx, singly detoxified; STAI, State and Trait Anxiety Inventory.
IQ is taken from NART (National Adult Reading Test).
Main group effect.
Significantly different from SDTx.
Behavioral Data
All groups showed high accuracy in the implicit task and did not differ in fear recognition in the explicit task for the 100% fear condition; groups differed only with regard to number of fear-biased recognition responses for 50% fear morphs in the explicit task (Supplementary Table S1). Both SDTx and MDTx patients produced significantly fewer such responses than controls (F(2,57)=3.601, p=.034). There were no group differences on any measurements in the match to sample visual search task.
Functional MRI Results
Emotion and task effects
In response to fearful faces, a large cluster of activation was found in the left fusiform gyrus (Supplementary Figure S3 and Supplementary Table S2) with greatest BOLD signal response to 100%, and little difference between 50% fear and neutral faces (main emotion effect).
Furthermore, during the explicit task condition, SLEA and amygdala proper were more activated with 100% fear than with 50% fear or neutral images; no activation was seen during the implicit task condition (Supplementary Figure S4; Supplementary Table S3). The frontal cortical regions, OFC/anterior insula (Brodmann area 47), and the ACC were activated with both 100 and 50% fearful faces compared with neutral (Supplementary Figure S4; Supplementary Table S3); no activation was seen in the implicit task. In both cases, the findings were consistent with a significant emotion by task interaction.
Group effects
A main effect of group (Figure 1a and c; Supplementary Table S4) was seen in the primary motor cortex, indicating greatest BOLD signal in healthy controls and the least in MDTx patients. Similarly, the OFC parameter estimates were smallest in the MDTx group (deactivation was present), but there was no reduction in the SDTx group. A significant group effect was also found for anterior insula. Finally, at an uncorrected threshold, there was evidence for all three factors interacting in SLEA (task by emotion by group interaction; Figure 1b, d, and e; Supplementary Table S4). As we had predicted involvement of amygdala and associated structures, a small volume correction was applied using a 15-mm radius sphere around coordinates drawn from an independent publication identifying a role of SLEA in fear ((Liberzon et al, 2003) coordinates (8 10−7). Applying this correction, the interaction remained significant. The pattern of BOLD signals for the amygdala indicated that this interaction was driven by a lack of activation in both SDTx and MDTx compared with controls in the explicit task, and a decrease in activation in MDTx compared with both SDTx and controls in the 100% condition in the implicit task, whereas in the same task an increased activation was seen in the same group in the presence of the ambiguous stimulus (fear 50%). There was no reduction in BOLD activation when performance on the 50% fear trials, which showed group differences, was entered as a covariate in the analysis.
Figure 1.
Brain regions identified with a main effect of group ((a) yellow) or a significant three-way interaction ((b) yellow). Clusters are shown at an uncorrected voxel p-value of 0.001, with a minimum cluster size of 50 voxels. Primary motor cortex and orbitofrontal cortex (OFC) survived FWE corrections; insula was thresholded following correction for multiple comparisons on the basis of spatial extent. Sublenticular extended amygdala (SLEA) was identified based on region of interest analysis. Bar graphs depict changes of BOLD signal (mean±SEM) for the main effect of group (c) and the interaction involving group (d, e). MDTx, multiply detoxified; SDTx, singly detoxified.
Connectivity analyses
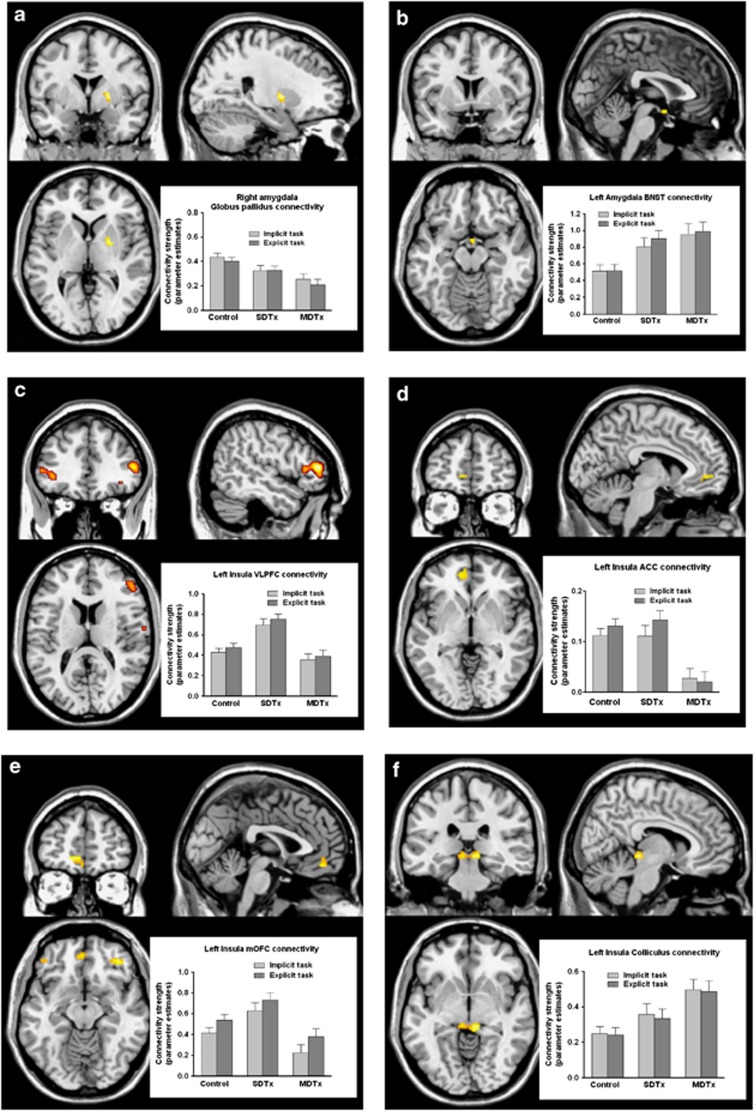
There were significant main effects of group in connectivity pattern for only the amygdala and insula. Connectivity between amygdala and globus pallidus (pallidum/putamen) was reduced in SDTx and even more so in MDTx compared with controls (Figure 2a; Supplementary Table S5). The left insula's connectivity with the anterior cingulate gyrus (ACC), ventrolateral PFC (VLPFC), and medial OFC/ventromedial PFC (VMPFC) was also reduced in MDTx compared with SDTx and controls (Figure 2c–e, see also Supplementary Table S6). Left insula–frontal pole connectivity was also reduced in MDTx compared with SDTx and controls (Supplementary Table S6). An increased connectivity between left insula and the colliculus neuronal cluster (right and left), and also between amygdala and the Bed Nucleus of Stria Terminalis (right BNST/hypothalamus) was seen in MDTx patients compared with SDTx and controls (MDTx>SDTx>controls; see Figure 2b and f; Supplementary Tables S5 and S6).
Figure 2.
Brain regions showing a group effect in connectivity of amygdala (a, b) and insula (c–f) as seed chosen regions. For display purposes, maps are shown at an uncorrected threshold of p<0.001. Bar graphs depict changes of BOLD signal in the different groups for implicit and explicit condition for connectivity between right amygdala and right globus pallidus (a), left amygdala and right BNST/hypothalamus (b), left insula and right VMPFC (c), left insula and left anterior cingulate cortex (d), left insula and left orbitofrontal cortex (OFC) (e), and left insula and right colliculus (f). MDTx, multiply detoxified; SDTx, singly detoxified; VMPFC, ventromedial PFC
Regression analysis
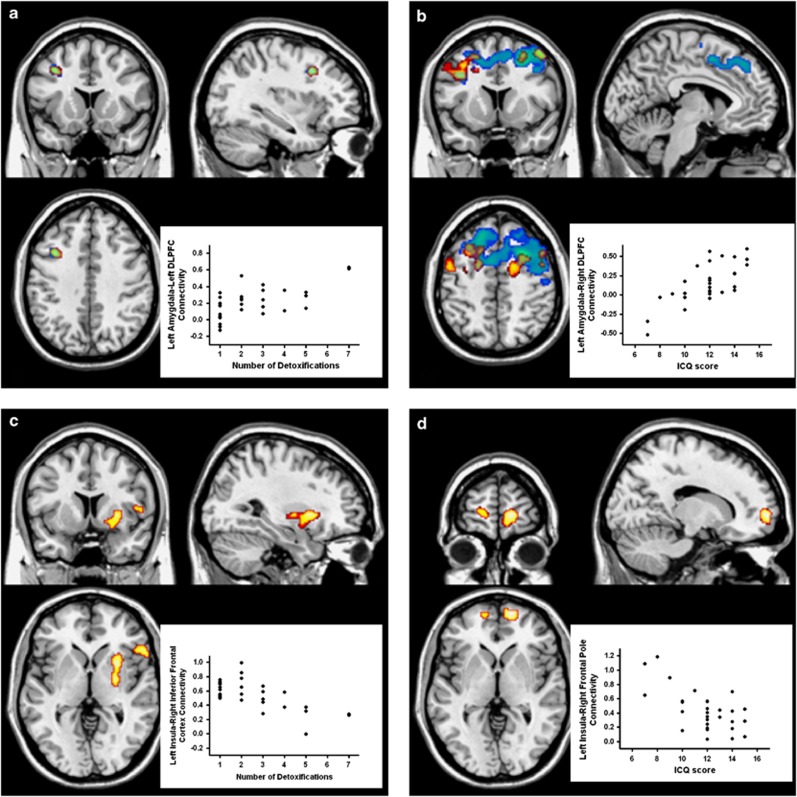
The voxel-wise regression analysis for the implicit fear processing within the alcoholic group identified a significant positive relationship between the number of detoxifications as well as the ICQ score and the strength of connectivity between amygdala and a large cluster identified as dorsolateral prefrontal cortex (DLPFC) (Figure 3a and b; see also Supplementary Table S7). No relationships were found with SADQ score (Supplementary Table S7).
Figure 3.
Brain regions where connectivity of left (yellow) and right (blue) amygdala, and left (yellow) insula are presented. For display purposes, maps are shown at an uncorrected threshold of p<0.001. Scatterplots show the relationship between left amygdala–left dorsolateral prefrontal cortex and number of multiple detoxifications (a), left amygdala–right dorsolateral prefrontal cortex (DLLPFC) and Impaired Control Questionnaire (ICQ) scores (b) in the implicit condition, and between left insula–right inferior frontal cortex connectivity and number of multiple detoxifications (c), as well as between left insula–right frontal pole and ICQ scores (d) in the explicit condition.
During. explicit fear processing, a negative relationship was found between the number of detoxifications, as well as the ICQ score, and the strength of connectivity between left insula and inferior frontal cortex (Figure 3c; Supplementary Table S8). Furthermore, the ICQ score correlated negatively with insula connectivity to the frontal pole bilaterally (although only connectivity with right frontal pole reached significance after multiple comparisons correction; Figure 3d; Supplementary Table S8). In addition, left insula connectivity to the frontal pole and medial orbitofrontal gyrus was negatively correlated with the SADQ score (Supplementary Table S8).
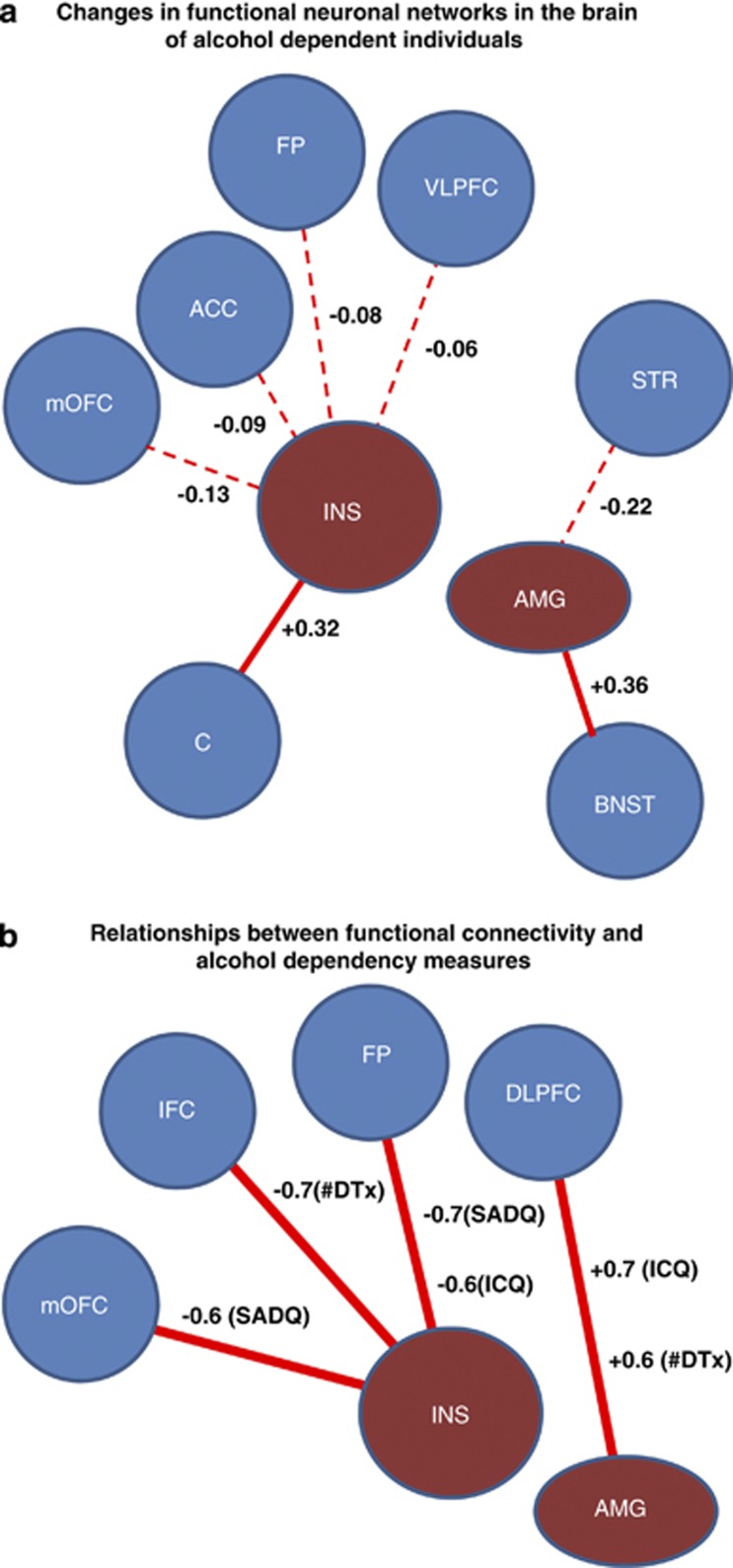
Figure 4 summarizes the connectivity data. Part a presents the relative differences between controls and MDTx for the seed areas: insula, amygdala, and other brain areas, whereas part b presents the values of correlations between these connections and the three clinical measures of alcohol dependency.
Figure 4.
(a) Relative differences (broken line: less, non-broken line greater) between controls and multiply detoxified (MDTx) patients in functional neuronal connections between insula and amygdala as seed regions, and other brain areas. The sign and magnitude of the difference (patient beta coefficient minus control beta coefficient) is given for each neuronal connection. (b) Relationships (r values) between intensity of connectivity and measures of dependency (number of detoxifications (#DTx), Severity of Alcohol Dependency Questionnaire (SADQ) score, and DCQ score). For lateralization of brain areas see Supplementary Table S5, S6, S7 and S8 in the Supplementary Material). DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; VLPFC, ventrolateral PFC.
DISCUSSION
In the present experiments we found that alcoholic patients with increased incidence of detoxification/relapse show less activation in prefrontal areas during performance of a task in which we have previously shown alcoholic patients to be impaired in recognizing fearful facial expressions in ambiguous morphs (Townshend and Duka, 2003). The overall pattern of activation in the task resembled that described in the literature (Chaudhry et al, 2009; Vuilleumier and Pourtois, 2007; Winston et al, 2003), suggesting that our approach is reliable. In the same group of alcoholic patients, increased connectivity was identified between amygdala and the BNST, as well as insula and colliculus. In contrast, decreased connectivity was present between amygdala and globus pallidus, as well as insula and prefrontal regions (eg, ACC, OFC and VLPFC). Areas showing decreased activation included primary motor cortex, and importantly, cortical affective structures including OFC and insula, which are involved in identifying the emotional significance of stimuli and in generating an affective response (Adolphs et al, 2003; Phillips et al, 2003, 2004). The lower activation was independent of whether the task was implicit or explicit, and of the degree of fear present (50 or 100% morphs), and was more robust in the subgroup of alcoholic patients who had undergone multiple detoxifications. Reduced activations in prefrontal areas (ACC and medial frontal gyrus), as well as insula, of alcoholic patients viewing fearful expressions has been previously reported (Salloum et al, 2007). In contrast to our study, all six emotions were presented, potentially exerting higher load for the processing and evaluation of emotions, perhaps accounting for their findings of ACC changes.
We found no evidence to support our prediction that amygdalae of alcoholic patients would show increased activation, either independently of cortical activity (during presentation of fearful faces in the implicit condition), or as a result of a decrease in activation of prefrontal structures in the explicit condition. In the absence of changes in amygdala activation, changes found in amygdala projection areas known to control the expression of emotional behavior (eg, SLEA) (Davis and Whalen, 2001; Liberzon and Sripada, 2008) may be secondary to impaired prefrontal cortical function. However, it cannot be excluded that the apparent absence of changes in amygdala activation results from an elevation of amygdala basal activity in the patient groups, leading to a reduced ability of the subtractive/relative approach in BOLD fMRI to detect an effect. Future studies might use a more quantitative approach such as ASL to address this possibility. Nevertheless, a previous study found alcoholic patients to show reduced amygdala activation when viewing negative emotional expressions (Marinkovic et al, 2009), and in that study, amygdala response remained blunted when deep encoding of the emotional expression was required (a condition not present in our study). Interestingly, under this condition of high cognitive demand, there was a parallel increased activation of lateral prefrontal areas in the alcoholic patients.
The insula interacts with prefrontal areas, such as ACC and the OFC (Mesulam and Mufson, 1982a, 1982b; Ongur et al, 2003; Singer et al, 2009), so that the lower connectivity of insula with these areas in alcoholic patients with MDTx, when compared with either controls or SDTx patients, would be expected to lead to general deficits in emotional processing (eg, (Craig (2002); Singer et al (2009)). Lower connectivity between amygdala and globus pallidus, on the other hand, may reflect a reduction in emotionally driven motivational outputs. Lower connectivity of insula with the right VLPFC in MDTx patients could lead to reduced adaptive responses to abrupt changes in the environment (Levy and Wagner, 2011), thus compromising appropriate responding.
Although lower connectivity may reflect less gray matter (Duka et al, 2011), other neuronal networks showed greater connectivity. Thus, greater connectivity was found between insula and colliculus neuronal cluster, an important subcortical area for arousal mechanisms. There is evidence of a fast tracking network mediating early information processing, involving the colliculus, activated by subliminal signals of fear, which transfers the fear signal, bypassing the cortex, to activate the locus coeruleus (Liddell et al, 2005); such a system may activate attentional mechanisms and facilitate processing in cortical regions involved in automatic avoidance without conscious appraisal of threat (see also Baas et al (2006)). The increase in connectivity between insula and colliculus may potentiate such a subliminal excitatory input in MDTx patients. Greater connectivity was also found between amygdala and BNST. BNST is one of the key components of stress-induced relapse in animal models of addiction (Aston-Jones and Harris, 2004). Furthermore BNST is involved in sustained reactivity to threat (Somerville et al, 2010) and is associated with high trait anxiety (Fox et al, 2008; Oler et al, 2009). As BNST activation is associated with continuous monitoring of potential threat (Somerville et al, 2010), we suggest that greater connectivity between amygdala and BNST seen in alcoholic patients facilitates neural integration of these structures, allowing threatening stimuli to be more efficiently processed within the extended amygdala.
The relative strength of some connections, but weakness of others, in MDTx patients might be explained as a consequence of changes in glutamate signaling. Ethanol acts as an antagonist of glutamatergic NMDA receptors (Samson and Harris, 1992), and compensatory effects include increased glutamate release and upregulation of NMDA receptors (Roberto et al, 2004, 2006) that persist following withdrawal and are likely to lead to facilitation of NMDA receptor-mediated calcium flux into postsynaptic neurons. Such flux may give rise to increased synaptic strengthening (Lack et al, 2007), but can also result in neurotoxicity, and loss of connectivity (Stephens and Duka, 2008).
Both the number of detoxifications and patients' perceived inability to control their drinking were associated with strength of neuronal connectivity between amygdala and insula, and prefrontal regions. In the explicit condition, strength of connectivity between insula and areas involved in control of behavior and regulation of emotion (ie, inferior frontal cortex, the frontal pole) was negatively correlated with the number of detoxification, the severity of dependency in general (SADQ), and the control over drinking subscore (ICQ). In the implicit condition, the number of detoxifications, and ICQ correlated positively with connectivity between the amygdala and a large cluster of prefrontal cortex areas involved in attentional and executive processes (eg, DLPFC).
Although the number of detoxifications experienced by the patients was highly associated with brain responsivity, it cannot be excluded that the reduced activation in prefrontal areas and changes in connectivity reflect other aspects of alcohol dependence. Relationships with the numbers of detoxifications might then arise from the more dependent patients having undergone more detoxifications. However, this seems unlikely, as other aspects of alcohol dependence (eg, alcohol units drunk during the last 6 months or age at which heavy drinking started) were not different between the two subgroups of patients. Another possibility could be that our findings reflect differences preceding (and perhaps contributing to) alcohol dependence. There is evidence for a decreased responsiveness to emotionally salient stimuli, as measured by their startle response, of children at risk for alcoholism (Miranda et al, 2002, 2003). Thus, for instance, the reduced activation of SLEA, in both SDTx and MDTx in the explicit condition and in MDTx in the implicit condition, could represent a condition existing before alcohol abuse (Glahn et al, 2007; Miranda et al, 2002, 2003) rather than a consequence of repeated detoxifications. Such potential confounds are difficult to resolve within a patient population, but animal behavioral data (Stephens et al, 2001) consistent with the current findings suggest consequences of repeated episodes of withdrawal on emotional behaviour.
Taking the present data together, we can summarize that in alcoholic patients prefrontal structures involved in the regulation of emotion show less activation under emotional task demands. Second, connectivity between insula and other cortical structures underlying a top–down control of emotional input is diminished. In contrast, connectivity between insula and subcortical regions (colliculus), as well as between amygdala and other subcortical regions (BNST) underlying a bottom up emotional input, is exaggerated. Such changes in connectivity in alcoholic patients may contribute to altered anxiety reactions and, thus, to stress-induced relapse.
Data from regression analyses revealed positive relationships between numbers of detoxifications, as well as between control over drinking score, and connectivity strength in the network involving amygdala and prefrontal structures. This positive relationship was found only when cognitive demands were not present (ie, in the implicit condition). On the other hand, when cognitive demands were present (ie, in the explicit condition), the number of detoxifications, severity of dependency, and control over drinking score were negatively correlated with connectivity strength in a network involving insula and prefrontal structures. Thus, it seems that during a passive view of threat stimuli (implicit condition), amygdala-related neural networks become more strongly integrated as severity of alcoholism increases. In contrast, when recognition and regulation of emotion is required (explicit condition), insula-related networks are less integrated as severity of alcoholism increases. Again, increased connectivity in amygdala-related networks would lead to an increased emotional reactivity (Davis and Whalen, 2001), whereas decreases in the network integrity of insula-related networks would lead to inappropriate analysis of the emotional input (Bechara and Van Der Linden, 2005).
We recently reported that abstaining MDTx patients are severely impaired in a task requiring processing of conflicting motivational information (Duka et al, 2011); this impairment was associated with loss of gray matter in areas of the brain including the ventromedial prefrontal cortex and superior frontal gyrus. The present findings indicate additional deficits in brain areas involved in evaluating and expressing emotion. Together they point to adverse effects of repeated detoxification on two functions crucial for successful abstinence (on the one hand making appropriate decisions to avoid risk, and on the other allowing for emotions to be perceived and expressed accurately in order to support a social network).
Acknowledgments
Supported by Medical Research Council Programme Grant G0400568 to DNS, TD, MLP and SSCW, and by the EU InterReg grant ‘AlcoBinge'.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adinoff B. Double-blind study of alprazolam, diazepam, clonidine, and placebo in the alcohol withdrawal syndrome: preliminary findings. Alcohol Clin Exp Res. 1994;18:873–878. doi: 10.1111/j.1530-0277.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. Dissociable neural systems for recognizing emotions. Brain Cogn. 2003;52:61–69. doi: 10.1016/s0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association: Washington, DC; 1994. [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47 (Suppl 1:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Baas JM, Milstein J, Donlevy M, Grillon C. Brainstem correlates of defensive states in humans. Biol Psychiatry. 2006;59:588–593. doi: 10.1016/j.biopsych.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Chaudhry AM, Parkinson JA, Hinton EC, Owen AM, Roberts AC. Preference judgements involve a network of structures within frontal, cingulate and insula cortices. Eur J Neurosci. 2009;29:1047–1055. doi: 10.1111/j.1460-9568.2009.06646.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Derntl B, Habel U, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, et al. General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neurosci. 2009;10:91. doi: 10.1186/1471-2202-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Trick L, Nikolaou K, Gray MA, Kempton MJ, Williams H, et al. Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biol Psychiatry. 2011;70:545–552. doi: 10.1016/j.biopsych.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SC, et al. Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry. 2003;53:226–232. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, et al. A direct brainstem-amygdala-cortical ‘alarm' system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O'Reilly CE, Howard JA, Sawyer K, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol. 1982b;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Buchanan TW, Lovallo WR. Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcohol Clin Exp Res. 2002;26:441–448. [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Myers RR, Lovallo WR. Altered affective modulation of the startle reflex in alcoholics with antisocial personality disorder. Alcohol Clin Exp Res. 2003;27:1901–1911. doi: 10.1097/01.ALC.0000099263.71214.F9. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, et al. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci. 2009;29:9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D, et al. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Harris RA. Neurobiology of alcohol abuse. Trends Pharmacol Sci. 1992;13:206–211. doi: 10.1016/0165-6147(92)90065-e. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur J Neurosci. 2001;14:2023–2031. doi: 10.1046/j.0953-816x.2001.01824.x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T. Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2008;363:3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, et al. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: alcoholics' impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- WHO 1993International Classification of Diseases (ICD-10)10th ednWorld Health Organisation: Geneva [Google Scholar]
- Winston JS, O'Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.