Abstract
The purpose of this study is to review the current literatures on breast cancer (BC) in the Arctic, especially the trends in incidence during the last decades and the possible explanations. The design of this study is a literature review. The scientific literature concerning BC were reviewed, especially focusing on the Arctic and the special conditions that exist in this region. Breast cancer incidence is increasing all over the world, including in the Arctic. The enormous transition in health conditions and lifestyle in the Arctic might be contributing to the known risk factors. In Greenland, the age at menarche has diminished by 3 years during the course of 100 years, and the number of children per women as well as the duration of breastfeeding is decreasing. Obesity and intake of saturated fat is increasing and the intake of traditional food rich in unsaturated fat and vitamin D decreasing. Smoking and alcohol consumption in the Arctic has been relatively high but is now decreasing. More focus on genetic susceptibility in relation to BC has identified the specific BRCA1 founder mutation in the Greenlandic population, which might appear to be an important risk factor. However, the known established risk factors alone cannot account for the increasing trend observed. Studies suggest that environmental contaminants such as persistent organic pollutants (POPs) including perfluorinated compounds increase the risk of BC possibly in conjunction with certain genetic polymorphisms involved in carcinogen activation. The lipophilic POPs such as polychlorinated biphenyls and organochlorine pesticides are found at very high levels in the Arctic population. Several factors can explain the increasing incidence of BC in the Arctic. The transition in lifestyle and health conditions unfortunately increases the known risk factors of BC. Moreover, the population of the Arctic might show up to be especially vulnerable because of the contemporary high burden of POPs and genetic susceptibility.
Keywords: breast cancer, incidence, Arctic, risk factors, persistent organic pollutants
Breast cancer (BC) has been a rare disease in the Arctic until approximately 50 years ago (1). Over a 25-year period between 1950 and 1974, only 57 cases of BCs were diagnosed in Greenland (2). However, in recent decades we have witnessed a considerable increase in the incidence. Although still lower, the incidence is now approaching incidences recorded in Western populations (3), and today about 12–15 women are diagnosed every year in Greenland (4). From 1988 to 1997, the age-adjusted incidence rate (world-standard) for women in Greenland was reported as 46.4 per 100,000 (3). For comparison, the age-adjusted (world-standard) incidence in the USA is 124 per 100,000 women based on numbers from 2001 to 2008 (5) and in Denmark approximately 100 per 100,000 women in 2010 (6).
Different factors might affect this increase in BC incidence. Underreporting in the past, diagnostic improvements and more focus on the disease might contribute to the increase in incidence of BC, but would be expected to affect the incidence in all age groups, whereas the increase seems to be most pronounced in postmenopausal women (7) as in Western countries.
The incidence of BC is increasing in almost all countries. The differences that still exist between different ethnic groups (8) might be explained by differences in reproductive and hormonal factors, genetic background, lifestyle and environmental exposures. Changes in reproductive behaviour have been pronounced in the Arctic over the last decades. The birth rate in Inuit populations has been steadily decreasing, especially in Greenland (9) and Arctic Canada (7), and the duration of breastfeeding has been decreasing (10). Both factors are known to increase the risk of BC.
Genetic inheritance plays a major role in BC risk. Approximately 8–10% of all BCs are inherited, mainly by mutations in BRCA1 and/or BRCA2. These mutations occur all over the world and in Greenland a specific founder mutation has been identified. Recent studies have shown a high frequency of this BRCA1 founder mutation, especially at the East coast of Greenland with the highest population frequency of a BRCA1 mutation ever described (11,12). These findings suggest that genetics is a potential risk factor associated to the risk of developing BC in Inuit women in certain parts of Greenland. Increased risk of BC has also been associated with height, obesity after the menopause and large weight gains after the age of 18 years (3). An array of studies have supported the hypothesis that exposure to environmental contaminants can contribute to the risk of BC, and that this exposure risk is further increased with certain genetic polymorphisms.
This review aims to evaluate and review the present literature on BC in the Arctic, and draw a steady-state conclusion about trends in incidence and possible explanations. The papers used for this review were found by using different online libraries, especially PubMed. Moreover, we have visited several homepages describing statistics on the subject. The literature though is very sparse concerning BC in the Arctic. The focus is primarily on Greenland because the statistics is best described in this region (9).
Epidemiology
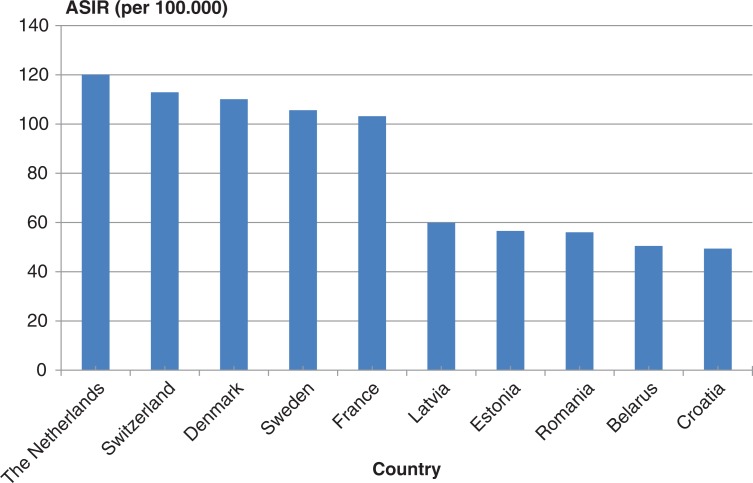
Breast cancer is the most common cancer for women in the Western world, and the incidence has been increasing since 1940 (13). The highest incidence rates are observed in North America, and the lowest risk is found in Asia and Africa (14). BC is also the most common cancer in females in Europe, with the highest incidence in The Netherlands, Switzerland and Denmark and lowest in the eastern part of Europe (15) (Fig. 1).
Fig. 1.
Age-standardised BC incidence rates (ASIR) per 100,000 women by country in Europe 1995. Adapted from (15).
From very few cases an extraordinary increase in BC is observed in the Inuit population of Greenland and Canada (2,7). A study on the incidence of BC among American Indians (AI), Alaska Natives (AN) and women in the USA from 1999 through 2004 reported that BC rates in general were lower among AI and AN than among the US non-Hispanic whites (NHWs). However, in Alaska, the BC incidence rate (134.8)1 for AI and AN women was nearly identical to the rate for NHW women in Alaska (136.5)2 (16). It could be speculated whether the Alaskan Natives have a higher BC risk because of their genetic background or exposure to environmental factors together with factors associated to their lifestyle.
During the 1970s, the cancer pattern among Inuit was characterised by high frequencies of carcinomas of the nasopharynx, salivary glands and esophagus, and low frequencies of tumours common in Western countries (7). Now, the traditional cancer types in the Arctic are decreasing, whereas the cancers common for the Western world such as lung cancer and BC are increasing in the Arctic. Thus, the distribution of cancer types in Greenland has changed during the last decade (3,4).
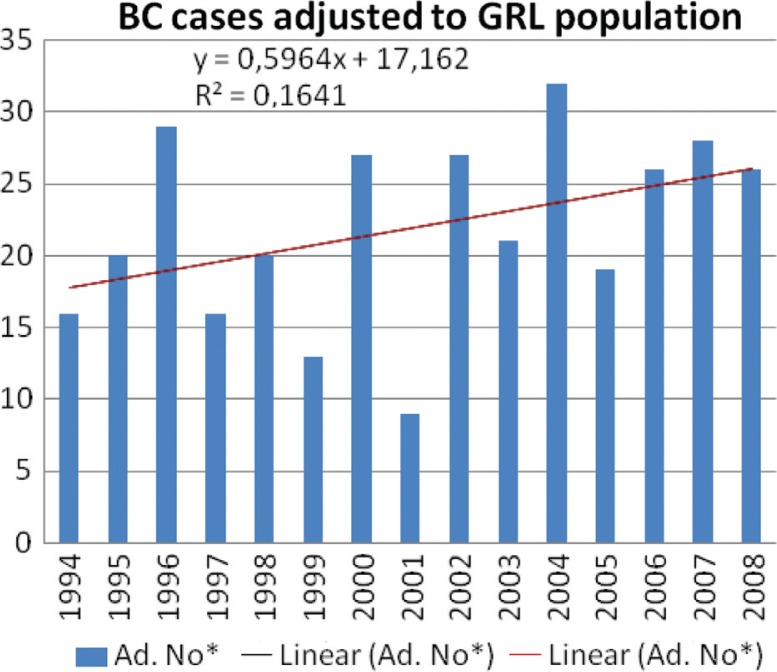
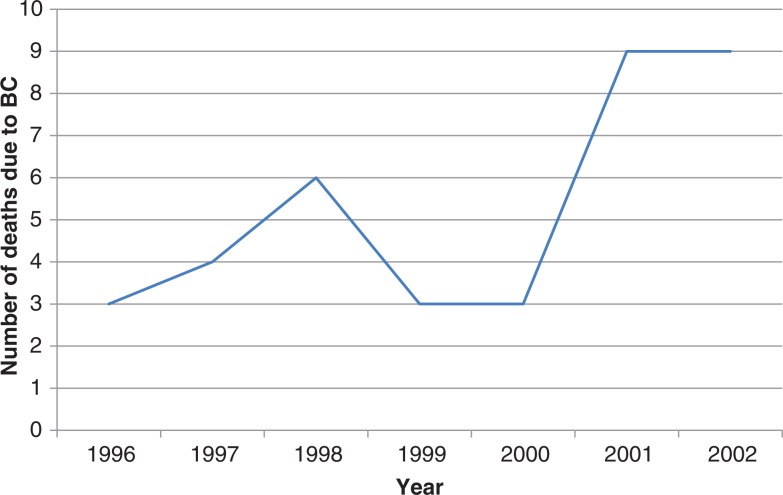
As shown in Fig. 2, an increase in BC cases in the period 1994–2008 with a yearly increase of 0.60 was found in Greenland. Moreover, the latest data from Greenland Statistics report that BC as a cause of death is increasing, despite the fact that treatment is improving (Fig. 3) (9).
Fig. 2.
Increase in breast cancer incidence in the period 1994–2008. The number of cases per year was adjusted as a ratio to the population size (no/population) and then multiplied with 100,000. The diagram and trend was performed using the MS Excel programme (9). GRL: Greenland.
Fig. 3.
Number of deaths in Greenland due to breast cancer, 2010. Adapted from (9).
However, the BC incidence in Greenland is still low being 13–17% of all cancers, compared to 23–34% in the Nordic countries during 2000–2008 (17; Table I).
Table I.
Number of breast cancers and percentage of all cancers in the Nordic countries
| Total number of cancersa | Number of breast cancers | Per cent of total | |
|---|---|---|---|
| Denmark | |||
| 2000–2004 | 5,162 | 1,426 | 28 |
| 2008 | 5,785 | 1,720 | 30 |
| Faroe Islandb | |||
| 2000–2004 | 3,027 | 897 | 30 |
| 2003–2007 | 3,110 | 812 | 26 |
| Greenlandb | |||
| 2000–2004 | 3,100 | 515 | 17 |
| 2002–2006 | 3,286 | 442 | 13 |
| 2004–2008 | 3,404 | 531 | 16 |
| Finland | |||
| 2000–2004 | 4,297 | 1,352 | 31 |
| 2005 | 4,449 | 1,505 | 34 |
| 2007 | 4,617 | 1,535 | 33 |
| Icelandb | |||
| 2000–2004 | 3,945 | 1,108 | 28 |
| 2004–2008 | 4,082 | 1,238 | 30 |
| Norway | |||
| 2000–2004 | 4,666 | 1,163 | 25 |
| 2005 | 4,978 | 1,198 | 24 |
| 2008 | 5,070 | 1,151 | 23 |
| Sweden | |||
| 2000–2004 | 4,530 | 1,365 | 30 |
| 2005 | 5,602 | 1,529 | 27 |
| 2008 | 5,718 | 1,575 | 28 |
Source: Adapted from (17). http://www-dep.iarc.fr/NORDCAN/DK/frame.asp
The total covers all cancers diagnosed according to the ICD-10 criteria, chapter C, except C44 and C46.0 (including D09.0, D32, D33, D41.4, D42 and D43).
Based on 5-year average.
Also among other Arctic populations, the BC incidence is lower compared to the non-Arctic populations in the same region. In the Canadian population, BC accounts for more than a quarter (28%) of new cancer cases in women (18), whereas the number in Arctic Canada is 17% (1988–2004) (19). In the Arctic Russia, BC constituted 17.5% (2009) among all cancers in women (20,21). The age-standardised BC incidence in Russia as a whole has increased annually by 3.25% from 1994 to 2004 being 38.3 per 100,000 women in 2000 and comprising 19.3% of all new cancers. The highest rates are observed in Moscow, where BC accounts for 23.9% of all new cancers (21).
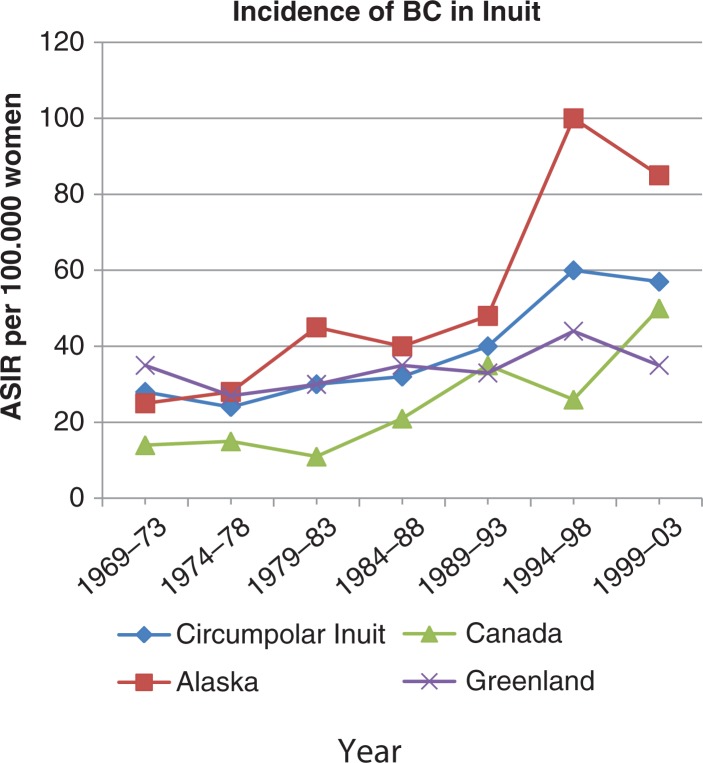
Currently, very few statistical data on the BC incidence in the Arctic exist. Due to small population sizes, it is difficult to foresee the evolution of a disease like BC. The number can fluctuate widely from year to year, and therefore one can be led to believe that things are moving in one direction, although it is not. However, viewed over the last decades, there is no doubt that the incidence of BC is increasing in the Arctic (22; Fig. 4).
Fig. 4.
Trends in age-standardised breast cancer incidence (per 100,000 women) among Inuits, 1969–1973 to 1999–2003. Adapted from (22).
Known BC risk factors
Results from many studies have confirmed that BC is not a single disease with variable morphologic features and biomarkers, but rather a group of molecularly distinct neoplastic disorders (23). Although it is known that parity is a strong protective factor against the development of BC, presently approximately 5–10% of new BCs are attributable to hereditary syndromes, and other well-established risk factors accounted for approximately 30% of cases (23). Thus, the risk of BC is not only influenced by the reproductive history and the genetic background but is also thought to be affected by lifestyle factors and exposure to environmental contaminants.
This section has been divided into the following subsections: Known established BC risk factors (ad 1 to ad 5), followed by factors believed to reduce the BC risk (ad 6 and ad 7) and finally, factors studied and being discussed as BC risk factors (ad 7 to ad 10).
Factors known to increase the BC risk: 1. early menarche and late menopause (lifelong exposure to oestrogens); 2. genetic inheritance in mutations in the BRCA-1 and BRCA-2 genes and other genetic polymorphisms relevant for BC risk; 3. obesity after menopause and high intake of fat; 4. alcohol; and to a less extent, 5. smoking.
Factors believed to reduce the BC risk: 6. low age at first birth and large number of full-term pregnancies; 7. long duration of breastfeeding and physical activity (see also ad 3).
Factors studied and discussed as BC risk factors: 8. women's birth weight; 9. vitamin D level; 10. exposure to environmental contaminants.
Ad 1: menarche and menopause
Breast cancer risk is related to several reproductive factors. Specifically, risk increases with early age at menarche (24) and late age at menopause (25), meaning many menstrual cycles. These associations are consistent with the hypothesis that BC risk is related to the total extent of breast mitotic activities, driven by oestrogen and progesterone exposure during the luteal phase of the menstrual cycle, which will determine the probability of tumourigenic somatic events (26). Thus, early age at menarche increases the period during which the breast is mitotically active, particularly the period before first full-term pregnancy during which breast cells undergo differentiation.
The incidence of breast cancer increases rapidly during the reproductive years and then it slows down after about age 50 years, the average age at menopause (27), and it seems that the association with age is explicable in terms of the marked reduction in steroid hormones leading up to the menopause (26). A study tried to mimic this by exposing rhesus monkeys in captivity to a variety of synthetic oral contraceptive steroids. The exposure resulted in a variety of proliferative changes including atypical hyperplasia and carcinoma in the mammary duct system (28).
Today, menarche in Greenlandic women appears 3 years earlier than 100 years ago, and the menarcheal age is now 3 months lower than in Denmark (29). A study from 2008 on European and Inuit women suggests that it is unlikely that exposure to polychlorinated biphenyls (PCBs) and Dichlorodiphenyldichloroethylene (DDE), is a main cause of menstrual disturbances. Disparities in the relation between organochlorine exposure and menstrual cycle were observed between countries, suggesting that genetic differences and/or dietary factors may be involved (30). To our knowledge, no publications on the menopausal age among Inuit are published to date.
Ad 2: genetic susceptibility
BRCA1 and BRCA2 are tumour suppressor genes that are involved in repairing the mutated and damaged DNA and/or lead the cell into a programmed cell death (apoptosis) if the DNA is not repaired. If the BRCA1 and BRCA2 genes themselves are mutated/damaged, the function of the genes might be disrupted, and if the damaged DNA in other genes is not repaired properly the risks of breast (and ovarian) cancer are increased. Approximately 8–10% of all BCs are inherited, mainly by mutations in BRCA1 and/or BRCA2 (31).
BRCA1 founder mutations
Most mutations in BRCA1 are uniquely occurring mutations, but a specific founder mutation in Greenlandic Inuit has been found and described in two different studies (11,12). Founder mutations appear in the DNA of one or more individuals who are founders of a distinct population. They initiate with changes that occur in the DNA and can be passed to coming generations.
In one of the two studies it is described that 13 families exhibited a BRCA1 exon 3 nucleotide 234 T>G mutation (pCys39Gly), which is not previously reported in the BC information core database and thus only demonstrated in the Inuit population. All 13 families shared a 4.5-Mb genomic fragment containing the BRCA1 gene, showing that the mutation originates from a founder (11). In the other study, 2,869 persons from Greenland were screened for the presence of a BRCA1 mutation (p.Cys39Gly). The overall carrier frequency was 1.6% in the general population, but the frequency differed geographically from 0.6% at the West coast to 9.7% in the previously isolated population at the East coast. This is the highest population frequency of a BRCA1 mutation ever described (12).
Recently, we studied the BC risk in Greenlandic Inuit and found that BC cases compared with their controls had a higher frequency of the Greenlandic BRCA-1 founder mutation; however, the BRCA-1 mutation alone could not at all explain the frequency of BC cases (32).
Gene polymorphisms in other genes related to BC risk
Genetic polymorphism and BC risk have been extensively analysed, and differences in genotype frequencies between cases and controls have been reported (33–35). Polymorphism in the genes involved in the maintenance of the endogenous hormonal balance such as oestrogen biosynthesis (e.g. CYP17 and CYP19) and oestrogen metabolism (e.g. CYP1A1, CYP1A2, CYP1B1 and catechol-O-methyltransferase) may affect the synthesis and degradation of oestrogens and consequently have possible adverse health effects.
There are several publications on the association between CYP17 and/or CYP19 polymorphisms, and BC risk and some controversial data are reported (36,37). Several factors might explain the controversial data including interactions between different gene polymorphisms, interactions with other known BC risk factors, a general different genetic background and different susceptibility of geographical/ethnic groups to, for example, exposure to environmental contaminants. Data show differences in these polymorphisms between Asians and Caucasians (35,38), and we have data showing that it also applies to Greenlandic Inuit vs. European Caucasian (Ghisari, Long, Bonefeld-Jorgensen, manuscript submitted).
The CYP1A1, 1A2, 1B1 genes are also involved in the P450 phase I metabolism of a wide range of environmental pollutants. The phase I P450-associated enzymes catalyse the oxidative conversion of lipophilic xenobiotics via phase II enzymes into entities, which are more water soluble and thus can be readily detoxified and excreted. However, an imbalance between the phase I and phase II metabolism can increase the level of reactive radicals, increasing the risk of oxidative stress and carcinogenic processes. High chemical exposure and/or genetic polymorphisms can increase the risk of oxidative cellular stress and thus carcinogenicity (39).
Studies have reported an association between PCB concentrations and CYP1A1 gene polymorphism in women BC (34); and an association between the CYP1B1 val/leu polymorphism and BC risk (40) has been suggested. A huge meta-analysis on data from 26 studies did not support any association of the CYP1B1 val432leu polymorphism with BC risk (41). However, recently it has been suggested that race-specific associations between CYP 1B1 Val432Leu polymorphism and BC risk may exist (42).These controversial reported data call for more studies on CYP1B1 polymorphism combined with other gene polymorphisms and potential BC risks vs. exposure to persistent organic pollutants (POPs).
We have compared the frequency of the CYP1A1 and 1B1 polymorphism between Greenlandic Inuit and Europeans and found significant differences (manuscript submitted). Future studies might elucidate whether these genetic polymorphisms affect the risk of BC in Inuit.
Ad 3: obesity, diet and physical activity
The transition in the societies of Inuit includes lower levels of physical activity and a significant change in diet (3). The traditional Inuit diet is rich in unsaturated fatty acids (uFAs) from fish and marine mammals such as seal and whale. These uFAs are suggested to have a protective effect against the development of BC (43). In the Arctic, fruits and vegetables have never been a big part of the diet, and in the recent years the Inuit have changed their diet to include more saturated fat (sFA). Furthermore, the Inuit have become less physically active over the last decades (3). This transition is very unfortunate and has contributed to increasing problems with lifestyle-associated diseases such as obesity that is increasing in Inuit population, especially among women (7,44).
Obesity is known to be of importance in the risk of BC. Oestrogen, which is also produced by the adipose tissue, is held responsible for the elevated risk of BC in obese menopausal women (45). Moreover, the adipose tissue also secretes hormones and adipokines such as leptin and IGF-I, and these substances can also contribute to an increased BC risk in obese women (45). In contrast, there is a decreased risk of BC among overweight premenopausal women (46). The mechanisms underlying this association are not well understood.
A very-low-fat, high-fibre diet combined with daily exercise results in reduced serum oestradiol and also major reductions in known BC risk factors such as obesity (47). The in vitro serum changes observed in the women was demonstrated in vivo to slow the growth and induced apoptosis in serum-stimulated BC cell lines (47). A high intake of foods rich in fat and animal protein and a large body weight are factors that increase the risk of BC (48). It has been speculated that high fat intake in childhood and puberty further increases the risk of BC (49).
Exercise decreases the risk of BC (50) both in younger (51) and older women. A study found a 14% decreased risk among postmenopausal women with high physical activity compared with women with low physical activity, being most evident for ER-positive and PR-negative tumours (52). Recently another study concluded that strenuous but not moderate physical activity at age 12 was inversely associated with premenopausal and postmenopausal BC risk for nearly all subtypes, and that moderate physical activity in the previous 5 years was significantly associated with a decrease in risk for postmenopausal BC, however, for ER- and PR-positive tumours only (53). Inverse relation between physical activity and BC is found both among white (including Hispanics), black (54) and Japanese women (55).
In summary, BC risk seems to be influenced by diet, obesity and exercise. Noteworthy, the increase in BC incidence in Inuit populations is parallel with the increasing prevalence of obesity and type-2 diabetes and lower level of physical activity (7,50).
Ad 4: alcohol intake
The correlation between alcohol and BC has been discussed widely over the last many years, but many studies conclude that the evidence on the association between alcohol drinking and BC risk remains controversial (56).
As with many of the other risk factors, evidence suggests that some populations are more susceptible than others. A study from 1980 included 89,538 American nurses who were questioned about their alcohol consumption. A follow-up showed that women who consumed 5–14 g alcohol daily (about 3–9 drinks per week) had an age-adjusted relative risk of BC at 1.3 [95% confidence interval (CI), 1.1–1.7], whereas consumption of 15 g alcohol or more per day was associated with a relative risk of 1.6 (95% CI, 1.3–2.0), and there was evidence of a highly significant increase in risk with further increase in alcohol consumption (57). A Mexican study concluded that there is an increased risk of BC with increasing intake of alcohol (58), whereas no association between alcohol and BC risk was found for Japanese (59). To date, no studies about alcohol and BC risk in the Arctic have been reported. Judging from the fact that Inuit descend from the same tribe as Asians and Mongolians, one can be tempted to hypothesise that alcohol might not be a BC risk factor among Inuit.
Another factor that seems to influence BC risk in relation to alcohol consumption is the type of BC developed. Results suggest that alcohol use may be more strongly associated with risk of hormone-sensitive BCs than hormone-insensitive subtypes (60). Alcohol consumption seems not to increase the BC risk in women carrying a BRCA gene mutation (61). Compared to non-drinkers, exclusive consumption of wine was reported associated with a significant reduction in the risk of BC among BRCA1 carriers (61). The protective effect has been attributed to polyphenols, and particularly to resveratrol, an antioxidant found in red wine.
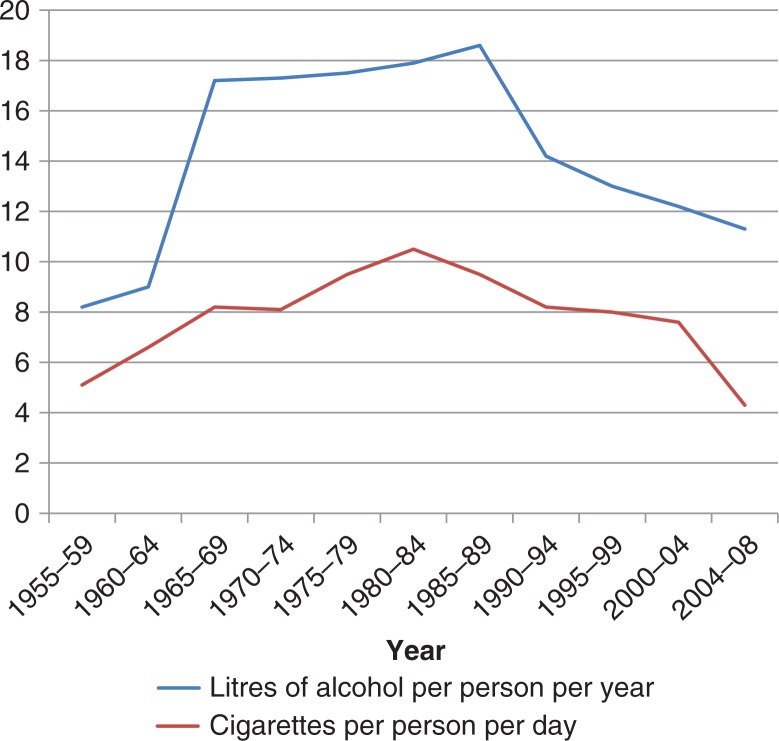
Generally, it is difficult to make any conclusions on the association between alcohol use and BC risk. The alcohol consumption in Greenland was known to be high in the past (1960–1990), but actually it has been markedly decreasing over the last decades (7,9) (Fig. 5), and is presently at the same level as in Denmark per citizen (62). The consumption is decreasing for the population as a whole, but actually statistics point out that the decreasing consumption is most evident for men compared to women (7). The current BC incidence may reflect the former high intakes of alcohol, and it can be speculated whether the actual trend will be reflected in the BC incidence of the future.
Fig. 5.
Consumption of cigarettes and alcohol in Greenland per capita, 1955–2008. Adapted from (7,9).
Ad 5: smoking
Tobacco smoke contains carcinogens, which may increase the risk of BC. Conversely, cigarette smoking also has anti-oestrogenic effects (63), which hypothetically may reduce the risk of BC. The association between smoking and BC remains controversial.
Results of epidemiological studies, investigating the association between smoking and BC, have been conflicting. Of 19 case–control and cohort studies reviewed in 1993, a weak positive association was observed in 7, 11 showed no relation and 1 reported a reduced risk. Results of recent studies are also inconclusive; some indicating no association, and some suggesting a positive relation or a protective effect. Competing carcinogenic and anti-oestrogenic effects of smoking on risk of BC could account for these discrepancies (64).
Many studies have shown that BC risk increases upon exposure to radiation and other carcinogens between 10 years and 16 years of age (65). In nulliparous women, BC risk was strongly related to smoking intensity and duration. Although numbers were few, premenopausal women who started to smoke after a first full-term pregnancy did not have an increased risk of BC, a result consistent with the reduced susceptibility of breast tissue to chemical carcinogens documented in animals after a first full-term pregnancy (66). Another recent study also concluded that active smoking, especially smoking before the first birth, may be associated with a modest increase in the risk of BC (67).
Polycyclicaromatic hydrocarbons (PAHs) are ubiquitous in the environment and also occur in cigarette smoke. PAHs are metabolised via enzymes coded by the cytochrome P450 (e.g. CYP1A1) gene, which contributes to aryl hydrocarbon hydroxylase (AHH) activity. Genotypic variants of CYP1A1 have been associated with increased AHH activity. Several studies suggest that women with the variant genotype(s) are at increased risk for BC (35). Another study also concludes that cigarette smoking early in life is a modifiable cause of BC in a subpopulation of genetically susceptible women with respect to CYP1A1, but that the proportion of BCs attributable to cigarette smoking at a young age among Caucasian women with the variant form of the polymorphisms is low (68).
In summary, the risk of BC and smoking might be influenced by the genetic background and depends on the time of exposure. As for alcohol, the consumption of cigarettes in Greenland has been decreasing over the last decades, being 4.3 cigarettes per capita per day in 2008 (Fig. 5) (7,9), and the statistics indicate that Greenlandic women smoke less than Greenlandic men. In Denmark, the number of cigarettes per capita per day was 4.1 in 2008 (62).
Ad 6: pregnancy and births
The number of births and the mother's age at the first and second birth also seems to be important for the risk of BC development (69). Early full-term pregnancy affords lifetime protection against the development of BC. This phenomenon can be mimicked in rat and mouse models of mammary cancer in which the hormones oestrogen and progesterone are given for 21 days. Carcinogen-induced proliferation is blocked as a consequence of hormone pretreatment (70). P53 is a tumour suppressor gene that is involved in the regulation of the cell cycle in humans and therefore it is a factor in cancer prevention. Both immune histochemical and Western blot studies indicate that p53 protein expression is increased in hormone-pretreated mice and rats (70), indicating that pregnancy (or the simulation thereof) might protect against BC.
It is concluded that BC diagnosed within 5 years of childbirth should be regarded as a negative prognostic factor, particularly if pregnancies have been multiple (71). Another study also observed a short-term increase in the risk of BC after a full-term pregnancy (with a maximum 3–4 years after delivery) followed by a long-lasting decrease in risk (72). The most commonly postulated biological process for the dual effect of pregnancy on BC risk suggests that the hormonal milieu during pregnancy increases cell proliferation, including the proliferation of the already-initiated cells, yet at the same time inducing terminal differentiation of the mammary gland, rendering it less susceptible to the carcinogen stimuli (73).
Pregnancy among Inuit women occur earlier in life than it does in Western countries. In 2004, the median age for the birth of the first child was reported as 19 years for Canadian Inuit women compared to 26 years for women nationally (74). According to a report published in 2003, the average age of the Greenlandic mothers of the firstborn child was 24.7 years and the total fertility rate (TFR) among Greenlandic women was 2.36 (9). In overall, the fertility in Greenland has not changed during 1977–2012, but women in the outer districts (village) give, in general, birth to more children compared to women in towns (9). In Denmark, the average age was 29.1 years in 2009 (62), and the TFR among Danish women was 1.84 in 2009, being higher than in many other European countries. Thus, the TFR is still higher for Greenlandic women, whereas the age for the birth of the first born child is increasing and approaching the age of the Danish women, indicating that these factors might be contributing to the increase in BC incidence.
Ad 7: breastfeeding
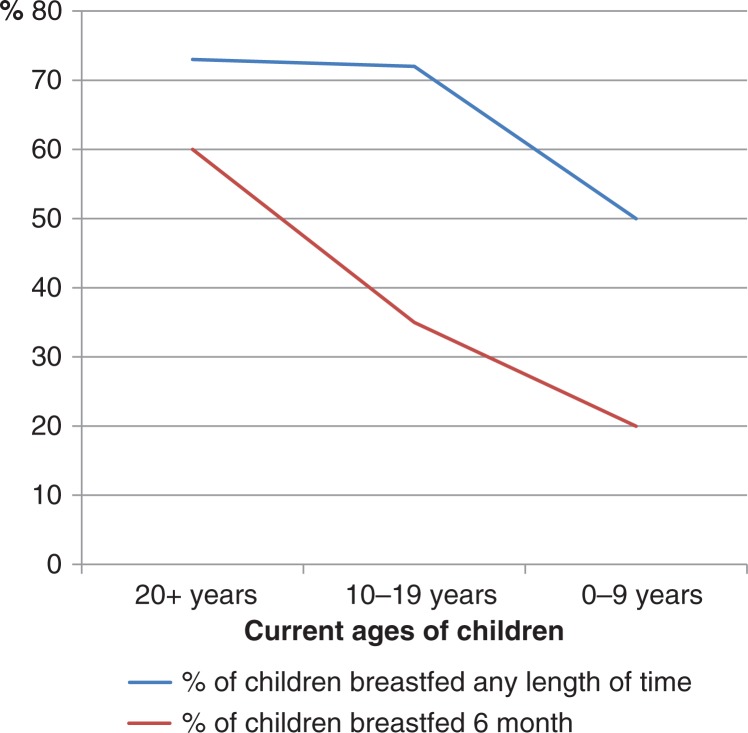
Long-standing lactation is associated with a lower BC risk (75). A theory is that lactation protects against BC, because it means fewer menstrual cycles. A study concludes that changes in lactation practices rather than in age at first pregnancy, multiparity or fat consumption can explain the changes in the frequency of BC in various Inuit populations (76), but more studies are needed to draw any conclusions. In the beginning of the twentieth century, extended periods of breastfeeding continuing through the childbearing years were common in Inuit, but decreased throughout the present century (Fig. 6) (10).
Fig. 6.
Percentage of Inupiat* children breastfed according to their current age**, 2001. Adapted from (10).
*The people of Alaska's Northwest Arctic and North Slope boroughs and the Bering Straits region.
**Age 20+ correspond approximately to birth year 1980, age 10–19 correspond to birth year 1980–1990, and age 0–9 correspond to birth year 1990–2000.
Studies have shown that the association between lactation and premenopausal BC is modified by the family history of BC. Among premenopausal women who had ever breastfed and had a first-degree relative with BC, the covariate-adjusted Hazard Ratio (HR) was 0.41 (95% CI, 0.22–0.75) compared with premenopausal women who had never breastfed, whereas no association was observed among women without a family history of BC (77).
It is known that one of the major routes for elimination of hydrophobic and lipophilic substances including POPs is through breast milk. With decreasing trends in breastfeeding in the Arctic it could be hypothesised that a lower level of POP elimination via breastfeeding causes a relative higher body burden of POPs, and thereby increases the BC risk and thus incidence. However, further studies are needed to elucidate this hypothesis.
Ad 8: birth weight
Several studies have observed an increased risk of BC related to a high birth weight. Women with their own birth weight >4,000 g or 8.5 lb have a higher risk of developing BC compared with birth weights below 2,500 g or 3,000 g [odds ratio (OR)=1.20; 95% CI 1.08, 1.34]. The findings are consistent with a weight-response effect. The summary effect estimated for BC risk per 1 kg increase in birth weight was statistically significant (78). The explanation for this phenomenon is difficult. Higher birth weight has been shown to predict an increased risk of overweight in 6- to 7-year-old girls, and because obesity is a strong predictor of development of the breast it is important to assess peripubertal obesity in girls (79). It is known that the relation between BMI values and age at menarche is inverse (80), meaning that higher BMI results in puberty at a younger age. However, suggestions have been made that a positive association between birth weight and adult BC is not likely to be mediated by early age at menarche, but possibly by increased body height in adolescence (81). A Mexican study supports this point of view by concluding that there is no correlation between early menarche (unset before 12 years) and the risk of BC (82). Another study concludes that height is an independent risk factor for postmenopausal BC, but that the relation is less clear in premenopausal women (83).
In summary, reported data support that birth weight and height can influence the risk of BC. The average birth weight among Greenlandic children in 2002 was 3,466 g for live born (9) and 3,514 g for Danish live born singletons in 2009 (62). These numbers have been stable during the last decade (9,62).
Ad 9: vitamin D
The association between BC and vitamin D has been widely studied over the past decades. Although individual studies are not entirely consistent, recent meta-analyses of all relevant, published epidemiological data support the concept that optimal vitamin D status has a protective effect against development of BC (84). Extensive laboratory data indicate that vitamin D acts directly in mammary tissue to suppress tumourigenesis (85), although it has been shown that there is no association between mammographic density and intake of vitamin D (86). The normal value range for vitamin D can vary slightly among different laboratories, but the common perception is that levels above 75 nmol/L are preferential (87). Vitamin D insufficiency is defined as 25-hydroksyvitamin D <50 nmol/L, vitamin D deficiency as a 25-hydroksyvitamin D<25 nmol/L, and severe vitamin D deficiency as a 25-hydroksyvitamin D <12.5 nmol/L.
In northern Norway, Tromsoe, levels of vitamin D are found to vary between winter (52.4 nmol/L and 49.3 nmol/L in 1994 and 2008, respectively) and summer (60.6 nmol/L and 65.2 nmol/L in 1994 and 2008, respectively) (88), and for this reason it has been speculated whether the levels are high enough to protect against BC (89). Knowing that Tromsoe is located north of the northern polar circle, this fact is perhaps valid for all regions in the Arctic. However, the data from North Norway do not take into consideration the higher intake of cod oil and thus higher intake of vitamin A that can compete with vitamin D (89); adjustment for cod liver intake and then a possible competition between vitamin D and vitamin A is needed for clarification. A study on 282 Inuit children from Nunavut found that 78.6% had low vitamin D status even at the end of summer (median, 48.3 (32.8–71.3) nmol/L), when plasma 25(OH)D concentrations should be at their highest compared to winter (median, 37.7 (21.4–52.0) nmol/L) (90).
As earlier mentioned the Inuit have reduced their consumption of traditional foods rich in vitamin D. Furthermore, infrequent use of vitamin D supplements, darker skin, higher obesity rates and northern latitude are risk factors predisposing Arctic Indigenous peoples to vitamin D deficiency or insufficiency. At northern latitudes, ineffective synthesis of vitamin D lasts from October through March (90). Moreover, the annual average air temperature in the Arctic is only around 1°C, and the population wears clothing that covers most of the body all year round, only leaving the skin of the face free to produce vitamin D. To our knowledge there is no report on whether there is any relation between vitamin D and the BC risk in the Arctic.
Ad 10: environmental contaminants
Persistent organic pollutants (POPs) are organic compounds that are resistant to environmental and metabolic degradation, including chemical, biological and photolytic processes. Most POPs are currently or were in the past used in industrial processes or as pesticides. The POPs can be divided into legacy POPs and perfluorinated compounds (PFCs).
The lipophilic legacy POPs including PCBs, dioxins, furans and hexachlorobenzenes and organochlorine pesticides were globally banned in the 1970s–1980s (91).
In 2001, it was discovered that the more hydrophilic POP group, the PFCs used in different industrial applications were accumulating in the environment, animals and human tissues with a global distribution. PFCs are a large group of chemicals used since the 1950s in, for example, fire-fighting foams, cleaners, lubricants, textile, carpets, furniture, shoes, food wrapping and various coatings (92).
The PFCs can further be subdivided into the perfluoroalkyl acids (PFAAs) including the perfluorocarboxylated acids (PFCAs) and perfluorosulfonated acids (PFSAs), and the PFAAs include the two most studied PFCs: the perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA).
Heavy metals and POPs are biomagnified in the Arctic food chain and have attracted substantial attention in recent decades. The biomagnification up the marine food chain results in high exposure of the Inuit and is consistent with the high intake of marine mammals in this population. There is variation in the POP levels measured in the different Inuit populations, but the concentrations are all above their respective non-Inuit national populations. Serum levels of legacy POPs and heavy metals are especially high in Greenlandic Inuit (91). A sleight but significant decline for most legacy POPs is found in blood over the last decade (1998–2006) within three Arctic regions studied. But still the Inuit continue to have the highest levels of almost all POPs and heavy metals among the ethnic groups studied (91,93).
Recently, we reported that serum PFAA levels are higher in Nuuk Inuit than in non-Nuuk Inuit, suggesting that urban populations are more exposed and that sources other than seafood intake might contribute to the observed higher PFAA levels (94). Within the same district, higher PFAA levels were observed for males, and (as for the lipophilic legacy POPs) an age-dependent increasing trend of serum PFAA levels in the period 1998–2005 was observed for Nuuk Inuit (94). The levels found in Greenlandic Inuit were similar to the levels observed in Inuit of the Arctic Canada (94).
Whether these contaminants affect cancer development in Inuit is still speculative. However, legacy POPs have immune and hormone disruptive potentials (91), and studies suggest that also PFCs can interfere with the function of immune system and endogenous hormones (95–97).
In a recent study on Greenlandic Inuit, we found that BC cases have a significant higher serum level of PFCs in all quartiles and also PCBs in the highest quartile, and the adjusted ORs indicated a significant risk for BC in relation to the level of serum PFOS and sumPFSA and total sum of legacy POP plus PFCs (32). Thus, future studies must further elucidate whether there is a link between the exposure to legacy POPs and PFC, and risk of BC in the Arctic may be because of an interaction between decreased immune defence and chemically induced carcinogenesis.
Studies also point to an association between PCBs and BC in conjunction with certain genetic polymorphisms involved in carcinogen activation (35,34) (see ad 2). Although POPs and heavy metals are shown to have mutagenic and carcinogenic potential in an abundance of experimental and observational studies, a review from 2003 concludes that the weight of evidence does not support a causal association for PCBs and human cancer (98). However, this statement is based on a meta-analyses not taking into consideration the exact exposure levels and profiles, different geographical locations and ethnic groups and neither the hypothesised increase in susceptibility to development of BC for the interaction between certain genetic polymorphisms and POP exposure.
Several recent studies in Arctic Canada confirm and support the relationship between contaminant exposure and depressed immunity (97). These effects on the immune system might contribute to an increased risk of BC. Whether there is an interacting effect of POPs via diet, outdoor and indoor air pollution and soil and drinking water contamination on the increasing incidences of BC in Inuit is difficult to assess, because synchronous changes in lifestyle factors such as diet, smoking, alcohol and the fertility pattern might also play an important role in BC risk.
Mammary gland development and exposure to environmental contaminants
The human tissue begins its ductal development early in gestation, about embryonic week 12–14 (99).
There are three phases of mammary gland growth that are suggested to be critical. These phases cover the times during which paramount developmental events occur. Phase 1, the prenatal development of the mammary epithelial sprout; phase 2, the peripubertal period, when mammary growth is exponential in nature. Several studies have determined that unique, highly proliferative, terminal end buds (TEBs) structures are sensitive to chemical carcinogens in rodent models, and in fact, TEB presence at the time of carcinogen exposure is positively associated with tumour multiplicity (the number of tumours per tumour-bearing animal); phase 3, the gland undergoes a third critical period of development during pregnancy (99). Full differentiation of the breast takes place after the first full-term pregnancy, at which time susceptibility to tumour induction is greatly reduced (64). Studies that focus solely on exposure of mature animals or adult women trying to evaluate exposures once the disease or adverse health outcome has occurred may be missing important details on developmental effects of the exposure.
High mammogram density is known to be a risk factor for BC. A breast is said to be dense if it consists mostly of ductal tissue (the glandular tissue) rather than fatty tissue. A breast consisting of more fat is less likely to becoming cancerous, because it is generally considered that the mature fat cell is unable to proliferate. Women with very dense breasts, as seen on a mammogram, are four to five times more likely to develop BC than women with low breast density (100). A study concluded that Alaskan Inuit women had higher mammogram density than Aleuts or Indians (101), suggesting that this race might be more susceptible to develop BC.
The time of exposure is critical for the dioxin effect on BC risk. In animal studies, it was shown that both gestational and postnatal exposure to dioxins can cause an impairment of TEB development and delay both their formation and differentiation (99), meaning that upon dioxin exposure the undeveloped TEBs are there for a longer period of time, and thus the TEBs constitute an increased number of targets for carcinogen action. This fact might explain the increased mammary tumours after carcinogen exposure to dioxin early in life. Interestingly, the stromal portion of the mammary gland is not only important for dioxin-induced developmental events (102), but mouse mammary fibroblasts lacking dioxin receptor demonstrate reduced tumourigenicity (nearly four times less than control) in a mouse xenograft model (103). These findings suggest that the dioxin receptor signalling pathway is involved in an angiogenic or extracellular matrix-mediated response needed for tumour formation. Although it should be said that dioxin is shown to be anti-oestrogenic and thereby possibly protective against BC by exposure in adult life (104).
Epigenetic changes in utero can also predetermine the susceptibility to BC through alterations of the hormonal environment caused by either maternal diet and/or exposure to environmental chemicals with endocrine activities that can modify the epigenome. These epigenetic modifications, inherited by somatic daughter cells, lead to changes in mammary gland development and increase the vulnerability for malignant transformation (105), suggesting that a breast tumour develops over many decades. Therefore, one could be tempted to hypothesise that the increase in BC observed today might be influenced by the emission of and exposure to these substances in the 1950s.
In all, we cannot ignore the environmental contaminants and their possible effect on BC development. Several different contaminants are suspected to influence BC risk. Knowing that Inuit have very high levels of almost all POPs, the rise in BC incidence in the Arctic might be explained by the fact in interplay with transitions in lifestyle. However, more studies are needed for further conclusions.
Discussion
A number of known risk factors for BC have changed in the Arctic community for the last generations. In Greenland, the menarche age is now 3 years lower compared to 100 years ago. Obesity especially among women is increasing, and the level of physical activity is lower than in previous decades. The number of children per women as well as the duration of breastfeeding is slightly decreasing. Intake of sFA is increasing and the intake of traditional food rich in uFA is decreasing. These trends could contribute to the increase in the risk of BC. Smoking and alcohol consumption has been high but are nowadays decreasing in the Arctic, which should mean a decrease in the BC risk. Genetic susceptibility to BC might be of great importance in the Arctic and need further investigation including the Greenlandic Inuit population carrying a specific BRCA1 founder mutation.
However, known established risk factors alone cannot account for the increasing BC trends observed. Low parity, late age at first birth and obesity are risk factors that are also associated to two other hormone-associated cancers, corpus uteri and ovarian cancers. Incidence rates of these two cancers among the Inuit have not increased in parallel with BC rates indicating that factors other than those associated with reproductive history may be important for the increasing incidence in BC.
Studies suggest that environmental contaminants such as POPs and PFCs can increase the risk of BC.
A better understanding of the sources of environmental exposures known to affect breast development and cancer risk was obtained during the last century. However, further research are needed to evaluate and understand the interplay between lifestyle changes, environmental exposures and genetic susceptibility.
Balancing the risks and benefits of the traditional diet of the Arctic populations is very difficult and called “The Arctic Dilemma” (97). The nutritional benefits of traditional food and its contribution to the total diet are substantial. The traditional food of Inuit contributes significantly more protein, iron, selenium and zinc to the diet of the consumers than the western food. The social, cultural, spiritual, nutritional and economic benefits of the traditional food must be considered in concert with the risks of exposure to environmental contaminants. Consequently, the chemical contamination of traditional food raises problems that go beyond the usual concerns of public health and might not be solved alone by risk-based health advisories or food substitutions.
We suggest that initiatives to prevent must be taken such as guidance of pregnant and lactating women and peripubertal adolescent by informing about the potential sources of POP exposure by recommending higher intake of teresstic animals and non-fatty marine diet including young animals with lower content of POPs. This must be done by general practitioners, paediatricians, obstetricians, lactation consultants or midwives. A substantial learning curve lies ahead to accomplish this goal; however, it is imperative that exposure during critical periods of development and growth of the mammary gland (as well as other reproductive tissues) must be limited.
Conclusion
The risk factors behind the increasing incidence in BC all over the world are multiple and the mechanisms still far from understandable. By studying the increased BC incidence in the Arctic population through their transition in lifestyle and exposure to various environmental contaminants we might come closer to an understanding.
The transition in health conditions and lifestyle in the Arctic has happened over a relatively short period of time, which makes the Inuit population unique to study. No longer than 50 years ago, a great proportion of this population lived in huts of turf, ate the food of the wild nature and had a high level of physical activity every day. Today, most of the Inuit live lives similar to that of Western populations. Together, the lifestyle changes and exposure to environmental chemicals might contribute to the increasing trend in BC incidence in the Arctic.
134,8/100.000 (Age-adjusted incidence rate (ASIR) per 100.000 persons).
136,5/100.000 (Age-adjusted incidence rate (ASIR) per 100.000 persons).
Conflict of interest and funding
The authors declare that there is no conflict of interest.
References
- 1.Lanier AP, Blot WJ, Bender TR, Fraumeni JF., Jr. Cancer in Alaskan Indians, Eskimos, and Aleuts. J Natl Cancer Inst. 1980;65:1157–9. [PubMed] [Google Scholar]
- 2.Nielsen NH, Hansen JP. Breast cancer in Greenland–selected epidemiological, clinical, and histological features. J Cancer Res Clin Oncol. 1980;98:287–99. doi: 10.1007/BF00410791. [DOI] [PubMed] [Google Scholar]
- 3.Friborg JT, Melbye M. Cancer patterns in Inuit populations. Lancet Oncol. 2008;9:892–900. doi: 10.1016/S1470-2045(08)70231-6. [DOI] [PubMed] [Google Scholar]
- 4.Nuuk: Government of Greenland; 2011. Landslægeembedets Årsberetning [Annual report of the Country Doctor's office] [cited 2011 Apr 18]. Available from: http://dk.nanoq.gl/Emner/Landsstyre/Departementer/Landslaegeembedet/Udgivelser/Aarsberetning.aspx. [In Danish] [Google Scholar]
- 5.U.S. National Institutes of Health, National Cancer Institute, Cancer Statistics. Breast cancer incidence rates by race. Bethesda: U.S. National Institutes of Health; U.S. National Institutes of Health. [cited 2011 Nov]. Available from: http://seer.cancer.gov/statfacts/html/breast.html#incidence-mortality. [Google Scholar]
- 6.Kræftens Bekæmpelse. Copenhagen: Kræftens Bekæmpelse; 2010. Statistik om brystkræft. [cited 2012 May 3]. Available from: http://www.cancer.dk/Hjaelp+viden/kraeftformer/kraeftsygdomme/brystkraeft/statistik+brystkraeft/. [In Danish] [Google Scholar]
- 7.Young TK, Bjerregaard P, editors. Health transitions in Arctic populations, part four. Toronto: University of Toronto Press; 2008. pp. 308–33.pp. 379–402. [Google Scholar]
- 8.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 9.Statistics Greenland. Nuuk: Statistics Greenland; 2011. [cited 2012 February 29]. Available from: http://www.stat.gl/. [In Danish] [Google Scholar]
- 10.Cutting S, Flanders-Stepans MB. Breastfeeding prevalence among an Alaskan Inupiat Eskimo population. J Perinat Educ. 2001;10:21–30. doi: 10.1624/105812401X88020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen TV, Ejlertsen B, Albrechtsen A, Bergsten E, Bjerregaard P, Hansen T, et al. A common Greenlandic Inuit BRCA1 RING domain founder mutation. Breast Cancer Res Treat. 2009;115:69–76. doi: 10.1007/s10549-008-0060-z. [DOI] [PubMed] [Google Scholar]
- 12.Harboe TL, Eiberg H, Kern P, Ejlertsen B, Nedergaard L, Timmermans-Wielenga V, et al. A high frequent BRCA1 founder mutation identified in the Greenlandic population. Fam Cancer. 2009;8:413–9. doi: 10.1007/s10689-009-9257-5. [DOI] [PubMed] [Google Scholar]
- 13.Newcomb PA, Lantz PM. Recent trends in breast cancer incidence, mortality, and mammography. Breast Cancer Res Treat. 1993;28:97–106. doi: 10.1007/BF00666422. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 15.Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 16.Wingo PA, King J, Swan J, Coughlin SS, Kaur JS, Erb-Alvarez JA, et al. Breast cancer incidence among American Indian and Alaska Native women: US, 1999–2004. Cancer. 2008;113(Suppl 5):1191–202. doi: 10.1002/cncr.23725. [DOI] [PubMed] [Google Scholar]
- 17.The Association of the Nordic Cancer Registries. The NORDCAN project. The Association of the Nordic Cancer Registries. 2009. [cited 2012 Aug 1]. Available from: http://www-dep.iarc.fr/NORDCAN/DK/frame.asp.
- 18.Canadian Cancer Society. Toronto: Canadian Cancer Society; 2012. General cancer statistics at a glance. [cited 2012 May 8] Available from: http://www.cancer.ca/Canada-wide/About%20cancer/Cancer%20statistics/Stats%20at%20a%20glance/General%20cancer%20stats.aspx?sc_lang=en#ixzz1JofN4kJQ. [Google Scholar]
- 19.Louchini R, Beaupre M. Cancer incidence and mortality among Aboriginal people living on reserves and northern villages in Quebec, 1988–2004. Int J Circumpolar Health. 2008;67:445–51. doi: 10.3402/ijch.v67i5.18355. [DOI] [PubMed] [Google Scholar]
- 20.Vaktskjold A, Ungurjanu TN, Klestsjinov NM. Cancer incidence in the Nenetskij Avtonomnyj Okrug, Arctic Russia. Int J Circumpolar Health. 2008;67:433–44. doi: 10.3402/ijch.v67i5.18359. [DOI] [PubMed] [Google Scholar]
- 21.Garin AM. Breast cancer in Russia. Breast Cancer. 2004;11:7–9. doi: 10.1007/BF02967993. [DOI] [PubMed] [Google Scholar]
- 22.Kelly J, Lanier A, Santos M, Healey S, Louchini R, Friborg J, et al. Cancer among the circumpolar Inuit, 1989–2003. II. Patterns and trends. Int J Circumpolar Health. 2008;67:408–20. [PubMed] [Google Scholar]
- 23.Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681–5. doi: 10.1093/jnci/87.22.1681. [DOI] [PubMed] [Google Scholar]
- 24.Peeters PH, Verbeek AL, Krol A, Matthyssen MM, de Waard F. Age at menarche and breast cancer risk in nulliparous women. Breast Cancer Res Treat. 1995;33:55–61. doi: 10.1007/BF00666071. [DOI] [PubMed] [Google Scholar]
- 25.Kelsey JL, Horn-Ross PL. Breast cancer: magnitude of the problem and descriptive epidemiology. Epidemiol Rev. 1993;15:7–16. doi: 10.1093/oxfordjournals.epirev.a036118. [DOI] [PubMed] [Google Scholar]
- 27.Chang-Claude J, Andrieu N, Rookus M, Brohet R, Antoniou AC, Peock S, et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16:740–6. doi: 10.1158/1055-9965.EPI-06-0829. [DOI] [PubMed] [Google Scholar]
- 26.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–40. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 28.Tavassoli FA. The influence of endogenous and exogenous reproductive hormones on the mammary glands with emphasis on experimental studies in rhesus monkeys. Verh Dtsch Ges Pathol. 1997;81:514–20. [PubMed] [Google Scholar]
- 29.Becker-Christensen FG. Growth in Greenland: development of body proportions and menarcheal age in Greenlandic children. Int J Circumpolar Health. 2003;62:284–95. doi: 10.3402/ijch.v62i3.17565. [DOI] [PubMed] [Google Scholar]
- 30.Toft G, Axmon A, Lindh CH, Giwercman A, Bonde JP. Menstrual cycle characteristics in European and Inuit women exposed to persistent organochlorine pollutants. Hum Reprod. 2008;23:193–200. doi: 10.1093/humrep/dem349. [DOI] [PubMed] [Google Scholar]
- 31.Mellon S, Berry-Bobovski L, Gold R, Levin N, Tainsky MA. Concerns and recommendations regarding inherited cancer risk: the perspectives of survivors and female relatives. J Cancer Educ. 2007;22:168–73. doi: 10.1007/BF03174331. [DOI] [PubMed] [Google Scholar]
- 32.Bonefeld-Jorgensen EC, Long M, Bossi R, Ayotte P, Asmund G, Krüger T, et al. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: a case control study. Environ Health. 2011;10:88. doi: 10.1186/1476-069X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyoshi Y, Noguchi S. Polymorphisms of estrogen synthesizing and metabolizing genes and breast cancer risk in Japanese women. Biomed Pharmacother. 2003;57:471–81. doi: 10.1016/j.biopha.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Moysich KB, Shields PG, Freudenheim JL, Schisterman EF, Vena JE, Kostyniak P, et al. Polychlorinated biphenyls, cytochrome P4501A1 polymorphism, and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:41–4. [PubMed] [Google Scholar]
- 35.Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(Suppl 12):2667–711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Pei J. Factors influencing the association between CYP17 T34C polymorphism and the risk of breast cancer: meta-regression and subgroup analysis. Breast Cancer Res Treat. 2010;122:471–81. doi: 10.1007/s10549-009-0690-9. [DOI] [PubMed] [Google Scholar]
- 37.Ma X, Qi X, Chen C, Lin H, Xiong H, Li Y, et al. Association between CYP19 polymorphisms and breast cancer risk: results from 10,592 cases and 11,720 controls. Breast Cancer Res Treat. 2010;122:495–501. doi: 10.1007/s10549-009-0693-6. [DOI] [PubMed] [Google Scholar]
- 38.Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–48. [PubMed] [Google Scholar]
- 39.Guengerich FP. Cytochromes P450, drugs, and diseases. Mol Interv. 2003;3:194–204. doi: 10.1124/mi.3.4.194. [DOI] [PubMed] [Google Scholar]
- 40.Yadav S, Singhal NK, Singh V, Rastogi N, Srivastava PK, Singh MP. Association of single nucleotide polymorphisms in CYP1B1 and COMT genes with breast cancer susceptibility in Indian women. Dis Markers. 2009;27:203–10. doi: 10.3233/DMA-2009-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao L, Fang F, Wu Q, Zhong Y, Yu L. No association between CYP1B1 Val432Leu polymorphism and breast cancer risk: a meta-analysis involving 40,303 subjects. Breast Cancer Res Treat. 2010;122:237–42. doi: 10.1007/s10549-009-0689-2. [DOI] [PubMed] [Google Scholar]
- 42.Economopoulos KP, Sergentanis TN. Does race modify the association between CYP1B1 Val432Leu polymorphism and breast cancer risk? A critical appraisal of a recent meta-analysis. Breast Cancer Res Treat. 2010;124:293–4. doi: 10.1007/s10549-010-1097-3. [DOI] [PubMed] [Google Scholar]
- 43.Maclennan M, Ma DW. Role of dietary fatty acids in mammary gland development and breast cancer. Breast Cancer Res. 2010;12:211. doi: 10.1186/bcr2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young TK, Bjerregaard P, Dewailly E, Risica PM, Jørgensen ME, Ebbesson SE. Prevalence of obesity and its metabolic correlates among the circumpolar inuit in 3 countries. Am J Public Health. 2007;97:691–5. doi: 10.2105/AJPH.2005.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lautenbach A, Budde A, Wrann CD, Teichmann B, Vieten G, Karl T, et al. Obesity and the associated mediators leptin, estrogen and IGF-I enhance the cell proliferation and early tumorigenesis of breast cancer cells. Nutr Cancer. 2009;61:484–91. doi: 10.1080/01635580802610115. [DOI] [PubMed] [Google Scholar]
- 46.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166:2395–402. doi: 10.1001/archinte.166.21.2395. [DOI] [PubMed] [Google Scholar]
- 47.Barnard RJ, Gonzalez JH, Liva ME, Ngo TH. Effects of a low-fat, high-fiber diet and exercise program on breast cancer risk factors in vivo and tumor cell growth and apoptosis in vitro. Nutr Cancer. 2006;55:28–34. doi: 10.1207/s15327914nc5501_4. [DOI] [PubMed] [Google Scholar]
- 48.Goodman MT, Nomura AM, Wilkens LR, Hankin J. The association of diet, obesity, and breast cancer in Hawaii. Cancer Epidemiol Biomarkers Prev. 1992;1:269–75. [PubMed] [Google Scholar]
- 49.Haslam SZ, Schwartz RC. Is there a link between a high-fat diet during puberty and breast cancer risk? Womens Health (Lond Engl) 2011;7:1–3. doi: 10.2217/whe.10.83. [DOI] [PubMed] [Google Scholar]
- 50.Brody JG, Rudel RA, Michels KB, Moysich KB, Bernstein L, Attfield KR, et al. Environmental pollutants, diet, physical activity, body size, and breast cancer: where do we stand in research to identify opportunities for prevention? Cancer. 2007;109(Suppl 12):2627–34. doi: 10.1002/cncr.22656. [DOI] [PubMed] [Google Scholar]
- 51.Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst. 1994;86:1403–8. doi: 10.1093/jnci/86.18.1403. [DOI] [PubMed] [Google Scholar]
- 52.Theroux R. Breast cancer menopause: do weight and exercise affect risk? Nurse Women’s. Health. 2007;11:319–21. doi: 10.1111/j.1751-486X.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki R, Iwasaki M, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, et al. Leisure-time physical activity and breast cancer risk by hormone receptor status: effective life periods and exercise intensity. Cancer Causes Control. 2010;21:1787–98. doi: 10.1007/s10552-010-9605-7. [DOI] [PubMed] [Google Scholar]
- 54.Bernstein L, Patel AV, Ursin G, Sullivan-Halley J, Press MF, Deapen D, et al. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst. 2005;97:1671–9. doi: 10.1093/jnci/dji374. [DOI] [PubMed] [Google Scholar]
- 55.Hirose K, Hamajima N, Takezaki T, Miura S, Tajima K. Physical exercise reduces risk of breast cancer in Japanese women. Cancer Sci. 2003;94:193–9. doi: 10.1111/j.1349-7006.2003.tb01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagata C, Mizoue T, Tanaka K, Tsuji I, Wakai K, Inoue M, et al. Alcohol drinking and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2007;37:568–74. doi: 10.1093/jjco/hym062. [DOI] [PubMed] [Google Scholar]
- 57.Boyle P, Boffetta P. Alcohol consumption and breast cancer risk. Breast Cancer Res. 2009;11(Suppl 3):S3. doi: 10.1186/bcr2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beasley JM, Coronado GD, Livaudais J, Angeles-Llerenas A, Ortega-Olvera C, Romieu I, et al. Alcohol and risk of breast cancer in Mexican women. Cancer Causes Control. 2010;21:863–70. doi: 10.1007/s10552-010-9513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawai M, Minami Y, Kakizaki M, Kakugawa Y, Nishino Y, Fukao A, et al. Alcohol consumption and breast cancer risk in Japanese women: The Miyagi Cohort Study. Breast Cancer Res Treat. 2011;128:817–25. doi: 10.1007/s10549-011-1381-x. [DOI] [PubMed] [Google Scholar]
- 60.Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010;102:1422–31. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dennis J, Ghadirian P, Little J, Lubinski J, Gronwald J, Kim-Sing C, et al. Alcohol consumption and the risk of breast cancer among BRCA1 and BRCA2 mutation carriers. Breast. 2010;19:479–83. doi: 10.1016/j.breast.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danmarks Statistik. Copenhagen: Danmarks Statistik; 2010. NYT Fra Danmarks Statistik Nr. 309, 2. Juli 2010. [cited 2012 Aug 1]. Available from: http://www.dst.dk/da/statistik/nyt/relateret.aspx?psi=1168. [Google Scholar]
- 63.Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol. 1990;162:502–14. doi: 10.1016/0002-9378(90)90420-c. [DOI] [PubMed] [Google Scholar]
- 64.Band PR, Le ND, Fang R, Deschamps M. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360:1044–9. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- 65.John EM, Kelsey JL. Radiation and other environmental exposures and breast cancer. Epidemiol Rev. 1993;15:157–62. doi: 10.1093/oxfordjournals.epirev.a036099. [DOI] [PubMed] [Google Scholar]
- 66.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 67.Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med. 2011;171:125–33. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishibe N, Hankinson SE, Colditz GA, Spiegelman D, Willett WC, Speizer FE, et al. Cigarette smoking, cytochrome P450 1A1 polymorphisms, and breast cancer risk in the nurses’ health study. Cancer Res. 1998;58:667–71. [PubMed] [Google Scholar]
- 69.Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92:167–75. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medina D. Breast cancer: the protective effect of pregnancy. Clin Cancer Res. 2004;10(1 Pt 2):380S–4S. doi: 10.1158/1078-0432.ccr-031211. [DOI] [PubMed] [Google Scholar]
- 71.Thalib L, Doi SA, Hall P. Multiple births and breast cancer prognosis: a population based study. Eur J Epidemiol. 2005;20:613–7. doi: 10.1007/s10654-005-5530-6. [DOI] [PubMed] [Google Scholar]
- 72.Albrektsen G, Heuch I, Kvåle G. The short-term and long-term effect of a pregnancy on breast cancer risk: a prospective study of 802,457 parous Norwegian women. Br J Cancer. 1995;72:480–4. doi: 10.1038/bjc.1995.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pathak DR. Dual effect of first full term pregnancy on breast cancer risk: empirical evidence and postulated underlying biology. Cancer Causes Control. 2002;13:295–8. doi: 10.1023/a:1015282916368. [DOI] [PubMed] [Google Scholar]
- 74.Archibald L. Ottawa: Pauktuutit Inuit Women's Association; 2004. Teenage pregnancy in Inuit Communities: issues and perspectives. cited 2012 Aug 2]. Available from: http://pauktuutit.ca/pdf/publications/pauktuutit/TeenPregnancy_e.pdf. [Google Scholar]
- 75.Jordan I, Hebestreit A, Swai B, Krawinkel MB. Breast cancer risk among women with long-standing lactation and reproductive parameters at low risk level: a case-control study in Northern Tanzania. Breast Cancer Res Treat. 2010 Nov 20; doi: 10.1007/s10549-010-1255-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.Hildes JA, Schaefer O. The changing picture of neoplastic disease in the western and central Canadian Arctic (1950–1980) Can Med Assoc J. 1984;130:25–32. [PMC free article] [PubMed] [Google Scholar]
- 77.Stuebe AM, Willett WC, Xue F, Michels KB. Lactation and incidence of premenopausal breast cancer: a longitudinal study. Arch Intern Med. 2009;169:1364–71. doi: 10.1001/archinternmed.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X, Dailey AB, Peoples-Sheps M, Talbott EO, Li N, Roth J. Birth weight as a risk factor for breast cancer: a meta-analysis of 18 epidemiological studies. J Womens Health (Larchmt) 2009;18:1169–78. doi: 10.1089/jwh.2008.1034. [DOI] [PubMed] [Google Scholar]
- 79.Deardorff J, Ekwaru JP, Kushi LH, Ellis BJ, Greenspan LC, Mirabedi A, et al. Father absence, body mass index, and pubertal timing in girls: differential effects by family income and ethnicity. J Adolesc Health. 2011;48:441–7. doi: 10.1016/j.jadohealth.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buyken AE, Karaolis-Danckert N, Remer T. Association of prepubertal body composition in healthy girls and boys with the timing of early and late pubertal markers. Am J Clin Nutr. 2009;89:221–30. doi: 10.3945/ajcn.2008.26733. [DOI] [PubMed] [Google Scholar]
- 81.Romundstad PR, Vatten LJ, Nilsen TI, Holmen TL, Hsieh CC, Trichopoulos D, et al. Birth size in relation to age at menarche and adolescent body size: implications for breast cancer risk. Int J Cancer. 2003;105:400–3. doi: 10.1002/ijc.11103. [DOI] [PubMed] [Google Scholar]
- 82.Lujan Irastorza JE, Garcia Rodriguez F, Figueroa Preciado G, Hernandez Marin I, Ayala AR. [Early menarche as a risk factor of breast cancer] Ginecol Obstet Mex. 2006;74:568–72. [In Spanish] [PubMed] [Google Scholar]
- 83.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–27. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 84.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46:2196–205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 85.Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol. 2011;347:55–60. doi: 10.1016/j.mce.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 86.Qureshi SA, Couto E, Hilsen M, Hofvind S, Wu AH, Ursin G. Mammographic density and intake of selected nutrients and vitamins in Norwegian women. Nutr Cancer. 2011;63:1011–20. doi: 10.1080/01635581.2011.605983. [DOI] [PubMed] [Google Scholar]
- 87.Gueli N, Verrusio W, Linguanti A, Di Maio F, Martinez A, Marigliano B, et al. Vitamin D: drug of the future. A new therapeutic approach. Arch Gerontol Geriatr. 2012;54:222–7. doi: 10.1016/j.archger.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–8. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 89.Grant WB, Juzeniene A, Lagunova Z, Porojnicu AC, Moan JE. Vitamin D levels in Norway may be inadequate to reduce risk of breast cancer. Int J Cancer. 2011;128:2249–50. doi: 10.1002/ijc.25552. [DOI] [PubMed] [Google Scholar]
- 90.El Hayek J, Egeland G, Weiler H. Vitamin D status of Inuit preschoolers reflects season and vitamin D intake. J Nutr. 2010;140:1839–45. doi: 10.3945/jn.110.124644. [DOI] [PubMed] [Google Scholar]
- 91.Bonefeld-Jorgensen EC. Biomonitoring in Greenland: human biomarkers of exposure and effects – a short review. Rural Remote Health. 2010;10:1362. [PubMed] [Google Scholar]
- 92.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–4. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 93.Donaldson SG, Van Oostdam J, Tikhonov C, Feeley M, Armstrong B, Ayotte P, et al. Environmental contaminants and human health in the Canadian Arctic. Sci Total Environ. 2010;408:5165–234. doi: 10.1016/j.scitotenv.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 94.Long M, Bossi R, Bonefeld-Jørgensen EC. Level and temporal trend of perfluoroalkyl acids in Greenlandic Inuit. Int J Circumpolar Health. 2012;71:17998. doi: 10.3402/ijch.v71i0.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Mølbak K, Weihe P, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, et al. Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation. Environ Health Perspect. 2006;114:100–5. doi: 10.1289/ehp.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilman A, Ayotte P, Dewailly E, Dudarev A, Bonefeld-Jørgensen EC, Muckle G, et al. The International Arctic Monitoring and Assessment Report (AMAP), Human health in the Arctic 2009. Oslo: Arctic Monitoring and Assessment Programme (AMAP); 2009. Chapter 8. Public Health and the Effects of Contaminants; pp. 143–90. [cited 2012 Apr 15] Available from: http://www.amap.no. [Google Scholar]
- 98.Golden R, Kimbrough R. Weight of evidence evaluation of potential human cancer risks from exposure to polychlorinated biphenyls: an update based on studies published since 2003. Crit Rev Toxicol. 2009;39:299–331. doi: 10.1080/10408440802291521. [DOI] [PubMed] [Google Scholar]
- 99.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(Suppl 6):S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 100.Schreer I. Dense breast tissue as an important risk factor for breast cancer and implications for early detection. Breast Care (Basel) 2009;4:89–92. doi: 10.1159/000211954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roubidoux MA, Kaur JS, Griffith KA, Stillwater B, Novotny P, Sloan J. Relationship of mammographic parenchymal patterns to breast cancer risk factors and smoking in Alaska Native women. Cancer Epidemiol Biomarkers Prev. 2003;12:1081–6. [PubMed] [Google Scholar]
- 102.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 103.Mulero-Navarro S, Pozo-Guisado E, Perez-Mancera PA, Alvarez-Barrientos A, Catalina-Fernandez I, Hernandez-Nieto E, et al. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J Biol Chem. 2005;280(31):28731–41. doi: 10.1074/jbc.M504538200. [DOI] [PubMed] [Google Scholar]
- 104.Yoshizawa K, Heatherly A, Malarkey DE, Walker NJ, Nyska A. A critical comparison of murine pathology and epidemiological data of TCDD, PCB126, and PeCDF. Toxicol Pathol. 2007;35:865–79. doi: 10.1080/01926230701618516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–8. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]