Abstract
Basal bodies are freed from cilia and transition into centrioles to organize centrosomes in dividing cells. A mutually exclusive centriole/basal body existence during cell cycle progression has become a widely accepted principle. Contrary to this view, we show here that cilia assemble and persist through two meiotic divisions in Drosophila spermatocytes. Remarkably, all four centrioles assemble primary cilia-centriole complexes that transit from the plasma membrane encased in a packet of membrane, recruit centrosomal material into microtubule-organizing centers, and persist at the spindle poles through division. Thus, spermatocyte centrioles organize centrosomes and cilia simultaneously at cell division. These findings challenge the prevailing view that cilia antagonize cell cycle progression, and raise the possibility that cilium retention at cell division may occur in diverse organisms and cell types.
Introduction
The elegant nine-fold radially symmetric design of the centriole is truly one of nature’s aesthetic wonders. Centrioles are found at the core of microtubule-organizing centers in most animal cells. Despite their requirement for the spatial organization of the centrosomal material to nucleate cytoplasmic and spindle microtubules, centrioles are dispensable for somatic cell division where acentrosomal pathways of microtubule organization can suffice (Azimzadeh and Marshall, 2010; Debec et al., 2010). Centrioles have an additional, and perhaps more critical function: as templates for cilia and flagella, a context in which they are called basal bodies. The ability of centrioles to function both as centrosomal organizers at the spindle poles, and also as basal bodies to template primary cilia, was originally proposed in the Henneguy-Lenhosseck hypothesis (1898), which predicted the functional equivalence of these organelles (Bloodgood, 2009).
The cilium is an essential antenna-like projection that consists of the microtubule-based axoneme and a surrounding membrane that is continuous but distinct from the cell’s plasma membrane. The axoneme is the structural backbone of the cilium and mirrors the nine-fold radial symmetry conveyed by the basal body (Riparbelli et al., 2009b). Generally, motile cilia contain a pair of axial singlet microtubules at the center of the axoneme surrounded by the nine doublet microtubules of the axoneme: a “9+2” structure. Non-motile primary cilia lack the central pair (“9+0”). Primary cilia are sensory organelles for intercellular signalling involving hedgehog, Wnt and PDGF pathways. Defects in cilia have pleiotropic effects on development and physiology, depending on the molecules affected and the alleles involved, and lesions in affected human genes are collectively called ciliopathies (Hildebrandt et al., 2011).
Centrioles have a double life: as centrioles within the centrosomes, and as basal bodies at the cell membrane in differentiated or resting cells (Bettencourt-Dias and Carvalho-Santos, 2008; Kobayashi and Dynlacht, 2011). In cycling cells, centrioles are found within the centrosomal material during cell division, but then move to the cell periphery in quiescent cells to nucleate a ciliary axoneme after docking at the plasma membrane. Upon cell cycle entry the centriole is released from the axoneme and/or the cilium resorbs, and then the centriole migrates into the cytoplasm, and recruits pericentriolar material to organize the centrosome. Although cell-cycle dependent cilium assembly and disassembly is typical of vertebrate cells, it remains unclear how this process is executed and regulated. The switch between centriole and basal body is accompanied by morphological and molecular changes (for reviews see (Bettencourt-Dias and Carvalho-Santos, 2008; Ishikawa and Marshall, 2011; Kobayashi and Dynlacht, 2011). Moreover, there is considerable evidence that the presence of a cilium is incompatible with cell division, and that cells will not divide until their centrioles are freed from cilia (Kim and Tsiokas, 2011; Kobayashi and Dynlacht, 2011; Santos and Reiter, 2008; Seeley and Nachury, 2010). It is generally assumed that the centriole cannot function simultaneously to assemble cilia and mitotic centrosomes. The vast majority of studies of cilium dynamics in the cell cycle were done in cell culture.
Contrasting evidence suggested that cilia and flagella might be maintained at cell division. It was reported that “flagellar” structures were maintained during meiotic divisions of some Lepidopteran (Friedlander and Wahrman, 1970; Henneguy, 1898; Meves, 1903; Yamashiki and Kawamura, 1998), Neuropteran (Friedlander, 1980), and Dipteran (Fritz-Niggli, 1972; LaFountain, 1976) species. Cilia and flagella may also be retained during the division of some protozoa (Kirk, 1998). The seminal EM work by Tates showed the detailed changes that centrioles undergo during spermatogenesis, and in retrospect revealed the presence of short cilia in spermatocytes during division (Tates, 1971).
Here, we investigated the timing and ultrastructure of cilium biogenesis during Drosophila spermatogenesis using molecular markers for basal bodies and cilia, coupled with unequivocal ultrastructural imaging using electron microscopy (EM). We show that cilia assemble in premeiotic prophase, and remain intact through two rounds of cell division in vivo in spermatocytes, a cell type with a discrete developmental program involving two rounds of division followed by differentiation.
Results
During male gametogenesis in Drosophila melanogaster (Fuller, 1993), the germline stem cells divide repeatedly to produce spermatogonial cells. Each spermatogonium undergoes four mitotic divisions to produce a cyst of 16 interconnected spermatocytes. Spermatocytes then embark on a terminal differentiation program involving two meiotic divisions followed by spermatid differentiation. Centrioles duplicate in early “polar spermatocytes” and then both centriole pairs transit to the plasma membrane at the “apolar” stage where they remain through the “mature” stage until just prior to metaphase (Tates, 1971). These stages encompass an extended growth period (~90 hr) during G2 phase, most often referred to as meiotic prophase, during which time the centrioles elongate 10-fold. The two meiotic divisions produce 64 haploid spermatids that differentiate into elongated sperm cells with exceptionally long flagella approximately 1.8 mm long.
Centrioles are not required for most of Drosophila development (Basto et al., 2006). Germ stem cells and spermatogonia do not require centrosomes to organize a functional mitotic spindle and divide. Spermatocytes, however, fail to successfully undergo cytokinesis in the absence of centrioles (Riparbelli and Callaini, 2011).
Spermatocytes assemble cilia from all four prophase centrioles
To define the assembly of cilia during spermatogenesis, we examined the recruitment of cilium-specific proteins to centrioles. First, we examined the dynamics of uncoordinated (UNC), a potential ortholog of orofaciodigital syndrome 1 (Ofd1, MIM 311200) (Baker et al., 2004), a distal centriole protein required for proper cilium formation and whose gene is mutated in OFD1 ciliopathy (Singla et al., 2010). UNC is involved in centriole-to-basal body conversion, and localizes just distal to the basal body (Baker et al., 2004; Ma and Jarman, 2011). We examined expression of UNC-GFP fusion protein, to track the onset of cilium assembly.
In agreement with previous studies (Baker et al., 2004), UNC-GFP was not detected at centrioles in germ line stem cells or other early stages (Figure 1Aa–c), but first appeared on centrioles in the youngest apolar spermatocytes (G2 phase) (Figure 1A and S1). Co-staining for centriolar markers revealed that UNC-GFP localized predominantly to the distal ends of each pair of orthogonally-arranged centrioles in spermatocytes (Figures 1 and S1). As spermatocytes mature and centrioles elongate, UNC-GFP persists at the distal tips of all four centrioles (Figure 1B and S1). These data show that UNC, a basal body protein, is localized to the distal tips of all four centrioles in late-stage spermatocytes, indicating that cilium assembly occurs on all four membrane-docked centrioles in these cycling cells concurrent with centriole elongation.
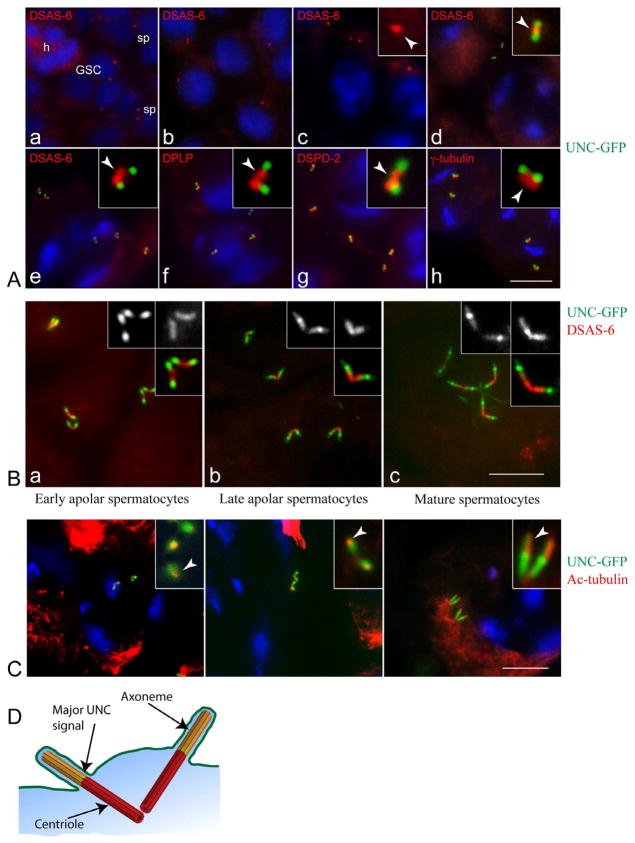
Figure 1. Primary cilia assemble in post-S phase spermatocytes.
(A) UNC-GFP is recruited to post-replicative centrioles in early spermatocytes. Centrioles are labelled red by immunostaining for DSAS-6 in panels (a–e), DPLP (pericentrin-like protein) in (f), DSPD-2 in (g) and γ-tubulin in (h); DNA is blue. (a) The stem cell niche at the apical tip of the pupal testis: h, hub; GSC, germline stem cells; sp, spermatogonia. (b) Early primary spermatocytes. (c) Polar spermatocytes (G2 phase): only the DSAS-6 labelling is detected (arrowhead in inset). (d–h) Young apolar spermatocytes: UNC-GFP localizes to distal ends of short centriole pairs; centriole and centrosome markers do not overlap with UNC-GFP (arrowheads in insets d–h).
(B). UNC-GFP persists in elongating centrioles during premeiotic prophase. Localization of UNC-GFP (green) in early apolar spermatocytes (a), later apolar spermatocytes (b) and mature primary spermatocytes (c) stained with DSAS-6 (red). Monochrome insets show UNC-GFP (left) and DSAS-6 (right).
(C). Axonemal microtubules project distally from the UNC-GFP compartment. UNC-GFP (green) and acetylated-tubulin (red) in growing primary spermatocytes. DNA is blue. A small spot of acetylated-tubulin overlaps the distal UNC-GFP signal in young (a, arrow in inset) and later (b, arrow in inset) apolar spermatocytes. In mature spermatocytes acetylated-tubulin marks elongated cilia (c, arrow in inset).
(D) Cartoon depicting one of the two pairs of mature spermatocyte cilia. Centriole, red; cilium, yellow; transition zone, green.
See also Figure S1. Scale bar = 5 μm in (A) and (B); 5.5 μm in (C).
To assess whether the structures at the distal tips of spermatocyte centrioles are cilia, we examined the pattern of acetylated α-tubulin (ac-tub), a modified form of stable tubulin that is concentrated in axonemal microtubules in Drosophila (Piperno and Fuller, 1985). Antibodies specific for acetylated tubulin mark the distal tips of centrioles during all stages of spermatocytes following recruitment of UNC-GFP (Figure 1C). Ac-tub was detected at the dot-like centriole pairs when they resided near the nucleus of young polar spermatocytes (not shown). By contrast, when the centrioles had moved to the cell periphery, a small dot of ac-tub appeared beyond their distal ends (Figure 1Ca). As the centrioles elongated toward the end of prophase, the ac-tub labelling also increased (Figure 1Cc). Moreover, the signal for ac-tub localizes adjacent but distal to UNC-GFP. UNC localization therefore straddles the centriole-cilium boundary, consistent with UNC localization at the distal centriole near the transition zone. These data indicate that cilia assemble from all four membrane-docked centrioles in spermatocytes (Figure 1D).
Altogether, the patterns of UNC-GFP and ac-tub distribution indicate that cilia assemble at the distal ends of all four centrioles in post-S phase spermatocytes. To corroborate these findings, we next sought definitive ultrastructural evidence of cilia in dividing spermatocytes using EM.
In young apolar spermatocytes, when the centrioles have docked at the membrane and are beginning to elongate, EM reveals short axonemes projecting into bulges at the cell membrane (Figures 2a and S2). As centrioles elongate in growing spermatocytes, the cilium also elongates (Figure 2). These structures are consistent with the findings of Tates, who described these centrioles and referred to the axonemes as “distal centrioles” (Tates, 1971). In young spermatocytes centrioles are short with a canonical 9-fold symmetry, composed of nine triplet microtubules and a central cartwheel (Riparbelli et al., 2009a). The centriole maintains the cartwheel throughout spermatogenesis, unlike most cells where it is only present in immature centrioles. The ultrastructure of the centriole remains largely unchanged during its growth in the extended spermatocyte G2 phase (see Figs. 2 and 3).
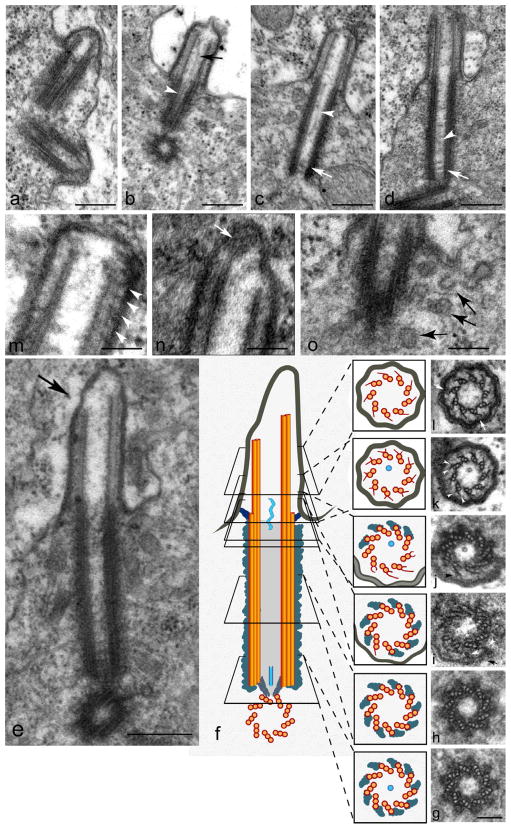
Figure 2. Centrioles and cilia elongate in growing spermatocytes.
Ultrastructure of the centriole-primary cilium complex. (a) Orthogonal centriole pair at the beginning of primary cilia formation. (b,c,d,) Variable presence of tubule-like structure in growing cilia within the lumen of the axoneme (arrow) and the centriole (white arrowhead); see also (j) and (k). The central tubule appears to meander along the length of the lumen, and is not fastened centrally. White arrows in (c) and (d) indicate three dense transverse rows in the basal centriole lumen that may be remnants of the cartwheel. (e) Longitudinal section at the end of prophase: note the asymmetric extension of the doublets at the ciliary tip (arrow). (f) Schematic of a cilium like that in (e) aligned with serial cross sections (g–l). Arrowheads in (i) point to fibrous material connecting microtubule triplets to the ciliary membrane (arrow) near the transition zone. (j) Transition zone: note that the triplets that have lost the C tubule develop radial projections (arrowhead). Basal (k) and distal (l) regions of the ciliary axoneme: Y-shaped radial projections (arrowheads) originate from the B-tubule of each doublet and contact small clusters of moderately dense material at the ciliary membrane (arrow). (m) Longitudinal section of a primary cilium showing regularly spaced clusters of electron-dense material (arrowheads) positioned between the doublets and the plasma membrane. (n) Apical tip of a cilium like that in (e) where the more elongated microtubules end in a granular cluster at the tip (arrow). (o) The presence of many vesicles (arrows) at the basal region of a cilium. Scale bar = 300 nm in a–e; 100nm in g–n, 100 nm in l,m; 150 nm in o. See also Figure S2.
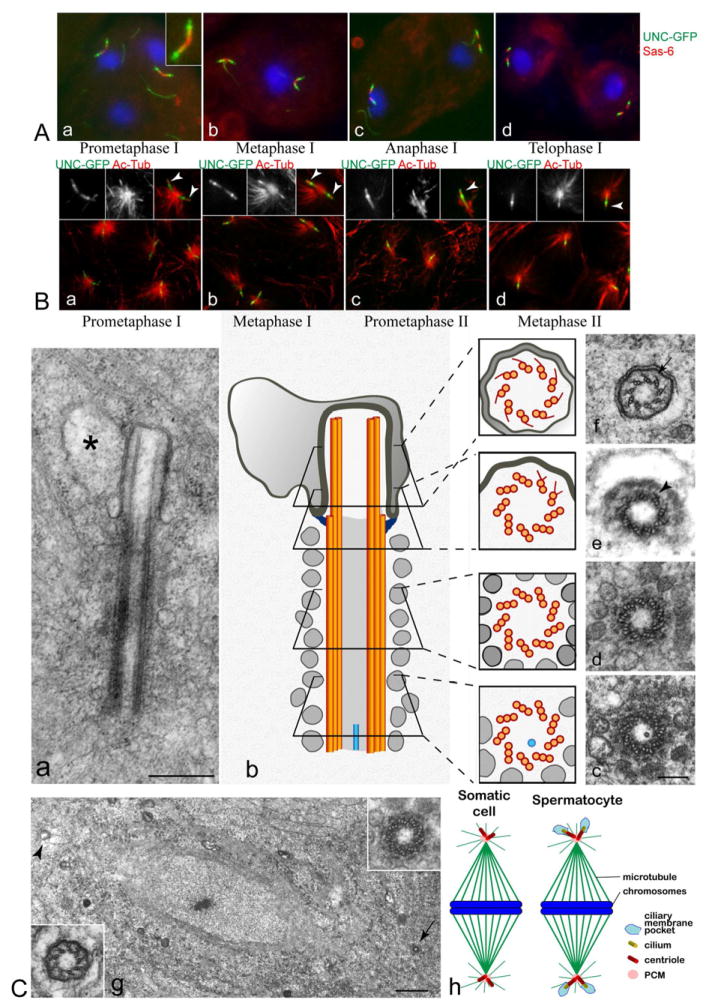
Figure 3. Cilia persist through two meiotic divisions.
(A) UNC-GFP remains at centrioles during two meiotic divisions. Colocalization of UNC-GFP (green) and DSAS-6 (red) in dividing spermatocytes. DNA is blue. The pattern of UNC-GFP localization is unchanged during spindle assembly and cell division at meiosis I and II (a–d, and Figure S3A), even after centrioles disengage in meiosis I (d).
(B). Distal acetylated-tubulin persists during the meiotic divisions. Acetylated-tubulin overlaps with distal UNC-GFP localization, and also extends more distally (insets a–d). UNC-GFP is green, acetylated-tubulin is red, DNA is blue. Acetylated tubulin signals at cilia are labelled with arrows in insets.
(C) Ultrastructure of cilia in dividing spermatocytes.
Anaphase of the second meiosis (a): the ciliary complex is inside the cytoplasm and the primary cilium is contained in a cytoplasmic membrane-bound pocket (asterisk) (See also Figure S3Bd). (b) Schematic of a cilium like that in (a) aligned with serial cross sections (c–f). Lateral projections are found in both the transition region (arrowhead, in e) and within the axoneme (arrow in f). (g) Low magnification of a metaphase spindle during the second meiotic division showing two opposite poles in which a centriole (arrow, and right inset) and a primary cilium (arrowhead, and left inset) are seen in cross sections. (h) Model showing four cilia participating in spindle assembly in a primary spermatocyte. Scale bar = 400 nm in e; 150 nm in c–f; 1 μm in g, and 200 nm in insets. See also Figure S3.
Structural features of spermatocyte cilia
Each centriole pair was positioned orthogonally and nucleated axonemes concurrently (Figures 2a and 1D) that increased in length during prophase progression (Figure 2b–d). At the end of prophase, cilia were composed of a long centriole (basal body) and a short axoneme (Figure 2d). These cilia appear identical to the “distal centriole” structures shown by Tates (Tates, 1971). Longitudinal sections often revealed an asymmetric extension of the axonemal apical microtubules at the end of prophase (Figure 2e). Although the centriole elongated during prophase it retained its structural features: the nine microtubule triplets were still immersed in a dense material that projected laterally (Figure 2g,h) and a cartwheel was still visible (Figure 2g).
Mother and daughter centrioles are easily distinguishable in vertebrates (Azimzadeh and Marshall, 2010), but this is not the case in Drosophila (Callaini et al., 1997). However, despite the absence of ultrastructural distinctions between centrioles by EM, a subtle asymmetric distribution of the centriole biogenesis factor Ana2 on centriole pairs (Stevens et al., 2010) and the daughter centriole-specific localization of centrobin (Januschke et al., 2011) establishes molecular distinctions associated with centriole maturation. While only mother centrioles are known to assemble primary cilia in vertebrate cells, here we show that both mothers and both daughters assemble cilia in G2 spermatocytes. This is not a general rule in Drosophila, because ciliated neurons assemble only one cilium from the centriole pair, presumably from the mother.
Together, the above data show that all four Drosophila spermatocyte centrioles assemble and maintain cilia in G2/prophase. The prevailing view is that primary cilia assemble in cells in G1/G0, which have exited the cell cycle, and that resorption of the primary cilium is a necessary prerequisite for re-entry into the cell cycle (Kim and Tsiokas, 2011; Kobayashi and Dynlacht, 2011). To examine if this cilium restriction to cell division is present, we next examined spermatocytes as they proceeded through two divisions.
Persistence of cilia in dividing spermatocytes
UNC-GFP localization at distal centrioles remained unchanged from prophase to telophase of primary spermatocytes and continued throughout the second meiotic division too (Figure 3A,B and S3A). Note that centrioles do not replicate in the second meiotic cycle. Acetylated-tubulin still colocalized with UNC-GFP at distal centrioles during meiotic progression (Figure 3B). However, these centrioles, still marked as basal bodies by UNC-GFP, and containing cilia as indicated by ac-tub, migrated away from the plasma membrane at prometaphase. This suggests that cilia do not resorb or detach from centrioles at the onset of meiotic spindle assembly.
Since cilia typically do not persist at mitosis, we examined the ultrastructure of centriole complexes by EM (Figure 3C). These images reveal that before the onset of metaphase, when the basal bodies dislodged from the plasma membrane and moved into the cytoplasm, they retained their association with the primary cilium, which remained intact, yet was encased in a membrane pocket as it transited through the cytoplasm as a basal body-cilium complex (Figures 3Ca,b and S3B). This cilium-membrane pocket complex was also seen by Tates, who interpreted it as invaginated plasma membrane (Tates, 1971). Thus, during progression through the first meiotic division (Figure 3Cb) and into meiosis II (Figure 3Ca–f) the primary cilia were embedded in a membrane-bound pocket deep within the cell cytoplasm while the centrioles were engaged at the poles to organize meiotic spindle microtubules (Figure 3Cg and h). The general architecture of the centriole (Figure 3Cc,d) and the gross morphology of the ciliary axoneme (Figure 3Ce,f) experienced no evident changes during these developmental stages. These data show that cilia can persist during two complete rounds of cell division, and that the double life of centrioles is not necessarily mutually exclusive.
Centrosome function is essential for spermatocyte division (Riparbelli and Callaini, 2011), but it is unclear if cilia are necessary. Mutations in Klp64D/Kif3A, an anterograde kinesin motor protein required for cilium assembly, and unc did not impede spermatocyte division, consistent with previous reports (Baker et al., 2004; Sarpal et al., 2003). However, the structure of cilia was partially disrupted in unc2 mutant spermatocytes, but showed no apparent disruption in the Klp64D mutant (Figure S3). Therefore, we cannot exclude a requirement for cilia in spermatocyte division.
Discussion
Here we show that Drosophila spermatocytes have primary cilia, and that the cilia in these cells are retained during cell division. These findings challenge the principle that cilia are inconsistent with progression through the cell cycle, and provide a system to study this regulation. Remarkably, rather than dissociating basal bodies from the cilia, spermatocyte basal body-cilia complexes are internalized with an associated “pocket” of plasma membrane as their centrioles assemble PCM into centrosomes that participate in spindle assembly. The detailed dynamic changes in the centriole-cilium structures from spermatocytes to spermatid (summarized in Figure 4) will serve as vital features for future analysis of cilium regulation during cell division.
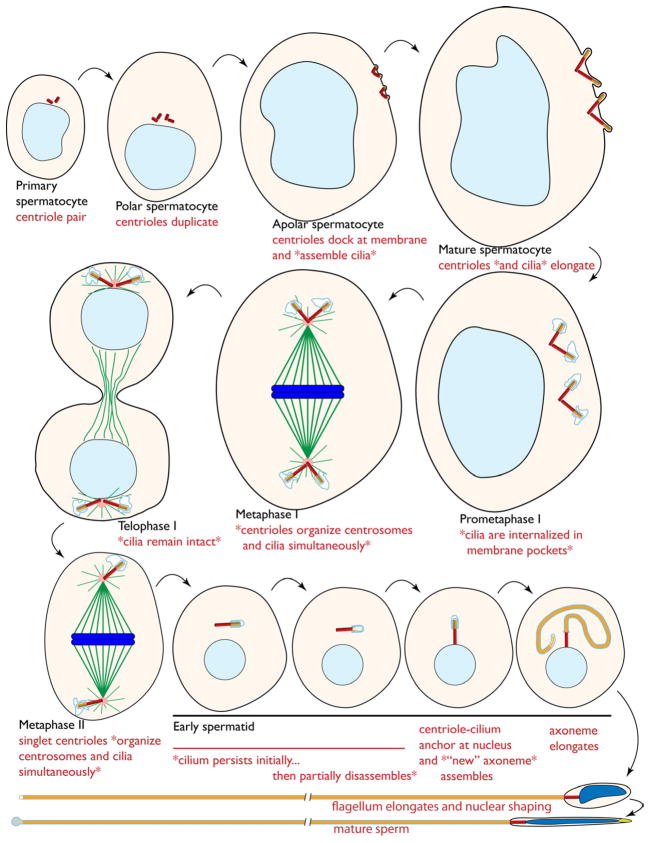
Figure 4. Model for primary cilium dynamics during spermatogenesis.
Cartoon depicting the stages of spermatogenesis from the very early spermatocyte to the mature sperm. Nuclei are shown in shades of blue, centrioles are red, axonemes are yellow, microtubules are green, centrosome PCM is pink, and ciliary membrane is light blue. The features relevant to this study are described in red font with asterisks (*) surrounding the findings reported here. Centrioles are not drawn to scale.
Our results clearly define two opposite scenarios in cilia biogenesis: 1) that of most known animal cells in which the primary cilium dynamics appear governed by cell cycle and are exclusive of dividing cells, and 2) that of Drosophila spermatocytes in which the primary cilium is stable through meiotic divisions. These observations shift the general point of view that the centriole must be freed from the cilium to organize a functional centrosome for spindle organization, and that dividing cells are unable to form primary cilia (Kim and Tsiokas, 2011; Kobayashi and Dynlacht, 2011). Nevertheless, the ability of spermatocytes to assemble cilia and then maintain this structure at the spindle poles during division could be a specialized feature of spermatocytes and perhaps other cell types. On the other hand, this could be a feature of progenitor cells that are programmed to divide a discrete set of rounds before differentiating into a ciliated cell type. There are few such studies performed in vivo to support or counter this possibility at present.
Primary cilia in vertebrate cells are antenna-like projections that detect mechanical and chemical cues from the environment to signal differentiation, proliferation and other responses (Hildebrandt et al., 2011; Ishikawa and Marshall, 2011). Drosophila spermatocytes develop within a cyst that is separated by the environment by a thin cellular envelope. Cytoplasmic bridges that ensure communication and synchrony interconnect the sixteen spermatocytes within the cyst. In this condition the function of primary cilia as sensory organs may be redundant or their role unclear. While centrosomes are essential for accurate division of spermatocytes, the importance or requirement of the cilia for division is unclear. Such a requirement is difficult to test at present, as there are no known mutants that completely disrupt spermatocyte cilia that also leave the centrioles intact.
A surprising property of spermatocyte cilia is their independence from IFT for their assembly. This could therefore be a special class of cilia, similar to the sperm flagellum with its independence on IFT for assembly. However, while the spermatid axoneme assembles in the cytoplasm, it is actually encased in membrane that invaginates from the plasma membrane to form a 5–10 μm long cilium which retains this length at the distal tip of the axoneme as it grows (Tokuyasu, 1975). A requirement on IFT for assembly of this distal cilium has not been examined. On the other hand, the spermatocyte cilium could be an elaborated transition zone, which can assemble independent of IFT (Williams et al., 2011).
Questions on the function of spermatocyte cilia are also raised by some of their peculiarities. Spermatocyte cilia are nucleated by all four centrioles, whereas in vertebrate cells only the mother centriole is able to do this. Vertebrate cells assemble a single primary cilium during G1 phase, but Drosophila spermatocytes assemble cilia during G2 phase. Moreover, the ciliary axonemes are nucleated by centrioles that continue to grow while the cilium grows, whereas in vertebrate cells the mother centriole has reached its definitive length at maturity before it organizes the primary cilium. How can the centriole and the cilium grow simultaneously? We can suppose that the cilium elongates by addition of subunits at its distal tip. But how can the centriole elongate if its distal extremity is committed to form the ciliary axoneme? While a necessary function is not demonstrated, spermatocyte cilia could be precursors for building the flagellar axoneme for the sperm, despite axonemal reorganization in early spermatids (data not shown).
Collectively, these results show that cilia are not incompatible with cell division as a general rule. While cilium resorption initiates at cell cycle entry in most cell culture models, it is not clear that disassembly of cilia are necessary for cell division to occur. In spermatocytes, on the other hand, it is possible that a potential cilium block to cell cycle progression is repressed. Alternatively, the assembly of cilia in these cells in G2 phase might be a unique mechanism to circumvent a G1-specific cell cycle block imposed by cilia in quiescent cells. Yet another possibility is that spermatocyte cilia have distinct properties from other primary cilia that are permissive for cell cycle progression. Consistent with this possibility is the finding that these cilia appear to assemble independent of IFT. These intriguing clues provide a unique framework to further probe the interdependence of cilia with cell cycle control.
Materials and Methods
Drosophila strains
The stock containing the UNC-GFP transgene and the unc2 mutant was described in (Baker et al., 2004). The Klp64D alleles were described in (Sarpal et al., 2003). Flies were raised on standard Drosophila medium at 24°C.
Antibodies
We used the following antibodies from Rodriguez-Martins et al., 2007: chicken anti- DSAS-6 (1:1000), rabbit anti-DSPD-2 (1:500), and chicken anti-DPLP (1:1500). Mouse anti-γ-tubulin-GTU88 (1:100) and mouse anti-acetylated tubulin (1:100) were from Sigma-Aldrich. Alexa Fluor 488- and 555- secondary antibodies (1:800) were purchased from Invitrogen.
Immunofluorescence preparations
Testes from third instar larvae and pupae were dissected in phosphate buffered saline (PBS) and placed in a small drop of 5% glycerol in PBS on a glass slide. Testes were squashed under a small cover glass and frozen in liquid nitrogen. After removal of the coverslip the samples were immersed in methanol for 10 min at −20°C.
For localization of microtubules or centriole/centrosomal components, the samples were washed 15 minutes in PBS and incubated for 1 hour in PBS containing 0.1% bovine serum albumin (PBS-BSA) to block non specific staining. The samples were incubated overnight at 4°C with the specific antisera in a humid chamber. After washing in PBS-BSA the samples were incubated for one hour at room temperature with the appropriate secondary antibodies. In all cases DNA was visualized with incubation of 3–4 minutes in Hoechst. Testes were mounted in small drops of 90% glycerol in PBS.
Image acquisition
Images were taken by using an Axio Imager Z1 (Carl Zeiss) microscope using 100X objective, and equipped with an HBO 50-W mercury lamp for epifluorescence and with an AxioCam HR cooled charge-coupled camera (Carl Zeiss). Gray-scale digital images were collected separately and then pseudocolored and merged using Adobe Photoshop 7.0 software (Adobe Systems).
Transmission electron microscopy
Testes dissected in PBS from larvae and pupae were fixed overnight at 4°C in 2.5% glutaraldehyde in PBS. After rinsing 30 minutes in PBS the samples were post-fixed in 1% osmium tetroxide in PBS for 2 hours. After extensive washing in distilled water the samples were dehydrated in a graded series of alcohols, and then embedded in an Epon-Araldite mixture and polymerized at 60°C for 48 hr. Ultrathin sections obtained with a LKB ultratome Nova were stained with uranyl acetate and lead citrate and observed with a Philips CM 10 operating at 80 kV.
Supplementary Material
Highlights.
Cilia assemble and persist in dividing spermatocytes
Cilia-centrosome complexes participate in spindle assembly
All four spermatocyte centrioles assemble cilia during prophase
Cilia within spermatocytes are surrounded by a membrane envelope
Acknowledgments
We thank Monica Bettencourt-Dias, Pedro Machado, Joel Rosenbaum, Robert Bloodgood and Julie Brill for helpful suggestions and sharing unpublished data. We are grateful to Ana Rodrigues-Martins for generously providing antibodies, and Tomer Avidor-Reiss and Maurice Kernan for mutant stocks. Special thanks to Charles Badland for help with graphical figures and David Mercati with confocal microscopy. This work was supported by NIH grant GM068756 to TLM and PRIN2009 to GC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azimzadeh J, Marshall WF. Building the centriole. Current biology. 2010;20:R816–825. doi: 10.1016/j.cub.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JD, Adhikarakunnathu S, Kernan MJ. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development (Cambridge, England) 2004;131:3411–3422. doi: 10.1242/dev.01229. [DOI] [PubMed] [Google Scholar]
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, et al. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Carvalho-Santos Z. Double life of centrioles: CP110 in the spotlight. Trends in cell biology. 2008;18:8–11. doi: 10.1016/j.tcb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Bloodgood RA. From central to rudimentary to primary: the history of an underappreciated organelle whose time has come. The primary cilium. Methods in cell biology. 2009;94:3–52. doi: 10.1016/S0091-679X(08)94001-2. [DOI] [PubMed] [Google Scholar]
- Callaini G, Whitfield WG, Riparbelli MG. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Experimental cell research. 1997;234:183–190. doi: 10.1006/excr.1997.3618. [DOI] [PubMed] [Google Scholar]
- Debec A, Sullivan W, Bettencourt-Dias M. Centrioles: active players or passengers during mitosis? Cell Mol Life Sci. 2010;67:2173–2194. doi: 10.1007/s00018-010-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. The sperm centriole persists during early egg cleavage in the insect Chrysopa carnea (Neuroptera, Chrysopidae) Journal of cell science. 1980;42:221–226. doi: 10.1242/jcs.42.1.221. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Wahrman J. The spindle as a basal body distributor. A study in the meiosis of the male silkworm moth, Bombyx mori. Journal of cell science. 1970;7:65–89. doi: 10.1242/jcs.7.1.65. [DOI] [PubMed] [Google Scholar]
- Fritz-Niggli H. Meiosis and spermatid formation in non-irradiated and irradiated male germ cells in Drosophila melanogaster. Rev Suisse Zool. 1972;(Suppl):245–265. [PubMed] [Google Scholar]
- Fuller MT. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. Spermatogenesis; pp. 71–147. [Google Scholar]
- Henneguy LF. Sur les rapports des cils vibratiles avec les centrosomes. Archives d’Anatomie Microscopique. 1898;1:481–496. [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nature reviews Molecular cell biology. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nat Commun. 2011;2:243. doi: 10.1038/ncomms1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Tsiokas L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle. 2011;10:2683–2690. doi: 10.4161/cc.10.16.17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk DL. Volvox: molecular genetic origins of multicellularity and cellular differentiation. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. The Journal of cell biology. 2011;193:435–444. doi: 10.1083/jcb.201101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFountain JR., Jr Analysis of birefringence and ultrastructure of spindles in primary spermatocytes of Nephrotoma suturalis during anaphase. J Ultrastruct Res. 1976;54:333–346. doi: 10.1016/s0022-5320(76)80020-2. [DOI] [PubMed] [Google Scholar]
- Ma L, Jarman AP. Dilatory is a Drosophila protein related to AZI1 (CEP131) that is located at the ciliary base and required for cilium formation. Journal of cell science. 2011;124:2622–2630. doi: 10.1242/jcs.084798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves F. Ueber oligopyrene und apyrene Spermien und uber ihre Entstehung, nach Beobachtungen an Paludina und Pygaera. Arch Mikrosk Anat. 1903;61:1–84. [Google Scholar]
- Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. The Journal of cell biology. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli MG, Callaini G. Male gametogenesis without centrioles. Developmental biology. 2011;349:427–439. doi: 10.1016/j.ydbio.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Riparbelli MG, Colozza G, Callaini G. Procentriole elongation and recruitment of pericentriolar material are downregulated in cyst cells as they enter quiescence. Journal of cell science. 2009a;122:3613–3618. doi: 10.1242/jcs.049957. [DOI] [PubMed] [Google Scholar]
- Riparbelli MG, Dallai R, Mercati D, Bu Y, Callaini G. Centriole symmetry: a big tale from small organisms. Cell motility and the cytoskeleton. 2009b;66:1100–1105. doi: 10.1002/cm.20417. [DOI] [PubMed] [Google Scholar]
- Santos N, Reiter JF. Building it up and taking it down: the regulation of vertebrate ciliogenesis. Developmental dynamics. 2008;237:1972–1981. doi: 10.1002/dvdy.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, et al. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Current biology. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. Journal of cell science. 2010;123:511–518. doi: 10.1242/jcs.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Developmental cell. 2010;18:410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens NR, Dobbelaere J, Brunk K, Franz A, Raff JW. Drosophila Ana2 is a conserved centriole duplication factor. The Journal of cell biology. 2010;188:313–323. doi: 10.1083/jcb.200910016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tates AD. PhD thesis. Rijksunivrsiteit de Leiden; Netherlands: 1971. Cytodiferentiation during spermatogenesis in Drosophila melanogaster : An electron microscopy study. [Google Scholar]
- Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. VI. Significance of “onion” nebenkern formation. J Ultrastruct Res. 1975;53:93–112. doi: 10.1016/s0022-5320(75)80089-x. [DOI] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. The Journal of cell biology. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiki N, Kawamura N. Behavior of centrioles during meiosis in the male silkworm, Bombyx mori (Lepidoptera) Dev Growth Differ. 1998;40:619–630. doi: 10.1046/j.1440-169x.1998.t01-4-00006.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.