Summary
We show that R-Ras, a small GTPase of the Ras family, is essential for the establishment of mature, functional blood vessels in tumors. The genetic disruption of R-Ras severely impaired the maturation processes of tumor vessels in mice. Conversely, the gain of function of R-Ras improved vessel structure and blood perfusion and blocked plasma leakage by enhanced endothelial barrier function and pericyte association with nascent blood vessels. Thus, R-Ras promotes normalization of the tumor vasculature. These findings identify R-Ras as a critical regulator of vessel integrity and function during tumor vascularization.
INTRODUCTION
Tumor vasculature is structurally and functionally abnormal (Jain, 2005). These vessels are highly permeable, tortuous, dilated and saccular, and poorly covered by mural cells (pericytes). These properties, which are mainly attributed to the impaired maturation process of the tumor vessels, cause inadequate blood circulation and poor oxygenation of the tumors. Although this could slow down tumor growth, it also reduces the sensitivity of the tumors to ionizing radiation and impedes the delivery of chemotherapeutic agents, allowing the tumors to become resistant to cytotoxic therapies (Jain, 2005). The plasma leakage from tumor vessels elevates the interstitial fluid pressure within the tumor and significantly reduces the diffusion of therapeutic agents (Jain, 2005). Cerebral edema caused by leaky vessels is a serious complication in brain cancer. The vessel leakiness also allows invasive tumor cells to penetrate into circulation and facilitates distant metastasis (Weis et al., 2004). The functionality of tumor blood vessels, therefore, critically impacts tumor progression and tumors responses to therapeutic regimens (Bergers and Hanahan, 2008; Carmeliet and Jain, 2011b).
Previous studies suggested that VEGF-targeted cancer therapies lead not only to inhibition of tumor angiogenesis but also to transient maturation and normalization of tumor vasculature, thereby improving delivery of chemotherapeutic compounds, enhancing the efficacy of chemotherapy (Carmeliet and Jain, 2011b). However, recent reports have demonstrated that VEGF-targeted therapies also result in increased tumor invasiveness and distant metastasis in mice, potentially explaining the lack of lasting clinical responses to the current antiangiogenic treatments (Ebos et al., 2009; Paez-Ribes et al., 2009). These studies highlight the importance of a better understanding of the biology of tumor angiogenesis and its implications in tumor malignancy and therapies. The molecular and cellular mechanisms that govern vessel maturation, especially in tumor vasculature, remain elusive at present. The currently held view is that the processes of vessel formation and maturation are regulated by the balance between pro- and antiangiogenic signals (Carmeliet and Jain, 2011a; Darland and D'Amore, 1999).
Previously, we identified an antiangiogenic activity of a small GTPase, R-Ras (Komatsu and Ruoslahti, 2005). R-Ras is strongly expressed in fully differentiated, quiescent vascular smooth muscle cells and endothelial cells (EC) of normal adult vasculature. Unlike the prototypic Ras oncoproteins such as K-Ras and H-Ras, R-Ras inhibits vascular cell proliferation and invasion, and promotes vascular quiescence via yet unidentified pathways. Challenging R-Ras knockout mice with arterial injury or tumor implantation induced exaggerated neointimal thickening and increased angiogenesis in the tumors. Thus, R-Ras signaling primarily affects vessel remodeling and regeneration by counterbalancing the vessel activation. The effect of R-Ras is, however, distinct from the effect of classic antiangiogenic agents: R-Ras does not induce EC death. Instead, it induces quiescence of ECs while supporting their survival–activities that promote vessel maturation. R-Ras expression level correlates with the maturation status of the vessels suggesting a role of R-Ras in the cellular processes essential for the vessel maturation. Little expression of R-Ras was detected in the developmentally growing or pathologically regenerating vessels such as angiogenic vessels in the tumor and hyperplastic neointimal lesion in acute arterial injury in mice. In comparison, abundant expression was found in mature functional vessels of normal adult tissues and in dormant arterial lesions (Komatsu and Ruoslahti, 2005). Based on these observations, we hypothesized that R-Ras promotes maturation of regenerating adult vasculature and that chronically reduced R-Ras expression in tumor vessels causes the immaturity and poor functionality of these vessels. In the present study, we tested this hypothesis by analyzing the effect of R-Ras deficiency or upregulation on the structural and functional integrity of tumor vessels.
RESULTS
R-Ras Deficiency Severely Impairs Maturation Process of Tumor Blood Vessels
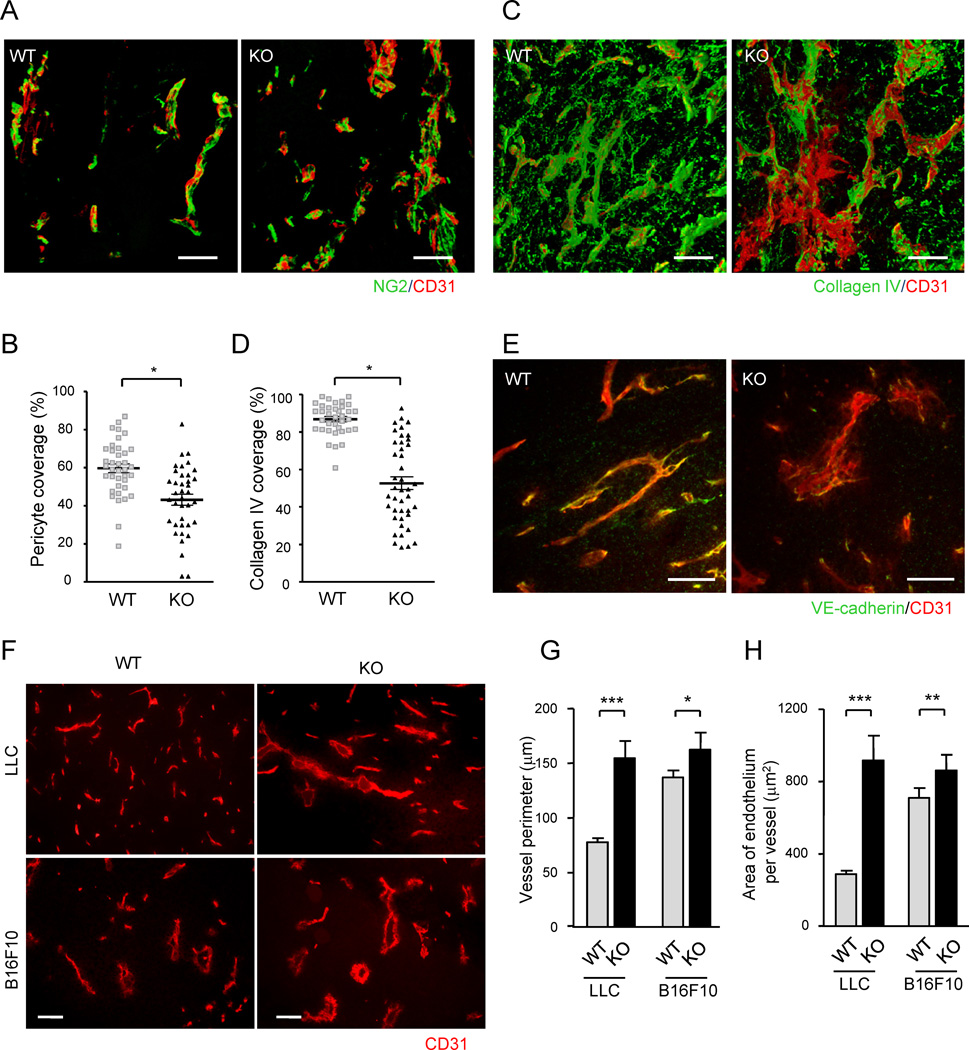
Close interaction between nascent endothelium and pericytes is crucial for vessel maturation, and tumor vasculature is characterized by insufficient pericyte association (Jain, 2003). Using a computer-assisted 3-D image reconstruction (Videos S1A and S1B), we found a significant reduction of the direct physical contact between pericytes and the endothelium of blood vessels developed in subcutaneous (s.c.) tumor implants in R-Ras knockout (KO) mice (Figures 1A and 1B). A similar 3-D analysis of these vessels revealed severely impaired coverage by collagen IV, a major constituent of normal endothelial basement membrane (Figures 1C and 1D). Reflecting a weakened pericyte and basement membrane support, these vessels were often found to be more dilated or saccular, and their endothelium exhibited more severe deformations compared with that of the wild type tumor vessels (Figures 1E–1H). Our analysis suggests that the vessel deformity caused by the R-Ras deficiency is also linked to the abnormality in the VE-cadherin-mediated vessel stabilization. VE-cadherin is essential for the structural integrity of blood vessels, and its genetic disruption leads to leaky vessels, hemorrhaging, and prenatal lethality (Crosby et al., 2005; Gory-Faure et al., 1999). The intensity of VE-cadherin immunostaining was diminished in the R-Ras-deficient vessels (Figure 1E), suggesting that the endothelial adherens junctions of the tumor vessels are disrupted or unstable in the absence of R-Ras. Taken together, these results indicate an important role of R-Ras in establishing close EC-pericyte interaction, basement membrane formation, and endothelial integrity, which are essential for the maturation of blood vessels. Consequently, the R-Ras deficiency greatly exacerbates the tumor-associated immature and abnormal characteristics of blood vessels.
Figure 1. Pronounced structural abnormalities of tumor vessels caused by the R-Ras deficiency.
(A) Endothelium and associated pericytes were visualized by CD31 (red) and NG2 (green) immunofluorescence staining of B16F10 tumor implants. Representative images of wild type (WT) and R-Ras KO (KO) tumor vessels are shown. See also Video S1. (B) Pericyte coverage was quantified by calculating the % fraction of vessel surface (CD31) area that overlapped with NG2 staining in the 3-D image of the vessels to determine direct contact between the two cell types. *p = 0.001, ±SEM. (C and D) A 3-D analysis of collagen IV coverage. CD31, red; collagen IV, green. *p = 4×10−13 (E) Double staining of LLC tumors for CD31 (red) and VE-cadherin (green). (F) CD31-staining of LLC and B16F10 tumor sections. Scale bars, 100 µm (A, C, F), 50 µm (E). (G and H) The perimeter and thickness of the endothelium (assessed by average CD31+ area per vessel) of tumor vessels were increased in R-Ras KO mice, reflecting the augmented vessel deformations. *p = 0.01, **p < 0.001, ***p < 1×10−4
R-Ras Dictates Blood Vessel Phenotype in Tumors
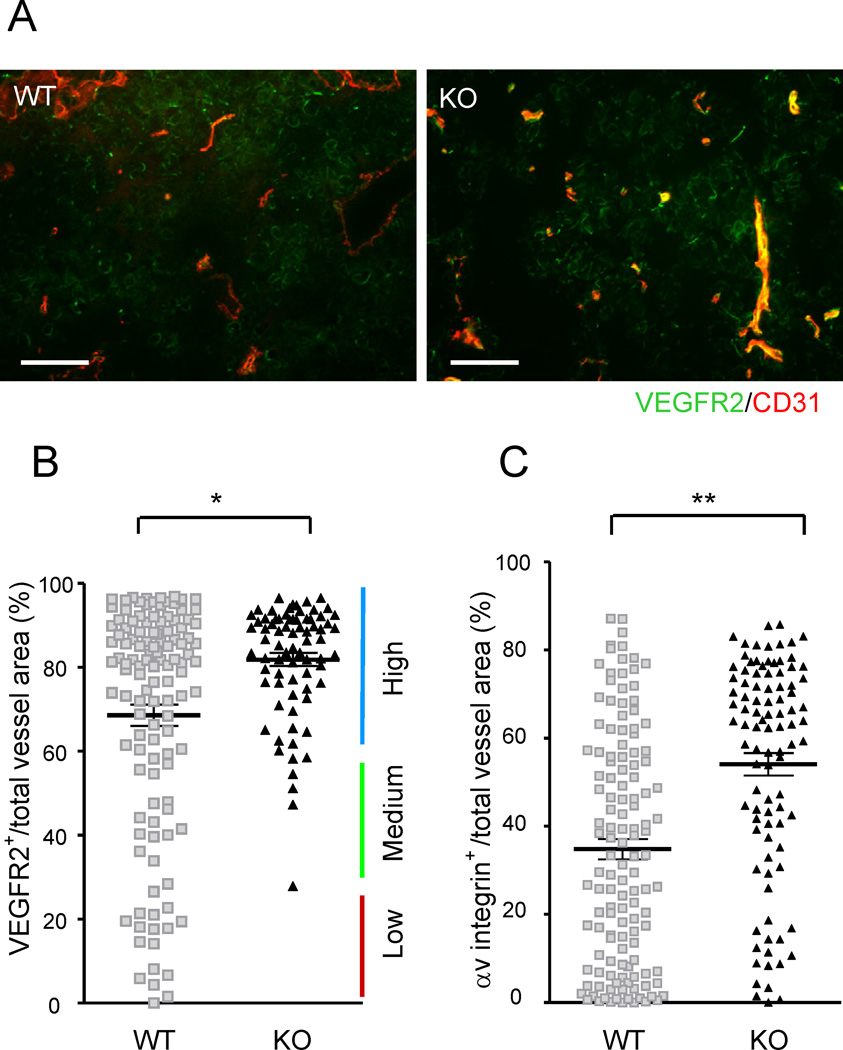
To further evaluate the ability of R-Ras to regulate blood vessel maturation in the tumor microenvironment, we determined the expression status of VEGF receptor 2 (VEGFR2), a molecular marker highly expressed by the endothelium of growing, immature vessels (Heidenreich et al., 2000; Kappel et al., 1999). VEGFR2 and R-Ras show opposite temporal expression patterns during mouse development: developing prenatal vessels are VEGFR2high, R-Rasundetected whereas quiescent adult vessels are VEGFR2low, R-Rashigh (Heidenreich et al., 2000; Kappel et al., 1999; Komatsu and Ruoslahti, 2005). In the present study using B16F10 melanoma implants, tumor vasculature in wild type control mice contained a mixed population of vessels expressing high to low levels of VEGFR2, with the majority of the vessel population being VEGFR2high (Figures 2A and 2B). This result reflects the fact that tumor vessels are generally immature, while containing a small subpopulation of relatively mature vessels formed by VEGFR2low endothelium (Heidenreich et al., 2000; Mancuso et al., 2006) (Figure 2B). These relatively established vessels are a minority, but functionally important, because they can deliver blood and drugs more efficiently to the tumor than immature vessels and are resistant to antiangiogenic treatment by VEGF blockade (Helfrich et al., 2010; Jain, 2005; Mancuso et al., 2006). In R-Ras KO mice, this VEGFR2low subpopulation was absent from the tumor vasculature. Instead, the vessel population has largely shifted to VEGFR2high, immature phenotype (Figure 2B) demonstrating that R-Ras is required for maturation of tumor blood vessels. Analysis of the angiogenic EC marker, αv integrin subunit expression (Stupack and Cheresh, 2002), also demonstrated a major shift of vessel populations to a highly angiogenic αv integrinhigh phenotype in the R-Ras-deficient vasculature (Figure 2C), again indicating a state of un-attenuated vessel activation, consistent with their failure to mature. Similar results were obtained in s.c. implants of LLC tumors (Figures S1).
Figure 2. R-Ras regulates tumor vessel phenotype.
(A) Immunostaining of B16F10 tumor implants for CD31 (red) and VEGFR2 (green). Yellow, double-stained area. (B) The VEGFR2 expression status of the tumor vasculature was assessed by the fraction of VEGFR2-expressing vessel area (VEGFR2+CD31+ double-positive) as a percentage of the total vessel area (total CD31). Each dot represents the average of several vessels found in a micrograph. Fifteen micrographs were obtained randomly from multiple tumor sections. Eight wild type and five R-Ras KO mice were examined. *p = 1×10−5, ±SEM. (C) A similar analysis with αv integrin staining as a marker for angiogenic vessels. Scale bar, 100 µm. **p = 2×10−11. See also Figure S1.
Malfunctions of Tumor Vessels Are Augmented by the R-Ras Deficiency
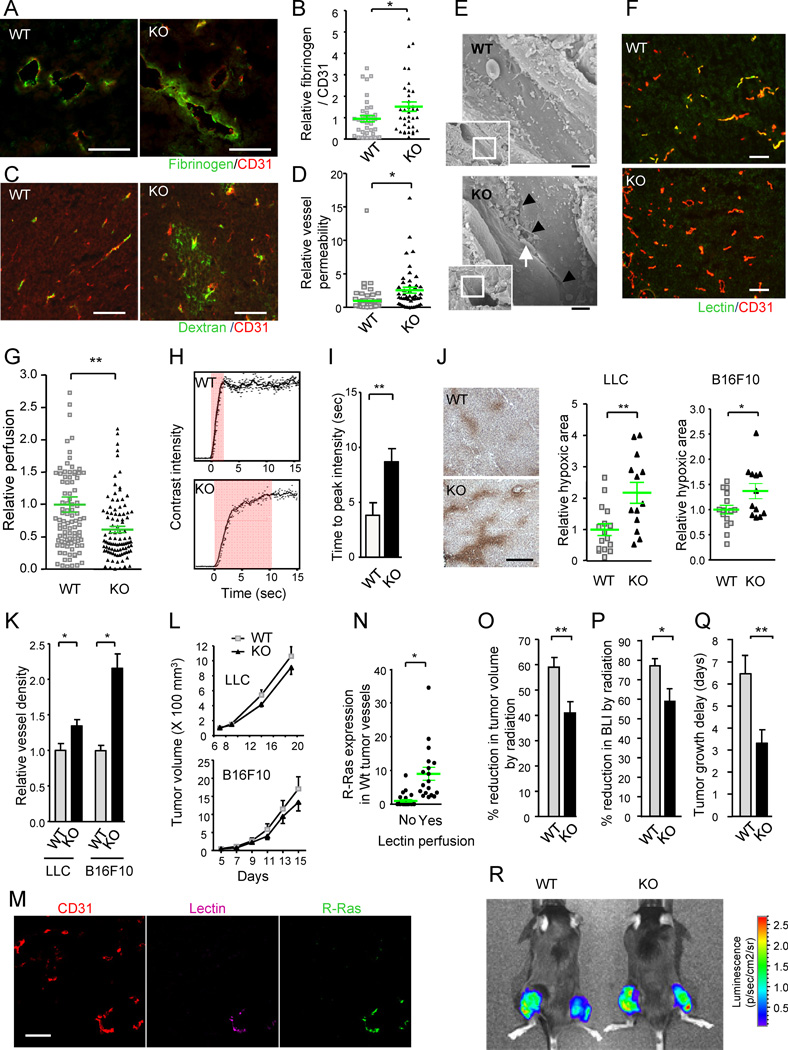
The defects in pericyte support, aberrant basement membrane, and structural deformity of blood vessels are associated with poor functionality of the vasculature (Jain, 2005). Consistent with this notion, tumors in the R-Ras KO mice showed increased vessel permeability and plasma leakage, as indicated by elevated levels of provisional matrix (fibrinogen/fibrin) deposition around the tumor vessels (Figures 3A and 3B). Further indicating abnormal permeability, extensive leakage of intravenously administered FITC-dextran was found in these tumors (Figures 3C and 3D). The increased permeability of the R-Ras-deficient tumor vessels is consistent with the reduced immunoreactivity to VE-cadherin of these vessels (Figure 1E), suggesting that the disruption of R-Ras destabilizes endothelial adherent junctions of tumor vessels increasing their permeability. Supporting the role of R-Ras in endothelial integrity, ultrastructural analysis of the tumor vessels revealed significantly increased presence of disrupted endothelial cell-cell junctions in the R-Ras KO mice, causing spatial gaps between adjacent endothelial cells (Figure 3E).
Figure 3. R-Ras deficiency diminishes tumor vessel function.
(A–D) Analyses of vessel leakiness. Fibrinogen and CD31 staining for plasma leakage in B16F10 tumors (A). To quantify the level of plasma leakage and standardize it for tumor vessel area, the ratio of fibrinogen (fibrin)-stained area to CD31 area was determined and is presented as relative values (B). *p = 0.035, ±SEM. Dextran-FITC was injected i.v. into mice bearing LLC tumors (C). The fluorescence intensity of extravasated FITC in the tumors was standardized for the perfused vessel counts to assess vessel permeability (D). *p = 0.002 (E) Ultrastructure of tumor vessels by scanning electron microscopy. The R-Ras KO tumor endothelium shows significant special gaps at the cell-cell junctions (arrowheads). Arrow, a red blood cell extravasating between the cellular gap. Scale bar, 2 µm. (F and G) Blood perfusion efficiency. Lectin perfusion and CD31 staining of B16F10 tumor sections (F). Yellow color indicates double-stained vessel area. Percent lectin+CD31+ double-positive area/total CD31+ area was determined to assess perfusion efficiency of tumor vasculature (G). **p = 2×10−4 (H and I) Study of tumor vessel perfusion by contrast-enhanced ultrasound imaging. Examples of raw contrast kinetics acquired after bolus i.v. injection of microbubble contrast agent (H). Delayed time-to-peak-intensity in KO mice indicating poor perfusion efficiency (I). **p = 0.02 (J) Analysis of tumor hypoxia. The ratio of the hypoxic area/total area of the tumor section was determined and presented as relative values. Representative anti-Hypoxyprobe staining of LLC tumors is shown. *p = 0.03, **p = 0.003 (K) Number of tumor vessels per unit area (vessel density) is presented relative to the control group. *p < 0.05 (L) Growth of LLC and B16F10 tumors. (M and N) Analysis of R-Ras expression in wild type vessels. Sections of lectin-perfused tumors were stained for CD31, lectin, and R-Ras (M). The tumor vessels were classified as lectin-perfused and non-perfused vessels, and the level of R-Ras expression was determined for individual vessels in each group (N). *p = 7×10−4 (O–R) Effect of radiotherapy. (O) The percent reduction in the tumor volume (as compared with untreated tumors) was determined at 7 days after a local 12 Gy irradiation of LLC tumors (O). **p < 0.01 (P) Percent inhibition of tumor growth was also assessed by bioluminescence tumor imaging at day 7. BLI, bioluminescence intensity. *p = 0.02 (Q) Tumor growth delay as defined by delay in tumor volume quadrupling time as a result of irradiation treatment. The average delay time (in days) is presented. **p < 0.01 (R) Bioluminescence imaging of Luc2-transfected LLC tumors. Tumors on the right thigh received 12Gy irradiation whereas tumors on the left did not. WT, wild type; KO, R-Ras KO mice. Scale bars, 100 µm (A, C, F, M), 200 µm (J). See also Figure S2 and Table S1.
Disruption of endothelial adherens junctions allows increased penetration of tumor cells into the circulation (Weis et al., 2004). Pericyte coverage of tumor vessels is also critical for limiting blood-bone metastasis (Gerhardt and Semb, 2008). In our study, tumor cells were rarely detected by PCR in the peripheral blood of control mice bearing subcutaneous tumors. In contrast, circulating tumor cells were readily detectable in the R-Ras KO mice bearing primary tumors of similar sizes, suggesting that significantly increased number of tumor cells escaped into the blood circulation in the KO mice (Table S1). This finding is consistent with the impaired endothelial barrier function and poor pericyte support of the R-Ras-deficient tumor vasculature. Interestingly, we found a 4-fold increase in metastatic foci counts in the lungs of KO mice after tail vein injection of tumor cells, suggesting that R-Ras may also regulate the penetration of circulating tumor cells or tumor establishment at secondary target tissues (Figure S2A).
We next determined the efficiency of blood perfusion through the tumor vasculature by intravenously injecting biotin labeled tomato lectin, which highlights vessel lumens. Vessel perfusion was markedly reduced in tumors growing in R-Ras KO mice compared to tumors in wild type mice (Figures 3F, 3G, and S2B). Furthermore, a contrast-enhanced ultrasonography study showed delayed time to peak intensity of the contrast perfusion in tumors in the KO mice (Figures 3H and 3I), an observation consistent with more irregular and deformed vascular structure (Kersting et al., 2009). The reduced tumor vessel perfusion coincided with elevated levels of tumor hypoxia in R-Ras KO mice (Figure 3J) despite a significant increase in the vessel density in these tumors (Figure 3K). In addition, the tumor size was not increased in R-Ras KO mice despite the increased vascularization (Figure 3L). These observations indicate that the new vessels are immature to an extent that renders them functionally defective. Importantly, in wild type mice, R-Ras was expressed at a 10-fold higher level by the blood-perfused tumor vessel population than the nonperfused, nonfunctional vessels (Figures 3M and 3N). A correlation between increased R-Ras expression and vessel perfusion was also found in human prostate tumor xenografts grown in nude mice (Figure S2C). Thus, R-Ras expression is associated with better blood flow in tumors, further reinforcing the link between R-Ras expression and tumor vessel functions.
Since poor vascular function in tumors could lessen the effectiveness of radiation treatments, we tested how R-Ras deficiency affects the efficacy of local irradiation to the subcutaneous LLC tumor implants. In the wild type control group, a single dose of irradiation (12 Gy) resulted in 59% tumor volume reduction compared with the volume of untreated group at 7 days post irradiation. In comparison, only 41% reduction was observed in the R-Ras KO mice (Figure 3O). A similar decrease in the treatment effect was demonstrated by in vivo bioluminescence intensity (BLI) measurement, which quantifies the amount of live tumor cell mass (Figures 3P and 3R). Accordingly, the tumor growth delay by irradiation was reduced in R-Ras KO mice compared with wild type mice (Figure 3Q). These results indicate that R-Ras deficiency negatively affects the effectiveness of radiotherapy. This finding was consistent with the poor vessel function and elevated hypoxia level in tumors of the R-Ras KO mice (Figures 3A–3J).
Next, we examined the effect of VEGF blockade on R-Ras-deficient tumor vessels. A single dose DC101 (anti-VEGFR2) antibody treatment resulted in 50% reduction in the tumor vessel density in the KO mice while the same treatment had minimal effect in wild type mice at 3 days post treatment (Figure S2D). Because R-Ras deficiency exacerbates immaturity of tumor vessels (Figures 1, 2, and S1), our result is consistent with the previous findings indicating that immature vessels are vulnerable to VEGF blockade (Benjamin et al., 1999). At the dosage and treatment schedule we used, DC101 had little effect on the growth of LLC tumors in wild type or R-Ras KO mice (Figure S2E).
R-Ras is Downregulated in Human Tumor Vasculature
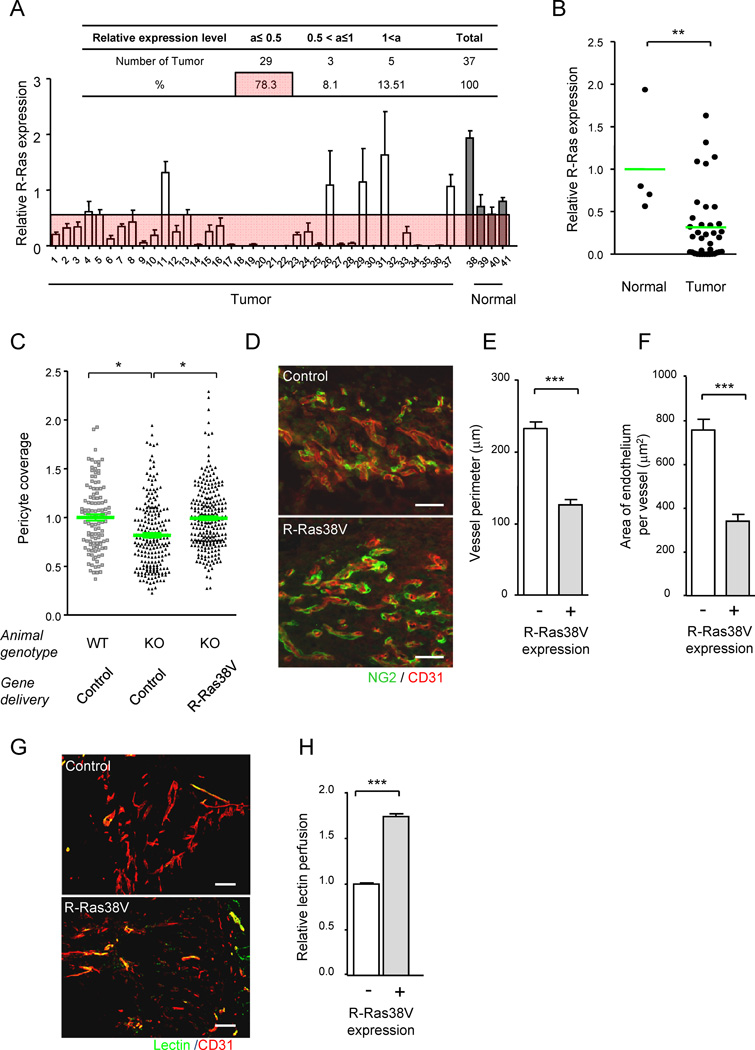
In mice, R-Ras is highly expressed in mature functional vessels of normal tissues while it is down-regulated to a level undetectable by immunofluorescence in the majority of tumor vessels (Komatsu and Ruoslahti, 2005), except for the blood-perfused functional vessels (Figures 3M and 3N). Notably, significant downregulation of R-Ras was also observed in tumor vessels in the majority of human breast cancers we examined, compared with blood vessels in normal breast tissue (Figures 4A and 4B, Table S2). This result corroborates the previous finding in gene expression profiling study, which revealed significantly reduced R-Ras mRNA level in the endothelium of human breast carcinoma compared with the normal counterpart (Allinen et al., 2004). Thus R-Ras downregulation in tumor vasculature is found in both mice and humans supporting the hypothesis that R-Ras expression is chronically reduced in tumor vasculature contributing to immaturity and poor functionality of these vessels.
Figure 4. R-Ras promotes normalization of VEGF-induced pathologically regenerating vasculature.
(A and B) R-Ras is downregulated in human tumor vasculature. The level of R-Ras expression in tumor vessels was analyzed in a human breast cancer tissue array by immunostaining for R-Ras and CD31 (A). Dot plot representation of A (B). **p = 0.004. See also Table S2. (C–H) R-Ras signaling improves structure and function of VEGF-induced vessels. VEGF-induced angiogenic vessels developed in Matrigel plugs were transduced in vivo with R-Ras38V or control lentivirus. Six days later, pericyte association with the endothelium was determined (C) by NG2 and CD31 immunofluorescence (D). The data is presented as relative values. (E–H) The vessels in the R-Ras38V virus-infected plugs were classified as R-Ras-positive (successfully transduced) or negative (non-transduced) vessels. The in vivo R-Ras transduction reduced the vessel perimeter (reduced vessel dilation) in R-Ras KO mice (E). The thickness of the endothelium (assessed by average CD31+ area per vessel) was also reduced in the KO mice by R-Ras transduction (F). The histological sections of Matrigel implants in R-Ras KO mice, which received control or R-Ras38V virus, were stained for perfused lectin, and CD31 to determine the blood perfusion of the vessels (G). Blood perfusion efficiency was compared between the R-Ras-transduced and non-transduced vessels (H). *p < 0.05, ***p < 1×10−3, ±SEM. Scale bars, 100 µm. See also Figure S3.
Upregulation of R-Ras Signaling Leads to Normalization of Pathologically Regenerating Blood Vessels
The above observations prompted us to examine whether restoring R-Ras signaling to pathologically regenerating blood vessels will promote vessel maturation and improve structure and functions of the defective vessels. For this purpose, lentivirus carrying activated R-Ras (R-Ras38V) or mock control virus was injected into pre-implanted, VEGF-containing Matrigel plugs that have developed angiogenic vessels in this tumor-like environment in R-Ras KO mice (Figure S3A). Following forced expression of R-Ras38V (Figure S3B), the pericyte-EC association of the newly formed microvessels in the R-Ras KO mice increased to a level comparable to that in wild type animals (Figures 4C and 4D). This effect was accompanied by reduced vessel dilation and thickness of the endothelium, minimizing the deformation of the VEGF-induced vessels in Matrigel (Figures 4E and 4F). Concomitantly, the efficiency of blood perfusion improved by 74% in the R-Ras KO vessels that were successfully transduced with R-Ras38V compared with non-transduced vessels within the same Matrigel plugs (Figures 4G and 4H). Thus, the in vivo upregulation of R-Ras signaling reverses the vessel phenotype and restores vessel function in the pathologically developing vasculature.
Role of Endothelial R-Ras in Controlling Vessel Sprouting and Maturation
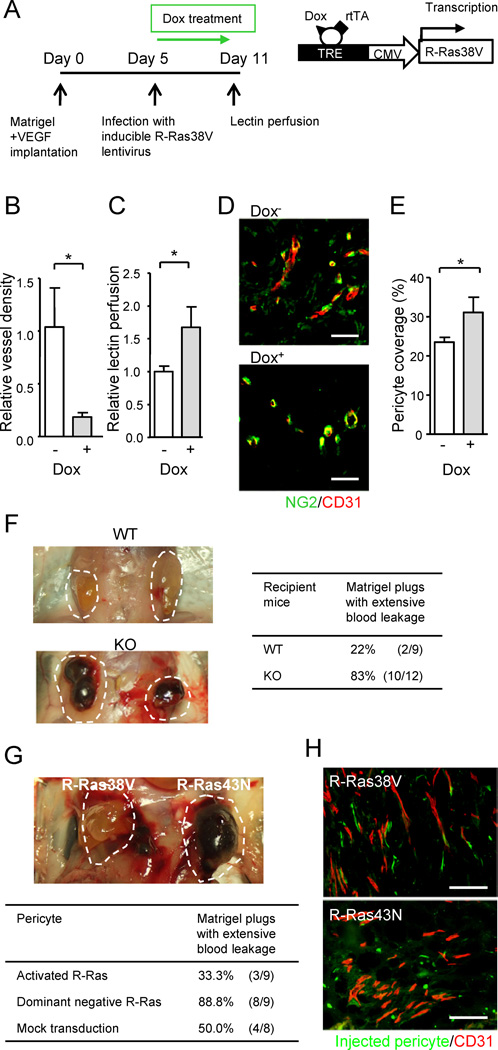
R-Ras is expressed in both the endothelium and mural cells (smooth muscle cells and pericytes). To determine the endothelial cell-specific contribution of R-Ras, we used a technique that combines lentivirus-mediated in vivo gene transduction and Tie-2 promoter-driven gene expression. In this model, VEGF-containing Matrigel was implanted into Tie2-rtTA mice, which express reverse transactivator (rtTA) in an endothelial cell-specific manner (Bao et al., 2010). Lentivirus carrying a vector for rtTA-dependent expression of activated R-Ras (pLVX-Tight-Puro/R-Ras38V) was then injected into the pre-implanted Matrigel plugs that were developing new vessels in response to VEGF (Figure 5A). The mice received doxycycline daily to induce the expression of R-Ras38V in a Tie2-rtTA-dependent manner (Figures S4A and S4B). In this study, Dox-treated (Dox+) mice showed significantly reduced vessel density in the Matrigel plugs compared with the plugs of Dox− animals demonstrating a strong inhibitory activity of endothelial R-Ras against vessel sprouting induced by VEGF (Figure 5B). Doxycycline itself did not have any noticeable effect on the neovascularization in the control mice without R-Ras38V virus infection (data not shown). Importantly, the vessels developed in Matrigel were significantly better perfused in Dox+ mice than in Dox− mice indicating improved circulation in these vessels following R-Ras38V induction (Figure 5C). Furthermore, pericyte coverage of these vessels was also improved upon R-Ras38V induction in the endothelium (Figures 5D and 5E). Because Matrigel itself contains large amount of matrix components, it was not possible to do specific immunostaining to assess basement membrane coverage of these vessels; however, we observed enhanced extracellular matrix depositions by cultured ECs upon R-Ras38V expression supporting a role of R-Ras in basement membrane formation (Figure S4C). These observations demonstrate an important activity of endothelial R-Ras, promoting the formation of functional vessels after VEGF-induction while inhibiting further vessel sprouting.
Figure 5. The effect of cell type specific upregulation of R-Ras on vessel maturation and functions.
(A–E) The effect of endothelial specific R-Ras. A timetable of Matrigel implantation in Tie2-rtTA transgenic mice, lentivirus infection, R-Ras38V induction by doxycycline (Dox), and lectin i.v. perfusion is shown (A). Vessel density in the Matrigel plugs at Day 11 (B). Relative perfusion efficiency of i.v. injected lectin at Day 11 (C). (D and E) Matrigel sections were stained for NG2 and CD31 (D), and pericyte coverage of the vessels was determined by image analysis (E). (F) VEGF-induced Matrigel angiogenesis produces extensively ‘bloody’ plugs in R-Ras KO mice demonstrating enhanced leakiness of pathologically regenerating vessels in the absence of R-Ras. (G and H) The effect of pericyte specific R-Ras. Fluorescently labeled, ex-vivo-transduced pericytes were implanted with VEGF-containing Matrigel into the flank of R-Ras KO mice. Matrigel plugs were examined 7 days later (indicated by dashed lines) (G). Histological analysis of the plugs (H). The ex vivo-transduced, implanted pericytes were visualized by green fluorescence. Microvessels were stained with CD31 (red). Scale bars, 100 µm (H), 50 µm (D). *p < 0.05, ±SEM. See also Figure S4.
Role of R-Ras in Pericytes for Enhancing Vessel Integrity
We next used an ex-vivo gene manipulation/cell transplantation approach to determine the role of R-Ras signaling in pericytes in vivo. Microvascular pericytes were isolated from wild type neonatal mouse brain (Wu et al., 2003) (Figures S4D–S4F) and transduced ex vivo with cDNA for activated R-Ras (R-Ras38V) or dominant negative R-Ras (R-Ras43N). R-Ras KO mice typically exhibit extensive hemorrhage in Matrigel plugs during VEGF-induced angiogenesis (Figure 5F). However, implantation of pericytes, which were ex vivo-transduced with activated R-Ras, significantly prevented blood leakage in the plugs implanted into R-Ras KO mice (Figure 5G). Histological analysis of the plugs revealed association of the implanted pericytes (green) with the tips of newly formed R-Ras-deficient vessels (red) (Figure 5H). Pericytes transduced with the dominant negative R-Ras43N failed to show this effect, and the implanted pericytes were mostly unassociated with the vessels (Figure 5H). These findings are consistent with the fact that pericyte association with endothelium is crucial for vessel integrity (Lindahl et al., 1997; McDonald and Choyke, 2003). Thus, R-Ras activity in pericytes alone can significantly contribute to the vessel maturation process independently from endothelial R-Ras signaling. However, R-Ras38V expression in the transplanted pericytes was not very effective at improving blood circulation in the plugs as measured by lectin perfusion (Figure S4G). This result suggests that R-Ras signaling in pericytes contributes to vessel maturation by promoting pericyte-EC interaction thereby improving vessel integrity (reducing permeability), whereas endothelial R-Ras is necessary to significantly improve blood perfusion in VEGF-induced vessels (Figure 5C). Collectively, our results demonstrate that R-Ras activity in ECs and in pericytes individually contribute to maturation and normalization of pathologically regenerating blood vessels.
R-Ras Does Not Affect PHD2 or HIF-2 Levels during Hypoxia
A recent study by Mazzone et al. showed that haplodeficiency of the oxygen-sensing prolyl hydroxylase domain protein 2 (PHD2) leads to normalization of tumor vasculature through stabilization of endothelial HIF-2α and a consequent upregulation of soluble VEGFR1 and VE-cadherin expression in ECs (Mazzone et al., 2009). The effect of PHD2 haplodeficiency and HIF-2α stabilization exemplifies the significance of oxygen sensing by ECs for vessel integrity (Coulon et al., 2010). We examined a potential role of R-Ras in regulating the levels of these oxygen sensors. The expression of R-Ras38V did not affect the levels of PHD2 or HIF-2α in cultured ECs in hypoxia or normoxia (Figure S5A). Likewise, downregulation of endogenous R-Ras by shRNA knockdown had no effect on the levels of these proteins (Figure S5B). These data do not support the idea that R-Ras modulates PHD2/HIF-2 pathway or that the vessel normalization effect of R-Ras is mediated via HIF-2α stabilization. In addition, the expression of endogenous R-Ras was not affected by hypoxia either in ECs or in pericytes (Figure S5C).
In vivo, we did not find any evidence to suggest that HIF-2α level is decreased in the tumor vessels in R-Ras KO mice compared with wild type control (Figure S5D). Furthermore, unlike the PHD2/HIF-2 model (Mazzone et al., 2009), the expression levels of HIF-2 target genes, VE-cadherin and Flt1, were not increased (Figure S5E) concurrently with the vascular normalization promoted by endothelial R-Ras in the doxycycline-inducible model (Figures 5A–5E). These observations strongly suggest that R-Ras is not involved in oxygen-sensing mechanisms of vessel regulations mediated by the PHD2/HIF-2 pathway.
R-Ras Enhances Endothelial Barrier Function via Suppression of VE-Cadherin Internalization
One possible mechanism of R-Ras-induced vessel normalization is stabilization of endothelial adherens junctions via stabilization of VE-cadherin. VE-cadherin is essential for the integrity of blood vessel wall and for the regulation of vessel permeability (Fukuhara et al., 2005). We observed disruption of endothelial cell-cell junction (Figure 3E), elevated plasma leakage (Figures 3A–3D), and reduced VE-cadherin immunostaining of the tumor vessels in R-Ras KO mice (Figure 1E). Therefore, we next examined the effect of R-Ras signaling on VE-cadherin.
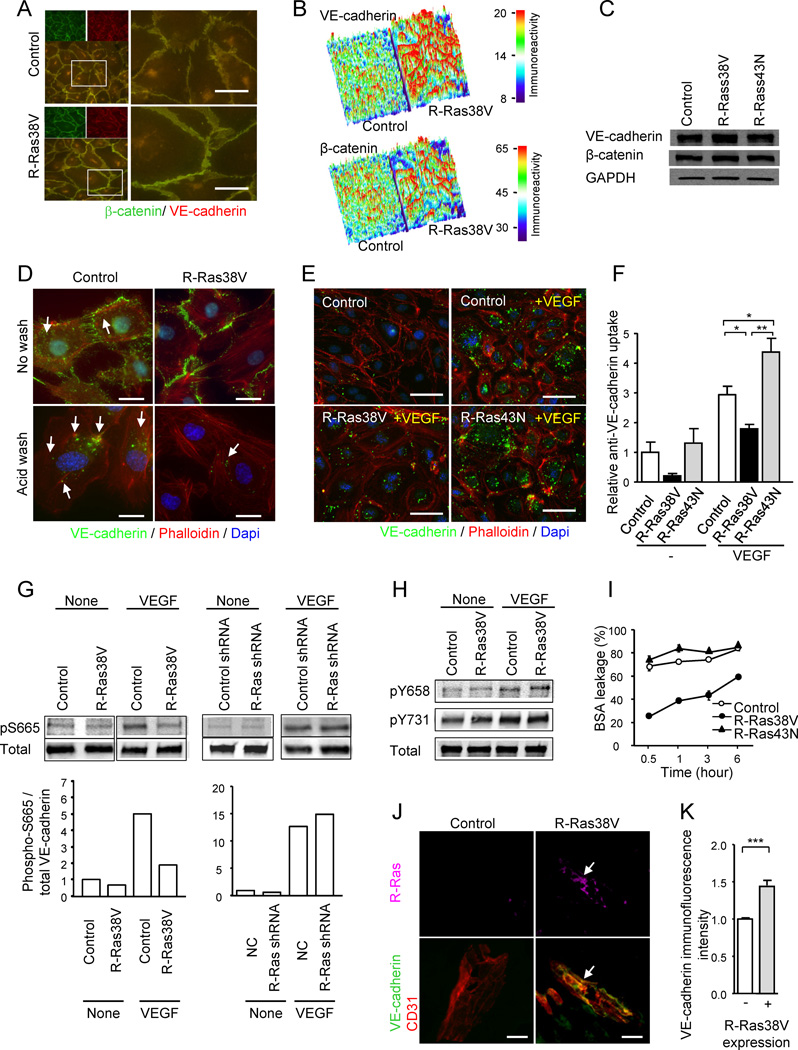
In confluent EC cultures, R-Ras38V expression induced marked accumulation of VE-cadherin and β–catenin at the cell-to-cell interface producing prominent and continuous barrier walls of the adherens junctions (Figures 6A and 6B). Despite this effect, the total expression level of neither VE-cadherin nor β-catenin protein in the cell was altered by activated or dominant negative R-Ras (Figure 6C). In vivo, the VE-cadherin immunofluorescence intensity was diminished in R-Ras-deficient tumor vasculature (Figure 1E) although the VE-cadherin protein level was unaltered (Figure S5F). This may be because the detectable fluorescence signal declines with the loss of VE-cadherin clustering at the cell-cell junctions. We, therefore, hypothesized that R-Ras signaling may affect the localization of VE-cadherin rather than regulating its gene expression. VEGF stimulation of ECs induces internalization of VE-cadherin (Gavard and Gutkind, 2006). VE-cadherin localization upon VEGF stimulation was analyzed using a monoclonal antibody that recognizes the extracellular domain of VE-cadherin (Figure 6D). The antibody displayed cell surface staining of VE-cadherin that is sensitive to a mild acid wash. The acid-resistant vesicular staining pattern represented the intracellular vesicles containing VE-cadherin molecules that are internalized (endocytosed) upon VEGF stimulation (Gavard and Gutkind, 2006). This analysis showed that R-Ras38V expression significantly reduces the internalization of VE-cadherin (Figures 6D–6F and S5G), causing an accumulation of VE-cadherin at the cell-cell junctions despite the VEGF stimulation. The R-Ras43N expression (Figures 6E and 6F) or knockdown of endogenous R-Ras (Figure S5H) showed an opposite effect, increasing VE-cadherin internalization during VEGF stimulation.
Figure 6. R-Ras stabilizes endothelial adherens junctions and improves endothelial barrier function via suppression of VE-cadherin internalization.
(A) R-Ras enhances adherens junction formation in EC monolayer. VE-cadherin (green) and β-catenin (red) staining of HUVEC monolayers transduced with R-Ras38V or control vector. Lower left panels and right panels are merged images of the double staining. Right panels show higher magnification at cell-cell junctions. (B) Surface plot of VE-cadherin and β-catenin immunofluorescence intensity shows marked accumulation of both proteins at the cell-cell interface upon R-Ras38V expression. The immunoreactivity is shown with arbitrary unit. (C) VE-cadherin and β-catenin immunoblotting of whole cell lysate. GAPDH is shown as a loading control. (D) Analysis of VEGF-induced VE-cadherin internalization. The monoclonal antibody (BV6) recognizes the extracellular domain of VE-cadherin (green). Following antibody incubation of the EC culture and stimulation with VEGF, the cell surface membrane-bound BV6 antibodies (upper panels) were removed by a mild acid wash (lower panels). The presence of internal acid-resistant vesicles stained with the BV6 antibody indicates the internalization of VE-cadherin. Phalloidin (actin), red; nucleus, blue; arrows, BV6-stained vesicles containing internalized VE-cadherin. (E) Dominant negative R-Ras43N enhances VE-cadherin internalization. (F) VE-cadherin internalization was quantified by the percentage of the cells showing internal acid-resistant vesicles. *p < 0.05, **p < 0.001, ±SEM. In the no-VEGF stimulation condition (−), statistical significance was not found between the control, R-Ras38V, and R-Ras43N. (G) Phosphorylation of VE-cadherin S665 was analyzed in the lysate of VEGF-stimulated ECs (100 ng/ml) using phospho-specific antibody. (H) Phosphorylation of VE-cadherin Y658 and Y731 residues. (I) The effect of R-Ras on the endothelial permeability was determined in HUVEC monolayer cultures. BSA leakage through the culture insert membrane without cells was set as 100%. (J) The in vivo transduction of R-Ras38V increased VE-cadherin immunoreactivity of angiogenic sprouts in Matrigel plugs implanted in R-Ras KO mice. Immunofluorescence showed accumulation of VE-cadherin at the endothelial cell-cell junctions upon R-Ras transgene expression (arrows). (K) Quantification of VE-cadherin immunoreactivity standardized with CD31 area was compared between R-Ras38V and mock transduced control plugs. ***p < 0.001. Scale bars, 20 µm (J), 25 µm (D), 50 µm (A, E). See also Figure S5.
It has been shown that the phosphorylation of VE-cadherin at Ser 665 in the cytoplasmic domain promotes VE-cadherin internalization upon VEGF stimulation (Gavard and Gutkind, 2006). Interestingly, we found that this serine phosphorylation was reduced by R-Ras38V expression almost to the level of the non-stimulated control during VEGF stimulation (Figure 6G). Thus, the reduction of Ser 665 phosphorylation observed here was consistent with the reduction of VE-cadherin internalization by R-Ras (Figures 6D–6F). The other VE-cadherin phosphorylation sites important for adherens junction disruption and endothelial permeability, Tyr 658 (Dejana et al., 2008) and Tyr 731 (Allingham et al., 2007) were unaffected by R-Ras (Figure 6H) or R-Ras knockdown (data not shown) during VEGF-stimulation. Consistent with the role of R-Ras in VE-cadherin regulation and adherens junction stabilization, the expression of R-Ras38V strongly suppressed the permeability of endothelial monolayer (Figure 6I). R-Ras knockdown had an opposite effect (Figure S5I). These results substantiated the in vivo observations of increased tumor vessel permeability in R-Ras KO mice (Figure 3A–D). Furthermore, the in vivo transduction of R-Ras38V enhanced VE-cadherin immunostaining at the endothelial cell-cell junctions of angiogenic sprouts induced by VEGF in R-Ras KO mice (Figures 6J and 6K), supporting the importance of R-Ras in the integrity of tumor vessels. Taken together, R-Ras plays a crucial role in reinforcing the VE-cadherin-dependent endothelial adherens junctions while counterbalancing the effect of VEGF stimulation in the tumor microenvironment.
R-Ras activation in Pericytes Induces Normal Capillary-Like Vessel Morphogenesis
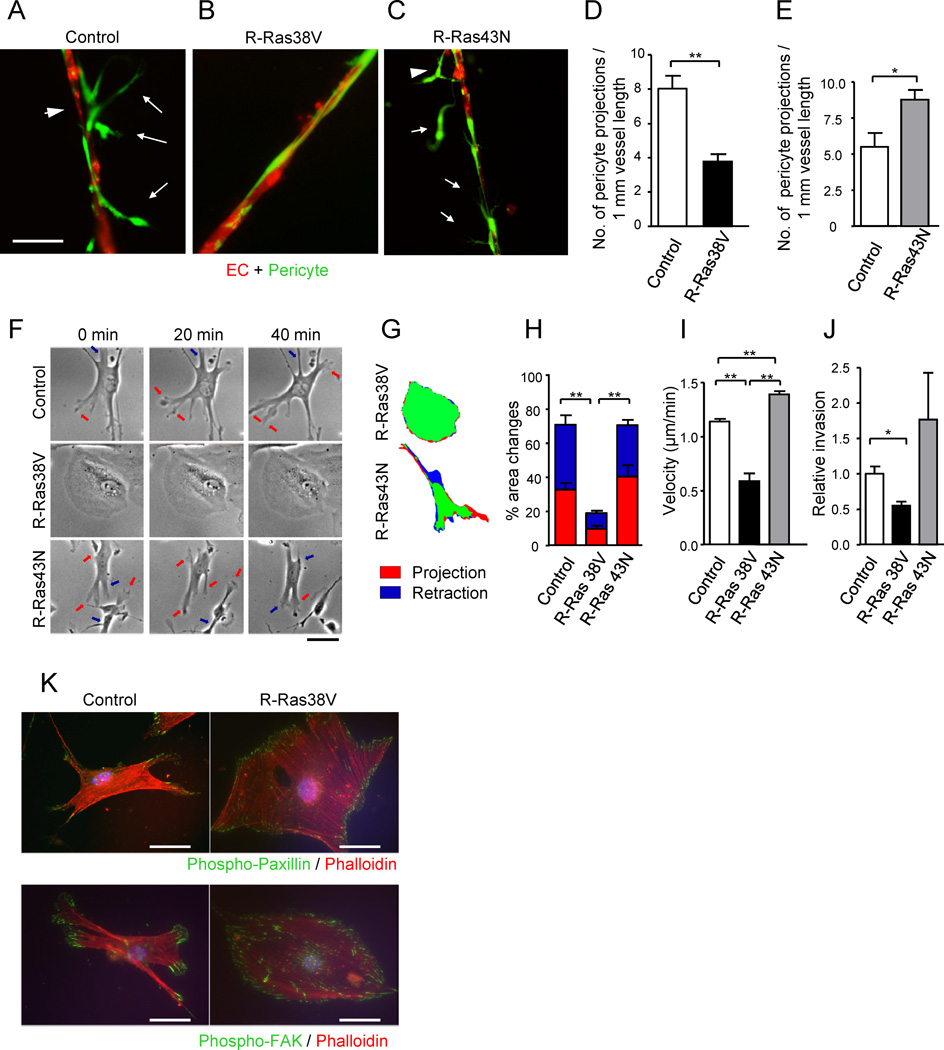
The physical interaction of pericytes with ECs is essential to vessel maturation and functions. Our in vivo studies showed that R-Ras activity in pericytes promotes the pericyte-EC interaction in regenerating vessels (Figure 5H). To recapitulate this phenomenon in vitro and to further analyze the relative contribution of the pericyte-expressed R-Ras to vessel formation, we used an EC/pericyte 3-D coculture that reconstitutes microvessels in Matrigel (Figures 7A–7E).
Figure 7. R-Ras regulates pericytes in the in vitro-reconstituted microvessels.
(A and B) Fluorescently labeled pericytes (green) were transduced with R-Ras38V or control vector and, at 3 hours post transduction, they were plated together with red-labeled ECs onto Matrigel at a 4:1 (EC : pericyte) ratio, cultured for 24 hours, and the resulting vessel-like structures were analyzed by fluorescent microscopy. In the control cocultures (A), pericytes were loosely attached to the vessel-like EC structures (arrowhead) with cytoplasmic processes (arrows). Upon R-Ras38V expression in pericytes, these abnormal characteristics were significantly diminished, and ECs and pericytes became tightly associated (B). (C) R-Ras43N expression in pericytes did not have this effect. (D and E) The number of pericyte projections from the reconstituted microvessels was significantly decreased by R-Ras38V (D) but increased by R-Ras43N expression (E). (F) Time-laps video microscopy of pericytes on the Matrigel-coated 2-D surface shows membrane projection/retraction activities of the pericytes. Arrows indicate dynamic membrane activities (orange, projections; blue, retractions). (G and H) The fractions of projecting (red) and retracting (blue) membrane area vs. total cell area (% area change) were determined between the time points 0 and 5 min. See also Figure S6. (I) The mean velocity of pericyte motility was calculated from 20 hour-long time-laps observations in 2-D culture. (J) Pericyte invasion through Matrigel-coated filter membrane. (K) Immunofluorescence of phospho-paxillin Y118 and phospho-FAK Y397 to highlight focal adhesions complexes (green) assembled throughout the cell perimeter upon R-Ras38V expression. Scale bars, 50 µm. *p < 0.05, **p < 0.001, ±SEM
In this model, the coculture produced networks of vessel-like, elongated endothelial structures with associated pericytes (Figures 7A–7C). In the control coculture with mock-transduced pericytes, the pericytes were found loosely or incompletely associated with ECs and appeared to be activated, invasive, and irregularly extended out from the main stalk of the vessels with long cytoplasmic processes (Figure 7A). These features are strikingly similar to the abnormalities in pericytes associated with tumor blood vessels (McDonald and Choyke, 2003). In contrast, such tumor-associated characteristics of pericytes were significantly diminished by the expression of activated R-Ras in pericytes (Figure 7B). Instead, most pericytes were flattened, widely spread or extended with long thin processes over the surface of the vessel-like structures, and were intimately associated with ECs as seen for the pericytes of normal capillary or microvessels. The dominant negative R-Ras did not show this effect (Figure 7C). The number of pericyte projections was also decreased by the activated R-Ras but increased by the dominant negative R-Ras signifying pericyte stabilization by R-Ras activity (Figures 7D and 7E). The ability of R-Ras to suppress invasive nature of pericytes was substantiated by the observation that R-Ras38V expression but not R-Ras43N or R-Ras knockdown strongly suppressed dynamic membrane projection/retraction activities (Figures 7F–7H and S6A), motility on the Matrigel-coated surface (Figures 7I and S6B), and invasion through Matrigel (Figure 7J) of the pericytes. These effects were also observed in cultured ECs (Figures S6C–S6E) but not in C2C12 myoblasts or NIH 3T3 fibroblasts demonstrating cell type-dependent effect of R-Ras (Figures S6F and S6G).
Pericytes interacts closely with ECs by adhering to endothelial basement membrane that is shared by both cell types (Armulik et al., 2005). The ability of R-Ras to promote intimate association of pericytes with ECs observed in vivo (Figure 5H) and in vitro (Figure 7B) may be attributable to enhancement of integrin-mediated cell adhesion to the extracellular matrix (ECM) (Kinbara et al., 2003; Zhang et al., 1996). R-Ras38V expression in pericytes resulted in enhanced pericyte adhesion to endothelial basement membrane components, collagen IV and laminin (Figure S6H). In the control pericytes with mock-transduction or shRNA knockdown of endogenous R-Ras, the presence of focal adhesion complexes was limited to the leading edge of migrating cells or protruding membranes (Figure 7K). In contrast, R-Ras38V expression resulted in assembly of numerous focal adhesion complexes throughout the cell perimeter, facilitating the flattening and wide spreading of cell membrane over the ECM-coated surface (Figure 7K). These results demonstrated the profound effect of R-Ras on pericyte-ECM interaction and support the importance of pericyte-expressed R-Ras for pericyte association with nascent microvessels and the stabilization of these vessels as observed in vivo (Figure 5H).
DISCUSSION
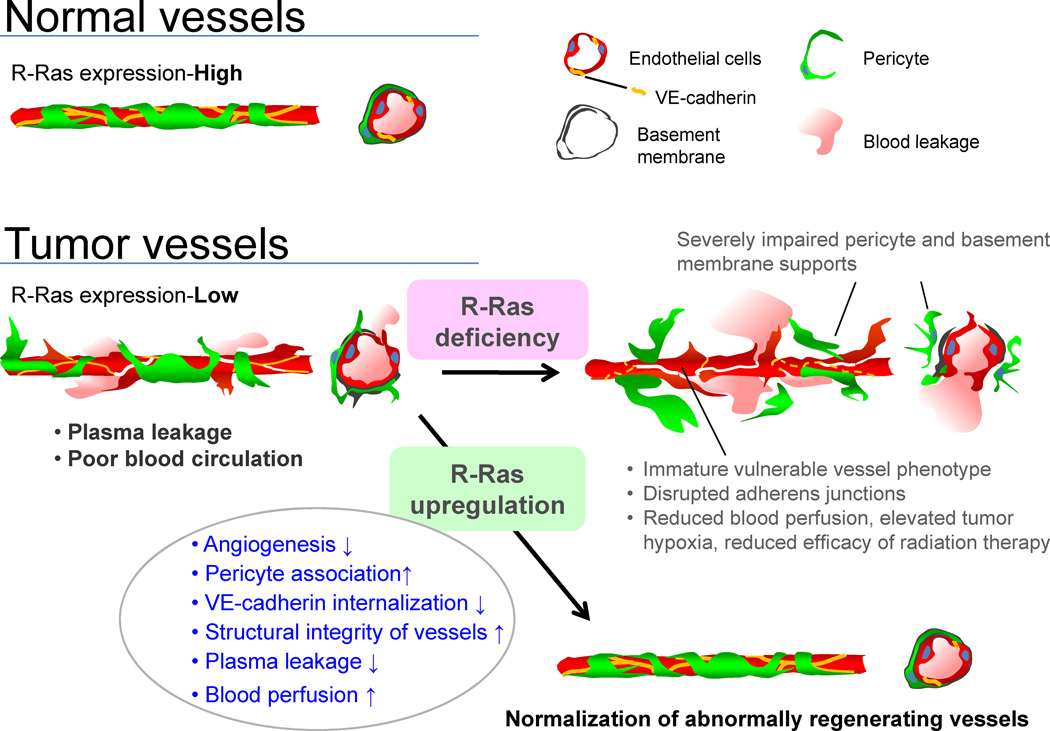
Studies by others have elucidated the importance of early-acting signaling pathways for vessel maturation, such as angiopoietin-1/Tie-2 and PDGF (Andrae et al., 2008; Thomas and Augustin, 2009). However, it has been unclear what directs the maturation process further to establish functional vessels during tumor angiogenesis. This is an important question because tumor progression and the efficacy of therapeutic regimens both depend on blood circulation in the tumors. In this study, we showed that R-Ras promotes the maturation of blood vessels in the tumor microenvironment, and that through this activity, R-Ras governs integrity and functionality of tumor vasculature (Figure 8).
Figure 8. Regulation of tumor vessel maturation by R-Ras.
Schematic summary of the consequences of R-Ras disruption and upregulation in tumor blood vessels. The disruption of R-Ras severely impairs structural and functional maturation of tumor vessels. These vessels exhibit the poorly pericyte-supported ‘vulnerable’ immature phenotype, which highly expresses VEGFR2 and αv integrins. The structural abnormalities are associated with extensive blood leakage, reduced blood perfusion, and elevated hypoxia within tumors. On the other hand, upregulation of R-Ras signaling enhances pericyte association and stabilizes VE-cadherin at the endothelial adherens junctions, leading to improved vessel structure and endothelial barrier function with improved blood perfusion. Thus, R-Ras signaling promotes normalization of pathologically regenerating blood vessels.
Malformation and malfunction of tumor vessels are exacerbated by genetic disruption of R-Ras. Increased tumor cell penetration into the circulation in R-Ras KO mice also supports the role of R-Ras in controlling the permeability of tumor vessels. The reduced perfusion of tumor vessels was consistent with elevated tumor hypoxia, which in turn led to reduced efficacy of radiotherapy in R-Ras KO mice. In addition, R-Ras KO tumor vessels were sensitized to VEGF blockade, as expected from exacerbated immaturity of these vessels (Benjamin et al., 1999). However, the anti-tumor effect of DC101 was not enhanced in the KO mice. A possible explanation for this may be that DC101 at the dosage we used mainly destroyed highly immature nonfunctional vessels, which did not significantly contribute to tumor growth.
In contrast to the R-Ras gene disruption, the gain of function of R-Ras improved the structure and perfusion of blood vessels induced by VEGF in tumor ECM extract (Matrigel) implants. Other cell types such as infiltrating macrophages and fibroblasts may have also contributed to the observed effects. However, the studies using EC-specific or pericyte-specific expression of R-Ras gave insights into how R-Ras in each vascular cell type contributes to the overall vessel formation process. For instance, R-Ras activity in ECs was sufficient to inhibit VEGF-induced vessel outgrowth. This effect coincided with improved pericyte coverage and enhanced blood perfusion of newly formed vessels. These observations suggest that endothelial R-Ras activity redirects nascent vessel formation from angiogenic process to maturation process. On the other hand, R-Ras activity in pericytes prevented severe leakiness of the R-Ras KO endothelium through facilitating pericyte association with the sprouting vessels. This result indicates that R-Ras activity in pericytes alone can significantly contribute to the vessel maturation process independently from endothelial R-Ras. However, enhanced R-Ras signaling in pericytes was not very effective at improving perfusion of the vessels to which the pericytes are attached. Therefore, the role of pericyte R-Ras in vessel maturation may be primarily to promote pericyte association with ECs thereby providing integrity to the vessel structure and reducing permeability. The endothelial R-Ras activity appears to be more effective in improving blood perfusion of newly formed vessels. However, we can not rule out the possibility that the ex vivo R-Ras transduction and pericyte transplantation model we used in this study may have not been fully recapitulating the in vivo functions of R-Ras in pericytes. A transgenic mouse model for pericyte-specific R-Ras expression will be needed to clarify this point in future studies.
Recent study by Mazzone et al. demonstrated vascular normalization phenomenon caused by PHD2 haplodeficiency and consequential HIF-2 upregulation (Mazzone et al., 2009). Our data did not support the PHD2/HIF-2-dependent EC regulation as a mechanism by which R-Ras promotes vascular normalization. In the study by Mazzone et al., haplodeficiency of PHD2 resulted in elevated HIF-2α level in ECs during hypoxia, which led to HIF-2-driven upregulation of VE-cadherin and soluble-VEGFR1 expressions in ECs. This cascade, in turn, led to vascular normalization (Mazzone et al., 2009). In our study, constitutively activated R-Ras did not affect VE-cadherin expression in ECs. Instead, it inhibited VE-cadherin internalization induced by VEGF-stimulation of ECs. This resulted in VE-cadherin accumulation at adherens junctions and stabilization of the endothelial barrier. In our previous study of transcriptional regulation of human R-Ras gene, we did not find HIF response elements in the promoter region or the 5’ upstream cis-regulatory sequences (Xu and Komatsu, 2009). This suggests that, unlike many hypoxia induced angiogenesis regulators, R-Ras expression is not controlled by oxygen sensing mechanism involving HIFs. Indeed, R-Ras expression level did not increase in ECs or pericytes under hypoxia. There is an additional important difference between the vessel regulations by PHD2/HIF-2 and by R-Ras. PHD2 haplodeficiency does not affect vessel density, area, or vessel dilation while it normalizes the endothelial barrier and stability (Mazzone et al., 2009). In comparison, R-Ras does normalize all of these vessel parameters while halting angiogenic sprouting. Based on these observations, we propose that R-Ras-dependent mechanism is distinct from the oxygen sensing mechanism of vascular normalization. The chronic low-oxygen environment is a hallmark of solid tumors, and the levels of HIF proteins are elevated in the cells in the tumor microenvironment. Yet, tumor vessels are chronically abnormal instead of being normalized. The observations reported in this study suggest an alternative, oxygen sensingin-dependent vascular normalization phenomenon.
This study also identified a cellular function of R-Ras: enhancement of endothelial cell-cell adhesion via regulation of VE-cadherin internalization induced by VEGF. R-Ras inhibits VE-cadherin phosphorylation at Ser 665 residue that is critical for the VE-cadherin internalization process upon VEGF stimulation, suggesting the modulation of this site by R-Ras. Additional mechanism(s) independent of Ser 665 may also be involved in the R-Ras-mediated VE-cadherin regulation. Identification of the signaling pathways for these regulations is an important goal of future work that could further advance our understanding in the vascular normalization process.
The upregulation of R-Ras activity in pericytes resulted in enhanced pericyte adhesion to ECM and formation of focal adhesion complexes around cell perimeter facilitating the flattening and wide spreading of pericyte cell membrane over the adhering surface. Consistent with this effect, the activated R-Ras facilitated close association of pericytes with nascent microvessel sprouts in vivo, and this phenomenon was recapitulated in a 3-D co-culture system. These studies show that R-Ras plays important roles in both pericytes and ECs. Thus, R-Ras in each cell type is vital to tumor vessel maturation process.
Our results suggest that means to upregulate R-Ras would be useful for normalizing tumor vasculature. In contrast to VEGF blockade, which only induces transient vascular normalization with the narrow window of therapeutic opportunity (Carmeliet and Jain, 2011b), the R-Ras effect is sustained. Also, unlike VEGF blockade, R-Ras does not cause subsequent destruction of the newly formed vessels. Instead, R-Ras stabilizes these vessels. This may be a significant advantage in improving drug delivery to the tumors and in other situations where stable, functional vascularization is beneficial, e.g. angiogenesis in ischemic heart. R-Ras may also be useful as a biomarker for the vessel maturity and functionality, for instance, to assess vessel normalization, and for drug screens/validation. As maturation of blood vessels is essential for the establishment of functional vasculature in regenerating adult tissues, our findings suggest the significance of R-Ras to a broad range of clinical challenges, from cancer to therapeutic angiogenesis and tissue engineering.
EXPERIMENTAL PROCEDURES
Analyses of tumor blood vessels
All animal experiments were performed in accordance with protocols approved by the institutional animal care and use committees of Sanford-Burnham Medical Research Institute (SBMRI) and University of Alabama at Birmingham. The R-Ras KO mouse line has been described previously (Komatsu and Ruoslahti, 2005). The mice received s.c. implantations of 1 × 106 B16F10 mouse melanoma cells or Lewis lung carcinoma cells (LLC) at the flank, and the tumors were excised at day 10 and 14, respectively. 3 × 106 PPC-1 human prostate tumor cells in 50%-diluted Matrigel were inoculated s.c. at the flank of athymic nude mice. The tumors were excised 2 weeks later. Tumors were cryosectioned and tumor vessels were analyzed by immunofluorescence (see Supplemental Information). Six to seven mice per group were used, and vessels in several micrographs from each tumor section were analyzed unless indicated otherwise.
In vivo gene transfer
Matrigel (BD Bioscience; 0.5 ml) containing 60 ng/ml VEGF (R&D systems) and 60 U/ml heparin (Sigma) was injected into the flanks of wild type or R-Ras KO mice. Five days later, lentivirus carrying activated R-Ras (R-Ras38V) or control vector was injected into the Matrigel plugs at 1×106 TU. At 6 days post lentivirus inoculation, mice received intravenous injection of biotinylated lycopersicon esculentum (tomato) lectin to analyze vessel perfusion. Five to six mice per group were used. Matrigel plugs were collected and the sections stained for CD31, NG2, R-Ras, and streptavidin to analyze the vessel structure, pericyte association, and perfusion efficiency.
EC-specific tetracycline-inducible expression of R-Ras
The Tie2-rtTA transgenic mouse line has been described previously (Bao et al.). Matrigel containing 60 ng/ml VEGF and 60 U/ml heparin was injected into the flanks of Tie2-rtTA transgenic mice. Five days later, lentivirus harboring pLVX-Tight-Puro/R-Ras38V expression vector was injected into the Matrigel plugs at 106 TU. Doxycycline was administrated i.p. daily at 5 mg/kg from Day 5 to Day10. At Day 11, mice were perfused with biotinylated tomato lectin. Four mice per group were examined. To confirm the induction of R-Ras38V, mRNA was isolated from the plugs, and RT-PCR was conducted using a primer set for human R-Ras: 5’- GACCCCACTATTGAGGACTCC -3' and 5’-CTACAGGAGGACGCAGGG-3’. RT-PCR for cyclophilin A was used as control.
Pericyte implantation into R-Ras KO mice
Microvascular pericytes were isolated from wild type neonatal mouse brain (Wu et al., 2003) and transduced ex-vivo with R-Ras38V, R-Ras43N, or control lentivirus. These pericytes were fluorescently labeled with 8 µM CellTracker® green for 30 min, and 3 × 105 cells were implanted into the flank of R-Ras KO mice after mixed with 0.5 ml Matrigel containing VEGF and Heparin. Seven days later, the Matrigel plugs were grossly examined for the extent of blood leakage in the plugs. The association of the implanted pericytes (green-labeled) with vessels was analyzed in histological sections by fluorescence microscopy.
Statistics
Statics were performed using the 2-tailed Student’s t test to compare wild type with R-Ras KO mice and the 1-way ANOVA with Tukey’s multiple comparison test for in vitro experiment. Error bars represent S.E.M.
Supplementary Material
Highlights.
Normalization of pathologically regenerating vasculature by a Ras homolog
A crucial role of R-Ras for endothelial integrity
R-Ras regulates pericytes and their association with tumor vessels
A potential O2 sensing-independent vascular normalization phenomenon identified
Significance.
Newly formed blood vessels in tumors fail to mature into fully functional vessels due to the chronically angiogenic microenvironment. The functional impairment of these vessels hampers drug delivery, thereby diminishing the efficacy of anti-tumor therapies. Excessive vessel permeability associated with tumors also causes clinical complications such as cerebral edema in brain cancer patients. The ability to control vessel maturity in tumors, therefore, provides a potential therapeutic opportunity. This study revealed a key role for R-Ras in promoting tumor vessel maturation and normalization. Consistent with this role, R-Ras expressed in endothelial cells and in pericytes both contribute individually to the vessel regulation. This study suggests R-Ras as a potential target for controlling blood circulation and vascular permeability in solid tumors.
ACKNOWLEDGEMENT
Anti-NG2 antibody was a gift from Dr. William Stallcup of SBMRI. The antibody for phospho-specific VE-cadherin Ser 665 was a gift from Drs. Silvio Gutkind and Julie Garvard of NIH. We thank Drs. Sara Courtneidge and Sunyoung Lee for their advices on the manuscript. Scanning electron microscopy was done with a help of Dr. Qi Zhang at Advanced Materials Processing and Analysis Center, University of Central Florida. Histological preparations were done at the Histology and Imaging core facilities, and RT-qPCR at the Analytical Genomics Core of SBMRI at La Jolla and at Lake Nona. This work was supported by the National Cancer Institute grants CA125255, CA30199, CA13148, CA104898, the American Cancer Society grant IRG6000147, Bankhead-Coley Cancer Research Program 1BD11-34213, Florida Breast Cancer Foundation Fellowship Award, and departmental funds provided by the Department of Pathology, University of Alabama at Birmingham and by SBMRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional Experimental Procedures are available in online Supplementary Information.
REFERENCES
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Bao X, Moseman EA, Saito H, Petryanik B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011a;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011b;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- Coulon C, Georgiadou M, Roncal C, De Bock K, Langenberg T, Carmeliet P. From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxiainducible factor oxygen-sensing machinery. Arterioscler Thromb Vasc Biol. 2010;30:2331–2336. doi: 10.1161/ATVBAHA.110.214106. [DOI] [PubMed] [Google Scholar]
- Crosby CV, Fleming PA, Argraves WS, Corada M, Zanetta L, Dejana E, Drake CJ. VE-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood. 2005;105:2771–2776. doi: 10.1182/blood-2004-06-2244. [DOI] [PubMed] [Google Scholar]
- Darland DC, D'Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Semb H. Pericytes: gatekeepers in tumour cell metastasis? J Mol Med. 2008;86:135–144. doi: 10.1007/s00109-007-0258-2. [DOI] [PubMed] [Google Scholar]
- Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- Heidenreich R, Kappel A, Breier G. Tumor endothelium-specific transgene expression directed by vascular endothelial growth factor receptor-2 (Flk-1) promoter/enhancer sequences. Cancer Res. 2000;60:6142–6147. [PubMed] [Google Scholar]
- Helfrich I, Scheffrahn I, Bartling S, Weis J, von Felbert V, Middleton M, Kato M, Ergun S, Schadendorf D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Kappel A, Ronicke V, Damert A, Flamme I, Risau W, Breier G. Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood. 1999;93:4284–4292. [PubMed] [Google Scholar]
- Kersting S, Konopke R, Kersting F, Volk A, Distler M, Bergert H, Saeger HD, Grutzmann R, Bunk A. Quantitative perfusion analysis of transabdominal contrastenhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology. 2009;137:1903–1911. doi: 10.1053/j.gastro.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins' friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–776. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Ruoslahti E. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat Med. 2005;11:1346–1350. doi: 10.1038/nm1324. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci STKE. 2002;2002:PE7. doi: 10.1126/stke.2002.119.pe7. [DOI] [PubMed] [Google Scholar]
- Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods. 2003;130:53–63. doi: 10.1016/s0165-0270(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Xu L, Komatsu M. Promoter cloning and characterization of the anti-vascular proliferation gene, R-ras: role of Ets- and Sp-binding motifs. J Biol Chem. 2009;284:2706–2718. doi: 10.1074/jbc.M808184200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.