Abstract
Glucans are natural product carbohydrates that stimulate immunity. Glucans are internalized by the pattern recognition receptor, Dectin-1. Glucans were thought to be trafficked to phagolysosomes, but this is unproven. We examined the internalization and trafficking of soluble glucans in macrophages. Incubation of macrophages with glucan resulted in internalization of Dectin-1 and glucan. Inhibition of clathrin blocked internalization of the Dectin-1/glucan complex. Lipid raft depletion resulted in decreased Dectin levels and glucan uptake. Once internalized, glucans colocalized with early endosomes at 0 to 15 min, with the Golgi apparatus at 15 min to 24 h, and with Dectin-1 immediately (0 h) and again later (15 min-24 h). Glucans did not colocalize with lysosomes at any time interval examined. We conclude that the internalization of Dectin-1/glucan complexes in macrophages is mediated by clathrin and negatively regulated by lipid rafts and/or caveolin-1. Upon internalization, soluble glucans are trafficked via endosomes to the Golgi apparatus, not lysosomes.
Introduction
Glucans are glucose polymers that are major constituents of the cell wall of fungi and certain bacteria (Stone and Clarke, 1992). In purified form, glucans have been demonstrated to stimulate innate immunity (Williams, 1997). Pharmaceutical-grade glucans are currently being evaluated as agents to increase resistance to infections (Williams et al., 1996), facilitate wound repair (Wei et al., 2002), prevent myocardial ischemia/reperfusion injury (Li et al., 2004), and serve as adjuvants for antitumor responses (Hong et al., 2004). The underlying cellular and molecular mechanisms responsible for the in vivo activities of glucans are only now coming to light. Glucans are bound and internalized by pattern recognition receptors (PRRs) including the C-type lectin Dectin-1 (Ariizumi et al., 2000; Brown and Gordon, 2001; Brown et al., 2002, 2003; Taylor et al., 2002). Dectin-1 is expressed at high levels on blood and splenic monocytes, neutrophils, and alveolar and inflammatory macrophages and at lower levels on dendritic cells and subpopulations of T cells (Ariizumi et al., 2000; Brown et al., 2002; Taylor et al., 2002). Dectin-1 will bind free glucans as well as whole Candida albicans and Saccharomyces cerevisiae cells in a glucan-dependent manner and stimulate signal transduction cascades, resulting in an immune response. These cascades are currently under investigation; however, we do know that phosphorylation of the immunoreceptor tyrosine-based activation motif in the cytoplasmic tail of Dectin-1 results in the activation of the Src homology 2-domain containing protein spleen tyrosine kinase (Syk) (Rogers et al., 2005; Underhill et al., 2005). Syk then mediates the induction of the respiratory burst and the Card9-dependent activation of NF-κB (Rogers et al., 2005; Underhill et al., 2005; Gross et al., 2006). Dectin-1 also activates NF-κB in a myeloid differentiation factor 88-dependent manner via Toll-like receptor 2 (Brown et al., 2003; Gantner et al., 2003). Finally, Dectin-1 is also able to mediate an inflammatory response to glucans by stimulating NF-κB activity and tumor necrosis factor α production in a Toll-like receptor 2-independent manner; however, this pathway is poorly understood (Brown et al., 2003).
Dectin-1 mediates the rapid internalization of the Dectin-1/glucan complex (Ariizumi et al., 2000; Brown and Gordon, 2001; Brown et al., 2002, 2003; Taylor et al., 2002; Herre et al., 2004). It has been reported that internalization of the Dectin-1/glucan complex is not necessary for signal transduction; however, the only outcomes investigated were NF-κB activation and cytokine production (Brown et al., 2003). Furthermore, two independent laboratories have shown that Dectin-1-mediated signaling is modulated by internalization of the Dectin-1/glucan complex (Rosas et al., 2008; Hernanz-Falcón et al., 2009). Hernanz-Falcón et al. demonstrated that Dectin-1 mediated mitogen-activated protein kinase activation is increased with inhibition of internalization, and Rosas et al. found an increase in Dectin-1-mediated cytokine production with “frustrated phagocytosis.” It is not known whether internalization of the Dectin-1/glucan complex is necessary for activation of Syk or other glucan-induced signaling.
Herre et al. (2004) have reported that the fate of Dectin-1 depends on the type of glucan ligand that is bound. If Dectin-1 binds and internalizes a glucan that is biologically inactive, such as laminarin, Dectin-1 is recycled to the cell surface within 24 h (Herre et al., 2004). In striking contrast, if the glucan that is bound is biologically active, then Dectin-1 does not recycle to the cell surface (Herre et al., 2004). In this case, new Dectin-1 must be synthesized and trafficked to the cell surface (Herre et al., 2004). These differences may relate to the structure/activity relationships of the glucans (Herre et al., 2004). We have confirmed and extended the in vitro observations of Herre et al. by demonstrating a similar effect after a single in vivo administration of glucans with diverse structures and physicochemical characteristics (Ozment-Skelton et al., 2006). These data clearly demonstrate that the internalization/trafficking of Dectin-1, after glucan binding, is complex and multifaceted.
Despite significant advances in our understanding of glucan/Dectin-1 biology, relatively little is known about the cellular biology of glucans. For more than 40 years it has been assumed that glucans are internalized via the endosomal pathway and trafficked to the lysosome and/or phagolysosome, although there is limited evidence in support of this assumption. McCann et al., 2005 have reported that water-insoluble, microparticulate glucans are internalized by a noncaveolin-dependent mechanism into endosomes and are trafficked to lysosomes. Those investigators also reported that modulation of cellular activity by glucan did not depend on internalization. However, they did not establish the mechanisms of internalization, nor did they investigate internalization/trafficking of water-soluble glucans, such as those that circulate in the plasma of patients with active fungal infections (Mori et al., 1997; Nakamura et al., 1998; Hiyoshi et al., 1999; Digby et al., 2003) or the soluble glucans that have been investigated as biopharmaceuticals (Aarsaether et al., 2006). In addition, it is well known that some fungal pathogens, whose cell walls are rich in glucans, are capable of parasitizing and, indeed, living within macrophages where they avoid lysosomal degradation and intracellular killing (Bodey, 2000). Thus, there is evidence to suggest that internalized glucans are not necessarily trafficked to the phagolysosome. Therefore, the goal of the present study was to elucidate the cellular mechanisms by which highly purified, water-soluble, biologically active glucans are internalized and trafficked in murine macrophages.
Materials and Methods
Mice.
Age- and weight-matched adult male ICR/HSD mice were obtained from Harlan (Indianapolis, IN). Male caveolin-1 knockout mice [Cav(−/−)] and the recommended wild-type (WT) control B6129SF2/J were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were maintained on standard laboratory chow and water ad libitum with a 12-h light/dark cycle. Serologic testing confirmed that the mice were virus free. All animal procedures were reviewed and approved by the institutional review board at the James H. Quillen College of Medicine, East Tennessee State University.
Materials and Antibodies.
Methyl β cyclodextran (MβCD) and density gradient-grade sucrose were purchased from Sigma (St. Louis, MO). Both were dissolved in RPMI medium, incubated overnight in prewashed polymyxin-coated agarose beads (BioRad Laboratories, Hercules, CA) to remove endotoxin, and filter-sterilized before use. Anti-EEA1 early endosome marker, caveolin-1, and Lamp-1 lysosome marker were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Anti-Golph4 Golgi marker was purchased from Abcam Inc. (Cambridge, MA). Anti-Dectin-1 antibody and its isotype control were purchased from R&D Systems (Minneapolis, MN). Purified anti-goat and anti-rabbit secondary antibodies were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA) and were labeled with Alexa Fluor (AF) 488 or 555 succinimidyl ester (Molecular Probes, Eugene OR) according to the manufacturer's instructions.
Glucans.
Water-soluble β-glucan was kindly donated by Biotec Pharmacon (Tromso, Norway) (Aarsaether et al., 2006). Water-soluble glucan phosphate (GP) was derived from S. cerevisiae as described previously (Williams et al., 1991). The glucans were chemically characterized as described previously (Williams et al., 1991; Ensley et al., 1994; Müller et al., 1994,1995; Lowman et al., 1998).
Derivatization and Fluorescent Labeling of Glucans.
β-Glucan and GP were derivatized with diaminopropane at the reducing terminus as described previously(Rice et al., 2004, 2005; Ozment-Skelton et al., 2006). The derivatized glucans were labeled with AF647 or AF555 (Molecular Probes) as described previously (Rice et al., 2004, 2005; Ozment-Skelton et al., 2006). In brief, the glucans were hydrated in 0.1M borate buffer, pH 8.5. The succinimidyl ester form of AF647 or AF555 was dissolved in dimethyl sulfoxide (100 μg/ml) and added to the glucan/borate buffer solution. The mixture was incubated in the dark (18 h) on a rotary shaker set to slow speed at ambient temperature. Excess dye was removed by dialyzing against water until dye was no longer visible in the diasylate. Conjugation and binding activity were confirmed by incubation of the labeled glucan with Dectin-1-transfected cells for 30 min at 4°C, followed by washing (3×) with Dulbecco's phosphate-buffered saline solution (PBS; Sigma) and analyzed by flow cytometry.
Experimental Protocol.
Mice were injected with 2 ml of thioglycollate intraperitoneally. Seventy-two hours later, the elicited macrophages were harvested by peritoneal lavage with RPMI-100 media supplemented with fetal bovine serum, newborn calf serum, and antibiotics. Macrophages were cultured in the same media on glass coverslips in 12-well plates at 2.5 × 105 cells/well and allowed to adhere overnight. All of the following procedures were performed in the dark. Cells were incubated with the labeled β-glucan (100 mg/ml) for 3 h at 4°C. The cells were washed, the media were replaced, and the cells were incubated at 37°C for 0 to 24 h. Cells were washed in PBS and fixed with 4% paraformaldehyde. Cells were blocked in 5% normal horse serum with 0.3% Triton-X 100 (Sigma) for 1 h. The primary antibodies were diluted in 0.3% Triton-X 100 in PBS and incubated at 4°C overnight. The cells were incubated with the appropriate secondary antibody diluted in 0.3% Triton in PBS with 5 μM Draq5 nuclear counterstain (Biostatus, Leicestershire, UK) for 1 h. Coverslips were mounted with Prolong Antifade (Molecular Probes).
Confocal Microscopy.
The slides were evaluated on a Leica DM IRBE inverted confocal microscope with the TCS SP2 confocal system (Leica, Exton, PA). Images were evaluated by using multicolor/two-dimensional cytofluorogram software from Leica Microsystems, Inc. (Bannockburn, IL). The software quantifies the extent of colocalization by creation of a binary mask of the image data in the cytofluorogram. The binary mask is created by masking all of the pixels that are double positive for both the glucan fluorescence and the organelle fluorescence. Colocalization for each individual cell in the image was then assessed by using the mask intensity rate for the colocalized glucan versus the overall intensities of the glucan in the image. A mask intensity rate of ≥40% was used to confirm colocalization.
Flow Cytometry.
Thioglycollate-elicited macrophages were plated in six-well plates and allowed to adhere overnight. The cells were pretreated with media alone, media containing 5 mM MβCD, or media containing 0.5 M sucrose for 1 h at 37°C. Fluorescent labeled β-glucan was added at 10 μg/ml, and the cells were incubated for 3 h at 37°C. The cells were washed with PBS, scraped into Pharmingen Stain Buffer (BD Biosciences Pharmingen, San Diego, CA), pelleted by centrifugation, and suspended in Fc blocking solution (5% rabbit serum, 0.5% bovine serum albumin, and 5 mM EDTA with anti-murine CD16/32 (BD Biosciences Pharmingen). The cells were stained with biotinylated goat anti-Dectin-1 according to the protocol described by BD Biosciences Pharmingen. Biotinylated antibodies were detected by streptavidin-phycoerythrin (BD Biosciences Pharmingen). Cells were suspended in Pharmingen Stain buffer and analyzed for mean fluorescent intensity (MFI) by using a FACScalibur flow cytometer with CellQuest software (BD Biosciences, San Jose, CA).
Immunoprecipitation.
Confluent T-75 flasks of murine Raw cells that overexpress Dectin-1 (CHTA) were incubated with unlabeled GP for 0 to 180 min. The cells were washed and lifted with lidocaine. The cells were pelleted and then lysed in 20 mM Tris, pH 8 with 1% Triton X-100, 60 mM octylglucoside (Calbiochem, San Diego, CA), and protease inhibitor cocktail tablets (Santa Cruz Biotechnology, Inc.) for 30 min on ice. The lysates were homogenized by 10 passages through 26-gauge needles. Protein concentrations were measured by the BCA method (Thermo Fisher Scientific, Waltham, MA). To 750 μg of protein, 10 μl of anti-goat Dectin-1 antibody or 15 μl of anticaveolin antibody were added. Two hours later, 20 μl of Protein G beads were added and incubated overnight at 4°C with rotation. The beads were pelleted and washed three times with 20 Mm Tris with 1% Triton X-100 and protease inhibitor cocktail. After the addition of 4× SDS loading buffer, the samples were boiled for 5 m and then separated by SDS-polyacrylamide gel electrophoresis. The proteins were transferred to nitrocellulose membranes and blocked for 1 h in blocking buffer (Tris buffer saline, pH 7.4 with 5% skim milk and 1% Tween 20). The membranes were incubated overnight at 4°C and 30 min at 37°C with anticaveolin or biotinylated rat anti-Dectin-1 antibodies diluted in blocking buffer. After three washes in blocking buffer, the membranes were incubated for 1 h in anti-rabbit or SAv horseradish peroxidase at room temperature. After three washes in 1% Tween 20 in Tris-buffered saline, the antibodies were detected by using Western blotting detection reagents purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK).
Statistics.
Each flow experiment was performed in triplicate (n = 3 or 4). Dectin-1 and glucan data were summarized by the mean and S.E.M. Group mean responses were compared by analysis of variance and pair-wise multiple comparison testing (the least significant difference procedure or Tukey's procedure for cases where analysis of variance was not significant). Probability levels of 0.05 or smaller were considered significant.
Results
The Dectin-1/Glucan Complex Is Internalized by a Clathrin-Dependent Mechanism.
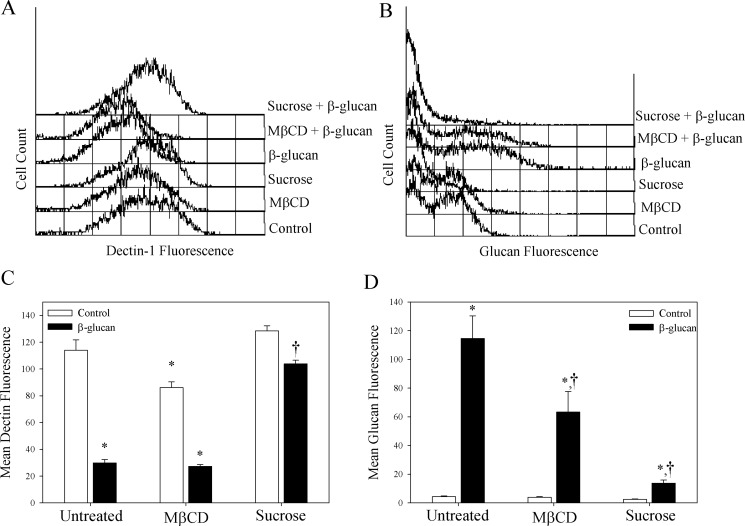
We first sought to determine the mechanism of internalization of the Dectin-1/glucan complex. The two primary mechanisms for internalization of receptor/ligand complexes are clathrin- and caveolin-1/lipid raft-mediated (Le Roy and Wrana, 2005). To determine which of these mechanisms is responsible for Dectin-1/glucan internalization, macrophages were incubated with MβCD, which depletes cholesterol and inhibits uptake by lipid rafts, or hyperosmotic sucrose, which inhibits clathrin-mediated uptake. Upon treating the cells with fluorescent-labeled β-glucan, the amount of cell surface Dectin-1, as expressed by MFI, was decreased by 73.8% (Fig. 1A), whereas the MFI representing glucan uptake was increased by 2535% compared with untreated cells (Fig. 1B). This indicates internalization of the Dectin-1/glucan complex. MβCD treatment significantly decreased Dectin-1 MFI by 24.4% compared with untreated cells (p < 0.05; Fig. 1A). β-Glucan-treated MβCD cells showed a 68.3% decrease in Dectin-1 surface expression compared with MβCD alone (p < 0.05; Fig. 1A) and a 76.0% decrease compared with untreated control cells (p < 0.05; Fig. 1A). It is noteworthy that the MβCD-treated cells internalized 44.8% less β-glucan compared with untreated macrophages, i.e., a 1506% increase in the mean glucan fluorescence compared with controls (p < 0.05; Fig. 1B). This suggests that the decrease in Dectin-1 expression in MβCD-treated cells leads to decreased glucan uptake. In contrast, pretreatment with hyperosmotic sucrose alone did not significantly alter the amount of Dectin-1 on the macrophage membrane compared with controls (Fig. 1A). Furthermore, although incubation of the sucrose-treated cells with β-glucan decreased Dectin-1 MFI (19.2%, p < 0.05), the reduction was significantly less than treatment with β-glucan alone (247.7%) (Fig. 1A). However, β-glucan fluorescence was significantly decreased in sucrose-treated macrophages compared with macrophages treated only with β-glucan (88.0%, Fig. 1B, p < 0.05). This indicates that the sucrose treatment inhibited Dectin-1/glucan internalization, suggesting that β-glucan is being internalized by a clathrin-mediated mechanism.
Fig. 1.
Macrophage internalization of the Dectin-1/glucan complex is mediated via a clathrin-dependent mechanism. Thioglycollate-elicited macrophages were incubated with media alone or fluorescent-labeled β-glucan for 3 h. Macrophages were incubated with MβCD (5 mM) or sucrose (500 mM) for 1 h before and after addition of β-glucan. Macrophages were stained with anti-Dectin antibody and analyzed by flow cytometry for Dectin-1 (A and C) and glucan (B and D) MFI. C and D show the mean ± S.E.M. of three independent experiments (n = 4). *, p < 0.05 compared with untreated control. †, p < 0.05 compared with glucan alone.
Caveolin-1 Is Not Required for Internalization of the Dectin-1/Glucan Complex.
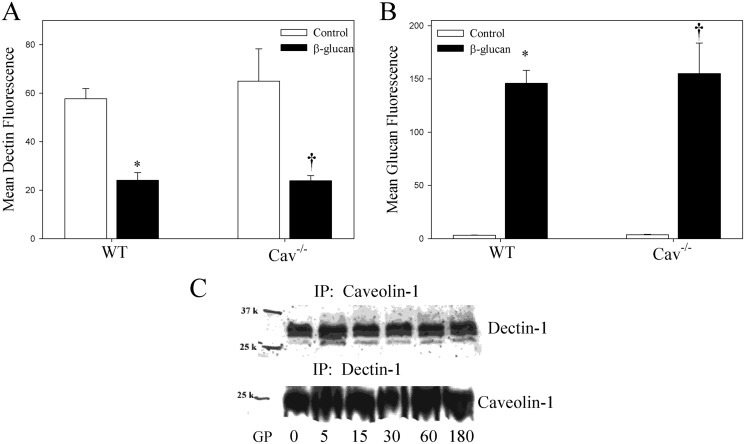
Because the lipid raft-depleted macrophages had lower levels of surface Dectin-1, and caveolin-1 and caveolae are an important lipid raft-containing domain, we sought to determine the effect of caveolin-1 on Dectin-1 levels and internalization of the Dectin-1/glucan complex. Macrophages from Cav(−/−) and WT mice were incubated with fluorescent-labeled β-glucan, stained for Dectin-1, and analyzed by flow cytometry to determine Dectin-1 and glucan MFI. Macrophages from Cav(−/−) and WT mice expressed similar levels of membrane Dectin-1 (57.7 versus 64.9, respectively) (Fig. 2A). Coincubation with glucan did not significantly alter Dectin-1 membrane levels in Cav(−/−) versus WT macrophages (23.8 versus 24.0, respectively) (Fig. 2A). Glucan uptake was similarly unchanged between Cav(−/−) and WT macrophages (155.1 versus 145.8, respectively). These data suggest that caveolin-1 and caveosomes play no role in the uptake of the Dectin-1/glucan complex, despite the lipid raft being involved in Dectin-1 cell surface expression. It is noteworthy that immunoprecipitation of Dectin-1 and caveolin-1 from CHTA cells treated with GP for 0 to 180 min reveals that the two immunoprecipitate together (Fig. 2C). Caveolin-1 was present in the lipid raft-rich caveolae, suggesting that Dectin-1 is also present in caveolae and probably associates with caveolin-1, although caveolin-1 has no effect on the internalization of the Dectin-1/glucan complex.
Fig. 2.
Internalization of the Dectin-1/glucan complex is negatively regulated by caveolin-1. Thioglycollate-elicited macrophages from Cav(−/−) and WT mice were incubated with fluorescent-labeled β-glucan (10 μg/ml) for 3 h. A and B, the cells were stained with anti-Dectin-1 antibody and analyzed for Dectin-1 (A) and glucan (B) MFI by flow cytometry. Shown are the mean ± S.E.M. of three independent experiments (n = 3). *, p < 0.05 compared with WT control. †, p < 0.05 compared with Cav(−/−) control. C, RAW cells that overexpress Dectin-1 were incubated with GP (10 mg/ml) for 0 to 180 min. The cells were lysed, and equal amounts of protein were incubated with anticaveolin-1 antibody or anti-Dectin-1 antibody. The proteins were precipitated with Protein G beads and separated by SDS-polyacrylamide gel electrophoresis. After transfer to nitrocellulose membranes, the proteins were detected by Western blot. Representative images of three independent experiments are shown. IP, immunoprecipitation.
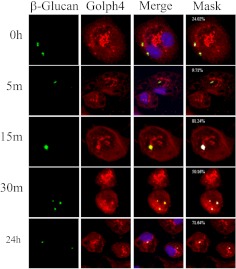
Glucan Is Trafficked to the Golgi Apparatus in Macrophages by Endosomes.
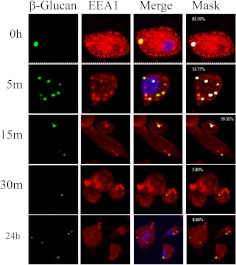
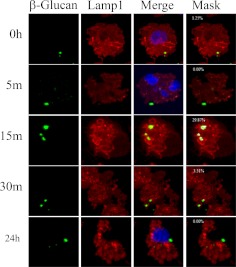
The previous data demonstrate that glucans are internalized by a clathrin-mediated mechanism that is negatively regulated by caveolin-1/caveosomes; however, it is not known how the glucans are trafficked after internalization. Conventional wisdom implies that glucans are trafficked via endosomes to lysosomes (McCann et al., 2005). To determine whether this is the case, macrophages were incubated with fluorescent-labeled β-glucan (green in Figs. 3 and 4), and stained with antibodies directed against endosomes (EEA1) (red in Fig. 3) or lysosomes (Lamp1) (red in Fig. 4). Confocal microscopy analysis revealed that glucans colocalized with the early endosome marker EEA1 (yellow and white in Fig. 3, Merge and Mask, respectively). β-Glucan was found in association with endosomes at 0, 5, and 15 min (yellow and white in Fig. 3, Merge and Mask, respectively). Thus, upon internalization, β-glucan is transported within the cells by the early endosome. However, β-glucan colocalized with lysosomes in fewer than 30% of cells imaged at any time interval examined (yellow and white in Fig. 4, Merge and Mask, respectively). Therefore, β-glucan is not consistently trafficked to lysosomes. Because other pathogen-associated molecular patterns and PRRs are known to be trafficked to the Golgi apparatus (Triantafilou et al., 2004), we asked whether glucan was trafficked to the Golgi. Macrophages were treated with fluorescent-labeled β-glucan (green in Fig. 5) and stained with the Golgi marker Golph4 (red in Fig. 5). β-Glucan was found to colocalize with the Golgi apparatus marker Golph4 at 15 min, 30 min, and 24 h (yellow and white in Fig. 5, Merge and Mask, respectively). This indicates that bioactive glucans are trafficked to the Golgi apparatus in macrophages, where they remain for at least 24 h.
Fig. 3.
Murine macrophages transport glucans via endosomes. Thioglycollate-elicited macrophages were incubated with fluorescent-labeled β-glucan for 3 h at 4°C, and then for 0 to 24 h at 37°C. The cells were stained for early endosomes by using EEA1 antibody, and the images were acquired with confocal microscopy. The numbers shown in white in the masked images (Mask column) are the mask intensity rate for the images and indicate the degree of colocalization. A mask intensity rate of ≥40% was considered indicative of colocalization. Representative maximum projection images of four to eight replicates from two to four independent experiments are shown.
Fig. 4.
Murine macrophages do not transport glucans via lysosomes. Thioglycollate-elicited macrophages were incubated with fluorescent-labeled β-glucan for 3 h at 4°C, and then for 0 to 24 h at 37°C. The cells were stained for lysosomes by using Lamp1 antibody, and the images were obtained by confocal microscopy. The numbers shown in white in the masked images (Mask column) are the mask intensity rate for the images and indicate the degree of colocalization. A mask intensity rate of ≥40% was considered indicative of colocalization. Representative maximum projection images of four to eight replicates from two to four independent experiments are shown.
Fig. 5.
Glucans are trafficked to the Golgi apparatus in murine macrophages. Thioglycollate-elicited macrophages were incubated with fluorescent-labeled β-glucan for 3 h at 4°C, and then for 0 to 24 h at 37°C. The cells were stained for Golgi by using Golph4 antibody, and the images were obtained by confocal microscopy. The numbers shown in white in the masked images (Mask column) are the mask intensity rate for the images and indicate the degree of colocalization. A mask intensity rate of ≥40% was considered indicative of colocalization. Representative maximum projection images of four to eight replicates from two to four independent experiments are shown.
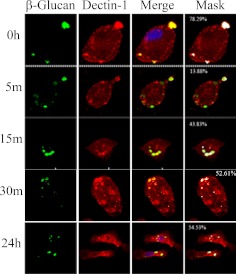
Glucan and Dectin-1 Dissociate after Internalization of the Dectin-1/Glucan Complex by Macrophages.
Previous work has shown that Dectin-1 is degraded by the lysosome upon internalization with glucans (Herre et al., 2004). Because our data indicate that glucans were not trafficked to the lysosome, we sought to determine whether the glucan/Dectin-1 complex remained intact upon internalization. Macrophages were treated with fluorescent-labeled β-glucan (green in Fig. 6) and then stained for Dectin-1 (red in Fig. 6). At 0 time, β-glucan colocalized with Dectin-1 (yellow and white in Fig. 6, Merge and Mask, respectively); however, the number of cells in which the Dectin-1 and β-glucan were colocalized decreased substantially after 5-min incubation (yellow and white in Fig. 6, Merge and Mask, respectively). Colocalization between Dectin-1 and β-glucan was observed again at 15 min and up to 24 h (yellow and white in Fig. 6, Merge and Mask, respectively). This suggests that the Dectin-1/glucan complex is disrupted upon internalization, and the Dectin-1 from the cell surface is then degraded while the glucan remains within the macrophage. Because β-glucan is found associated with the Golgi apparatus at these time intervals, it is reasonable to assume that the glucan colocalizes with Dectin-1 that has yet to be released from the Golgi.
Fig. 6.
The Dectin-1/glucan complex dissociates after internalization in murine macrophages. Thioglycollate-elicited macrophages were incubated with fluorescent-labeled β-glucan for 3 h at 4°C, and then for 0 to 24 h at 37°C. The cells were stained for Dectin-1, and the images were obtained by confocal microscopy. The numbers shown in white in the masked images (Mask column) are the mask intensity rate for the images and indicate the degree of colocalization. A mask intensity rate of ≥40% was considered indicative of colocalization. Representative maximum projection images of four to eight replicates from two to four independent experiments are shown.
Discussion
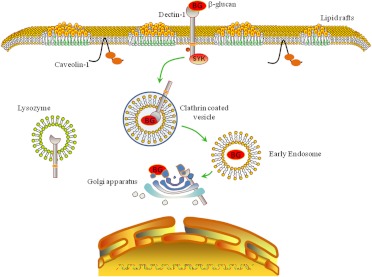
This is the first study to characterize the internalization and trafficking of soluble β-glucans in murine macrophages. Several new and novel observations have emerged from this research. First, our data demonstrate that Dectin-1/glucan complexes are internalized by a clathrin-mediated mechanism (Fig. 7). Second, we show that internalization of the Dectin-1/glucan complex is negatively regulated by lipid rafts, although not by caveolin-1. Third, we present evidence that Dectin-1 and caveolin-1 interact within the macrophage in the presence or absence of glucans (Fig. 7). Fourth, our data clearly show that glucans are trafficked by early endosomes to the Golgi apparatus after internalization (Fig. 7). Fifth, we observed that soluble glucans do not colocalize with macrophage lysosomes. Finally, we present evidence that the Dectin-1/glucan complex dissociates early after internalization in the macrophage endosome, but when the glucan is trafficked to the Golgi apparatus it again colocalizes with Dectin-1 (Fig. 7).
Fig. 7.
Schematic showing the proposed mechanisms for internalization and trafficking of soluble glucans by murine macrophages. In this model, soluble glucan is bound by membrane-associated Dectin-1, and the Dectin-1/glucan complex is rapidly internalized via a clathrin-dependent mechanism. The Dectin-1/glucan complex is then trafficked to the Golgi apparatus via endosomes. However, the Dectin-1/glucan complex dissociates after early internalization, followed by glucan colocalizing with Golgi-associated Dectin-1 at later time points. BG, β-glucan.
The evidence for internalization of the Dectin-1/GP complex via a clathrin-dependent mechanism is reasonable and clear cut; however, the role of lipid rafts and caveolin-1/caveosomes in internalization of the receptor/ligand complex is more complex. Our data demonstrate that the loss of lipid rafts results in a reduction in cell surface Dectin-1 and a decrease in glucan internalization. These data suggest that lipid rafts regulate macrophage Dectin-1 cell surface expression and thus glucan internalization. Caveolin-1 is known to interact with signaling molecules (Jayanthi et al., 2004; Williams and Lisanti, 2004). We present the first evidence that caveolin-1 interacts with the PRR Dectin-1. It is possible that Dectin-1 is normally sequestered into caveolae/lipid rafts via its interaction with caveolin-1. Disruption of lipid rafts may lead to a decrease in overall membrane Dectin-1 levels. Maintenance of cell surface Dectin-1 levels by lipid rafts may have important consequences for the Dectin-1-mediated cellular responses to glucan. Further studies are necessary to determine the effect of lipid rafts on the immune response mediated by Dectin-1 in response to glucans or fungal infection.
This study also demonstrated that soluble β-glucans are transported by early endosomes to the Golgi apparatus where they remain for at least 24 h. It is interesting to note that soluble glucans are transported to the Golgi apparatus after internalization rather than to lysosomes as has been reported previously for particulate glucans (McCann et al., 2005). It is not clear why soluble and particulate glucans would be differentially processed by macrophages, but this may relate to the differences in the physical state of the glucans, i.e., soluble versus particulate, and the mechanisms by which each macromolecule is internalized, i.e., endocytosis versus phagocytosis. Regardless, by bypassing lyosomal structures, pathogens can avoid intracellular killing mechanisms (Norkin, 2001). This could be interpreted to mean that glucans exposed in the cell wall of fungal pathogens may be a mechanism whereby the pathogen can co-opt macrophage cellular processes to avoid intracellular killing by macrophages. As an example, certain fungi, including C. albicans, are able to avoid lysosomal degradation to survive and multiply within macrophages after phagocytocis (Bodey, 2000). Endosomal trafficking of glucans to the Golgi apparatus, rather than lysosomes, might explain how fungal pathogens are able to survive within macrophages after internalization. It is also possible that our results differ from those of McCann et al. (2005) because of the method used to detect localization. We used confocal microscopy, which assesses colocalization with greater accuracy than the wide field fluorescence microscopy used by McCann et al. With wide-field fluorescent microscopy the entire depth of the specimen is illuminated and out-of-focus signals can interfere. Confocal micros copy illuminates a single, focused area of the specimen, so only signals from the focused plane are detected. This results in a much crisper image and reduces the possibility of “false positive” colocalization. Further studies using highly purified particulate glucan and confocal microscopy are necessary to determine whether particulate and soluble glucans are differentially internalized and trafficked within macrophages.
We observed that soluble glucans are trafficked to the Golgi apparatus. Localization to the Golgi apparatus is not an uncommon fate for pathogen-associated molecular patterns, because both lipoteichoic acid and lipopolysaccharide are transported to the Golgi apparatus after internalization (Thieblemont and Wright, 1999; Latz et al., 2002; Triantafilou et al., 2004). Indeed, Le Roy and Wrana, 2005 have reported that there are distinct caveolar endocytic pathways that target the Golgi (Le and Nabi, 2003). As part of this study, we asked the question what happens to the glucans once they have reached the Golgi? Mammalian cells do not possess the enzymes necessary for glucan catabolism (Stone and Clarke, 1992). We speculated that there are two possibilities for the fate of glucans after they reach the Golgi. The glucans may remain in the Golgi or another cellular compartment for the life of the cell, or they may be transported out of the macrophage. We found that glucans associate with the Golgi apparatus for at least 24 h, and we found no evidence for glucan release by the cell during this time. This suggests that glucans remain within macrophages, and perhaps other cells, for prolonged periods of time. This could explain, in part, why the biological effects of soluble glucans have been observed for up to 1 week after a single in vivo administration, even though the pharmacokinetic data indicate that soluble glucans are rapidly cleared from the systemic circulation after parenteral administration (Rice et al., 2004).
Herre et al. (2004) have demonstrated that upon internalization of the Dectin-1/glucan complex Dectin-1 is degraded within lysosomes and new receptor must be synthesized before the return of Dectin-1 to the cell surface. The present study has shown that glucans are not trafficked to lysosomes, which was an unanticipated finding. Another unanticipated finding was that Dectin-1 and glucan separate as soon as 5 min after internalization. However, we did find that glucan and Dectin-1 colocalized at later time intervals. At these later time points the glucans and Dectin-1 were found in association with the Golgi apparatus. It is possible that this second phase of Dectin-1/glucan association represents Golgi-associated Dectin-1 that may be newly synthesized and has not yet been transported to the cell surface. It is not clear whether the Golgi-associated glucan is bound to the Golgi-associated Dectin-1 or whether the two are just in close proximity. Thus, we cannot say with certainty whether Dectin-1 binds glucans intracellularly, but these data suggest the possibility that this may occur.
In conclusion, we present evidence for a new and novel mechanism by which β-glucans are internalized and trafficked within macrophages. Our data demonstrate that water-soluble glucans, derived from a fungal source, are internalized within macrophages and trafficked to the Golgi apparatus via a clathrin-dependent mechanism that is negatively regulated by lipid rafts. These data also demonstrate that the intracellular processing of fungal glucans by macrophages is much more complex than previously thought.
This work was supported, in part, by the National Institutes of Health National Institute of General Medical Sciences [Grant GM53522] (to D.L.W.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- PRR
- pattern recognition receptor
- Syk
- spleen tyrosine kinase
- NF-κB
- nuclear factor κB
- GP
- glucan phosphate
- MβCD
- methyl β cyclodextran
- AF
- Alexa Fluor
- Cav(−/−)
- caveolin-1 knockout
- WT
- wild type
- EEA1
- early endosomal antigen 1
- PBS
- phosphate-buffered saline
- MFI
- mean fluorescent intensity.
Authorship Contributions
Participated in research design: Ozment and Williams.
Conducted experiments: Ozment and Goldman.
Contributed new reagents or analytic tools: Williams.
Performed data analysis: Ozment and Kalbfleisch.
Wrote or contributed to the writing of the manuscript: Ozment and Williams.
References
- Aarsaether E, Rydningen M, Einar Engstad R, Busund R. (2006) Cardioprotective effect of pretreatment with β-glucan in coronary artery bypass grafting. Scand Cardiovasc J 40:298–304 [DOI] [PubMed] [Google Scholar]
- Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, 3rd, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A. (2000) Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem 275:20157–20167 [DOI] [PubMed] [Google Scholar]
- Bodey GP. (2000) Fungal infections in immunocompromised patients-candidiasis. Infect Dis Rev 1:4–8 [Google Scholar]
- Brown GD, Gordon S. (2001) Immune recognition: A new receptor for β-glucans. Nature 413:36–37 [DOI] [PubMed] [Google Scholar]
- Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. (2003) Dectin-1 mediates the biological effects of β-glucans. J Exp Med 197:1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. (2002) Dectin-1 is a major β-glucan receptor on macrophages. J Exp Med 196:407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D. (2003) Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin Diagn Lab Immunol 10:882–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley HE, Tobias B, Pretus HA, McNamee RB, Jones EL, Browder IW, Williams DL. (1994) NMR spectral analysis of a water-insoluble (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr Res 258:307–311 [DOI] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Gewies A, Finger K, Schäfer M, Sparwasser T, Peschel C, Förster I, Ruland J. (2006) Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442:651–656 [DOI] [PubMed] [Google Scholar]
- Hernanz-Falcón P, Joffre O, Williams DL, Reis e Sousa C. (2009) Internalization of Dectin-1 terminates induction of inflammatory responses. Eur J Immunol 39:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. (2004) Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104:4038–4045 [DOI] [PubMed] [Google Scholar]
- Hiyoshi M, Tagawa S, Hashimoto S, Sakamoto C, Tatsumi N. (1999) Evaluation of a new laboratory test measuring plasma (1→3)-β-d-glucan in the diagnosis of Candida deep mycosis: comparison with a serologic test. Kansenshogaku Zasshi 73:1–6 [DOI] [PubMed] [Google Scholar]
- Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. (2004) Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol 173:797–806 [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Ramamoorthy S. (2004) Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization.J Biol Chem 279:19315–19326 [DOI] [PubMed] [Google Scholar]
- Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. (2002) Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem 277:47834–47843 [DOI] [PubMed] [Google Scholar]
- Le PU, Nabi IR. (2003) Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci 116:1059–1071 [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. (2005) Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol 6:112–126 [DOI] [PubMed] [Google Scholar]
- Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, Williams DL. (2004) Modulating Toll-like receptor mediated signaling by (1→3)-β-d-glucan rapidly induces cardioprotection. Cardiovasc Res 61:538–547 [DOI] [PubMed] [Google Scholar]
- Lowman D, Ensley H, Williams D. (1998) Identification of phosphate substitution sites by NMR spectroscopy in a water-soluble phosphorylated (1–3)-β-D-glucan. Carbohydr Res 306:559–562 [Google Scholar]
- McCann F, Carmona E, Puri V, Pagano RE, Limper AH. (2005) Macrophage internalization of fungal β-glucans is not necessary for initiation of related inflammatory responses. Infect Immun 73:6340–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Ikemoto H, Matsumura M, Yoshida M, Inada K, Endo S, Ito A, Watanabe S, Yamaguchi H, Mitsuya M, et al. (1997) Evaluation of plasma (1→3)-β-d-glucan measurement by the kinetic turbidimetric Limulus test, for the clinical diagnosis of mycotic infections. Eur J Clin Chem Clin Biochem 35:553–560 [PubMed] [Google Scholar]
- Müller A, Mayberry W, Acuff R, Thedford S, Browder W, Williams D. (1994) Lipid content of macroparticulate (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Microbios 79:253–261 [PubMed] [Google Scholar]
- Müller A, Pretus HA, McNamee RB, Jones EL, Browder IW, Williams DL. (1995) Comparison of the carbohydrate biological response modifiers Krestin, schizophyllan and glucan phosphate by aqueous size exclusion chromatography with in-line argon-ion multi-angle laser light scattering photometry and differential viscometry detectors. J Chromatogr B Biomed Appl 666:283–290 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tazawa S, Tsutiya M. (1998) The Clinical significance of plasma (1→3)-β-d-glucan measurement by the kinetic turbidimetric limulus test for fungal febrile episodes. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 9:33–39 [PubMed] [Google Scholar]
- Norkin LC. (2001) Caveolae in the uptake and targeting of infectious agents and secreted toxins. Adv Drug Deliv Rev 49:301–315 [DOI] [PubMed] [Google Scholar]
- Ozment-Skelton TR, Goldman MP, Gordon S, Brown GD, Williams DL. (2006) Prolonged reduction of leukocyte membrane-associated Dectin-1 levels following β-glucan administration. J Pharmacol Exp Ther 318:540–546 [DOI] [PubMed] [Google Scholar]
- Rice PJ, Adams EL, Ozment-Skelton T, Gonzalez AJ, Goldman MP, Lockhart BE, Barker LA, Breuel KF, Deponti WK, Kalbfleisch JH, et al. (2005) Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther 314:1079–1086 [DOI] [PubMed] [Google Scholar]
- Rice PJ, Lockhart BE, Barker LA, Adams EL, Ensley HE, Williams DL. (2004) Pharmacokinetics of fungal (1–3)-β-d-glucans following intravenous administration in rats. Int Immunopharmacol 4:1209–1215 [DOI] [PubMed] [Google Scholar]
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, et al. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22:507–517 [DOI] [PubMed] [Google Scholar]
- Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, Williams DL, Brown GD, Taylor PR. (2008) The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol 181:3549–3557 [DOI] [PubMed] [Google Scholar]
- Stone BA, Clarke AE. (1992) Chemistry and Biology of (1–3)-β-d-Glucan. La Trobe University Press, Melbourne, Australia [Google Scholar]
- Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. (2002) The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 169:3876–3882 [DOI] [PubMed] [Google Scholar]
- Thieblemont N, Wright SD. (1999) Transport of bacterial lipopolysaccharide to the Golgi apparatus. J Exp Med 190:523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Manukyan M, Mackie A, Morath S, Hartung T, Heine H, Triantafilou K. (2004) Lipoteichoic acid and Toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J Biol Chem 279:40882–40889 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Zhang L, Williams DL, Browder IW. (2002) Glucan stimulates human dermal fibroblast collagen biosynthesis through a nuclear factor-1 dependent mechanism. Wound Repair Regen 10:161–168 [DOI] [PubMed] [Google Scholar]
- Williams DL. (1997) Overview of (1→3)-β-d-glucan immunobiology. Mediators Inflamm 6:247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, McNamee RB, Jones EL, Pretus HA, Ensley HE, Browder IW, Di Luzio NR. (1991) A method for the solubilization of a (1–3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr Res 219:203–213 [DOI] [PubMed] [Google Scholar]
- Williams DL, Mueller A, Browder W. (1996) Glucan-based macrophage stimulators: a review of their anti-infective potential. Clin Immunother 5:392–399 [Google Scholar]
- Williams TM, Lisanti MP. (2004) The Caveolin genes: from cell biology to medicine. Ann Med 36:584–595 [DOI] [PubMed] [Google Scholar]