Abstract
N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl) benzamide (ADL5859) and N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5747) are novel δ-opioid agonists that show good oral bioavailability and analgesic and antidepressive effects in the rat and represent potential drugs for chronic pain treatment. Here, we used genetic approaches to investigate molecular mechanisms underlying their analgesic effects in the mouse. We tested analgesic effects of ADL5859 and ADL5747 in mice by using mechanical sensitivity measures in both complete Freund's adjuvant and sciatic nerve ligation pain models. We examined their analgesic effects in δ-opioid receptor constitutive knockout (KO) mice and mice with a conditional deletion of δ-receptor in peripheral voltage-gated sodium channel (Nav)1.8-expressing neurons (cKO mice). Both ADL5859 and ADL5747, and the prototypical δ agonist 4-[(R)-[(2S,5R)-4-allyl-2,5-dimethyl-piperazin-1-yl]-(3-methoxyphenyl)methyl]-N,N-diethyl-benzamide (SNC80) as a control, significantly reduced inflammatory and neuropathic pain. The antiallodynic effects of all three δ-opioid agonists were abolished in constitutive δ-receptor KO mice and strongly diminished in δ-receptor cKO mice. We also measured two other well described effects of δ agonists, increase in locomotor activity and agonist-induced receptor internalization by using knock-in mice expressing enhanced green fluorescence protein-tagged δ receptors. In contrast to SNC80, ADL5859 and ADL5747 did not induce either hyperlocomotion or receptor internalization in vivo. In conclusion, both ADL5859 and ADL5747 showed efficient pain-reducing properties in the two models of chronic pain. Their effects were mediated by δ-opioid receptors, with a main contribution of receptors expressed on peripheral Nav1.8-positive neurons. The lack of in vivo receptor internalization and locomotor activation, typically induced by SNC80, suggests agonist-biased activity at the receptor for the two drugs.
Introduction
Opiates produce their strong analgesic and addictive properties by acting at opioid receptors, classified as μ, δ, and κ receptors. μ-Opioid agonists, such as morphine, have been the most powerful analgesics widely used in the clinic for more than a century. These compounds, however, produce side effects, including constipation, respiratory depression, and sedation (McNicol et al., 2003), and are often ineffective under chronic pain conditions such as neuropathic pain (Glajchen, 2001). Furthermore, 15 to 25% adults suffer from chronic pain (Brennan et al., 2007), and the treatment of persistent pain remains a true challenge. In this context, δ-opioid receptors have emerged as a promising therapeutic target, in particular because they exhibit analgesic activity in models of chronic pain and lack classic morphine-like side effects (Gavériaux-Ruff and Kieffer, 2011). No δ-opioid agonist has yet reached the clinic, but progress is being made toward the development of novel useful compounds.
In the past decade, preclinical pharmacological tools and genetic approaches have provided a comprehensive view of δ-receptor function in rodent models (Pradhan et al., 2011). The development of several selective nonpeptidic δ-opioid agonists with small molecular weights and the analysis of novel mouse lines with selected mutations targeting the δ-opioid receptor have clarified the role of δ receptors in pain control and mood disorders (Gavériaux-Ruff and Kieffer, 2011; Pradhan et al., 2011). In brief, pharmacological data in rodents have demonstrated that δ-opioid agonists weakly influence acute pain perception (Gallantine and Meert, 2005), but efficiently decrease persistent pain, including inflammatory pain (Fraser et al., 2000; Brandt et al., 2001; Dondio et al., 2001), cancer (Brainin-Mattos et al., 2006; Otis et al., 2011), and neuropathic pain (Dondio et al., 2001; Holdridge and Cahill, 2007; Kabli and Cahill, 2007). The analysis of conventional knockout (KO) mice showed the existence of an endogenous δ-opioid receptor tone, which reduces chronic pain (Nadal et al., 2006; Gavériaux-Ruff et al., 2008) and improves mood (Filliol et al., 2000; Gavériaux-Ruff and Kieffer, 2002). Further analysis of pain responses and δ-opioid analgesia in conditional KO (cKO) animals has highlighted a critical role of peripheral receptors in chronic pain control (Gavériaux-Ruff et al., 2011).
The novel nonpeptidic compounds N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5859) (Le Bourdonnec et al., 2008) and N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5747) (Le Bourdonnec et al., 2009) were developed with structures distinct from other δ-agonist classes to obtain molecules with higher selectivity for δ receptors (Fig. 1). They showed δ-opioid selectivity based on nanomolar affinities and signaling potencies for the receptor in vitro, with no detectable binding at more than 100 nonopioid receptors, channels, or enzymes (Le Bourdonnec et al., 2008, 2009). In rats, the two drugs display good oral bioavailability and produce antidepressant (ADL5859) and antihyperalgesic effects in a model of inflammatory pain (Le Bourdonnec et al., 2008, 2009). Here, we took advantage of several existing mutant mouse lines targeting the δ-opioid receptor gene to investigate the molecular bases of ADL5747 and ADL5859 effects in vivo. We first tested analgesic activities of the two compounds in models of inflammatory and neuropathic pain in wild-type (WT) mice. We then tested whether these drugs produce analgesic effects in mice lacking δ receptors, either throughout the body (Filliol et al., 2000) or specifically in peripheral Nav1.8-positive nociceptive neurons (Gavériaux-Ruff et al., 2011). Nav1.8 is a voltage-gated sodium channel expressed specifically in more than 85% of primary nociceptive neurons (Stirling et al., 2005; Liu and Wood, 2011) that include both unmyelinated C and thinly myelinated Aδ fibers (Foulkes and Wood, 2008). Nav1.8-expressing neurons are involved in sensing cold and mechanical pressure and inflammatory and visceral hyperalgesia (Liu and Wood, 2011). Our data show that, like 4-[(R)-[(2S,5R)-4-allyl-2,5-dimethyl-piperazin-1-yl]-(3-methoxyphenyl)methyl]-N,N-diethyl-benzamide (SNC80), both ADL5747 and ADL5859 efficiently reduce inflammatory and neuropathic pain mainly by recruiting δ-opioid receptors expressed by peripheral Nav1.8-expressing neurons.
Fig. 1.
Chemical structures of SNC80 (left), ADL5747 (center), and ADL5859 (right).
Finally, our previous work has revealed the existence of biased agonism at the δ-opioid receptor in vivo. We showed that the two δ agonists, SNC80 and N,N-diethyl-4-(phenylpiperidin-4-ylidene-methyl)-benzamide (AR-M100390), display comparable analgesic properties, but distinct locomotor and trafficking effects (Pradhan et al., 2009), and these differential agonist properties lead to highly distinct forms of tolerance (Pradhan et al., 2010). Because biased agonism has important implications for receptor biology and drug design (Galandrin et al., 2007), we further characterized ADL effects in vivo and examined here the locomotor and internalizing properties of the ADL5747 and ADL5859 compounds. We show that, like the AR-M100390 compound and in contrast to SNC80, the ADL compounds are ineffective on these two in vivo integrated and cellular responses, respectively.
Materials and Methods
Animals
Mice were housed in a temperature-controlled (21 ± 1°C) and humidity-controlled (55 ± 10%) room with a 12-h light/dark cycle (light on between 8:00 AM and 8:00 PM). Food and water were available ad libitum except during behavioral observations. For behavioral studies, all mice were habituated to their new experimental environment and handled for 1 week before starting the experiments. Researchers were blind to genotype and treatment during behavioral experiment. All data are presented as means ± S.E.M. All mice were generated at Institut Clinique de la Souris-Institut de Genetique et Biologie Moleculaire et Cellulaire.
All experimental procedures and animal husbandry were conducted according to standard ethical guidelines (European Community Guidelines on the Care and Use of Laboratory Animals 86/609EEC) and approved by the local ethical committee (Comité d'Éthique pour l'Expérimentation Animale, Institut de Génétique et Biologie Moléculaire et Cellulaire-Institut Clinique de la Souris).
Conventional Knockout Mice.
δ-Opioid receptor KO mice and their WT counterparts with a mixed genetic background (C57BL6/J × SV129Pas), aged 7 to 10 weeks, were used (Filliol et al., 2000). Eight to 10 mice were used in each group.
Nav1.8-δ Receptor Conditional KO Mice.
Floxed mice, Nav1.8-δ receptor conditional KO (Nav1.8-cKO) mice and total KO (CMV-KO) mice with a mixed genetic background (C57BL6/J × SV129Pas) were used. The δ-receptor floxed mice were generated at the Institut Clinique de la Souris-Institut de Genetique et Biologie Moleculaire et Cellulaire (Gavériaux-Ruff et al., 2011). These floxed mice were interbred with Nav1.8-Cre mice from the John Wood laboratory at University College London (Stirling et al., 2005) or CMV-Cre mice to generate the Nav1.8-cKO or CMV-KO mice, respectively (Gavériaux-Ruff et al., 2011). Six to eight mice, aged 7 to 10 weeks, were used in each group.
DOR-eGFP Mice.
Knock-in mice with δ receptor in fusion to δ-opioid receptor (Scherrer et al., 2006) (four mice per drug, two male and two female) with a mixed genetic background (C57BL6/J × SV129Pas), aged 10 to 13 weeks, were used for imaging experiments.
Induction of Inflammatory Pain.
Complete Freund's adjuvant (CFA) was used to induce the inflammatory pain on the hindpaws or tails of the mice. The hindpaw CFA model was used to evaluate the analgesic properties of ADL compounds. Tail CFA mice were used to examine the effect of inflammatory pain on δ agonist-induced locomotor activity. After the measurement of baseline pain threshold, 8 or 20 μl of CFA (Sigma-Aldrich, Saint-Quentin Fallavier, France) were injected subcutaneously into the plantar surface of the left hindpaw or 3 cm from the tip of the tail, under inhalation anesthetic (2% flothane; CSP Centre de Spécialité Pharmaceutique, Cournon-d'Auvergne, France), respectively. Pain testing was conducted 2 or 3 days after CFA injection.
Induction of Neuropathic Pain.
Sciatic nerve ligation (SNL)-induced neuropathic pain was produced by a tight ligation of approximately half the diameter of the left common sciatic nerve by 7-0 braid silk suture under deep ketamine/xylazine anesthesia (100/10 mg/kg mixture; ketamine; Virbac, Carros, France; xylazine, Rompun, Bayer Healthcare, Puteaux, France) according to the surgical method described previously (Malmberg and Basbaum, 1998). Experiments with sham mice followed the same surgical procedure except for the nerve ligation. Baseline pain threshold was measured before the surgery. Pain measurement was performed on week 2 postsurgery.
Behavioral Assessment
Assessment of Mechanical Allodynia.
Von Frey test (up-down method; Chaplan et al., 1994) was used to determine the mechanical sensitivity (50% withdrawal threshold) on CFA-treated mice. In brief, eight Von Frey filaments were chosen (0.008, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, and 2.0g force; Bioseb, Valbonne, France), and the test was initiated with the 0.4g hair. Clear paw withdrawal, shaking the paw, or licking the paw indicated a positive response and promoted the use of the next weaker filament. Absence of a paw withdrawal response prompted the use of the next stronger filament. This procedure was stopped four measures after the first change in animal responding. The threshold of response was calculated by using the up-down Excel program generously provided by the Basbaum laboratory (University of California, San Francisco, CA).
Kinetics for analgesia was performed 45 min and 2 h after SNC80 injection and 30 min and 1, 2, and 4 h after ADL5747 or ADL5859 injection. Systemic analgesia was measured 45 and 60 min after SNC80 and ADL compound administration, respectively. Local ADL5747-induced analgesia was tested 30 min after treatment.
Assessment of Thermal Hyperalgesia.
On the tails of CFA mice, thermal sensitivity was measured by immersing the tail (5 cm from the tip) into a water bath at 46°C. Each individual mouse was lightly restrained in a 50-ml cylinder. Tail withdrawal latencies were determined, and a cutoff of 30 s was established.
Assessment of Locomotor Activity.
Locomotor activity during exploratory behavior was determined in acrylic cages (21 × 11 × 17 cm) on an actimetry platform. Assessment of locomotor activity was carried out for 30 min preinjection (habituation to the cage) and 90 min postinjection, and locomotion (total distance of the movement) was measured in 5-min windows by a video camera with an automatic tracking system (Viewpoint, Lyon, France).
Perfusion and Microscopy
Mice were anesthetized with a ketamine/xylasine mixture (100/10 mg/kg) and intracardially perfused with 9.25% sucrose/double-distilled H2O followed by 4% paraformaldehyde/0.1 M phosphate buffer (PB), pH 7.4. Perfusion was conducted 45 min (SNC80) or 60 min (ADL compounds) after drug administration. Brain, spinal cord, and dorsal root ganglia (DRG) were dissected, postfixed for 2 h at 4°C in 4% paraformaldehyde/0.1 M PB, and cryoprotected at 4°C in 30% sucrose/0.1 M PB solution until the tissue sank. Tissues were then frozen in isopentane and cut to 30-μm-thick sections in a cryostat, by using freely floating sections for hippocampus and striatum. Sections were mounted on glass slides, and DOR-eGFP receptor distribution in striatum, hippocampus, spinal cord, and DRG was observed under a Leica (Wetzlar, Germany) confocal microscope (SP2UV; 63× objective and numerical aperture of 1.32). LCS (Leica) software was used for image acquisition.
Quantification of cell surface mean fluorescence intensity was determined by using ImageJ software (National Institutes of Health, Bethesda, MD). Nuclear fluorescence defined the background level. Fluorescence intensity of cell membrane and cytoplasm was used to calculate the ratios of surface (Df surf) versus cytoplasmic (Df cyto) fluorescence densities (for more details, see Scherrer et al., 2006). Df surf/Df cyto value of 1.0 results from equal densities of DOR-eGFP at the cell surface and in the cytoplasm. In total, two to three neurons per region per mouse were analyzed, and there were four mice per group.
Drugs
SNC80 (10 mg/kg; Tocris Bioscience, Bristol, UK) was dissolved in saline and injected intraperitoneally. Control groups for SNC80 received intraperitoneal saline. ADL5747 and ADL5859 (10–300 mg/kg, Adolor Corporation, Exton, PA) were dissolved in distilled water with 0.5% hydroxypropyl methylcellulose/0.1% Tween 80 and administered by gavage orally as described previously for rats (Le Bourdonnec et al., 2008, 2009) for systemic administration. In a clinical phase I study with ADL5859, this route was well tolerated and suitable for daily dosing. Control groups received distilled water with 0.5% hydroxypropyl methylcellulose/ 0.1% Tween 80 (orally). For local administration, ADL5747 in saline or saline control were injected 2 days after CFA intraplantarly into the inflamed hindpaw in a 5-μl volume.
Data Analysis
All data are presented as means ± S.E.M. Pharmacokinetics of drug were analyzed by using repeated-measures ANOVA followed by Student's t test for individual time points when appropriate. The analysis of pharmacological effect was performed by using two-way ANOVA for drug effect and genotype, followed by Bonferroni test to determine statistically significant differences.
Results
ADL5747 and ADL5859 Produce Dose-Dependent Analgesia in Both Inflammatory Pain and Neuropathic Pain Mouse Models.
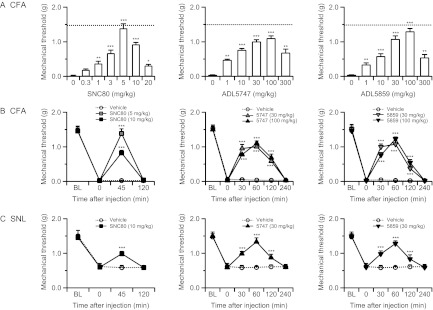
We first examined the analgesic effects of ADL5747 and ADL5859 in C57BL6/J × SV129Pas wild-type mice matching our δ-opioid receptor knockout lines. SNC80, the prototypical nonpeptidic δ agonist, was used as a reference compound. In the classic CFA-induced inflammatory pain model, CFA induced strong mechanical allodynia, which was fully reversed by SNC80 (P = 0.692 versus baseline threshold; not significant) at the 5 mg/kg dose (Fig. 2A). Administration of either ADL5747 or ADL5859 also produced significant antiallodynia, with the best efficacy within the 30 to 100 mg/kg dose range for the two compounds (Fig. 2A). All three agonists produced a bell-shape-type dose response for antihyperalgesia. We then tested the kinetics of δ-agonist effects by using optimal doses (SNC80, 5 and 10 mg/kg; ADL compounds, 30 and 100 mg/kg). The SNC80 effect disappeared within 2 h, whereas the two ADL compounds were still active after 120 min and analgesia terminated 4 h after drug administration (Fig. 2B).
Fig. 2.
ADL5747-, ADL5859-, and SNC80-induced analgesia in CFA-induced inflammatory (A and B) or SNL-induced neuropathic (C) pain models in wild-type mice. Nociceptive thresholds were determined by the Von Frey test. A, dose-dependent analgesia of SNC80 and ADL compounds. Broken lines indicate basal mechanical thresholds. SNC80 analgesia was measured 45 min postinjection, whereas both ADL5747 and ADL5859 were tested 60 min postadministration. Significant drug effects are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA followed by Bonferroni post hoc test (n = 8–10 mice/group). B and C, time course of SNC80- or ADL compound-induced analgesia in CFA (B) and SNL (C) mice. ADL compounds and SNC80 showed significant antiallodynic effects in both CFA and SNL pain models. Significant drug effects are indicated: ***, P < 0.001, two-way repeated-measures ANOVA followed by Bonferroni post hoc test (n = 8–10 mice/group).
We also tested ADL5747- and ADL5859-induced analgesia in the SNL neuropathic pain model in these mice. SNL decreased the mechanical threshold, as classically described, and this effect was significantly attenuated by SNC80 (Fig. 2C). Administration of either ADL5747 or ADL5859, at a dose optimal to reduce inflammatory pain, also efficiently reversed mechanical allodynia in SNL mice (Fig. 2C). Finally, there was no allodynia in contralateral paws animals for both CFA and SNL animals, and no ADL compound effects in sham-operated SNL controls (data not shown). Altogether, the data indicate that ADL5747 and ADL5859 reduced mechanical hypersensitivity in both inflammatory and neuropathic pain models with their action terminated at 4 h after administration.
ADL5747 and ADL5859 Are Ineffective in δ-Opioid Receptor Knockout Mice.
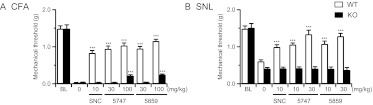
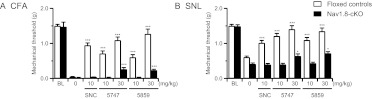
We examined the in vivo selectivity of ADL compounds for the δ-opioid receptor by comparing analgesic effects in δ-opioid receptor KO mice and their WT controls. In WT mice, and in both CFA (Fig. 3A) and SNL (Fig. 3B) models, administration of ADL5747 and ADL5859 potently reversed mechanical allodynia 60 min postinjection. In particular, SNL-induced mechanical allodynia was fully reversed by the two ADL compounds at the analgesic dose of 30 mg/kg (ADL5747, P = 0.167; ADL5859, P = 0.078, versus baseline threshold; not significant). These effects were comparable with those obtained with SNC80 45 min postinjection (Fig. 2). At the same optimal analgesic dose of 30 mg/kg, ADL5747 and ADL5859 did not modify pain thresholds in KO mice. Like SNC80, therefore, the δ-opioid receptor is necessary for ADL compound-induced analgesia.
Fig. 3.
Effect of total receptor knockout on antinociceptive effect of SNC80 and ADL compounds. Conventional δ-receptor KO mice and their WT counterparts were used for testing. Chronic pain was induced by CFA (A) or SNL (B). The nociceptive threshold was determined before induction of pain (BL), 45 min after SNC80 (10 mg/kg) or vehicle injection, and 60 min after the ADL compound by Von Frey filaments. Both SNC80 and ADL compounds induced analgesic effect in WT mice, whereas these antinociceptions were abolished in KO mice, except for ADL compounds at dose of 100 mg/kg. Data are expressed as means ± S.E.M. of 8 to 10 mice/group. Significance is indicated: ***, P < 0.001, drug-treated group versus vehicle-treated group (two-way ANOVA followed by Bonferroni post hoc test).
This result also indicates the ADL compounds' effect is selective at this 30 mg/kg dose (Fig. 3). We further tested ADL compounds at the 100 mg/kg dose in the inflammatory pain model and found a slight analgesic effect in KO mice (Fig. 3A). At this high dose, therefore, ADL compounds may show off-target effects in vivo, and this dose was not further used in our experiments. Finally, pain thresholds were unchanged and ADL compounds showed no activity at the contralateral paws of CFA and SNL animals or sham-operated animals (data not shown).
ADL5747 and ADL5859 Show Strongly Reduced Analgesic Effects in Peripheral Nav1.8-δ Receptor Conditional KO Mice.
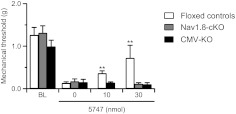
To examine whether peripheral opioid δ receptors may be involved in ADL compound-induced analgesia, we administered ADL5747 locally into the hindpaw of CFA-inflamed mice. Local ADL5747 induced a dose-dependent decrease of mechanical allodynia in control mice (Fig. 4), with no effect at the contralateral paws (data not shown). This effect was δ receptor-selective, because local ADL5747-induced analgesia was abolished in KO mice. These results suggest that the ADL compound-induced analgesia may be mediated by peripheral δ receptors. We then analyzed the effects of ADL5747 in conditional knockout mice lacking δ receptors in Nav1.8 primary nociceptive neurons (Nav1.8-cKO; see Gavériaux-Ruff et al., 2011). The intraplantar injection of 30 nmol of ADL4757 did not produce any analgesia in the Nav1.8-cKO mice.
Fig. 4.
Analgesic effect of intraplantar ADL5747 under CFA-induced mechanical allodynia. Injection of 10 and 30 nmol ADL5747 in the hindpaw of floxed mice induced a dose-dependent antiallodynic effect. The analgesic effect of local ADL5747 was abolished in δ-receptor total knockout (CMV-KO) and conditional knockout (Nav1.8-KO) animals (n = 7–8/group). **, P < 0.01 ADL5747 versus saline; two-way ANOVA followed by Bonferroni post hoc test.
To further determine the implication of peripheral δ receptors in both ADL5747- and ADL5859-induced analgesia, we administered both compounds systemically and examined their effects in both inflammatory and neuropathic pain models, using Nav1.8-cKO mice. In control floxed mice, as with WT mice in the previous experiment (Fig. 3), SNC80 (10 mg/kg) significantly suppressed mechanical allodynia in both the CFA (Fig. 5A) and SNL (Fig. 5B) models. Administration of either ADL5747 or ADL5859 (10–30 mg/kg) also produced strong antiallodynia in floxed mice, as it did for control WT mice (Fig. 3). In Nav1.8-cKO mice, the ADL compounds were ineffective at the 10 mg/kg dose, indicating that peripheral δ receptors expressed on Nav1.8 neurons are mandatory for mediating analgesia at this dose. At the 30 mg/kg dose, however, there was a significant analgesic (10–20%) effect for both compounds, suggesting that other δ-receptor populations may also contribute to ADL compound analgesia. Finally, pain scores on the contralateral paws of CFA, SNL, and sham-operated animals were also unchanged, and ADL compounds showed no activity (data not shown).
Fig. 5.
Involvement of peripheral δ receptor on antinociceptive effect induced by either SNC80 or ADL compounds. Conditional δ receptor knockout (Nav1.8-KO) mice and floxed mice were used for testing. Chronic pain was induced by CFA (A) or SNL (B). The nociceptive threshold was determined before induction of pain (BL), 45 min after SNC80 (10 mg/kg) or vehicle injection, and 60 min after the ADL compound (10–30 mg/kg) injection by Von Frey filament. Both SNC80 and ADL compounds induced analgesic effect in floxed mice, whereas these antiallodynic effects were completely abolished in Nav1.8-KO mice, except ADL compounds at doses of 30 mg/kg. Data are expressed as means ± S.E.M. of 8 to 10 mice/group. Significance is indicated: *, P < 0.05; ***, P < 0.001, drug-treated group versus vehicle-treated group (two-way ANOVA followed by Bonferroni post hoc test).
ADL5747 and ADL5859 Show No Locomotor-Activating Effect.
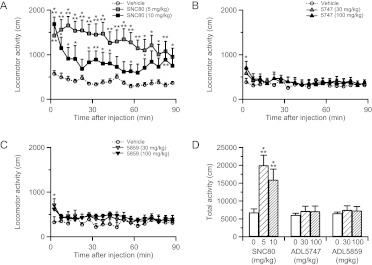
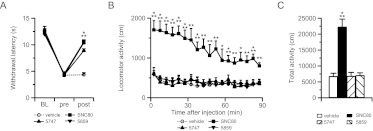
SNC80 is well known to increase locomotor activity in rats and mice (Jutkiewicz et al., 2005; Scherrer et al., 2006; Ito et al., 2008; Pradhan et al., 2010), whereas ADL compounds did not induce hyperlocomotion in rats (Le Bourdonnec et al., 2008, 2009). Therefore, we investigated the locomotor effects of ADL compounds in naive WT mice. As described previously (Jutkiewicz et al., 2005; Scherrer et al., 2006; Ito et al., 2008; Pradhan et al., 2010), SNC80 at 5 and 10 mg/kg induced a strong hyperlocomotor effect from 5 to 90 min postinjection (Fig. 6, A and D). However, ADL5859 and ADL5747 had no effect when tested at 30 and 100 mg/kg (Fig. 6, B–D) except for a marginal effect 5 min after administration. In addition, we examined whether inflammatory pain may influence the locomotor properties of δ agonists. The tail-CFA model was used, and administration of CFA into the tail produced a significant heat hyperalgesia (Fig. 7A). SNC80 at 5 mg/kg and both ADL compounds at 30 mg/kg significantly alleviated thermal hypersensivity. Finally, in these CFA-pain mice, analgesic dose of SNC80 induced hyperlocomotion, whereas ADL5859 and ADL5747 did not stimulate locomotor activity (Fig. 7, B and C), as shown previously in naive mice.
Fig. 6.
Locomotor activity after administration of SNC80 and ADL compounds in naive mice. Locomotion (total distance of the movement) was measured automatically by the automatic tracking system immediately after drug administration. A to C, although SNC80 (A) induced significant hyperlocomotion, ADL 5747 (B) and ADL5859 (C) had no effect on locomotor activity. D, total activity shows the hyperlocomotor effect of SNC80 and the absence of effect of the ADL compounds. Data are expressed as means ± S.E.M. of 8 to 10 mice/group. Significance is indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001, drug-treated group versus vehicle-treated group for individual time points, two-way repeated-measures ANOVA followed by Bonferroni post hoc test (A–C) or Student's t test (D).
Fig. 7.
Locomotor activity after administration of SNC80 and ADL compounds in tail-CFA mice. A, analgesic effect of SNC80 (5 mg/kg) and ADL compounds (30 mg/kg) on tail-CFA mice. Pain threshold was determined by tail immersion test before the CFA injection (BL), at the time point of before and after injection of drugs 2 days after tail-CFA. Postinjection period for SNC80 was 45 min, whereas that for ADL5747 and ADL5859 was 60 min. Heat sensitivity was increased by CFA injection to tail, and all of the δ agonists induced antihyperalgesic effect under these conditions. Significant drug effects are indicated: ***, P < 0.001, one-way ANOVA followed by Bonferroni post hoc test (n = 8–10 mice/group). B, locomotion was measured by the automatic tracking system immediately after drug administration. SNC80 (5 mg/kg) produced significant hyperlocomotion, whereas ADL 5747 and ADL5859 (both 30 mg/kg) had no effect on locomotor activity. C, total activity indicates SNC80 hyperlocomotor effect, and the absence of effect of the ADL agonists. In B and C, data are expressed as means ± S.E.M. of seven to nine mice/group. Significance is indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001, drug-treated group versus vehicle-treated group for individual time points, two-way repeated-measures ANOVA followed by Bonferroni post hoc test (B) or Student's t test (C).
ADL5747 and ADL5859 Do Not Induce DOR-eGFP Internalization In Vivo.
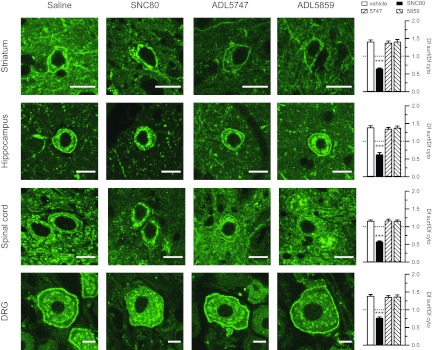
We reported previously that SNC80 induces strong δ-receptor internalization in vivo, whereas another δ-opioid agonist with similar analgesic potency (AR-M100390) produced no detectable receptor internalization in vivo (Pradhan et al., 2009). It is noteworthy that the latter compound did not desensitize the receptor acutely and produced an analgesia-specific tolerance, thereby contrasting with SNC80, which desensitized the receptor and triggered a tolerance to δ-opioid agonist-induced analgesia, hyperlocomotion, and anxiolytic effect (Pradhan et al., 2010). Because internalization properties of the ligand may be predictive of in vivo behavioral outcomes, we tested whether ADL compounds produce receptor sequestration in naive mice. As done earlier, we took advantage of a knockin mouse line expressing a functional δ receptor in fusion with the green fluorescent protein (DOR-eGFP). As expected, SNC80 (10 mg/kg) induced obvious receptor internalization in the hippocampus, striatum, spinal cord, and DRG (Fig. 8) of DOR-eGFP mice. However, neither ADL5747 nor ADL5859 tested at the optimal analgesic dose (30 mg/kg) had any visible effect on receptor distribution at a cellular level, because a strong fluorescent signal was detected at the cell surface in all tissue sections, similar to vehicle controls (Fig. 8). Therefore, ADL5747 and ADL5859 display activity profiles similar to AR-M100390, because the two compounds produce remarkable antiallodynic effects without increasing locomotor activity or triggering receptor internalization.
Fig. 8.
Confocal imaging of SNC80-, ADL5747-, and ADL5859-induced δ-receptor redistribution in DOR-eGFP mice. All mice were perfused 45 min after the drug administration. Confocal images were taken at striatum, hippocampus, spinal cord, and dorsal root ganglia. Bars on each image indicates the length of 10 μm. Ratio of mean fluorescence density on cell surface (Df surf) and cytoplasm (Df cyto) was defined by total fluorescence intensity of cell surface or cytoplasm. Data are expressed as means ± S.E.M. of four mice per group for each region or tissue. Empty bars, control group; filled bars, SNC80 group; left striped bars, ADL5747; right striped bars, ADL5859. Data were first averaged for each brain region or tissue for each mouse, and statistical comparison was performed between the four experimental groups. A significant effect of δ agonist versus vehicle control is indicated: ***, P < 0.001, drug-treated group versus vehicle-treated group (one-way ANOVA followed by Bonferroni post hoc test).
Discussion
In the present study, we show that ADL5747 and ADL5859 dose-dependently suppress CFA- or SNL-induced mechanical pain in WT mice after oral administration. The ability of ADL compounds to increase paw withdrawal thresholds represents true antiallodynic activity, because no effect was observed in contralateral paws or sham-operated animals. These results support and extend previous findings with these compounds in a rat model of inflammatory pain (Le Bourdonnec et al., 2008, 2009).
Analgesia produced by ADL5747 and ADL5859 in the mouse differed somehow from analgesia obtained in the rat study (Le Bourdonnec et al., 2009). First, the reduction of inflammatory pain in CFA mice seemed weaker compared with antiallodynia observed in the rat (3- to 10-fold higher doses required for an analgesic effect). In addition, the two ADL compounds showed similar dose-dependent effects in mice, whereas ADL5747 was less effective than ADL5859 in the rat. These discrepancies may be explained by distinct intensities of CFA-induced inflammatory pain between the two studies, differences in mechanical sensitivities of paw pressure (Le Bourdonnec et al., 2009) and Von Frey filament (this study) tests, and potentially distinct bioavailability of ADL compounds across the two species. In addition, differences in the ADL analgesic effects between mice and rats may be explained by distinct expression patterns of δ-opioid receptor between the two species (Mennicken et al., 2003). It is noteworthy that neuropathic pain was not examined in the rat studies. Here, we show almost complete reversal of mechanical pain in SNL mice, indicating that the two ADL compounds are at least as efficient in reducing chronic pain induced by nerve injury and tissue inflammation. It is also noteworthy that both ADL compounds had a longer duration of action compared with SNC80 in the two chronic pain models. This is consistent with the pharmacokinetic properties of the compounds evaluated in previous studies (rat and dog), indicating a long half-life for each compound (5.1 h for ADL5859 and 12.2 h for ADL5747) (Le Bourdonnec et al., 2009).
We found that the analgesic effects of ADL5747 and ADL5859 were abolished in conventional δ-receptor KO mice at doses up to 30 mg/kg, and this applied to both CFA and SNL pain models. These results provide a genetic demonstration for the in vivo selectivity of ADL compounds up to this dose. We also observed a slight, but significant, effect of both compounds at the dose of 100 mg/kg in KO mice, suggesting that, at this high dose, the two drugs may also induce analgesia partly via other mechanisms. In the CFA-induced inflammatory pain model, the three agonists seemed to produce a bell-shape antihyperalgesia dose response. This may be the result of the agonists' action on other receptors at the highest doses (20 mg/kg SNC80; 300 mg/kg ADL5747 and ADL5859) or the combination of relative activation of several signaling pathways after receptor activation depending on the dose. Because the ADL 30 mg/kg dose showed optimal effect, the data indicate that specific δ analgesia may be obtained with the two compounds without off-target effects.
Our further analysis using local and systemic injections in Nav1.8-KO mice showed the implication of peripheral δ receptors for ADL5747- and ADL5859-induced analgesia. ADL compound-induced analgesic effects are abolished after local injection and at the low systemic dose (10 mg/kg) and strongly reduced at the higher δ-receptor selective dose (30 mg/kg). These findings indicate that δ receptors on Nav1.8 neurons represent important targets for ADL compounds in the control of both inflammatory and neuropathic pain. Furthermore, as with ADL compounds, SNC80 analgesia was also abolished or almost abolished in Nav1.8 conditional KO mice and the two chronic pain paradigms (present study; Gavériaux-Ruff et al., 2011). Therefore, the importance of δ-opioid receptors on Nav1.8 neurons applies to all agonists tested so far, indicating that δ-opioid receptor-mediated blockade of primary nociceptive inputs is a main mechanism for δ-opioid analgesia. This does not exclude the participation of other receptor populations located in the peripheral or central nervous systems. One implication of these findings is that δ drugs that potentially poorly cross the blood-brain barrier may retain significant analgesic properties.
On the other hand, there was a small, but significant, remaining analgesic effect in δ-receptor peripheral conditional KO (Nav1.8-KO) mice for both ADL5747 and ADL5859 at the δ-receptor selective dose of 30 mg/kg. This indicates that δ-opioid receptors at other sites of nociceptive pathways also partly contribute to ADL agonist activities at this dose. Several pharmacological studies indicate that the local application of δ agonists in central regions such as the spinal cord (Bilsky et al., 1995; Kawaraguchi et al., 2004; Scherrer et al., 2009) or cerebral ventricle (Bilsky et al., 1995; Fraser et al., 2000; Cao et al., 2001) produces analgesia. The activation of peripheral receptors by ADL compounds is therefore required but may not be necessarily sufficient to reduce chronic pain (Gavériaux-Ruff and Kieffer, 2011).
Even though SNC80 and the two ADL compounds show similar in vitro receptor binding and signaling properties, as well as in vivo analgesic effects (this work; Le Bourdonnec et al., 2008, 2009), we found profound differences in their ability to trigger receptor internalization at the cellular level. As reported previously (Scherrer et al., 2006; Pradhan et al., 2009, 2010) and in this study, analgesic doses of SNC80 triggered massive receptor internalization at all δ receptor-expressing sites that were examined (hippocampus, striatum, spinal cord, and DRG), whereas analgesic doses of ADL compounds had no effect on receptor distribution in neurons. This observation parallels our previous set of studies (Pradhan et al., 2009, 2010), which compared behavioral and in vivo cellular effects of SNC80 with those of another δ-receptor agonist, AR-M100390. Like for ADL compounds, AR-M100390 showed binding affinities and signaling potencies similar to SNC80, but did not produce any detectable receptor internalization in vivo. Remarkably, the differential internalization potencies of SNC80 and AR-M100390 correlated with distinct adaptive responses of δ receptors to repeated drug injections (Pradhan et al., 2010). Chronic AR-M100390 produced analgesic tolerance without altering locomotor or anxiolytic receptor-mediated responses (Pradhan et al., 2010), whereas chronic SNC80 produced receptor down-regulation accompanied with tolerance to the analgesic, hyperlocomor, and anxiolytic responses to δ drugs, although no tolerance was obtained for chronic SNC80-induced antidepressant effect in rats (Jutkiewicz et al., 2005; Saitoh et al., 2008). It is noteworthy that SNC80 produced δ-receptor internalization in both naive (this work; Scherrer et al., 2006) and CFA-pain animals (Pradhan et al., 2009, 2010), and AR-M100390 induced no internalization in naive animals or CFA-pain animals (Pradhan et al., 2009, 2010), indicating that inflammatory pain did not change the internalizing properties of δ agonists. Altogether, our study demonstrates the existence of two additional δ agonists with high analgesic efficacy and low internalizing properties, extending the repertoire of biased agonists at the δ receptor (Pradhan et al., 2009, 2010).
There was another main difference between SNC80 and ADL compound activities in vivo. SNC80 significantly increased locomotor activity, as reported in many previous studies, whereas ADL5859 and ADL5747 did not affect locomotion in naive animals or CFA-pain animals. This shows that the locomotor properties of SNC80 and ADL agonists are not affected by inflammatory pain. Whether the inability of the two ADL compounds to stimulate animal activity is related to the inability to internalize the receptor is presently unknown. We may speculate that locomotor-activating effects of δ agonists require receptor internalization, in which case the behavioral activation would result from the specific activation of an internalization-dependent signaling pathway in vivo. The detailed mechanistic basis of this particular behavioral response, and whether β-arrestins are involved (Qiu et al., 2007; Urs et al., 2011), will be the subject of future studies.
In conclusion, our study shows that ADL5859 and ADL5747 efficiently reduce inflammatory and neuropathic pain in mice, there is δ-opioid receptor selectivity in vivo at doses up to 30 mg/kg, and δ receptors on peripheral Nav1.8 neurons are implicated to produce their analgesic effects. Furthermore, and in line with our previous study (Pradhan et al., 2010), our results suggest that ADL compounds show biased activity at the δ receptor and may activate signaling pathways distinct from SNC80 in vivo. It is likely that the number of δ drugs with biased activity will increase in the future as more compounds are synthesized and characterized for a broad set of signaling and biological activities (Galandrin et al., 2007; Zheng et al., 2010). Altogether, data obtained from ADL5859 and ADL5747 in this study increase our knowledge about δ-agonist properties in vivo at cellular and behavioral levels and strengthen the potential utility of these two molecules within the repertoire of novel δ-opioid agonists (Pradhan et al., 2011).
Acknowledgments
We thank Audrey Matifas and the staff at the Institut de Génétique et de Biologie Moléculaire et Cellulaire imaging platform for technical assistance; G. Scherrer for development of DOR-eGFP mice; John Wood for the Nav1.8-Cre mouse line; and Amynah A. A. Pradhan for helpful discussion.
This research was supported by the Centre National de la Recherche Scientifique; Institut National de la Santé et de la Recherche Médicale; the Université de Strasbourg; l'Agence Nationale de la Recherche Lymhopioid; Fondation pour la Recherche Médicale (C.N.); the National Institutes of Health National Institute on Drug Abuse [Grant DA05010]; the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant 016658]; and Adolor Corporation.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- ADL5859
- N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl)benzamide
- ADL5747
- N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl)benzamide
- ADL
- Adolor
- SNC80
- 4-[(R)-[(2S,5R)-4-allyl-2,5-dimethyl-piperazin-1-yl]-(3-methoxyphenyl)methyl]-N,N-diethyl-benzamide
- AR-M100390
- N,N-diethyl-4-(phenylpiperidin-4-ylidene-methyl)-benzamide
- CFA
- complete Freund's adjuvant
- DRG
- dorsal root ganglia
- Nav
- voltage-gated sodium channel
- DOR
- δ-opioid receptor
- eGFP
- enhanced green fluorescence protein
- KO
- knockout
- cKO
- conditional KO
- SNL
- sciatic nerve ligation
- WT
- wild type
- CMV
- cytomegalovirus
- PB
- phosphate buffer
- ANOVA
- analysis of variance
- Df surf
- fluorescence density on cell surface
- Df cyto
- fluorescence density on cytoplasm.
Authorship Contributions
Participated in research design: Nozaki, Windh, Little, Kieffer, and Gavériaux-Ruff.
Conducted experiments: Nozaki and Reiss.
Contributed new reagents or analytic tools: Le Bourdonnec, Windh, Little, and Dolle.
Performed data analysis: Nozaki and Reiss.
Wrote or contributed to the writing of the manuscript: Nozaki, Kieffer, and Gavériaux-Ruff.
References
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. (1995) SNC 80, a selective, nonpeptidic and systemically active opioid δ agonist. J Pharmacol Exp Ther 273:359–366 [PubMed] [Google Scholar]
- Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, Yaksh TL. (2006) Cancer-related bone pain is attenuated by a systemically available δ-opioid receptor agonist. Pain 122:174–181 [DOI] [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. (2001) Antinociceptive effects of δ-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther 296:939–946 [PubMed] [Google Scholar]
- Brennan F, Carr DB, Cousins M. (2007) Pain management: a fundamental human right. Anesth Analg 105:205–221 [DOI] [PubMed] [Google Scholar]
- Cao CQ, Hong Y, Dray A, Perkins M. (2001) Spinal δ-opioid receptors mediate suppression of systemic SNC80 on excitability of the flexor reflex in normal and inflamed rat. Eur J Pharmacol 418:79–87 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63 [DOI] [PubMed] [Google Scholar]
- Dondio G, Ronzoni S, Farina C, Graziani D, Parini C, Petrillo P, Giardina GA. (2001) Selective δ opioid receptor agonists for inflammatory and neuropathic pain. Farmaco 56:117–119 [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. (2000) Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25:195–200 [DOI] [PubMed] [Google Scholar]
- Foulkes T, Wood JN. (2008) Pain genes. PLoS Genet 4:e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PB, Ménard DP, Perkins MN. (2000) Antihyperalgesic effects of δ opioid agonists in a rat model of chronic inflammation. Br J Pharmacol 129:1668–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bouvier M. (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28:423–430 [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. (2005) A comparison of the antinociceptive and adverse effects of the μ-opioid agonist morphine and the δ-opioid agonist SNC80. Basic Clin Pharmacol Toxicol 97:39–51 [DOI] [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. (2008) Inflammatory pain is enhanced in δ opioid receptor-knockout mice. Eur J Neurosci 27:2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Kieffer BL. (2002) Opioid receptor genes inactivated in mice: the highlights. Neuropeptides 36:62–71 [DOI] [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Kieffer BL. (2011) δ-Opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol 22:405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, et al. (2011) Genetic ablation of δ opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain 152:1238–1248 [DOI] [PubMed] [Google Scholar]
- Glajchen M. (2001) Chronic pain: treatment barriers and strategies for clinical practice. J Am Board Fam Pract 14:211–218 [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. (2007) Spinal administration of a δ opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain 11:685–693 [DOI] [PubMed] [Google Scholar]
- Ito S, Mori T, Sawaguchi T. (2008) Dopamine-independent psychostimulant activity of a δ-agonist. Behav Pharmacol 19:113–119 [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Kaminsky ST, Rice KC, Traynor JR, Woods JH. (2005) Differential behavioral tolerance to the δ-opioid agonist SNC80 ([(+)-4-[(αR)-α-(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther 315:414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Cahill CM. (2007) Anti-allodynic effects of peripheral δ opioid receptors in neuropathic pain. Pain 127:84–93 [DOI] [PubMed] [Google Scholar]
- Kawaraguchi Y, Kawaguchi M, Takahashi M, Horiuchi T, Sakamoto T, Furuya H. (2004) δ-Opioid agonist SNC80 can attenuate the development of dynorphin A-induced tactile allodynia in rats. Anesthesiology 101:546–549 [DOI] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD, et al. (2008) Potent, orally bioavailable δ opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5859). J Med Chem 51:5893–5896 [DOI] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Leister LK, Zhou QJ, Ajello CW, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, et al. (2009) Spirocyclic δ opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl) benzamide (ADL5747). J Med Chem 52:5685–5702 [DOI] [PubMed] [Google Scholar]
- Liu M, Wood JN. (2011) The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med 12 (Suppl 3):S93–S99 [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Basbaum AI. (1998) Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain 76:215–222 [DOI] [PubMed] [Google Scholar]
- McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, Lau J, Carr D, and American Pain Society (2003) Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain 4:231–256 [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O'Donnell D. (2003) Phylogenetic changes in the expression of δ opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol 465:349–360 [DOI] [PubMed] [Google Scholar]
- Nadal X, Baños JE, Kieffer BL, Maldonado R. (2006) Neuropathic pain is enhanced in δ-opioid receptor knockout mice. Eur J Neurosci 23:830–834 [DOI] [PubMed] [Google Scholar]
- Otis V, Sarret P, Gendron L. (2011) Spinal activation of δ opioid receptors alleviates cancer-related bone pain. Neuroscience 183:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gavériaux-Ruff C, Kieffer BL. (2009) In vivo δ opioid receptor internalization controls behavioral effects of agonists. PLoS One 4:e5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL. (2011) The δ opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. (2010) Ligand-directed trafficking of the δ-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci 30:16459–16468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Loh HH, Law PY. (2007) Phosphorylation of the δ-opioid receptor regulates its β-arrestins selectivity and subsequent receptor internalization and adenylyl cyclase desensitization. J Biol Chem 282:22315–22323 [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yamada M, Yamada M, Takahashi K, Yamaguchi K, Murasawa H, Nakatani A, Tatsumi Y, Hirose N, Kamei J. (2008) Antidepressant-like effects of the δ-opioid receptor agonist SNC80 ([(+)-4-[(αR)-α-(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in an olfactory bulbectomized rat model. Brain Res 1208:160–169 [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gavériaux-Ruff C, et al. (2006) Knockin mice expressing fluorescent δ-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A 103:9691–9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, Nassar MA. (2005) Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain 113:27–36 [DOI] [PubMed] [Google Scholar]
- Urs NM, Daigle TL, Caron MG. (2011) A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology 36:551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2010) Agonist-selective signaling of G protein-coupled receptor: mechanisms and implications. IUBMB Life 62:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]