Abstract
In humans and rodents, paraoxonase (PON/Pon) 1 expression and activity in livers and serum are higher in females than in males, and some drugs increase paraoxonase's expression. However, the underlining mechanisms of gender-divergent expression and chemical regulation of Pon1 remain largely unknown. The present study determined the regulatory mechanisms contributing to gender-divergent and chemically altered Pon expression in mouse livers. Pon1 mRNA was much more abundant in the livers of mice than other tissues, with higher levels in female livers than male livers at mRNA and protein levels. Pon2 mRNA was ubiquitously expressed in mouse tissues, but minimally in mouse liver. Pon3 mRNA was most abundant in mouse lung and liver and less abundant in other tissues. Pon1 mRNA was lowest in fetal liver, markedly increased at parturition, and remained relatively constant thereafter. Pon2 and Pon3 mRNA are highly expressed in fetal liver and decreased after birth. Male-pattern growth hormone (GH) administration in hypophysectomized and lit/lit mice decreased Pon1 expression. Sex hormones and female-pattern GH administration had no effect on Pon1 expression, indicating the importance of male-pattern GH in regulating Pon1. Aryl hydrocarbon receptor, pregnane X receptor, and NF-E2-related factor activators had no effect on Pon1 mRNA. A constitutive androstane receptor (CAR) activator decreased Pon1 expression in wild-type but not CAR-null mice. In conclusion, Pon1 mRNA was most abundant in adult mouse livers, whereas Pon2 and Pon3 mRNAs were most abundant in fetal mouse livers. Female-predominant Pon1 expression in mouse livers is caused by the inhibitory effects of male-pattern GH secretion, and CAR activation decreases Pon1 expression.
Introduction
Oxidation of low-density lipoprotein (LDL) and high-density lipoprotein (HDL) is a critical risk factor in the development of atherosclerosis and other cardiovascular diseases. Paraoxonases (PONs/Pons) are a family of proteins that protect LDL and HDL from oxidation, thus preventing the development of atherosclerosis and other cardiovascular diseases (Aviram and Rosenblat, 2005).
Human PON1 was first reported to hydrolyze organophosphates, such as paraoxon (Mazur, 1946). PON1 was originally classified as an A-esterase because of its paraoxonase and arylesterase activities (Sorenson et al., 1995). In addition, PON1 has lactonase activity and can metabolize a number of drugs and prodrugs such as dihydrocoumarin and homocysteine thiolactone (Draganov and La Du, 2004). In contrast, PON2 and PON3 lack paraoxonase or arylesterase activities, but have lactonase activity capable of hydrolyzing aromatic and long-chain aliphatic lactones, such as dihydrocoumarin and 5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid lactone (Draganov et al., 2000; Ng et al., 2001).
PON1 and PON3 are synthesized primarily in liver and secreted into plasma, where they are associated with HDL (Mackness and Walker, 1983, 1988; Draganov et al., 2000). PON2 is not present in blood, but is expressed widely in a number of tissues, including liver, lung, brain, and heart (Ng et al., 2001).
In addition to its ability to hydrolyze paraoxon, PON1 attenuates the production and accumulation of lipoperoxides in LDL (Mackness et al., 1991, 1993) and protects phospholipids from oxidation in HDL (Costa et al., 2003). Similar to PON1, both PON2 and PON3 have antioxidant function (Draganov and La Du, 2004; Costa et al., 2005).
Serum Pon1 activity in mice and rats is 14 to 26% higher in females than males (Wehner et al., 1987; Costa et al., 2005; Thomàs-Moyà et al., 2006, 2007). Human serum PON1 activity is also higher in women than men (Mueller et al., 1983). Pon1 mRNA in livers of female mice is 40% higher than in males (bin Ali et al., 2003). Gonadectomy results in an increase in Pon1 mRNA expression in male, but not female mice (bin Ali et al., 2003), suggesting that endogenous androgens, but not endogenous estrogens, regulate Pon1 expression.
Dietary polyphenols (Gouédard et al., 2004) and aspirin (Jaichander et al., 2008) increase PON1 gene expression through the activation of the aryl hydrocarbon receptor (AhR). In addition, alcohol, wine consumption, resveratrol, pitavastatin, and probucol increase Pon1/PON1 expression and activity in rodents and humans (Rao et al., 2003; Ota et al., 2005; Hong et al., 2006; Rajdl et al., 2007; Curtin et al., 2008). In contrast, dietary taurocholate decreased Pon1 expression in mice through the activation of the farnesoid X receptor-Fgf15-Fgfr4 pathway (Gutierrez et al., 2006). Proinflammatory cytokines, such as interleukin-1β and tumor necrosis factor α, decreased Pon1 expression in HepG2 cells (Kumon et al., 2002).
In addition to sex hormones, growth hormone (GH) plays important roles in gender-divergent gene expression in livers (Waxman and O'Connor, 2006). Except for the AhR signaling pathway, the characterization of chemical regulation of Pon1 remains largely unknown. Therefore, the current study was performed to determine the tissue distribution, ontogeny, and hormonal and chemical regulation of Pon1, Pon2, and Pon3 in mice.
Materials and Methods
Materials
Micro-O-protect was purchased from Roche Diagnostics (Indianapolis, IN). Formaldehyde, 4-morpholinepropanesulfonic acid, sodium citrate, and NaHCO3 were purchased from Fischer Chemicals (Fairlawn, NJ). Chloroform, agarose, and ethidium bromide were purchased from AMRESCO (Solon, OH). Rat growth hormone was obtained from Dr. Albert F. Parlow at the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Disease (Torrance, CA). Pellets for subcutaneous release of the hormones used in this study, including 5α-dihydroxytestosterone (DHT), 17β-estradiol (E2), GH, and placebo, were purchased from Innovative Research of America, Inc. (Sarasota, FL). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a gift from Dr. Karl Rozman (University of Kansas Medical Center, Kansas City, KS). Oltipraz (OPZ) was a gift from Dr. Stephen Safe (Texas A&M University, College Station, TX). Polychlorinated biphenyl 126 (PCB126) was obtained from AccuStandard (New Haven, CT). All other chemicals, unless otherwise indicated, were purchased from Sigma (St. Louis, MO).
Anti-human PON1-3 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), β-actin antibody (Abcam Inc., Cambridge, MA), and goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody (Sigma) were commercially available. According to the manufacturer, anti-human PON1-3 antibody recognizes mouse Pon1, Pon2, and to a lesser extent PON3 protein. Because Pon1, but not Pon2, is generally highly expressed in mouse liver, this antibody will detect mainly Pon1 in mouse liver.
Animals and Treatment
Eight-week-old adult male and female C57BL/6 mice (n = 5/gender) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed according to the Association for Assessment and Accreditation of Laboratory Animal Care International.
Tissue Distribution Study.
Twelve tissues [liver, kidney, lung, stomach, duodenum, jejunum, ileum, colon, heart, brain, and gonad (testes in males and ovaries in females)] were collected from adult (approximately 8 weeks of age) male and female C57BL/6 mice (n = 5/gender). Placenta was removed from pregnant mice on gestation day 17. The intestine was longitudinally dissected, rinsed in saline, and divided into three equal-length sections (referred to as duodenum, jejunum, and ileum) before being snap-frozen in liquid nitrogen.
Ontogenic Study.
C57BL/6 mice were bred in the animal facilities at the University of Kansas Medical Center. Livers from male and female C57BL/6 mice were collected at −2, 0, 5, 10, 15, 22, 30, 40, and 45 days of age (n = 5/gender/age), snap-frozen in liquid nitrogen, and stored at −80°C.
Treatment of Mice with Microsomal Enzyme Inducers.
Adult (approximately 8 weeks of age) male C57BL/6 mice (n = 5/treatment) were separated into groups. Groups of five mice were administered one of the following chemicals once daily for 4 days: AhR ligands, TCDD (40 μg/kg i.p. in corn oil), β-naphthoflavone (BNF; 200 mg/kg i.p. in corn oil), and PCB126 (300 μg/kg p.o. in corn oil); constitutive androstane receptor (CAR) activators, phenobarbital (PB; 100 mg/kg i.p. in saline), 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP; 3 mg/kg i.p. in corn oil), and diallyl sulfide (DAS; 200 mg/kg i.p. in corn oil); pregnane X receptor (PXR) ligands, pregnenolone-16α-carbonitrile (PCN; 200 mg/kg i.p. in corn oil), spironolactone (SPR; 200 mg/kg i.p. in corn oil), and dexamethasone (DEX; 75 mg/kg i.p. in corn oil); peroxisome proliferator-activated receptor α (PPARα) ligands, clofibric acid (CLFB; 500 mg/kg i.p. in saline), ciprofibrate (CPFB; 40 mg/kg i.p. in saline), and diethylhexylphthalate (DEHP; 1000 mg/kg p.o. in corn oil); NF-E2-related factor (Nrf2) activators, butylated hydroxyanisole (BHA; 350 mg/kg i.p. in corn oil), ethoxyquin (ETHOXYQ; 250 mg/kg p.o. in corn oil), and OPZ (150 mg/kg p.o. in corn oil). Four different vehicle control groups (corn oil intraperitoneally, corn oil orally, saline intraperitoneally, and saline orally) were used. No statistical difference between these control groups was observed, thus these groups were averaged together as a single vehicle control group. All injections were administered in a volume of 10 ml/kg. Livers were removed on day 5, snap-frozen in liquid nitrogen, and stored at −80°C.
Hormone Replacement Treatment in Gonadectomized, Hypophysectomized, and lit/lit Mice.
Both gonadectomized (GNX) mice and hypophysectomized (HX) mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA). lit/lit mice (GH-releasing hormone receptor mutant heterozygous mice, C57BL/6J-Ghrhrlit) were purchased from The Jackson Laboratory. The doses and routes of treatment of sex hormones and growth hormone in these mouse models have been reported previously (Cheng et al., 2006). After treatment, livers were removed for total RNA isolation.
TCPOBOP Treatment of WT and CAR-Null Mice.
Breeding pairs of CAR-null mice in the C57BL/6 background were obtained from Dr. Ivan Rusyn (University of North Carolina, Chapel Hill, NC) and engineered by Tularik Inc. (South San Francisco, CA) as described previously (Ueda et al., 2002). CAR-null and C57BL/6 mice (n = 5) were administered TCPOBOP (3 mg/kg i.p. in corn oil) or the vehicle corn oil once daily for 4 days. Livers were removed on day 5, snap-frozen in liquid nitrogen, and stored at −80°C.
Total RNA Isolation
Total RNA was isolated by using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer's protocol. RNA pellets were resuspended in diethyl pyrocarbonate-treated deionized water. Total RNA concentrations were quantified spectrophotometrically at 260 nm.
Development of Specific Oligonucleotide Probe Sets for Branched DNA Analysis
Gene sequences of Pon1, Pon2, and Pon3 were accessed from GenBank. The strategy of multiple oligonucleotide probe sets design has been described previously (Hartley and Klaassen, 2000). Probe sets for each mouse paraoxonase [including capture extenders (CEs), label extenders (LEs), and blockers (BLs)] are shown in Table 1. Probe sets were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
TABLE 1.
Oligonucleotide probes generated for analysis of mouse paraoxonase mRNA expression by bDNA signal amplification assay
Function refers to the type of bDNA oligonucleotide probe represented by each sequence. GenBank accession numbers for each transcript are given with the gene name.
| Function | Probe Sequence | Function | Probe Sequence |
|---|---|---|---|
| Pon1 (NM_011134) | Pon3 (NM_173006) | ||
| CE | gagggtgagtgctagcagcttcTTTTTctcttggaaagaaagt | CE | tggtctagtgagactgtgattccgTTTTTctcttggaaagaaagt |
| CE | cggaaagcatttaatcttgtttgatTTTTTctcttggaaagaaagt | CE | ttcccagctgaatgaccttcaTTTTTctcttggaaagaaagt |
| CE | tgagggttaaatgaagatatatccaaTTTTTctcttggaaagaaagt | CE | cctggaggatcctcagggttataTTTTTctcttggaaagaaagt |
| LE | gcagcaacactggtcagtcctgTTTTTaggcataggacccgtgtct | CE | ccaaatataaagacgttttagaaagatctTTTTTctcttggaaagaaagt |
| LE | gccatggcctgagacagatgTTTTTaggcataggacccgtgtct | LE | tgttacatcagctacatagacaaacttcTTTTTaggcataggacccgtgtct |
| LE | ctcgattcctttaactaaattacagttaTTTTTaggcataggacccgtgtct | LE | ctggagttaaatcccaattatcatgTTTTTaggcataggacccgtgtct |
| LE | cttactgggatcgaaactttttattTTTTTaggcataggacccgtgtct | LE | caacggtcaagttatccactaaggTTTTTaggcataggacccgtgtct |
| LE | tgtatttcctataatttctaactctgacacTTTTTaggcataggacccgtgtct | LE | aaatatctcccgtggctggatTTTTTaggcataggacccgtgtct |
| LE | ggacgaggagtctggatggtttTTTTTaggcataggacccgtgtct | LE | tcctggatgcgtagtacttctgacTTTTTaggcataggacccgtgtct |
| BL | gacaggctgaggatggacaagt | LE | agaggtcacagtacagagctttgtgTTTTTaggcataggacccgtgtct |
| BL | agtgccaacaccagtcccac | LE | ttagcatacaaattacaattttacttttgTTTTTaggcataggacccgtgtct |
| BL | aggaagaccgatggttcttgtaa | LE | tgataattgtaaataattacagttccccTTTTTaggcataggacccgtgtct |
| BL | ggaagttctactggcgttacttca | BL | ttggcagaactgaatccttgg |
| BL | atctctaagtcttcagcacccgt | BL | ttttttcattatgtgaatgttcttagc |
| BL | aaagaaagttagtccattaggcaga | BL | gggtggcagcctgcca |
| BL | ccaggatactttagcccagtgct | BL | gatcaacagcttcatggggtta |
| BL | gtccatcagaagtatttttccagg | BL | ccctgggcttatctgacaaagag |
| BL | tgctggctccttcttgttcaa | BL | ttgttcgcatacagtgtgctca |
| BL | ttcatctgtgaatgtactaatccca | BL | gccctgaagcacagagccg |
| BL | accaccagtaggtacacagtgttatc | BL | atacacagaagccacggtgct |
| Pon2 (NM_183308) | BL | aaatatagtacctatgagcattctcttatg | |
| CE | gcctggagaccgaggcgTTTTTctcttggaaagaaagt | CE | tggtctagtgagactgtgattccgTTTTTctcttggaaagaaagt |
| CE | ccctctccagaggatttcccTTTTTctcttggaaagaaagt | CE | ttcccagctgaatgaccttcaTTTTTctcttggaaagaaagt |
| CE | aagagcccctttacatagagcttTTTTTctcttggaaagaaagt | CE | cctggaggatcctcagggttataTTTTTctcttggaaagaaagt |
| LE | aagcccctctgtgcaaactgTTTTTaggcataggacccgtgtct | CE | ccaaatataaagacgttttagaaagatctTTTTTctcttggaaagaaagt |
| LE | cagacctgtttaccacactccctTTTTTaggcataggacccgtgtct | LE | tgttacatcagctacatagacaaacttcTTTTTaggcataggacccgtgtct |
| LE | acactcctaacttgtaaatcagtaaacaTTTTTaggcataggacccgtgtct | LE | ctggagttaaatcccaattatcatgTTTTTaggcataggacccgtgtct |
| LE | ccctaggagtcacttctagcctcaTTTTTaggcataggacccgtgtct | LE | caacggtcaagttatccactaaggTTTTTaggcataggacccgtgtct |
| LE | tgattcctgtccaaccgaatgTTTTTaggcataggacccgtgtct | LE | aaatatctcccgtggctggatTTTTTaggcataggacccgtgtct |
| LE | cggttgttttattttatttttccaataTTTTTaggcataggacccgtgtct | LE | tcctggatgcgtagtacttctgacTTTTTaggcataggacccgtgtct |
| BL | ggaggcacggaggggg | ||
| BL | gcttcagtggactcttacgtggt | ||
| BL | aagcccagcaggcgca | ||
| BL | aagtgttatctatcagtagtcctgcac | ||
| BL | aagccggatgtagaaaacatcc | ||
| BL | cccggattttgattagtcttgaat | ||
| BL | ttgggaattaaacacaaacaactc | ||
| BL | tcatgagaaattaacatggccat |
bDNA Assay
Reagents required for RNA analysis (i.e., lysis buffer, amplifier/label probe dilution buffer, and substrate solution) were supplied in the Quantigene bDNA signal amplification kit (Panomics Inc., Fremont, CA). Each paraoxonase mRNA was analyzed according to the method of Hartley and Klaassen (2000). Data are presented as relative light units (RLUs) per 8 μg of total RNA.
Liver Protein Preparation
Liver (50–100 mg) was minced in 1 ml of ice-cold homogenizing buffer (0.25 M sucrose and 10 mM Tris-HCl at pH 7.5, containing 25 μg/ml leupeptin, 50 μg/ml aprotinin, 40 μg/ml phenylmethylsulfonyl fluoride, 0.5 μg/ml pepstatin, and 50 μg/ml antipain). The minced tissue was poured into a Dounce homogenizer (Kontes Glass, Vineland, NJ) and homogenized on ice for 10 strokes. The homogenate was centrifuged at 10,000g for 15 min at 4°C. The resulting supernatants were collected for Western blots. Protein concentration of each sample was determined with a Bradford protein assay kit (Sigma).
Western Blots
Liver protein samples mixed with sample loading buffer (75 μg protein/lane) were loaded after heating onto a 12.5% SDS-polyacrylamide gel. After electrophoresis, proteins in the gel were electrotransferred to a nitrocellulose membrane for 4.5 h at 30 V at room temperature. Membranes were blocked for 5 h at room temperature with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T). Blots were then incubated overnight with anti-human PON1-3 (H-300) antibody at 4°C. β-Actin antibody was used as a loading control. After thorough washing (three 20-min washes with excess TBS-T), blots were incubated with goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody (1:5000 dilution with 5% nonfat milk in TBS-T) for 1 h. Blots were washed again. Immunoreactive bands were detected with an enhanced chemical luminescence kit (Thermo Fisher Scientific, Waltham, MA). Pon1 and β-actin protein bands were visualized by exposure to Fuji (Tokyo, Japan) Medical X-Ray film. The intensity of the protein band on the films was quantified with Gel-Pro 3.1 image analysis software (Media Cybernetics, Inc., Bethesda, MD).
Statistical Analysis
Data were expressed as mean ± S.E.M. Data were analyzed by one-way analysis of variance, followed by Duncan's post hoc test using Statistica software (StatSoft, Tulsa, OK). Data of gender difference between male and female mouse tissue were analyzed by Student's t test. Statistical significance was considered at p < 0.05.
Results
Tissue Distribution of Mouse Paraoxonases.
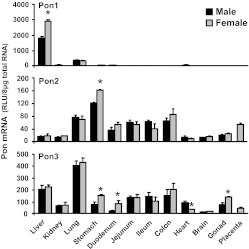
mRNA expression of mouse Pon1, Pon2, and Pon3 was quantified in 13 major tissues (Fig. 1). Expression of Pon1 mRNA (Fig. 1) was highest in mouse liver, detectable in mouse lung, and minimal in other tissues. A gender difference in Pon1 mRNA expression was observed in mouse liver with higher levels in females. Expression of Pon2 mRNA in mice was highest in stomach, followed by colon, lung, placenta, and small intestine (Fig. 1). Pon2 mRNA expression in mouse stomach was higher in females than males. Pon3 mRNA (Fig. 1) was ubiquitously expressed in mouse tissues, with the highest in lung, less in liver, ovary, and gastrointestinal tract (approximately 40–60% of that in lung), and least in kidney and brain (less than 20% of that in the lung). A gender difference of Pon3 expression was noted in mouse stomach, duodenum, and gonad, with higher mRNA in females. In contrast, Pon3 mRNA expression in mouse heart was higher in males than females.
Fig. 1.
Tissue distribution of Pon1 (top), Pon2 (middle), and Pon3 (bottom) mRNA in mice. Total RNA from both male and female C57BL/6 mouse tissues (n = 5/gender) was analyzed by bDNA assay for expression of each paraoxonase mRNA. Data are presented as mean ± S.E.M. *, statistical differences between male and female mice (p < 0.05).
Ontogenic Expression of Paraoxonases in Male and Female Mouse Liver.
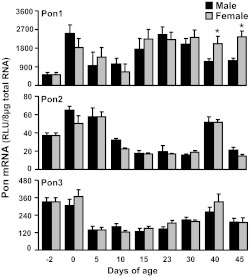
The neonatal and postnatal mRNA expression patterns of the three mouse paraoxonases in male and female livers are shown in Fig. 2. Pon1 is highly expressed in adult mouse liver (Fig. 1). Expression of Pon1 in mice was low 2 days before birth, but increased markedly at parturition to its highest level. After birth Pon1 mRNA decreased to half of that at birth. By 15 days of age Pon1 mRNA returned to the high levels seen at birth. After 30 days of age Pon1 mRNA decreased in male mice, but was maintained in female mice, leading to the gender differences in expression, with higher levels observed in female mice. Pon2 mRNA was low in the livers of adult mice (Fig. 1). The ontogeny study showed that Pon2 mRNA was expressed at high levels in mouse livers at birth and 5 days of age, but decreased rapidly to its lowest level and remained low at most time intervals thereafter (Fig. 2). It is noteworthy that at 40 days of age there was an unexpected peak in the mRNA expression of Pon2. The developmental pattern of Pon3 was somewhat similar to that of Pon2 (Fig. 2). Pon3 mRNA was highly expressed before and at birth. After birth Pon3 mRNA expression decreased by approximately 60% by 5 days of age and remained at relatively low levels thereafter. Similar to Pon2, at 40 days of age there was a peak in the mRNA of Pon3.
Fig. 2.
Ontogenic expression of mouse Pon1 (top), Pon2 (middle), and Pon3 (bottom) mRNA in mouse liver. Total RNA from C57BL/6 mice at each age (n = 5/gender) was analyzed by bDNA assay. Data are presented as mean ± S.E.M. *, statistical differences between male and female mice (p < 0.05).
Protein Levels of Pon1 in Adult Male and Female Mouse Livers.
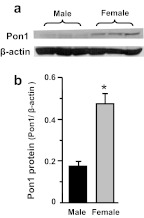
To determine whether gender differences in Pon1 mRNA expression result in a gender difference at the protein level, Pon1 protein levels were evaluated in the livers of adult male and female mice (Fig. 3). Similar to mRNA, Pon1 protein was 170% higher in female than male mouse livers.
Fig. 3.
Protein levels of Pon1 in adult male and female mouse livers. Total protein was isolated from adult C57BL/6 mouse livers. Protein samples (n = 3/gender) were analyzed by Western blotting. a, protein levels of Pon1 and β-actin in mouse liver homogenates were analyzed by Western blotting. b, protein levels of Pon1 and β-actin in mouse liver homogenates are expressed as a ratio of Pon1 to β-actin protein levels per 75 μg of total liver protein. Data are presented as mean ± S.E.M. *, statistical differences between male and female mice (p < 0.05).
Regulation of Gender Differences in Liver Pon1 by Sex Hormones and Growth Hormone.
Pon1 mRNA and protein are higher in adult female than male mouse livers (Figs. 1–3). Gender differences in gene expression may be the result of regulation by sex hormones and/or gender-dimorphic GH secretion patterns. Therefore, the effects of sex hormones and growth hormone replacement on Pon1 mRNA were determined in gonadectomized, hypophysectomized, and lit/lit mice.
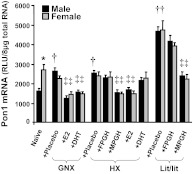
As shown in Fig. 4, castration of naive mice increased Pon1 mRNA, whereas ovariectomy of naive mice did not alter Pon1 mRNA expression, thus leading to a disappearance of the gender difference in Pon1 mRNA levels. In gonadectomized mice, both estrogen (E2) and androgen (DHT) replacement decreased Pon1 mRNA in both male and female mice.
Fig. 4.
Effects of GH and sex hormones on the gender differences in Pon1 mRNA expression in mouse liver from naive, GNX, HX, and lit/lit mice. Total liver RNA was isolated and analyzed by bDNA assay for Pon1 mRNA expression. The data are presented as mean RLU ± S.E.M. (n = 6–7). The study was separated into groups: GNX/HX/Lit + Plac (placebo administered to gonadectomized, hypophysectomized, or lit/lit mice); HX/Lit + MPGH (rat GH twice daily administered by intraperitoneal injection to hypophysectomized or lit/lit mice mimicking male-pattern GH secretion); HX/Lit + FPGH (continuous infusion of GH to hypophysectomized or lit/lit mice via subcutaneously implanted 21-day-release, 1-mg rat GH pellet mimicking female-pattern GH secretion); GNX/HX + DHT (DHT administered to gonadectomized or hypophysectomized mice); and GNX/HX + E2 (E2 administered to gonadectomized or hypophysectomized mice). *, statistical differences (p < 0.05) between male and female mice. †, statistical differences (p < 0.05) between naive mice and the same gender, placebo-treated gonadectomized, hypophysectomized, or lit/lit mice. ‡, statistical differences (p < 0.05) between placebo-treated gonadectomized, hypophysectomized, or lit/lit mice and the same gender, gonadectomized, hypophysectomized, or lit/lit mice after hormone replacement treatment.
The removal of pituitary glands, as indicated by the hypophysectomized mice (Fig. 4), increased Pon1 mRNA in male, but not female, mice, also resulting in the loss of the gender-divergent Pon1 mRNA pattern. In hypophysectomized mice, administration of male-pattern GH (MPGH) and estrogen (E2) decreased Pon1 mRNA in both male and female mice. In contrast, administration of female-pattern GH (FPGH) and androgen (DHT) did not alter Pon1 mRNA.
Loss of function of GH, as shown in lit/lit mice, in which the GH-releasing hormone receptor gene was mutated, caused a very high constitutive expression of Pon1 mRNA. Similar to that in hypophysectomized mice, male-pattern GH administration decreased Pon1 mRNA in both male and female lit/lit mice.
Regulation of Pon1, Pon2, and Pon3 by Prototypical Microsomal Enzyme Inducers.
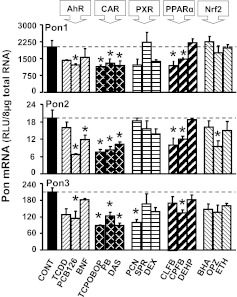
Drugs and other xenobiotics can alter target gene expression through the activation of nuclear receptors and other transcription factors such as AhR, CAR, PXR, PPARα, and Nrf2 (Klaassen and Aleksunes, 2010). To address the effects of distinct transcription factor pathways on the hepatic Pon mRNA expression, regulation of Pon1, Pon2, and Pon3 by well studied prototypical microsomal enzyme inducers was determined. As shown in Fig. 5, the AhR ligand PCB126 and all three CAR activators (PB, TCPOBOP, and DAS) decreased Pon1, Pon2, and Pon3 mRNA. Two PPARα activators (CLFB and CPFB) also decreased Pon1 and Pon2 mRNA.
Fig. 5.
Expression of Pon1 (top), Pon2 (middle), and Pon3 (bottom) mRNA in C57BL/6 mouse liver after administration of prototypical drug-metabolizing enzyme inducers: TCDD, BNF, PCB126, PB, TCPOBOP, DAS, PCN, SPR, DEX, CLFB, CPFB, DEHP, BHA, ETHOXYQ, and OPZ. Total RNA from five chemically treated male livers was analyzed by bDNA assay. All data are expressed as mean ± S.E.M. for five animals in each group, except for controls (CONT), which were combined from the four individual controls after it was determined that they were not statistically different. *, statistical differences between treated and control mice (p < 0.05).
Regulation of Pon1, Pon2, and Pon3 by TCPOBOP in CAR-Null and WT Mouse Livers.
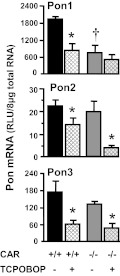
CAR-null mice were used to further determine whether down-regulation of Pon expression by TCPOBOP, the prototypical CAR activator, depends on CAR activation. As depicted in Fig. 6, TCPOBOP decreased Pon1, Pon2, and Pon3 mRNA in wild-type mice. Deficiency of CAR functional protein, as confirmed in the CAR-null mice, decreased basal mRNA of Pon1, but not Pon2 or Pon3. In CAR-null mice, TCPOBOP administration did not decrease Pon1 mRNA expression, but decreased Pon2 and Pon3 mRNA. Therefore, down-regulation of Pon1 by TCPOBOP is CAR-dependent, but regulation of Pon2 and Pon3 seems to be regulated by other mechanisms.
Fig. 6.
Effects of TCPOBOP on Pon1 (top), Pon2 (middle), and Pon3 (bottom) mRNA expression in wild-type and CAR-null mice. The black/gray bars depict the mRNA of each Pon after vehicle treatment, and the striated bars depict the mRNA of each Pon after TCPOBOP treatment. Adult C57BL/6 or CAR-null male mice (n = 5) were intraperitoneally administered TCPOBOP (3 mg/kg in corn oil) or the vehicle corn oil once daily for 4 days. Total RNA from untreated or treated mouse livers was analyzed by bDNA assay. All data are expressed as mean ± S.E.M. of five mice for each treatment. *, statistical differences between TCPOBOP-treated and control mice (p < 0.05). †, statistical differences (p < 0.05) between the vehicle-treated wild-type male mice and vehicle-treated CAR-null male mice.
Discussion
The present study systematically investigated the constitutive and chemical regulation of mouse Pons. Consistent with previous reports, Pon1 and Pon3 are highly expressed in mouse liver, and Pon2 and Pon3 are expressed in the gastrointestinal tract (Mackness and Walker, 1983, 1988; Draganov et al., 2000; Ng et al., 2001). In addition, the present study indicates that Pon1, Pon2, and Pon3 all are expressed in mouse lung (Fig. 1), indicating paraxonases may play detoxification functions or other physiological roles in the lung.
In mice and rats, serum and liver Pon1 enzyme activity is very low at birth and increases until 21 days of age, with a parallel increase in liver mRNA (Li et al., 1997; Moser et al., 1998). In humans, serum PON1 activity is minimal before and at birth, but gradually increases after birth, reaching a plateau between 6 and 15 months of age (Augustinsson and Barr, 1963; Ecobichon and Stephens, 1973; Mueller et al., 1983; Cole et al., 2003). In the present study in mice, Pon1 mRNA expression in mouse liver was very low in the fetus, but rapidly increased at birth, reaching adult levels at 15 days of age (Fig. 2). In contrast, Pon2 and Pon3 both were expressed in the livers of mouse fetuses, but decreased after birth (Fig. 2). Because Pon2 and Pon3 do not have paraoxonase activity (Draganov et al., 2000; Ng et al., 2001), low Pon1 level during early age contributes mainly to the increased susceptibility of young animals to the toxicity of some organophosphorus insecticides (Li et al., 1997; Moser et al., 1998; Karanth and Pope, 2000). Moreover, a recent study demonstrated that low expression of Pon3 in newborn mice resulted in increased rates of early fetal and neonatal death (Kempster et al., 2012).
Gender-divergent gene expression in liver usually is determined by sex hormones and/or growth hormone (Waxman and O'Connor, 2006). Gender-divergent growth hormone secretion, especially male-pattern growth hormone secretion, often contributes to sex-dependent mouse liver gene expression. In the present study, the lower Pon1 expression in male mouse livers was largely caused by the inhibitory effect of male-pattern growth hormone secretion (Fig. 4). Growth hormone produces the sex-dependent gene expression in liver via activation of signal transducer and activator of transcription 5b (STAT5b) (Clodfelter et al., 2006; Waxman and O'Connor, 2006). In silico analysis of the mouse Pon1 promoter sequence indicated that there is a putative STAT5b response element (5′-TGTCTTGGTTCTTTGAAAACGTACG-3′; consensus sequence of STAT5b response element underlined) located between 4881 and 4889 base pairs upstream of the transcription start site. Therefore, the male-pattern growth hormone decrease in mouse Pon1 gene expression probably is mediated via the STAT5b signaling pathway.
One interesting finding is that GH represses Pon1 expression and GH deficiency induces Pon1 in mice. Both Pons and growth hormone can prevent atherosclerosis and other cardiovascular diseases. Pons protect LDL and HDL from oxidation, thus preventing the development of atherosclerosis and other cardiovascular diseases (Aviram and Rosenblat, 2005). Adult patients with growth hormone deficiency are predisposed to premature atherosclerosis and other cardiovascular diseases (Twickler et al., 2000; Elhadd et al., 2001). Growth hormone replacement in GH-deficient adult patients improves their LDL-cholesterol abnormalities (Twickler et al., 2000; Abdul Shakoor and Shalet, 2003). Therefore, growth hormone also prevents atherosclerosis and other cardiovascular diseases. However, in the present study, we showed that male-pattern (intermittent injection) GH administration decreases Pon1 expression. Loss of GH function, as shown in the livers of lit/lit mice, remarkably increases Pon1 constitutive expression in both male and female phenotypes (Fig. 4). Thus, GH represses the expression of Pon1. Our explanation is that the actions of Pons and growth hormone are compensatory. During GH deficiency, Pon1 is induced further to prevent the body from developing atherosclerosis and other cardiovascular diseases.
In addition to growth hormone, DHT decreases Pon1 mRNA expression in gonadectomized mice (Fig. 4) (bin Ali et al., 2003), but not in hypophysectomized mice (Fig. 4), indicating that androgens indirectly decrease Pon1 mRNA in the livers of mice. Sex hormones have the capability of modifying gender-specific GH secretion and thus might influence gene expression in the liver (Legraverend et al., 1992; Painson et al., 1992; Cheng et al., 2006). Therefore, androgens probably regulate female-predominant Pon1 expression by enhancing male-pattern growth hormone secretion.
Administration of exogenous E2 decreased Pon1 mRNA in the livers of both gonadectomized and hypophysectomized mice (Fig. 3). However, in postmenopausal women estrogen replacement therapy increases serum PON1 (Sutherland et al., 2001; Kumru et al., 2005; Topçuoglu et al., 2005; Fenkci et al., 2006). In addition, menopause is an estrogen-deficient status, in which decreases in serum PON1 levels and activity are observed (Kumru et al., 2005; Topçuoglu et al., 2005). Therefore, the effect of estrogens on Pon1/PON1 expression is not consistent between mice and humans.
A major function of Pon1 is to prevent LDL oxidation; however, the effect of estrogens on LDL oxidation is controversial. In rats, 17β-estradiol treatment increases the peroxidation of LDL (Butterworth et al., 1998; Chiang et al., 2004; Eybl et al., 2004), but in humans estradiol at physiological concentrations is unlikely to act as an antioxidant and even as a pro-oxidant in women with high estradiol levels who have increased myeloperoxidase protein in their plasma (Santanam et al., 1998). However, there is evidence that estrogens protect against oxidation of LDL in humans (Arteaga et al., 1998; Brunelli et al., 2000; Ruiz-Sanz et al., 2001; Rontu et al., 2004). Therefore, it seems that estrogens can function to either decrease or increase Pon1/PON1 expression and activity and depends on the dosage of estrogens and species studied.
Ovariectomy depletes endogenous estrogens, but does not alter hepatic Pon1 mRNA in female mice (Fig. 4) (bin Ali et al., 2003), indicating that endogenous estrogens do not contribute to altering Pon1 expression. Therefore, exogenous E2 decreases hepatic Pon1 mRNA independent of its estrogenic activity. In addition to activating estrogen receptors, estrogens including E2, activate CAR (Mendelsohn and Karas, 1999; Kawamoto et al., 2000; Kretschmer and Baldwin, 2005; Hernandez et al., 2007). Administration of CAR activators, such as TCPOBOP, DAS, and PB, decrease Pon1 mRNA expression (Fig. 5). By using CAR-null mice, it was determined that TCPOBOP decreases Pon1 mRNA in a CAR-dependent manner (Fig. 6). Therefore, CAR activation decreases Pon1 expression, suggesting that exogenous E2 may decrease hepatic Pon1 mRNA in mice through CAR activation. In the present study, we also showed that TCPOBOP decreased Pon2 and Pon3 expression independently of CAR activation (Fig. 6). Pon2 is least expressed in the liver of mice, thus down-regulation of Pon2 in mouse liver is essentially not meaningful. However, we do not know how TCPOBOP, phenobarbital, and diallyl sulfide decrease Pon3 expression. This merits further investigation.
Previous reports have shown that dietary polyphenols (Gouédard et al., 2004) and aspirin (Jaichander et al., 2008) increase PON1 gene expression through activation of the AhR. However, in the present studies, AhR activators did not increase Pon1 mRNA. In contrast, the AhR activator PCB126 decreased Pon1 mRNA (Fig. 5).
Taken together, the current study provides important insight into the tissue-specific, gender-divergent, and ontogenic expression, as well as chemical alteration, of mouse paraoxonases. Female-predominant Pon1 mRNA expression in mouse liver is caused primarily by the inhibitory effect of male-pattern growth hormone secretion, whereas the activation of CAR decreases Pon1 expression. The present study provides useful information that helps to better predict the disposition of exogenous and endogenous compounds and suggests the alteration of Pon1 might affect the etiology and pathophysiology of atherosclerosis and other cardiovascular diseases.
Acknowledgments
We thank Drs. Youcai Zhang and Lauren M. Aleksunes for help with animal care and sample processing.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK081461]; the National Institutes of Health National Institute of Environmental Health Sciences [Grants ES09649, ES019487]; and the National Institutes of Health National Center for Research Resources [Grant RR021940].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- LDL
- low-density lipoprotein
- HDL
- high-density lipoprotein
- AhR
- aryl hydrocarbon receptor
- bDNA
- branched DNA
- BHA
- butylated hydroxyanisole
- BL
- blocker
- BNF
- β-naphthoflavone
- CAR
- constitutive androstane receptor
- CE
- capture extender
- CLFB
- clofibric acid
- CPFB
- ciprofibrate
- DAS
- diallyl sulfide
- DEHP
- diethylhexylphthalate
- DEX
- dexamethasone
- DHT
- 5α-dihydroxytestosterone
- E2
- 17β-estradiol
- ETHOXYQ
- ethoxyquin
- GH
- growth hormone
- GHRH
- GH-releasing hormone
- FPGH
- female-pattern GH
- MPGH
- male-pattern GH
- GNX
- gonadectomized
- HX
- hypophysectomized
- LE
- label extender
- Nrf2
- NF-E2 related factor
- OPZ
- oltipraz
- PB
- phenobarbital
- PCB126
- polychlorinated biphenyl 126
- PCN
- pregnenolone 16α-carbonitrile
- PON/Pon
- paraoxonase
- PPARα
- peroxisome proliferator-activated receptor α
- PXR
- pregnane X receptor
- RLU
- relative light units
- SPR
- spironolactone
- STAT5b
- signal transducer and activator of transcription 5b
- TBS-T
- Tris-buffered saline containing 0.1% Tween 20
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- TCPOBOP
- 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene.
Authorship Contributions
Participated in research design: Cheng and Klaassen.
Conducted experiments: Cheng.
Performed data analysis: Cheng.
Wrote or contributed to the writing of the manuscript: Cheng and Klaassen.
References
- Abdul Shakoor SK, Shalet SM. (2003) Effects of GH replacement on metabolism and physical performance in GH deficient adults. J Endocrinol Invest 26:911–918 [DOI] [PubMed] [Google Scholar]
- Arteaga E, Rojas A, Villaseca P, Bianchi M, Arteaga A, Durán D. (1998) In vitro effect of estradiol, progesterone, testosterone, and of combined estradiol/progestins on low density lipoprotein (LDL) oxidation in postmenopausal women. Menopause 5:16–23 [PubMed] [Google Scholar]
- Augustinsson KB, Barr M. (1963) Age variation in plasma arylesterase activity in children. Clin Chim Acta 8:568–573 [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M. (2005) Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences. Curr Opin Lipidol 16:393–399 [DOI] [PubMed] [Google Scholar]
- bin Ali A, Zhang Q, Lim YK, Fang D, Retnam L, Lim SK. (2003) Expression of major HDL-associated antioxidant PON-1 is gender dependent and regulated during inflammation. Free Radic Biol Med 34:824–829 [DOI] [PubMed] [Google Scholar]
- Brunelli R, Mei G, Krasnowska EK, Pierucci F, Zichella L, Ursini F, Parasassi T. (2000) Estradiol enhances the resistance of LDL to oxidation by stabilizing apoB-100 conformation. Biochemistry 39:13897–13903 [DOI] [PubMed] [Google Scholar]
- Butterworth M, Lau SS, Monks TJ. (1998) 2-Hydroxy-4-glutathion-S-yl-17β-estradiol and 2-hydroxy-1-glutathion-S-yl-17β-estradiol produce oxidative stress and renal toxicity in an animal model of 17β-estradiol-mediated nephrocarcinogenicity. Carcinogenesis 19:133–139 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Lu H, Klaassen CD. (2006) Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol 70:1291–1297 [DOI] [PubMed] [Google Scholar]
- Chiang K, Parthasarathy S, Santanam N. (2004) Estrogen, neutrophils and oxidation. Life Sci 75:2425–2438 [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. (2006) Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20:1333–1351 [DOI] [PubMed] [Google Scholar]
- Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, Tward A, Lusis AJ, Jack RM, Costa LG, et al. (2003) Expression of human paraoxonase (PON1) during development. Pharmacogenetics 13:357–364 [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Jarvik GP, Furlong CE. (2003) Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med 54:371–392 [DOI] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Cole TB, Furlong CE. (2005) Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 69:541–550 [DOI] [PubMed] [Google Scholar]
- Curtin BF, Seetharam KI, Dhoieam P, Gordon RK, Doctor BP, Nambiar MP. (2008) Resveratrol induces catalytic bioscavenger paraoxonase 1 expression and protects against chemical warfare nerve agent toxicity in human cell lines. J Cell Biochem 103:1524–1535 [DOI] [PubMed] [Google Scholar]
- Draganov DI, La Du BN. (2004) Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol 369:78–88 [DOI] [PubMed] [Google Scholar]
- Draganov DI, Stetson PL, Watson CE, Billecke SS, La Du BN. (2000) Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J Biol Chem 275:33435–33442 [DOI] [PubMed] [Google Scholar]
- Ecobichon DJ, Stephens DS. (1973) Perinatal development of human blood esterases. Clin Pharmacol Ther 14:41–47 [DOI] [PubMed] [Google Scholar]
- Elhadd TA, Abdu TA, Clayton R. (2001) Hypopituitarism and atherosclerosis. Ann Med 33:477–485 [DOI] [PubMed] [Google Scholar]
- Eybl V, Kotyzová D, Koutenský J, Glattre E. (2004) The effect of estradiol on the oxidative damage and trace element level determined in the liver of rats treated with dimethylarsinic acid. Cent Eur J Public Health 12 (Suppl):S26–S28 [PubMed] [Google Scholar]
- Fenkci IV, Serteser M, Fenkci S, Akyol AM. (2006) Effects of intranasal estradiol treatment on serum paraoxonase and lipids in healthy, postmenopausal women. Gynecol Obstet Invest 61:203–207 [DOI] [PubMed] [Google Scholar]
- Gouédard C, Barouki R, Morel Y. (2004) Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol 24:5209–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Ratliff EP, Andres AM, Huang X, McKeehan WL, Davis RA. (2006) Bile acids decrease hepatic paraoxonase 1 expression and plasma high-density lipoprotein levels via FXR-mediated signaling of FGFR4. Arterioscler Thromb Vasc Biol 26:301–306 [DOI] [PubMed] [Google Scholar]
- Hartley DP, Klaassen CD. (2000) Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab Dispos 28:608–616 [PubMed] [Google Scholar]
- Hernandez JP, Huang W, Chapman LM, Chua S, Moore DD, Baldwin WS. (2007) The environmental estrogen, nonylphenol, activates the constitutive androstane receptor. Toxicol Sci 98:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SC, Zhao SP, Wu ZH. (2006) Probucol up-regulates paraoxonase 1 expression in hepatocytes of hypercholesterolemic rabbits. J Cardiovasc Pharmacol 47:77–81 [DOI] [PubMed] [Google Scholar]
- Jaichander P, Selvarajan K, Garelnabi M, Parthasarathy S. (2008) Induction of paraoxonase 1 and apolipoprotein A1 gene expression by aspirin. J Lipid Res 49:2142–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth S, Pope C. (2000) Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol Sci 58:282–289 [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Kakizaki S, Yoshinari K, Negishi M. (2000) Estrogen activation of the nuclear orphan receptor CAR (constitutive active receptor) in induction of the mouse Cyp2b10 gene. Mol Endocrinol 14:1897–1905 [DOI] [PubMed] [Google Scholar]
- Kempster SL, Belteki G, Licence D, Charnock-Jones DS, Smith GC. (2012) Disruption of paraoxonase 3 impairs proliferation and antioxidant defenses in human A549 cells and causes embryonic lethality in mice. Am J Physiol Endocrinol Metab 302:E103–E107 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. (2005) CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact 155:111–128 [DOI] [PubMed] [Google Scholar]
- Kumon Y, Nakauchi Y, Suehiro T, Shiinoki T, Tanimoto N, Inoue M, Nakamura T, Hashimoto K, Sipe JD. (2002) Proinflammatory cytokines but not acute phase serum amyloid A or C-reactive protein, downregulate paraoxonase 1 (PON1) expression by HepG2 cells. Amyloid 9:160–164 [DOI] [PubMed] [Google Scholar]
- Kumru S, Aydin S, Aras A, Gursu MF, Gulcu F. (2005) Effects of surgical menopause and estrogen replacement therapy on serum paraoxonase activity and plasma malondialdehyde concentration. Gynecol Obstet Invest 59:108–112 [DOI] [PubMed] [Google Scholar]
- Legraverend C, Mode A, Wells T, Robinson I, Gustafsson JA. (1992) Hepatic steroid hydroxylating enzymes are controlled by the sexually dimorphic pattern of growth hormone secretion in normal and dwarf rats. Faseb J 6:711–718 [DOI] [PubMed] [Google Scholar]
- Li WF, Matthews C, Disteche CM, Costa LG, Furlong CE. (1997) Paraoxonase (PON1) gene in mice: sequencing, chromosomal localization and developmental expression. Pharmacogenetics 7:137–144 [DOI] [PubMed] [Google Scholar]
- Mackness MI, Arrol S, Abbott C, Durrington PN. (1993) Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 104:129–135 [DOI] [PubMed] [Google Scholar]
- Mackness MI, Arrol S, Durrington PN. (1991) Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett 286:152–154 [DOI] [PubMed] [Google Scholar]
- Mackness MI, Walker CH. (1983) Partial purification and properties of sheep serum “A'-esterases. Biochem Pharmacol 32:2291–2296 [DOI] [PubMed] [Google Scholar]
- Mackness MI, Walker CH. (1988) Multiple forms of sheep serum A-esterase activity associated with the high-density lipoprotein. Biochem J 250:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A. (1946) An enzyme in animal tissues capable of hydrolysing the phosphorus-fluorine bond of alkyl flurophosphates. J Biol Chem 164:271–289 [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. (1999) The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811 [DOI] [PubMed] [Google Scholar]
- Moser VC, Chanda SM, Mortensen SR, Padilla S. (1998) Age- and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol Sci 46:211–222 [DOI] [PubMed] [Google Scholar]
- Mueller RF, Hornung S, Furlong CE, Anderson J, Giblett ER, Motulsky AG. (1983) Plasma paraoxonase polymorphism: a new enzyme assay, population, family, biochemical, and linkage studies. Am J Hum Genet 35:393–408 [PMC free article] [PubMed] [Google Scholar]
- Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. (2001) Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem 276:44444–44449 [DOI] [PubMed] [Google Scholar]
- Ota K, Suehiro T, Arii K, Ikeda Y, Kumon Y, Osaki F, Hashimoto K. (2005) Effect of pitavastatin on transactivation of human serum paraoxonase 1 gene. Metabolism 54:142–150 [DOI] [PubMed] [Google Scholar]
- Painson JC, Thorner MO, Krieg RJ, Tannenbaum GS. (1992) Short-term adult exposure to estradiol feminizes the male pattern of spontaneous and growth hormone-releasing factor-stimulated growth hormone secretion in the rat. Endocrinology 130:511–519 [DOI] [PubMed] [Google Scholar]
- Rajdl D, Racek J, Trefil L, Siala K. (2007) Effect of white wine consumption on oxidative stress markers and homocysteine levels. Physiol Res 56:203–212 [DOI] [PubMed] [Google Scholar]
- Rao MN, Marmillot P, Gong M, Palmer DA, Seeff LB, Strader DB, Lakshman MR. (2003) Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism 52:1287–1294 [DOI] [PubMed] [Google Scholar]
- Rontu R, Solakivi T, Teisala K, Lehtimäki T, Punnonen R, Jokela H. (2004) Impact of long-term hormone replacement therapy on in vivo and in vitro markers of lipid oxidation. Free Radic Res 38:129–137 [DOI] [PubMed] [Google Scholar]
- Ruiz-Sanz JI, Navarro R, Martínez R, Martín C, Lacort M, Matorras R, Ruiz-Larrea MB. (2001) 17β-Estradiol affects in vivo the low density lipoprotein composition, particle size, and oxidizability. Free Radic Biol Med 31:391–397 [DOI] [PubMed] [Google Scholar]
- Santanam N, Shern-Brewer R, McClatchey R, Castellano PZ, Murphy AA, Voelkel S, Parthasarathy S. (1998) Estradiol as an antioxidant: incompatible with its physiological concentrations and function. J Lipid Res 39:2111–2118 [PubMed] [Google Scholar]
- Sorenson RC, Primo-Parmo SL, Kuo CL, Adkins S, Lockridge O, La Du BN. (1995) Reconsideration of the catalytic center and mechanism of mammalian paraoxonase/arylesterase. Proc Natl Acad Sci U S A 92:7187–7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland WH, Manning PJ, de Jong SA, Allum AR, Jones SD, Williams SM. (2001) Hormone-replacement therapy increases serum paraoxonase arylesterase activity in diabetic postmenopausal women. Metabolism 50:319–324 [DOI] [PubMed] [Google Scholar]
- Thomàs-Moyà E, Gianotti M, Lladó I, Proenza AM. (2006) Effects of caloric restriction and gender on rat serum paraoxonase 1 activity. J Nutr Biochem 17:197–203 [DOI] [PubMed] [Google Scholar]
- Thomàs-Moyà E, Gianotti M, Proenza AM, Lladó I. (2007) Paraoxonase 1 response to a high-fat diet: gender differences in the factors involved. Mol Med 13:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topçuoglu A, Uzun H, Aydin S, Kahraman N, Vehid S, Zeybek G, Topçuoglu D. (2005) The effect of hormone replacement therapy on oxidized low density lipoprotein levels and paraoxonase activity in postmenopausal women. Tohoku J Exp Med 205:79–86 [DOI] [PubMed] [Google Scholar]
- Twickler TB, Wilmink HW, Schreuder PC, Cabezas MC, van Dam PS, Koppeschaar HP, Erkelens DW, Dallinga-Thie GM. (2000) Growth hormone (GH) treatment decreases postprandial remnant-like particle cholesterol concentration and improves endothelial function in adult-onset GH deficiency. J Clin Endocrinol Metab 85:4683–4689 [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol 61:1–6 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C. (2006) Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Wehner JM, Murphy-Erdosh C, Smolen A, Smolen TN. (1987) Genetic variation in paraoxonase activity and sensitivity to diisopropylphosphofluoridate in inbred mice. Pharmacol Biochem Behav 28:317–320 [DOI] [PubMed] [Google Scholar]