Abstract
Mitochondrial-targeted analogs of coenzyme Q (CoQ) are under development to reduce oxidative damage induced by a variety of disease states. However, there is a need to understand the bioenergetic effects of these agents and whether or not these effects are related to redox properties, including their known pro-oxidant effects. We examined the bioenergetic effects of two mitochondrial-targeted CoQ analogs in their quinol forms, mitoquinol (MitoQ) and plastoquinonyl-decyl-triphenylphosphonium (SkQ1), in bovine aortic endothelial cells. We used an extracellular oxygen and proton flux analyzer to assess mitochondrial action at the intact-cell level. Both agents, in dose-dependent fashion, reduced the oxygen consumption rate (OCR) directed at ATP turnover (OCRATP) (IC50 values of 189 ± 13 nM for MitoQ and 181 ± 7 for SKQ1; difference not significant) while not affecting or mildly increasing basal oxygen consumption. Both compounds increased extracellular acidification in the basal state consistent with enhanced glycolysis. Both compounds enhanced mitochondrial superoxide production assessed by using mitochondrial-targeted dihydroethidium, and both increased H2O2 production from mitochondria of cells treated before isolation of the organelles. The manganese superoxide dismutase mimetic manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin did not alter or actually enhanced the actions of the targeted CoQ analogs to reduce OCRATP. In contrast, N-acetylcysteine mitigated this effect of MitoQ and SkQ1. In summary, our data demonstrate the important bioenergetic effects of targeted CoQ analogs. Moreover, these effects are mediated, at least in part, through superoxide production but depend on conversion to H2O2. These bioenergetic and redox actions need to be considered as these compounds are developed for therapeutic purposes.
Introduction
Oxidative damage contributes to a variety of disease states, many of them primarily of vascular etiology. Concern over this problem has led to attempts at antioxidant therapy, in particular, directed at mitochondria, because these organelles probably represent the predominant cellular source of reactive oxygen species (ROS) (Loschen et al., 1971; Boveris et al., 1972; Chance et al., 1979; Raha and Robinson, 2000). Efforts are underway to develop effective antioxidant compounds targeted to mitochondria (Murphy, 2001, 2004). One approach involves the synthesis of compounds linking redox forms of coenzyme Q analogs to alkylated triphenylphosphonium (TPP+) compounds. The resultant antioxidant compounds in the form of lipophilic cations are avidly taken up into the relatively negative mitochondrial matrix (Kelso et al., 2001; Tauskela, 2007). Although much attention has been directed at such targeted redox therapy, an important issue that has received less attention is the metabolic consequence of this approach.
In the work reported here, we focus on the bioenergetic actions of two mitochondrial-targeted antioxidant compounds, mitoquinol (MitoQ) and plastoquinonyl-decyl-triphenylphosphonium (SkQ1). Both have been characterized in vitro and in vivo for potential therapeutic effects (Smith et al., 2008; Skulachev et al., 2009). MitoQ (mitoquinone, mitoquinol, or a mixture of these two redox cycling compounds) consists of the quinone/quinol moiety of CoQ and a shortened (10 carbon or two 5-carbon prenyl units) side chain linked to triphenylphosphonium. SkQ1 arose from efforts to modify the MitoQ structure, improve the balance between antioxidant and pro-oxidant effects, and facilitate increased delivery. A potentially important modification, converting methoxy groups on the quinone moiety of MitoQ to methyl groups, led to SkQ1 (redox forms of plastoquinonyl-decyl-triphenylphosphonium), which demonstrated greater permeability across synthetic lipid bilayers than MitoQ (Skulachev et al., 2009).
MitoQ and SkQ1, here termed mitochondrial-targeted CoQ analogs (MTQAs), are effective antioxidants by virtue of inhibiting lipid peroxidation during redox cycling of the quinol/quinone forms of the compounds (James et al., 2004). The major protective reaction transfers a hydrogen atom (H·) from the quinol to a lipid radical (LO2·), forming LOOH and generating the semiquinone form of the MTQA (James et al., 2004; Skulachev et al., 2010). The respiratory chain then regenerates the MTQA through redox cycling similar to what occurs for native coenzyme Q. An example of the effectiveness of this process is the prevention of cardiolipin peroxidation in heart mitochondria exposed to iron and ascorbate (Skulachev et al., 2010). On the other hand, redox cycling generates electron leaks to oxygen, leading to pro-oxidant properties as demonstrated in past studies (James et al., 2004; O'Malley et al., 2006; Doughan and Dikalov, 2007).
Although CoQ analogs are promising as therapeutic agents there are certain concerns. In particular, there is a need for better understanding of the bioenergetic effects of targeted CoQ analogs and whether they are direct and/or consequent to their redox properties. There is evidence that MTQAs induce mild respiratory uncoupling through the reduction of mitochondrial membrane potential, possibly a protonophoric effect that may involve fatty acid transport (Skulachev et al., 2009, 2010). However, it is not clear to what extent this translates to bioenergetic action at the intact-cell level.
Here, we used recently available intact-cell respirometry technology to examine the dose-dependent effects of MitoQ and SKQ1 on mitochondrial function, extracellular acidification rate (ECAR), and reactive oxygen production in BAE cells. We show that both compounds have potent bioenergetic effects at the mitochondrial level, which are, at least in part, caused by their redox properties. We also provide evidence that MTQAs may, under conditions of cell stress, compromise mitochondrial substrate delivery.
Materials and Methods
Reagents and Supplies.
MitoQ was synthesized from commercially available 11-bromoundecanoic acid (Sigma-Aldrich, St. Louis, MO) and 2,3-dimethoxy-5-methyl-1,4-benzoquinone (Sigma-Aldrich) as described previously (Kelso et al., 2001; Asin-Cayuela et al., 2004). SkQ1 was synthesized from commercially available 2,3-dimethyl-1,4-hydroquinone (Sigma-Aldrich) by using a three-step procedure described previously (Antonenko et al., 2008). Structural integrity and purity were documented by using an Agilent liquid chromatography/mass spectrometry apparatus (Agilent Technologies, Santa Clara, CA). Both SkQ1 and mitoquinol eluted as single peaks in high-performance liquid chromatography. Moreover, 1H and 13C NMR demonstrated the single component nature of these samples. Figure 1 depicts the structures of MitoQ and SkQ1.
Fig. 1.
Reduced (quinol) forms of SkQ1 [10-(6′-plastoquinolyl)decyltriphenylphosphonium] (top) and MitoQ [10-(6′-ubiquinolyl)decyltriphenylphosphonium] (bottom).
Other reagents, kits, and supplies were as specified or purchased from standard sources.
Cell Culture.
BAE cells were grown in medium M199 (Invitrogen, Carlsbad, CA) supplemented with minimal essential medium amino acids (Invitrogen), minimal essential medium vitamins (Sigma-Aldrich), 1 mM sodium pyruvate (Invitrogen), and 20% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA) as described previously (Moser et al., 1992). Cells were grown to near confluence in 150-cm2 flasks and used between passages 5 and 10.
Respirometry.
Oxygen consumption rate (OCR) and ECAR were measured by using an intact-cell respirometer designed for adherent cells (Seahorse Bioscience, North Billerica, MA). BAE cells were grown in 24-well plates designed for respirometer analyses. OCR and ECAR were determined in assay medium consisting of medium M199 lacking sodium bicarbonate and pyruvate (Invitrogen) over time periods up to 95 min with assessments at 8- to 10-min intervals. Before analysis, cells within individual wells were exposed for 18 h or 30 min to 0.3% vehicle (ethanol, in well volume of 600 μl) or differing concentrations of MitoQ or SkQ1 (added in equivalent volume to vehicle alone) as described in the figure legends. In some experiments (see figure legends), cells were treated with manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP), N-acetyl cysteine (NAC), or vehicle for these compounds (water). During respirometry, wells were sequentially injected at the times indicated in the figures with oligomycin (2 μM) to block ATP synthase to assess respiration required for ATP turnover (OCRATP), carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone (FCCP; 2 μM), a proton ionophore, to induce chemical uncoupling and induce maximal respiration, or antimycin A (0.5 μM) plus rotenone (2 μM) to completely inhibit electron transport and measure nonmitochondrial respiration. The FCCP concentration used in these studies was determined by titration with differing amounts of the uncoupler by using the least amount required for maximal uncoupling in cells unexposed to MTQAs.
OCR (pmol per minute per microgram of DNA) and ECAR were determined as the average numbers recorded during time periods defined as intervals between the above sequential injections. ECAR is expressed as milli pH unit change per minute per microgram of DNA, where milli pH = 1/1000 pH unit. Basal OCR was determined as respiration before injection of any compounds minus nonmitochondrial OCR. OCRATP was determined as basal OCR minus OCR after oligomycin injection. OCR accountable by the proton leak was calculated as OCR in the presence of oligomycin minus nonmitochondrial OCR. Maximal uncoupled respiration was calculated as OCR after FCCP minus nonmitochondrial OCR. All values for OCR and ECAR were normalized to DNA content of the individual wells. ECAR was quantified simply as the recorded acidification rate during the respiratory conditions delineated above.
IC50 and EC50 Determinations.
Half-maximal inhibitory (IC50) or stimulatory (EC50) concentrations were determined by third-order polynomial curve fitting (r2 > 0.94 for all curve fits). Maximal inhibition of OCRATP and maximal leak respiration were considered as the theoretical maximal value. For both metrics, this was equal to the difference between basal OCR and nonmitochondrial respiration. Maximal FCCP respiration was considered as FCCP respiration in the presence of vehicle.
Quantification of DNA.
After respirometry, well contents were extracted in 0.4% SDS and diluted to 0.01%. DNA content of each well was determined by using a Sigma-Aldrich DNA Quantitation Kit (DNA-QF) using calf thymus DNA standards prepared in 0.01% SDS.
ROS Detection.
Superoxide was determined by fluorescent microscopy using the mitochondrial-targeted (TPP+ conjugated) dihydroethidium probe MitoSOX (Invitrogen). Cells were loaded for 15 min with 5 μM concentration of MitoSOX in 0.25 ml of medium 199 lacking bicarbonate and containing 5.55 mM d-glucose and 2 mM HEPES. Dyes were washed out twice with 0.3 ml of assay medium. Dye-loaded cells were then incubated at 37°C for 2.5 h and imaged by using an Olympus (Tokyo, Japan) IX71 microscope and tetramethylrhodamine isothiocyanate filter at 1-s exposure. A heat-controlled cell chamber was mounted in place on the microscope stage to maintain the cells at 37°C for the duration of photography. Excitation and emission were set at 555 and 604 nm with bandwidths of 25 and 45 nm, respectively. To document specificity MitoSOX fluorescence was determined with and without the addition of MnTMPyP (25 μM). Quantitative data were obtained by using the image analysis software application ImageJ (National Institutes of Health, Bethesda, MD).
H2O2 Production by Isolated BAE Mitochondria.
BAE cells were treated with MitoQ, SkQ1, or vehicle for 18 h before mitochondria were prepared. The organelles were isolated as described previously (O'Malley et al., 2006). After isolation, mitochondria remained unexposed to any further MTQA compound. Mitochondria isolated in this way were of good quality based on assay of cytochrome c oxidase (Sigma-Aldrich) revealing 5 to 10% outer membrane damage, which is well within an acceptable range compared with mitochondrial preparations from several sources (Wojtczak et al., 1972). There was also no difference in membrane damage between mitochondria from cells treated for 18 h with MitoQ or SkQ1 compared with vehicle-treated cells. H2O2 production was measured as described previously (O'Malley et al., 2006) using the probe 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red; Invitrogen), which is recognized as an optimal probe for isolated mitochondria (Brand, 2010). As we and others have previously shown, ROS detected in this way derives largely from superoxide converted to H2O2 by matrix manganese superoxide dismutase and released externally (O'Malley et al., 2006). Specificity was evident because fluorescence could be abolished by catalase. In addition, we determined fluorescence under no mitochondrial substrate conditions and subtracted that value to determine substrate-induced fluorescence. Samples were prepared in 96-well plates containing 0.06 ml per well of respiratory buffer. Fluorescence was measured as described previously (O'Malley et al., 2006) once every 60 s and carried out for 30 cycles. For quantification, a H2O2 standard curve ranging from 0 to 5 μM was prepared and included on each plate.
Statistics.
Data were analyzed by one-way ANOVA or unpaired, two-tailed t test as described in the figure legends. Analyses were performed by using Prism (GraphPad Software, Inc., San Diego, CA). Significant differences were determined as p < 0.05. Repetition numbers are given in the figure legends. For respirometer experiments, where indicated, repetitions refer to the number of repeated experiments each involving the average of two to three wells per 24-well experimental plate (experimental run). In other cases, the numbers refer to the total number of wells. In these cases, each condition studied was included on each of the multiple plates used, avoiding bias by experimental run, all of which were carried out under identical conditions using the same instrument.
Results
MitoQ and SkQ1 Alter Mitochondrial Respiration in Intact BAE Cells.
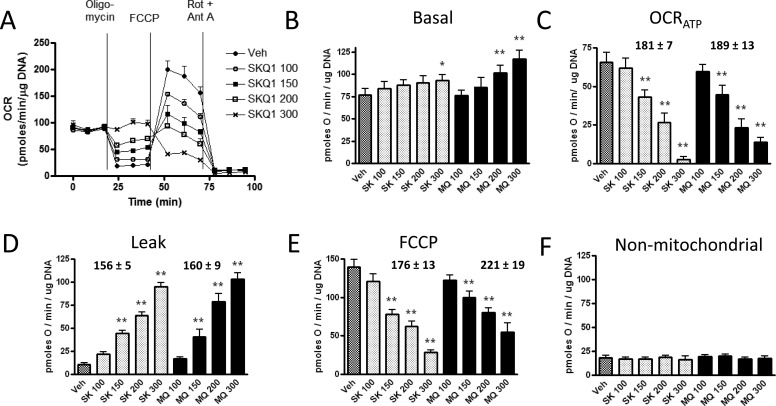
MitoQ and SkQ1 both showed dose-dependent effects on mitochondrial function (Fig. 2). As expected based on past studies we carried out in isolated mitochondria (Fink et al., 2009), untargeted coenzyme Q, at the same concentrations, had no effect (Supplemental Fig. 1). Data were normalized to DNA, although essentially the same results were obtained if oxygen consumption was normalized per well. Figure 2A illustrates a representative experiment depicting changes in OCR under different respiratory conditions defined by the sequential injections of oligomycin, FCCP, and rotenone plus antimycin A. As shown, MitoQ and SkQ1 altered respiratory parameters in a similar and dose-dependent fashion. There was a trend toward enhanced basal respiration, but it was significant only for the highest doses of MitoQ and SkQ1 (Fig. 2B). However, MitoQ and SkQ1 induced potent dose-dependent reductions in OCRATP with corresponding increases in the proton leak (Fig. 2, C and D). MitoQ and SkQ1 decreased respiration in the presence of the chemical uncoupler FCCP (Fig. 2E). There was no difference in nonmitochondrial respiration (Fig. 2F). IC50 and EC50 values (Fig. 2, C-E) did not significantly differ between MitoQ and SkQ1.
Fig. 2.
Effect of vehicle (Veh), MitoQ (MQ), and SkQ1 (SK) in nanomolar concentrations (numbers after abbreviations) on oxygen consumption measured under different respiratory conditions as described under Materials and methods. MitoQ or SkQ1 were added 18 h before respirometry. A, representative respirometer experiment indicating the dose-dependent effect of SkQ1 on OCR measured before and after sequential injections of the indicated compounds; oligomycin (2 μM), FCCP (2 μM), or antimycin A (Ant A; 0.5 μM) plus rotenone (Rot; 2 μM). MitoQ had similar effects (not shown for clarity). Each data point represents the mean of two to three determinations (individual wells) during basal, oligomycin, FCCP, and nonmitochondrial OCR. Data in subsequent panels represent this and five repetitions of this experiment (n = 6 for each data point; each data point representing the mean obtained in two to three wells per condition). B to F, basal OCR (B), OCRATP (C), OCR attributed to the proton leak (D), OCR after FCCP (E), and nonmitochondrial OCR (F) as affected by SkQ1 or MitoQ in the concentrations shown. Numbers above bars indicate IC50 ± S.E.M. for OCRATP and OCR after FCCP or EC50 for the proton leak. Data represent mean ± S.E.M. *, p < 0.05; **, p < 0.01 compared with vehicle by one-way ANOVA with repeated measures and Dunnett post tests. Note y-axis scale differences. IC50 and EC50 values did not differ significantly between SkQ1 and MitoQ.
If the above effects of MitoQ and SkQ1 were caused only by a protonophoric action, they would probably be observable much sooner than the 18-h time period used for the studies shown in Fig. 2. Therefore, we carried out analogous experiments adding MitoQ and SkQ1 either 18 h or 30 min before the experimental runs. As shown in Fig. 3A to D, after 30 min these agents, at concentrations of 200 nM, had no significant effects on basal OCR, OCRATP, or OCR related to the proton leak; however, FCCP uncoupled OCR was mildly reduced. This is in marked contrast to the effects of these agents when added 18 h before the experimental runs. At 300 nM concentrations (Fig. 3, E-G), acute (30 min) MitoQ and SkQ1 did alter OCRATP or FCCP respiration, but were less effective than when added 18 h before study.
Fig. 3.
Effect of MitoQ and SkQ1 added 30 min or 18 h before extracellular flux analyzer runs. A, bioenergetic profile in cells exposed to vehicle (Veh), 200 nM MitoQ (MQ), or 200 nM SkQ1 (SK) for the time periods indicated. Each data point represents mean ± S.E.M. of values determined in six individual wells. B to D, quantitative analysis of data in A demonstrating the effects of 200 nM MitoQ or 200 nM SkQ1 on basal OCR (B), OCRATP (C), and OCR during FCCP uncoupled respiration (D). E to G, quantitative effects of 300 nM MitoQ or 300 nM SkQ1 on basal OCR (E), OCRATP (F), and OCR during FCCP uncoupled respiration (G). Each data point represents mean ± S.E.M. of values determined in four to six individual wells. *, p < 0.05 or **, p < 0.01 compared with vehicle. †, p < 0.01 compared with corresponding compound at 30 min by one-way ANOVA and Tukey post tests.
MitoQ and SkQ1 Enhance Mitochondrial Superoxide Production in Intact BAE Cells.
Overnight exposure of BAE cells to either MitoQ or SkQ1 enhanced superoxide production as demonstrated by fluorescence of the mitochondrial-targeted dihydroethidium probe MitoSOX (Fig. 4). The fluorescent signal localized near the cell nucleus and cytoplasm, which was consistent with the distribution of mitochondria. The signal was abolished by the addition of the cell-permeable SOD mimetic MnTMPyP (25 μM), thus documenting specificity for the superoxide radical.
Fig. 4.
Superoxide release detected as MitoSOX fluorescence in BAE cells (magnification: 400×) treated with MTQAs (300 nM) or vehicle. A to E, cells treated with MitoQ (A), SkQ1 (B), vehicle (Veh) (C), MitoQ plus the superoxide dismutase mimetic MnTMPyP (D), or SkQ1 + MnTMPyP (E). F, quantitative results. Each data point represents the average light density per unit area over 10 representative cells in each of four experiments comparing cells exposed to each indicated condition. MQ, MitoQ; SK, SkQ1; adjacent numbers, concentration in nanomolar; M, MnTMPyP. Data represent mean ± S.E.M. normalized to the average density of vehicle-treated cells. *, p < 0.05; **, p < 0.01 compared with vehicle by one-way ANOVA and Dunnett post tests.
MitoQ and SkQ1 Enhance Mitochondrial H2O2 Production.
ROS production was increased in mitochondria isolated from cells exposed for 18 h to MitoQ or SkQ1 (Fig. 5). It is noteworthy that this increase was evident even though the mitochondria were not exposed to either agent after isolation or during in vitro incubation to assess H2O2 generation. Production rates were normalized to the mean value for vehicle-treated cells. Absolute rates of H2O2 production by mitochondria of vehicle-treated cells were 15.8 ± 1.3 pmol/min/mg mitochondria under state 4 conditions and 11.1 ± 0.9 under simulated state 3 conditions (see legend to Fig. 5). In these experiments, one of the MTQA concentrations (500 nM) was higher than used in other experiments. This was done in anticipation of some loss from mitochondria after isolation.
Fig. 5.
H2O2 production by mitochondria (0.1 mg/ml) isolated from BAE cells after 18-h treatment as indicated (MQ, MitoQ; SK, SkQ1). H2O2 production is expressed relative to the mean value for vehicle-treated cells (see Results). A, production rates were determined under state 4 conditions (no added ADP) in mitochondria respiring on 5 mM succinate + 5 mM glutamate + 1 mM malate (n = 10–12 determinations for each condition). B, production rates determined as in A, but under simulated state 3 conditions created by adding 20 μM ADP. Hexokinase (5 U/ml) and 2-deoxyglucose (5 mM) were added to recycle ATP (formed by oxidative phosphorylation) back to ADP, maintaining ADP availability through the incubation period (n = 10–12 determinations for each condition). *, p < 0.01 compared with vehicle by one-way ANOVA and Dunnett post tests.
Effect of an SOD Mimetic on the Bioenergetic Action of MTQAs.
MnTMPyP did not significantly alter OCR in vehicle-treated cells under any of the respiratory conditions studied (Fig. 6). The SOD mimetic also had no effect on basal OCR in the presence of MitoQ or SkQ1 (Fig. 6A). Although MnTMPyP did not affect OCRATP in the presence of vehicle, MnTMPyP reduced OCRATP in the presence of MitoQ and nonsignificantly (p = 0.06) in the presence of SkQ1 (Fig. 6B). Corresponding effects were observed on the proton leak (Fig. 6C). MnTMPyP did not alter the effects of the MTQA compounds on FCCP or nonmitochondrial OCR (Fig. 6, D and E).
Fig. 6.
Effect of the SOD mimetic MnTMPyP (25 μM; −, absent; +, present), on OCR in BAE cells treated for 18 h with vehicle (VEH), 200 nM MitoQ (MQ 200), or 200 nM SkQ1 (SK 200). MnTMPyP was added at the time of addition of the MTQA or vehicle. Basal OCR (A), OCRATP (B), OCR attributed to the proton leak (C), OCR after FCCP (D), and nonmitochondrial OCR (E) during respirometry were assessed as described in Fig. 2. Each bar represents mean values ± S.E.M. (n = 6 wells per data point). *, p < 0.05; **, p < 0.01 compared with the corresponding condition in the absence of MnTMPyP by two-tailed, unpaired t test.
Effect of N-Acetylcysteine on the Bioenergetic Action of MTQAs.
N-acetylcysteine (NAC) did not alter basal OCR either in the presence or absence of MitoQ or SkQ1 (Fig. 7A). However, NAC mitigated the effect of the MTQA compounds (200 μM) on OCRATP and OCR caused by the proton leak (Fig. 7, B and C). NAC also altered nonmitochondrial respiration for vehicle and MTQA-treated cells. Note that nonmitochondrial OCR did not affect the effect of the MTQA compounds on OCRATP or the calculated proton leak. This is because nonmitochondrial OCR did not enter into the oligomycin-sensitive calculation and was subtracted out of the proton leak calculation (see Materials and Methods).
Fig. 7.
Effect of 10 mM NAC (−, absent; +, present), on OCR in BAE cells treated for 18 h with vehicle (VEH), 200 nM MitoQ (MQ 200), or 200 nM SkQ1 (SK 200). NAC was added at the time of addition of the MTQA or vehicle. A to D, basal OCR (A), OCRATP (B), OCR attributed to the proton leak (C), and nonmitochondrial OCR (D) during respirometry were assessed as described in Fig. 2. Bars represent mean values ± S.E.M. (n = 8–10 wells per data point). *, p < 0.05; **, p < 0.01 compared with the corresponding condition in the absence of NAC by two-tailed, unpaired t test. E, effect of NAC on FCCP respiration. Cells were treated with NAC alone for 18 h or vehicle (water, for NAC) in the absence of exposure to either MitoQ or SkQ1 (n = 3 wells per data point). Data points (mean ± S.E.M.) are expressed as percentage of baseline OCR determined immediately before the addition of oligomycin.
It is noteworthy that NAC seemed to completely abolish any action of FCCP, which is possibly a chemical interaction between these compounds. This phenomenon did not involve MTQAs because it occurred even in cells with no exposure to MTQAs (Fig. 7E). Therefore, we assessed NAC effects on MTQA-modulated bioenergetics, this time using a different chemical uncoupler, 30 μM dinitrophenol (DNP), rather than FCCP (Fig. 8). These studies used 300 μM concentrations of MitoQ and SkQ1 rather than the 200 μM concentrations used in the study shown in Fig. 7. NAC significantly mitigated the effects of 300 μM SkQ1 on OCRATP, OCR attributed to the proton leak, and DNP respiration (Fig. 8, C-E). NAC had similar, but nonsignificant, effects to mitigate the action of 300 μM MitoQ on these parameters.
Fig. 8.
Effect of 10 mM NAC (−, absent; +, present), on OCR in BAE cells treated for 18 h with vehicle (VEH), 300 nM MitoQ (MQ 300), or 300 nM SkQ1 (SK 300). NAC was added at the time of addition of the MTQA or vehicle. A, bioenergetic profiles of cells treated with SkQ1, vehicle, or SkQ1 plus NAC. B, bioenergetic profiles of cells treated with MitoQ, vehicle, or MitoQ plus NAC. C to E, quantitative data showing the effect of NAC on MTQA-treated cells during respiration under OCRATP (C), proton leak (D), and DNP (E). Bars represent mean values ± S.E.M. (n = 4–5 wells per data point). *, p < 0.05; ** p < 0.01 compared with the corresponding condition in the absence of NAC by two-tailed, unpaired t test.
Effect of MTQAs on FCCP Respiration.
Of note is that MTQAs blunted or completely prevented the expected rise in OCR upon addition of FCCP after oligomycin (Fig. 2, A and E). This could, in part, be caused by a nonspecific interaction between the MTQAs and FCCP because the MTQAs had less effect on maximal DNP uncoupled respiration (Fig. 8). However, such interaction cannot explain the full effect of the MTQAs on FCCP respiration, because when the MTQAs were added 30 min before the respirometer runs (Fig. 3) there was much less reduction in FCCP respiration. Possibly oligomycin could deplete cellular ATP enough to impair the subsequent effect of FCCP to increase OCR (Brand and Nicholls, 2011). To determine whether this were the case, we examined the effect of the MTQAs on these parameters in the absence of prior oligomycin. However, MitoQ and SkQ1 (200 nM) had essentially the same effects independent of prior oligomycin (Fig. 9A).
Fig. 9.
Modulation of respiration by MitoQ (MQ), SkQ1 (SK), and pyruvate (all treatments administered for 18 h). Concentrations (nanomolar) are indicated after the designations MQ or SK. A, effects of MitoQ (200 nM) and SkQ1 (200 nM) on OCR as affected by FCCP (2 μM) added in the absence of prior oligomycin. B, effects of MitoQ (200 nM) and SkQ1 (200 nM) on OCR in the presence of pyruvate (5 mM) added to the respirometer (Seahorse Bioscience) medium. Each data point in A and B represents mean ± S.E.M. of values determined in six to seven individual wells. C, quantitative effect of pyruvate on maximal uncoupled (FCCP) respiration in the presence of SkQ1 or MitoQ. Data are expressed as percentage of FCCP respiration without added MTQA (only vehicle added) and represent mean ± S.E.M. of values determined in six to seven wells. *, p < 0.05; **, p < 0.005; ***, p < 0.001 compared with the absence of pyruvate by unpaired, two-tailed t test. D, lack of effect of pyruvate to mitigate OCRATP in the presence of SkQ1 or MitoQ (n = 6–7).
The effect of MTQAs to reduce or prevent the expected rise in OCR upon addition of FCCP could be caused by the limitation of substrate supply through glycolysis. FCCP per se may limit substrate supply through ionophoric action on endosomes and alterations in cytoplasmic calcium (Brand and Nicholls, 2011). We reasoned that MTQAs might further limit substrate supply, thereby reducing OCR in cells already stressed by FCCP. Therefore, we examined the effect of MitoQ and SkQ1 on OCR in the presence of added pyruvate. Pyruvate significantly mitigated the effect of the MTQAs to reduce uncoupled (FCCP) respiration (Fig. 9, B and C).
Pyruvate did not mitigate the effect of the MTQAs to reduce OCRATP (Fig. 9D).
Methyltriphenylphosphonium Does Not Alter BAE Bioenergetics.
The cationic component of the MTQAs might contribute to the bioenergetic effects. However, overnight (18 h) treatment with the cation moiety of the MTQAs, methyltriphenylphosphonium (MeTPP+), had no effect on the bioenergetic profile in BAE cells (Fig. 10). A repeat experiment showed the same result (data not shown).
Fig. 10.
Bioenergetic profiles of cells treated for 18 h with the cation moiety of the MTQAs, MeTPP. Data represent mean ± S.E.M. (n = 3 for each data point). This experiment was repeated with the same result. Ant A, antimycin A; Rot, rotenone.
Effects of MitoQ and SkQ1 on Extracellular Acidification.
Both MTQAs enhanced ECAR under basal conditions and at higher doses after oligomycin administration (Supplemental Fig. 2). As shown in Fig. 11, A and B, MitoQ and SkQ1 increased basal OCR and ECAR with a greater increase in ECAR at higher concentrations, which was consistent with progressive glycolysis (Ferrick et al., 2008; Nicholls et al., 2010). Administering either compound for 18 h, compared with 30 min, decreased the ratio of OCR to ECAR (Fig. 11C), which was consistent with a greater effect on glycolysis at 18 h.
Fig. 11.
Basal OCR compared with ECAR in BAE cells treated with MitoQ or SkQ1. A, cells were exposed to MitoQ for 18 h at the concentrations (nanomolar) indicated by the arrows. B, corresponding data for SkQ1. C, ratio of OCR to ECAR in cells exposed to 200 nM MitoQ or 200 nM SkQ1 for 30 min or 18 h. *, p < 0.01; **, p < 0.001 for differences between 30 min and 18 h by two-way ANOVA (time × MTQA × interaction). Data represent mean ± S.E.M. (n = 6 for each data point). Data were derived from that included in Figs. 2 and 3 and Supplemental Fig. 2.
Discussion
MTQAs have well documented antioxidant properties by preventing lipid peroxidation (James et al., 2004). However, we and others have described pro-oxidant effects caused by redox cycling with superoxide production by the semiquinone form of the compounds (James et al., 2004; O'Malley et al., 2006; Doughan and Dikalov, 2007). Moreover, because these agents are CoQ analogs targeted to mitochondria, metabolic effects should be anticipated. However, these have not been well described. Here, we examined the bioenergetic properties of two MTQA compounds in intact vascular endothelial cells. This cell type is of obvious importance to the problem of vascular disease, a state for which MTQAs are hypothesized to benefit. In fact, endothelial dysfunction is a well known independent predictor of atherosclerotic events in humans (Yeboah et al., 2009).
Here, we used recently available respirometer technology to assess the bioenergetic action of MTQAs. We show that higher MitoQ and SkQ1 mildly increase basal OCR, which is consistent with our past observation of increased oxygen use in BAE cells perfused on glass beads and exposed to a comparatively high concentration of MitoQ at 1000 nM (Fink et al., 2009). Of particular note is that, in spite of only mild change in basal OCR and no change in nonmitochondrial OCR, both MitoQ and SkQ1, in a dose-dependent fashion, markedly reduced OCRATP with corresponding increases in OCR resulting from the proton leak. These data indicate respiratory uncoupling, reaching a marked extent at MTQA concentrations over 150 nM, and imply that any attempt to use MTQAs for therapeutic purposes must remain cognizant of bioenergetic action and use dosing low enough to avoid critical limitation to cellular ATP generation. Of note is that most cellular studies demonstrating the benefits of MTQAs involved concentrations in the upper range or above what we examined herein, for example, reduced telomere shortening in fibroblasts exposed to oxidative stress (Saretzki et al., 2003), reduced glucose-induced oxidative damage in BAE cells (Dhanasekaran et al., 2004), and reduced endogenous DNA damage in human peripheral mononuclear cells (Marthandan et al., 2011).
The uncoupling effect of MTQAs could be caused by a protonophoric effect. If so, we would expect rapid action upon addition to cells, because these cations are highly permeable based on their positive charge and lipophilic side chains (Murphy and Smith, 2007). However, we observed much less effect when the MTQAs were added 30 min before the extracellular flux experiments (Fig. 3). Thus, it is unlikely that protonophoric action explains all of the metabolic effects of these compounds.
The uncoupling effect of MTQAs might be caused, in part, by their known pro-oxidant action (James et al., 2004; O'Malley et al., 2006; Doughan and Dikalov, 2007). Our current data support this, demonstrating increased mitochondrial superoxide production in intact BAE cells (Fig. 4) and increased H2O2 production from mitochondria isolated after 18-h antecedent treatment with MTQAs (Fig. 5). Because the isolated mitochondria were not further exposed to exogenous MTQAs the increase in H2O2 production resulted from the antecedent intact cell treatment.
We then asked whether the uncoupling effects might improve by administering antioxidant agents along with MitoQ or SkQ1. However, in spite of the action of MnTMPyP to reduce superoxide, this agent did not mitigate the effect of MitoQ or SkQ1 on OCRATP and the proton leak. In contrast, MnTMPyP actually enhanced the effect of MitoQ on these parameters with trends in the same direction for SkQ1 (Fig. 6). This could occur if the action of MTQAs on OCRATP was, at least in part, caused by generation of H2O2, because the SOD mimetic converts superoxide to H2O2.
The effects of NAC (Figs. 7 and 8) support a role for H2O2 in mediating the bioenergetic effects of MitoQ and SkQ1. NAC generates glutathione (Sen, 1998), which protects against H2O2-induced oxidation of thiol groups (Mallis et al., 2002). NAC also acts as a direct scavenger of hypochlorous and hydroxyl radicals and reacts slowly with H2O2 (but not with superoxide) (Aruoma et al., 1989). As shown in Figs. 7 and 8, NAC, opposite to MnTMPyP, partially reversed the effects of the MTQAs on OCRATP and the proton leak.
It is noteworthy that NAC completely abolished all action of FCCP even in the absence of an MTQA (Fig. 7E). This could represent the chemical interaction between NAC and FCCP compounds abolishing the ionophoric effect of FCCP. In fact, there is precedent for such direct reactivity with impaired ionophoric action of the chemical uncoupler (Sulo et al., 1985). Of further note, NAC has been reported to reduce FCCP-mediated glutathione depletion in malignant Calu-6 cells (Han et al., 2009) and As4.1 juxtaglomerular cells (Han and Park, 2011). Perhaps these are interactive chemical rather than physiologic effects.
The effects of MTQAs on uncoupling could be caused by the TPP+ cation moiety. However, this is not the case because the cation itself did not alter the bioenergetic profile (Fig. 10).
Reduced ADP or phosphorous availability (for example, through oxidative impairment of transport proteins) might impair ATP production and explain the decrease in OCRATP. However, this does not seem likely because it would impair basal OCR as well (OCRATP is a component of basal OCR).
Part of the effect of MTQAs on FCCP respiration could result from the limitation of substrate availability. FCCP itself can diminish substrate supply through nonmitochondrial cytoplasmic effects (Brand and Nicholls, 2011). MTQAs might exacerbate this leading to a reduction in OCR in cells already stressed with FCCP. This idea is supported by the effect of pyruvate to mitigate the effect of MTQAs on FCCP respiration (Fig. 9C). We acknowledge that this interpretation might be confounded by the possible antioxidant effects of pyruvate that may have improved FCCP respiration. However, adding pyruvate did not prevent the effect of MTQAs to reduce OCRATP (Fig. 9D). Moreover, this lack of effect of pyruvate to mitigate MTQA effects on OCRATP, as well as the relatively small effect of MTQAs on uncoupled respiration caused by DNP (Fig. 8, B and E), add further support to the concept that MTQAs limit substrate supply, at least under the stress condition imposed by FCCP. Although there may be little relevance to the effects of MTQA on substrate supply under usual physiologic conditions, it is possible that this effect may become important under certain cytoplasmic stress conditions.
As shown in Supplemental Fig. 2 and Fig. 11, MTQAs enhanced ECAR under basal respiratory conditions, suggesting that these compounds enhance glycolysis. This is consistent with our prior report indicating that MitoQ induces a dose-dependent (50–1000 nM) enhancement of glucose oxidation while reducing fatty acid oxidation (Fink et al., 2009). This is also consistent with an adaptive response to impaired capacity for ATP formation because glucose oxidation provides more ATP per unit oxygen consumed than fatty acids.
We can only speculate as to comparative effects between MitoQ and SkQ1. We observed no significant differences in IC50 for OCRATP or FCCP uncoupled respiration and no significant differences in EC50 for the proton leak. However, SkQ1 seemed to have less pro-oxidant effect both for superoxide (Fig. 4) and H2O2 production (Fig. 5), and the effects of SkQ1 may have been mitigated more by NAC. It has been reported that SkQ1, compared with MitoQ, manifests less pro-oxidant action in aqueous medium and greater antioxidant effect against lipid peroxidation (Skulachev et al., 2009). Thus, our current observations are compatible with this report.
Although our studies indicate pause as to the therapeutic use of MTQAs, these compounds may be of metabolic benefit at low enough doses. It is plausible that low doses could induce subtle uncoupling in vivo that could, over time, result in cumulative energy dissipation and weight loss. Furthermore, our past studies showed that MTQAs enhance glucose oxidation while reducing fatty acid oxidation (Fink et al., 2009). This effect might be advantageous to cells faced with an ischemic environment wherein glucose oxidation provides more ATP per unit oxygen consumed. In fact, a switch to glucose oxidation is a well known adaptive cardiac response to limited blood supply (Boudina and Abel, 2007). Finally, the prevention of lipid peroxidation remains a potential benefit of MTQA treatment (Kelso et al., 2002; Skulachev et al., 2009), and it is suggested that the cytotoxic effects of MitoQ may provide a form of anticancer therapy (Rao et al., 2010).
There are limitations to our study. Targeting of dihydroethidium to mitochondria in the form of MitoSOX depends on mitochondrial membrane potential for localization. However, we observed an increase in fluorescence, not a decrease, which would occur if MTQAs reduce potential. We were unable to determine EC50 values for the effect of the MTQA compounds on OCR or ECAR (Fig. 2 and Supplemental Fig. 2), because we do not know the maximal enhancement beyond 300 μM concentrations. However, this does not seem important given the near-maximal effect of the 300 μM concentrations on uncoupling and the proton leak, that is, effects that would probably render toxicity beyond tolerable limits. Another limitation is that we did not assess the effects of MTQAs on mitochondrial ultrastructure. Although we cannot rule this out, we doubt there is any significant impairment in mitochondrial structure because basal OCR was not adversely affected and we did not see any greater cytochrome c loss from mitochondria of control versus MTQA-treated cells (see Materials and Methods). Moreover, MitoQ actually protects mitochondria from structural changes caused by ischemic reperfusion (Adlam et al., 2005) and SkQ1 protects against age-related mitochondrial damage in Drosophila melanogaster flight wings (Anisimov et al., 2008), which is consistent with a general effect of MTQAs to protect against oxidative damage to lipid membranes (James et al., 2004; Skulachev et al., 2010). A further limitation is that we administered only the reduced forms of MitoQ and SkQ1. We would expect very similar effects caused by the regenerative redox cycling of these compounds (James et al., 2004; Skulachev et al., 2010). Moreover, in the past, we treated isolated BAE mitochondria with both mitoquinone and mitoquinol and observed similar effects on ROS production (O'Malley et al., 2006).
In summary, new findings are as follows. 1) MTQAs act in a dose-dependent fashion to reduce OCRATP while increasing proton leak and mildly increasing (higher doses) basal respiration. Higher doses lead to marked uncoupling and might not be tolerated in vivo depending on tissue concentrations achieved. 2) The bioenergetics effects of MTQAs on OCRATP and the proton leak are, in part, consequent to their pro-oxidant action. The pro-oxidant effect probably occurs through the generation of H2O2 or downstream effects of this radical to induce oxidative modification of molecules such as proteins or compounds containing thiol groups. 3) MTQAs enhance the basal rate of acidification, suggesting enhanced glycolysis. 4) MTQAs may limit substrate supply in cells subject to cytoplasmic stress.
We conclude that these effects of MTQAs and their dose dependence need to be considered as these or related compounds are developed for therapeutic purposes.
Supplementary Material
This work was supported by Veterans Affairs Medical Research Funds; the National Institutes of Health National Heart, Lung, and Blood Institute [Grant 5R01HL073166]; and the Iowa Affiliate Fraternal Order of the Eagles. A.M.F. received a predoctoral fellowship from the American Chemical Society Division of Medicinal Chemistry sponsored by Bristol-Myers Squibb and a National Institutes of Health National Institute of General Medical Sciences Training Grant in Pharmacological Sciences [Grant T32GM067795].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- ROS
- reactive oxygen species
- ANOVA
- analysis of variance
- BAE
- bovine aortic endothelial
- CoQ
- coenzyme Q
- MTQA
- mitochondrial-targeted CoQ analog
- DNP
- dinitrophenol
- ECAR
- extracellular acidification rate
- FCCP
- carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone
- MitoQ
- mitoquinol
- MnTMPyP
- manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin
- NAC
- N-acetylcysteine
- OCR
- oxygen consumption rate
- OCRATP
- OCR directed at ATP turnover
- SkQ1
- plastoquinonyl-decyl-triphenylphosphonium
- SOD
- superoxide dismutase
- TPP
- triphenylphosphonium
- MeTPP
- methyltriphenylphosphonium
- VEH/Veh
- vehicle.
Authorship Contributions
Participated in research design: Fink, Yorek, Kerns, and Sivitz.
Conducted experiments: Fink, Herlein, Fenner, and Sivitz.
Contributed new reagents or analytic tools: Fenner and Kerns.
Performed data analysis: Fink, Herlein, and Sivitz.
Wrote or contributed to the writing of the manuscript: Fink, Yorek, Kerns, and Sivitz.
References
- Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. (2005) Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. Faseb J 19:1088–1095 [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Bakeeva LE, Egormin PA, Filenko OF, Isakova EF, Manskikh VN, Mikhelson VM, Panteleeva AA, Pasyukova EG, Pilipenko DI, et al. (2008) Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 5. SkQ1 prolongs lifespan and prevents development of traits of senescence. Biochemistry (Mosc) 73:1329–1342 [DOI] [PubMed] [Google Scholar]
- Antonenko YN, Avetisyan AV, Bakeeva LE, Chernyak BV, Chertkov VA, Domnina LV, Ivanova OY, Izyumov DS, Khailova LS, Klishin SS, et al. (2008) Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc) 73:1273–1287 [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, Butler J. (1989) The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6:593–597 [DOI] [PubMed] [Google Scholar]
- Asin-Cayuela J, Manas AR, James AM, Smith RA, Murphy MP. (2004) Fine-tuning the hydrophobicity of a mitochondria-targeted antioxidant. FEBS Lett 571:9–16 [DOI] [PubMed] [Google Scholar]
- Boudina S, Abel ED. (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223 [DOI] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. (1972) The cellular production of hydrogen peroxide. Biochem J 128:617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD. (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 45:466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605 [DOI] [PubMed] [Google Scholar]
- Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B. (2004) Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem 279:37575–37587 [DOI] [PubMed] [Google Scholar]
- Doughan AK, Dikalov SI. (2007) Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9:1825–1836 [DOI] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, Beeson C. (2008) Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 13:268–274 [DOI] [PubMed] [Google Scholar]
- Fink BD, O'Malley Y, Dake BL, Ross NC, Prisinzano TE, Sivitz WI. (2009) Mitochondrial targeted coenzyme Q, superoxide, and fuel selectivity in endothelial cells. PLoS ONE 4:e4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YH, Kim SH, Kim SZ, Park WH. (2009) Carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) as an O2˙̄ generator induces apoptosis via the depletion of intracellular GSH contents in Calu-6 cells. Lung Cancer 63:201–209 [DOI] [PubMed] [Google Scholar]
- Han YH, Park WH. (2011) Intracellular glutathione levels are involved in carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone-induced apoptosis in As4.1 juxtaglomerular cells. Int J Mol Med 27:575–581 [DOI] [PubMed] [Google Scholar]
- James AM, Smith RA, Murphy MP. (2004) Antioxidant and pro-oxidant properties of mitochondrial coenzyme Q. Arch Biochem Biophys 423:47–56 [DOI] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. (2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276:4588–4596 [DOI] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Hughes G, Ledgerwood EC, Gane AM, Smith RA, Murphy MP. (2002) Prevention of mitochondrial oxidative damage using targeted antioxidants. Ann NY Acad Sci 959:263–274 [DOI] [PubMed] [Google Scholar]
- Loschen G, Flohé L, Chance B. (1971) Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett 18:261–264 [DOI] [PubMed] [Google Scholar]
- Mallis RJ, Hamann MJ, Zhao W, Zhang T, Hendrich S, Thomas JA. (2002) Irreversible thiol oxidation in carbonic anhydrase III: protection by S-glutathiolation and detection in aging rats. Biol Chem 383:649–662 [DOI] [PubMed] [Google Scholar]
- Marthandan S, Murphy MP, Billett E, Barnett Y. (2011) An investigation of the effects of MitoQ on human peripheral mononuclear cells. Free Radic Res 45:351–358 [DOI] [PubMed] [Google Scholar]
- Moser DR, Lowe WL, Jr, Dake BL, Booth BA, Boes M, Clemmons DR, Bar RS. (1992) Endothelial cells express insulin-like growth factor-binding proteins 2 to 6. Mol Endocrinol 6:1805–1814 [DOI] [PubMed] [Google Scholar]
- Murphy MP. (2001) Development of lipophilic cations as therapies for disorders due to mitochondrial dysfunction. Exp Opin Biol Ther 1:753–764 [DOI] [PubMed] [Google Scholar]
- Murphy MP. (2004) Investigating mitochondrial radical production using targeted probes. Biochem Soc Trans 32:1011–1014 [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47:629–656 [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. (2010) Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp pii:2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI. (2006) Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem 281:39766–39775 [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. (2000) Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25:502–508 [DOI] [PubMed] [Google Scholar]
- Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS, Keller PW, Joseph J, Kalyanaraman B, Shacter E. (2010) The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem 285:34447–34459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saretzki G, Murphy MP, von Zglinicki T. (2003) MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell 2:141–143 [DOI] [PubMed] [Google Scholar]
- Sen CK. (1998) Redox signaling and the emerging therapeutic potential of thiol antioxidants. Biochem Pharmacol 55:1747–1758 [DOI] [PubMed] [Google Scholar]
- Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, et al. (2009) An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta 1787:437–461 [DOI] [PubMed] [Google Scholar]
- Skulachev VP, Antonenko YN, Cherepanov DA, Chernyak BV, Izyumov DS, Khailova LS, Klishin SS, Korshunova GA, Lyamzaev KG, Pletjushkina OY, et al. (2010) Prevention of cardiolipin oxidation and fatty acid cycling as two antioxidant mechanisms of cationic derivatives of plastoquinone (SkQs). Biochim Biophys Acta 1797:878–889 [DOI] [PubMed] [Google Scholar]
- Smith RA, Adlam VJ, Blaikie FH, Manas AR, Porteous CM, James AM, Ross MF, Logan A, Cochemé HM, Trnka J, et al. (2008) Mitochondria-targeted antioxidants in the treatment of disease. Ann NY Acad Sci 1147:105–111 [DOI] [PubMed] [Google Scholar]
- Sulo PS, Liptaj T, Jakubik T, Antalik M. (1985) Structure characterization of reaction products from phenylhydrazonopropanedinitrile and thiols. Collect Czech Chem Commun 50:375–382 [Google Scholar]
- Tauskela JS. (2007) MitoQ–a mitochondria-targeted antioxidant. IDrugs 10:399–412 [PubMed] [Google Scholar]
- Wojtczak L, Zaluska H, Wroniszewska A, Wojtczak AB. (1972) Assay for the intactness of the outer membrane in isolated mitochondria. Acta Biochim Pol 19:227–234 [PubMed] [Google Scholar]
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. (2009) Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120:502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.