Abstract
Medullipin has been proposed to be an antihypertensive lipid hormone released from the renal medulla in response to increased arterial pressure and renal medullary blood flow. Because anandamide (AEA) possesses characteristics of this purported hormone, the present study tested the hypothesis that AEA or one of its metabolites represents medullipin. AEA was demonstrated to be enriched in the kidney medulla compared with cortex. Western blotting and enzymatic analyses of renal cortical and medullary microsomes revealed opposite patterns of enrichment of two AEA-metabolizing enzymes, with fatty acid amide hydrolase higher in the renal cortex and cyclooxygenase-2 (COX-2) higher in the renal medulla. In COX-2 reactions with renal medullary microsomes, prostamide E2, the ethanolamide of prostaglandin E2, was the major product detected. Intramedullarily infused AEA dose-dependently increased urine volume and sodium and potassium excretion (15–60 nmol/kg/min) but had little effect on mean arterial pressure (MAP). The renal excretory effects of AEA were blocked by intravenous infusion of celecoxib (0.1 μg/kg/min), a selective COX-2 inhibitor, suggesting the involvement of a prostamide intermediate. Plasma kinetic analysis revealed longer elimination half-lives for AEA and prostamide E2 compared with prostaglandin E2. Intravenous prostamide E2 reduced MAP and increased renal blood flow (RBF), actions opposite to those of angiotensin II. Coinfusion of prostamide E2 inhibited angiotensin II effects on MAP and RBF. These results suggest that AEA and/or its prostamide metabolites in the renal medulla may represent medullipin and function as a regulator of body fluid and MAP.
Introduction
The concept that the kidney is the most important organ for long-term blood pressure control and renal dysfunction is a major cause of hypertension has gained increasing acceptance. Although the prohypertensive hormonal system of the kidney, i.e., the renin-angiotensin system, is well established, the kidney has also been proposed to contain an antihypertensive endocrine system (Muirhead, 1993). According to this hypothesis, the renal medulla responds to increased medullary blood flow by secreting a vasodepressor lipid termed medullipin. The antihypertensive effects of medullipin were proposed to be caused by three specific biologic properties: vasodilator, inhibitor of sympathetic activation, and promoter of renal salt and water excretion. Others have speculated on a possible relationship between medullipin and the mechanism of pressure natriuresis, whereby sodium and water excretion by the kidneys are increased in proportion to elevated renal perfusion pressure (Mattson, 2003). With respect to pressure natriuresis, it is known that glomerular filtration, total RBF, and cortical blood flow are finely regulated and do not change significantly in response to increased renal perfusion pressure, whereas medullary blood flow is poorly autoregulated and increases in proportion to elevated renal perfusion pressure. Pressure natriuresis is a local renal adaptive mechanism to increased arterial blood pressure. However, the identity of medullipin has remained elusive, but its elucidation undoubtedly has great potential for leading to new therapies for hypertension.
The present study tested the hypothesis that anandamide (AEA) and/or its metabolites represent medullipin. AEA is the N-acyl ethanolamide of arachidonic acid (AA). Best known as a major endocannabinoid in the brain (Devane et al., 1992), AEA has been noted for its high concentration in kidney (Koga et al., 1997; Long et al., 2011), although its distribution in this organ has not been established. Although roles for AEA have been identified for various physiological responses, such as antinociception (Cravatt et al., 2001), drug dependence (Schlosburg et al., 2009a; Ramesh et al., 2011), and inflammation (Schlosburg et al., 2009b), its functions in the kidney are not well defined.
AEA has several characteristics consistent with the biological identity of medullipin. First, it is a neutral lipid and a derivative of arachidonic acid (Muirhead, 1993). After intravenous infusion into anesthetized rats, AEA elicited a prolonged vasodepressor response accompanied by bradycardia, consistent with inhibition of the baroreceptor reflex and sympathetic outflow (Varga et al., 1995). Indeed, the vasorelaxant properties of AEA have been demonstrated on various arteriolar beds (Mair et al., 2010) including afferent and efferent arterioles of the kidney (Deutsch et al., 1997; Koura et al., 2004). Deutsch et al. (1997) showed that AEA inhibits the KCl-stimulated release of norepinephrine from sympathetic nerves on renal arterioles, consistent with its ability to inhibit sympathetic effector function. Finally, methanandamide, a methylated analog of AEA, increased urine volume (UV) and decreased mean arterial blood pressure after intrarenal medullary infusion in anesthetized rats, implying that medullary AEA can regulate body volume homeostasis and blood pressure (Li and Wang, 2006). Although some of the latter effects were blocked by known antagonists of AEA receptors, others, such as the effect of intramedullarily infused methanandamide on diuresis and blood pressure, were not, suggesting a cannabinoid receptor-independent mechanism.
The kidney is also unique in its high activities of the AEA-metabolizing enzymes fatty acid amide hydrolase (FAAH) and cyclooxygenase-2 (COX-2). FAAH is a membrane-bound amidase that catalyzes the hydrolysis of AEA to arachidonic acid and ethanolamine (Cravatt et al., 2001). FAAH is usually considered as an AEA-inactivating enzyme, because its products are inactive at cannabinoid receptors. However, arachidonic acid is itself an enzyme substrate and signaling molecule. COX-2, the “inducible” form of cyclooxygenase, is expressed constitutively in the kidney (Harris et al., 1994), particularly in the renal medulla (Yang, 2003). COX-2 was demonstrated to metabolize AEA to N-ethanolamide analogs of prostaglandins (Yu et al., 1997), termed prostamides. Whether prostamides have a functional role in the kidney or elsewhere in the body remains unknown. However, several prostamides, including prostamide E2, were detected in the kidneys of mice treated with AEA (Weber et al., 2004).
The present study addressed the relationship among AEA, COX-2, and FAAH in the kidney, with a particular focus on the difference between cortex and medulla. The renal excretory effects of AEA after its infusion into the renal medulla were studied in the absence and presence of a COX-2 inhibitor. Finally, the pharmacokinetic and cardiovascular properties of prostamide E2 after intravenous infusion were characterized. Our data highlight several additional properties of AEA or its COX-2 metabolite, prostamide E2, consistent with their identity as renomedullary neutral antihypertensive lipids.
Materials and Methods
AEA, prostamide E2, and prostamide F2α were purchased from Cayman Chemical (Ann Arbor, MI). Arachidonic acid was from Sigma (St. Louis, MO). The deuterated analogs, d8-AEA, d8-2-arachidonoylglycerol, and d8-arachidonic acid, were from Cayman Chemical. All chemicals used in the study were of reagent grade or better.
Animals.
Two- to 4-month-old male mice with a C57BL6 background were used in this study. The mice were generated by using a breeding colony of cannabinoid receptor and FAAH knockout mice established at Virginia Commonwealth University. The knockout strains have been maintained by backcrossing onto the C57BL6 background for approximately 12 generations. In some cases, male C57BL6/J mice purchased from The Jackson Laboratory (Bar Harbor, ME) were used. All experiments involving mice were conducted according to protocols approved previously by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Liquid Chromatography/Tandem Mass Spectrometry for Quantitation of Endocannabinoids and Fatty Acyl Amides.
The endocannabinoids [AEA and 2-arachidonoyl glycerol (2-AG)] and the ethanolamides of the fatty acids (oleic acid and palmitic acid) were measured in renal cortex and medulla by LC-MS/MS as described previously (Ramesh et al., 2011). In brief, mice were euthanized, the kidneys were removed, and the renal cortex and medulla were rapidly dissected on ice, snap-frozen in liquid nitrogen, and stored at −80°C until the time of processing. On the day of processing, the preweighed tissues were homogenized in 1.4 ml of chloroform/methanol (2:1 v/v containing 0.2 M phenylmethylsulfonyl fluoride with 2 pmol d8-AEA, 1 nmol d8-2-arachidonoyl glycerol, 3.3 nmol d4-palmitoyl ethanolamide, 3 nmol d4-oleoyl ethanolamide, and 1 nmol d8-arachidonic acid as internal standards). After adding ice-cold normal saline (0.3 ml), the samples were vortexed for 1 min, and the tubes were then centrifuged at 3200g at 4°C for 10 min. The organic phase was collected, and the aqueous phase plus debris was extracted two more times with 0.8 ml of chloroform. The organic phases from the three extractions were pooled and dried under nitrogen gas, and the residue was reconstituted with 0.1 ml of chloroform and mixed with 1 ml of ice-cold acetone. After centrifuging at 1800g for 5 min at 4°C, the upper layer was collected and evaporated under nitrogen. Dried samples were reconstituted with 0.1 ml of methanol for analysis by LC-MS/MS in electrospray mode. The following ion transitions were monitored in positive mode, (348 > 62) and (348 > 91) for AEA; (356 > 62) for AEA-d8; (379 > 287) and (279 > 269) for 2-AG; (387 > 96) for 2-AG-d8; (300 > 62) and (300 > 283) for palmitoyl ethanolamine (PEA); (304 > 62) for PEA-d4; (326 > 62) and (326 > 309) for oleoyl ethanolamine (OEA); and (330 > 66) for OEA-d4; and in negative mode: (303 > 259) and (303 > 59) for AA and (311 > 267) for AA-d8. A calibration curve was constructed for each assay based on peak area ratios for the calibrators and internal standards. The extracted standard curves ranged from 0.03 to 40 pmol for AEA and 0.05 to 64 nmol for 2-arachidonoyl glycerol.
Immunohistochemical Analysis for COX-2 and FAAH in the Mouse Kidney.
The kidneys were removed, cut longitudinally, and fixed in 10% neutral buffered formalin. The fixed tissue was embedded in paraffin, and 4-μm sections were cut. Immunostaining was performed as described previously (Li et al., 2007) by using a rabbit anti-COX-2 polyclonal antibody (1:50, Cell Signaling Technology, Danvers, MA) or a rabbit monoclonal anti-FAAH antibody (1:2000 dilution; Cell Signaling Technology), respectively. For a negative control, normal goat or rabbit serum was used instead. The negative controls did not show positive immunoreactivity.
Prostamide-Forming Activity in Kidney Microsomes with AEA as Substrate.
Kidney cortical and medullary tissues from four to six mice were pooled and homogenized in ice-cold 0.25 M sucrose containing 25 mM Na-HEPES, pH 7.4, 1 mM EDTA, and Complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). After centrifuging at 10,000g for 10 min at 4°C, the supernatant was transferred and centrifuged at 100,000g for 1 h. The microsomes were resuspended in the same buffer, aliquotted into several tubes, snap-frozen in liquid nitrogen, and stored at −80°C. The microsomal protein concentration was determined by the Bradford method using a commercially available kit (Bio-Rad Laboratories, Hercules, CA).
Reactions (100 μl) contained 1 mg/ml microsomal protein, 0.1 M Tris-HCl, pH 8.0, 1 mM epinephrine, 1 mM glutathione, and 0.1 mM AEA. The reactions were incubated at 37°C for 30 min and stopped by the addition of 15 μl of 2 N HCl followed by 300 μl of 2:1 (v/v) chloroform/methanol. The samples were vortexed for 90 s and centrifuged for 3 min. The bottom layer was transferred to a glass conical tube, and AEA-d8 (5 ng) was added as external standard. The samples were dried under nitrogen gas and reconstituted in methanol for analysis by LC-MS/MS as described previously (Weber et al., 2004) with some modification. The flow rate was 0.2 ml/min. The column was a Luna C8 3 μm (100 × 2.0 mm) column (Phenomenex, Torrance, CA) maintained at 40°C temperature. The entire liquid chromatography eluent was directed into the electrospray ionization source. The mass spectrometer was switched between positive ion mode (for AEA, prostamides, and d8-AEA) and negative ion mode (for arachidonic acid). The ion transitions monitored were: (396 > 378) and (396 > 62) for prostamide E2; (356 > 62) for AEA-d8; (398 > 380) and (398 > 62) for prostamide F2α; and (303 > 259) for arachidonic acid. Calibration curves were constructed for prostamide E2 and arachidonic acid.
Western Blot Analysis.
Western blot analysis was performed as described previously (Zou et al., 2001). In brief, kidney cortical or medullary microsomal samples (20 μg) were electrophoresed through a SDS-polyacrylamide gel electrophoresis gel together with a protein size standard followed by electroblotting onto a nitrocellulose membrane. The membrane was probed for FAAH or COX-2 protein by using the anti-FAAH or anti-COX-2 antibodies described above at 1:2000 and 1:50 dilutions, respectively. After washing, the membranes were incubated for 1 h with 1:3000 horseradish peroxidase-labeled secondary antibody. Images were developed by using enhanced chemiluminescence detection solution (Thermo Fisher Scientific, Waltham, MA) followed by exposure to Kodak X-Omat Blue film (Eastman Kodak, Rochester, NY). The intensities of the protein bands on the image were quantified by using ImageJ analysis software (National Institutes of Health, Bethesda, MD). To normalize for small differences in protein loading, the blots were stripped and reanalyzed with an anti-β-actin primary antibody (1:2000 dilution; Sigma).
Surgical Preparation of Mice.
Mice were anesthetized and prepared surgically for each of the following protocols as described previously (Li et al., 2005). In brief, after anesthesia with ketamine (Ketaject, Phoenix Pharmaceutical, Inc., St. Charles, MO; 30 mg/kg i.p.) and thiobutabarbital (Inactin, Sigma; 50 mg/kg i.p.), the mice were placed on a thermostatically controlled warming table to maintain body temperature at 37°C. After tracheotomy, catheters were placed in the jugular vein and carotid artery for intravenous infusions and measurements of MAP, respectively. For experiments where RBF was measured, a flow probe (2 mm; Transonic Systems Inc., Ithaca, NY) was placed around the left renal artery, and RBF was measured as described previously.
Protocol 1: Intramedullary Interstitial Infusion of AEA and Measurement of MAP, Urinary Formation, and RBF.
The technique for construction and placement of a catheter for renal medullary infusion (Lu et al., 1992) is well established in our laboratory (Li et al., 2005; Zhu et al., 2011). In brief, the left kidney was immobilized by placement of its dorsal side up in a kidney cup. A catheter (tapered tip, 1 mm) was gently implanted into the renal outer medulla vertically from the dorsal surface and anchored into place on the kidney surface with Vetbond tissue adhesive (3M Co., Minneapolis, MN). The medullary catheter was infused with solution containing 90% phosphate-buffered saline containing 205 mM NaCl, 40.5 mM Na2HPO4, and 9.5 mM NaH2PO4 (pH 7.4, 550 mosM), 5% ethanol, and 5% Emulphor (BASF, Stamford, CT) at a rate of 2 μl/min to maintain the patency of the interstitial infusion line. For the collection of urine from the left kidney, the right ureter was ligated and then cut to keep open on the side proximal to the kidney, and the bladder was catheterized to collect urine. The UV was determined gravimetrically and urinary sodium (Na+) and potassium (K+) concentrations were measured by flame photometry. UV and urinary Na+ excretion were factored per gram of kidney weight. Mice received a continuous intravenous infusion of 0.9% NaCl solution containing 2% albumin at a rate of 1 ml · h−1 · 100 g of body weight−1 to replace fluid loss and maintain a constant hematocrit (≈42%). After a 1.5-h equilibration period and two 10-min control sample collection periods during infusion of the vehicle alone, AEA was infused for 30-min periods (10-min dead space-removing period and 20-min sample collection) at three doses (15, 30, and 60 nmol/kg/min). The AEA was first dissolved in ethanol and then in Emulphor and 550 mOsm saline-phosphate solution for final concentrations of 5, 5, and 90%, respectively. The vehicle without AEA was infused during the equilibration period. An additional group of mice was infused intravenously with the COX-2-selective inhibitor celecoxib (Sigma) at 0.1 μg/min/kg in the intravenous vehicle starting with the control infusion periods. This surgical preparation and protocol procedure have been widely used in the studies of renal physiology, and infusion of vehicle alone did not produce any significant change in renal function over the period of testing. At the end of the experiment, the mice were euthanized by an anesthetic overdose (Inactin). The left kidney was excised and weighed, and the position of the medullary catheter was confirmed.
Protocol 2: Effects of Intravenous Prostamide E2 on MAP and RBF.
After surgical preparation of the mice and a 1.5-h equilibration and 20-min control sample collection periods with intravenous infusion of vehicle alone, prostamide E2 dissolved in vehicle was infused intravenously at 30, 60 or 120 nmol/kg/min, and its effects on MAP and RBF were measured.
Protocol 3: Effects of Prostamide E2 on Angiotensin II-Induced Elevation of MAP and Decrease in RBF.
Mice were surgically prepared as described above. The effect of Ang II delivered intravenously either as a bolus (50 ng/kg) or continuously (10, 20, or 40 ng/kg/min) on MAP, and RBF was determined. The effect of prostamide E2 given intravenously (120 nmol/kg/min) on the Ang II responses was determined.
Pharmacokinetic Analysis of AEA and Prostamide E2 Plasma Elimination in Mice.
The plasma elimination of AEA and prostamide E2 was evaluated in conscious mice after intravenous administration of bolus doses of 3.6 μmol/kg of either compound by injection into the tail vein. The vehicle was 5% ethanol, 5% Emulphor, and 90% saline (1 ml/kg). Timed serial blood samples (50 μl) were removed from the tail vein at 5, 15, 30, 60, and 120 min after injection into EDTA tubes. Plasma was collected by centrifugation, and 25 μl was extracted and analyzed for AEA or prostamide E2 by LC-MS/MS as described above.
Statistical Analyses.
Data are presented as the mean ± S.E.M. For comparisons among multiple groups of data. The significant difference was determined by using one-way or two-way analysis of variance followed by the Tukey-Kramer post hoc test. For comparisons between two groups of data, a Students' t test was used to detect significant differences. Only comparisons having a p value ≤0.05 were considered statistically significant.
Results
Levels of AEA and 2-Arachidonoyl Glycerol in Mouse Renal Cortex and Medulla.
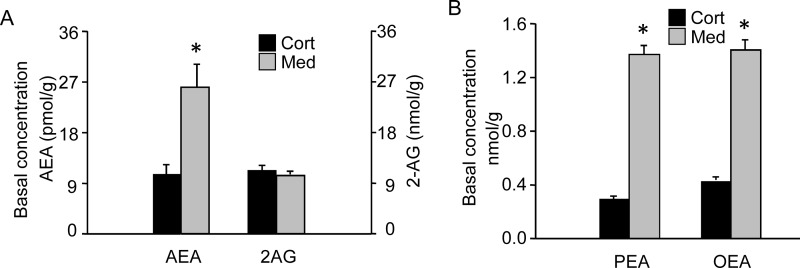
To assess the distribution of AEA in the kidney, the cortex and medulla from kidneys of C57BL6 mice were dissected and analyzed for endocannabinoids and other fatty acyl ethanolamides (Fig. 1). The AEA level was 2.4-fold higher in the renal medulla compared with the cortex (26.0 versus 10.7 pmol/g; p < 0.05). Two other fatty acyl ethanolamides, oleoyl ethanolamide and palmitoyl ethanolamide, were also higher in the renal medulla than in cortex (3.3- and 4.6-fold, respectively; p < 0.05). In contrast, 2-arachidonyl glycerol levels did not differ between the renal cortex and medulla (11.4 and 10.4 nmol/g, respectively).
Fig. 1.
Basal level of endocannabinoids and other fatty acid ethanolamine amides in the renal cortex and medulla. Lipids were extracted from samples of mouse renal cortex (Cort), and medulla (Med) were dissected from C57BL6 background mice and analyzed by LC-MS/MS. A, levels of AEA and 2-AG. Note the different scales for AEA and 2-AG. B, levels of PEA and OEA. The data represent the mean± S.E.M. of six mice per group. *, significant difference (p < 0.05) compared with the cortex sample.
Distribution of COX-2 and FAAH in the Renal Cortex and Medulla.
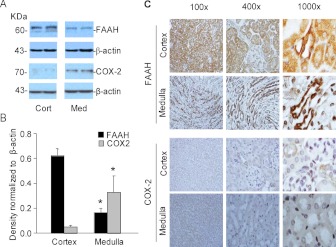
The distributions of the COX-2 and FAAH proteins in microsomes prepared from the kidney were studied by Western blot analysis and immunohistochemical localization (Fig. 2). In the Western analysis (Fig. 2A), immunoreactive bands consistent with FAAH (61 kDa) and COX-2 (74 kDa) were detected in the kidney microsomes. The two proteins showed opposite abundance profiles in the renal cortex compared with medulla with the FAAH immunoreactive band more intense in the cortex microsomes and the COX-2 band more intense in medulla microsomes. Quantitation using β-actin as a normalizing control indicated 3.6-fold higher FAAH protein in the cortex compared with medulla and 6.1-fold higher COX-2 in the medulla compared with cortex (Fig. 2B; p < 0.05). The results of immunohistochemical analyses of FAAH and COX-2 were in good agreement with the Western blot findings (Fig. 2C). FAAH immunostaining was most intense in the cortex region, with both proximal and distal tubule cells showing positive staining, in contrast to the weak or absent staining of cells in glomerular structures. In the medulla, FAAH staining was more selective, seeming to be limited to collecting ducts. The staining pattern with COX-2 in the cortex compared with the medulla was distinct from that of FAAH (Fig. 2C, bottom). In the renal cortex, positive COX-2 staining was limited to scattered cells or groups of cells in thick ascending limb tubules, particularly in regions where these cells came into contact with or were in proximity to glomeruli. This staining pattern is consistent with the reported expression of COX-2 in cells of the macula densa. In the medulla, COX-2-positive staining was detected in tubular segments with weak or otherwise minimal staining of collecting ducts.
Fig. 2.
Expression of FAAH and COX-2 in the kidney. A, Western blot analysis of FAAH and COX-2 in microsomes prepared from the renal cortex and medulla of C57BL6 mouse kidneys is shown. β-Actin antibody was used as a normalizing probe to control for differences in protein loading. B, summarized data for the Western blot analysis of FAAH and COX-2 protein in microsomes prepared from the renal cortex or medulla of mouse kidneys are presented as the mean ± S.E.M. of the target band intensity normalized to β-actin (n = 4 per group). *, significant difference compared with the corresponding cortex sample (p < 0.05). C, immunohistochemical localization of FAAH and COX-2 in the renal cortex and medulla of control mice is shown. Representative images are from analyses of kidneys from three mice at the indicated levels of magnification.
Hydrolysis and Prostamide E2 Formation from AEA by Cortical and Medullary Microsomes.
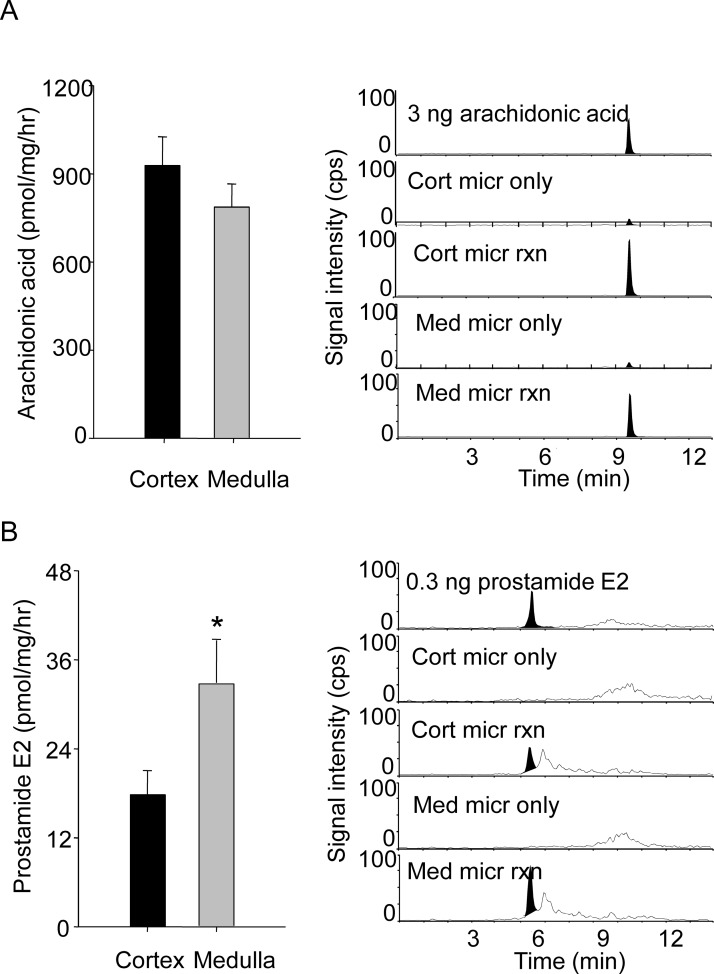
Figure 3 presents a comparison of the rates of AEA hydrolysis to arachidonic acid and oxidation to prostamide E2 by cortical and medullary microsomes with representative chromatograms. The kidney cortical microsomes showed a significantly higher rate of AEA-dependent arachidonic acid formation compared with medullary microsomes (928 ± 98 versus 787 ± 79 pmol/mg/h, respectively) (Fig. 3A), consistent with the cortex having higher FAAH activity. In contrast, the renal medulla microsomes were more active than cortical ones in forming prostamide E2 (33.4 ± 7.5 versus 20.4 ± 3.8 pmol/mg/h, respectively; p < 0.05) (Fig. 3B). Prostamide E2 was not detected in reactions containing only microsomes or only reaction components (data not shown).
Fig. 3.
FAAH and COX-2 activity in the renal cortex and medulla. Microsomes from the renal cortex (Cort) or medulla (Med) of C57BL6 mice were incubated with or without 100 μM AEA (micr rxn or micr only, respectively) for 30 min at 37°C. The reaction products were extracted and analyzed by LC-MS/MS as described under Materials and Methods. A, the rate of arachidonic acid formation, a measure of FAAH activity, was determined by subtraction of the arachidonic acid values from reactions with and without AEA (n = 6 per group). B, the rate of prostamide E2 formation in the same reactions was determined. To the right of each panel are representative chromatograms for arachidonic acid or prostamide E2/D2 in reactions with and without AEA and microsomes from the renal cortex or medulla. *, a significant difference compared with the cortex sample (p < 0.05).
Effect of Medullary Infusion of AEA on Blood Pressure, Urine Volume, and Sodium Excretion and Its Inhibition by a Selective COX-2 Inhibitor.
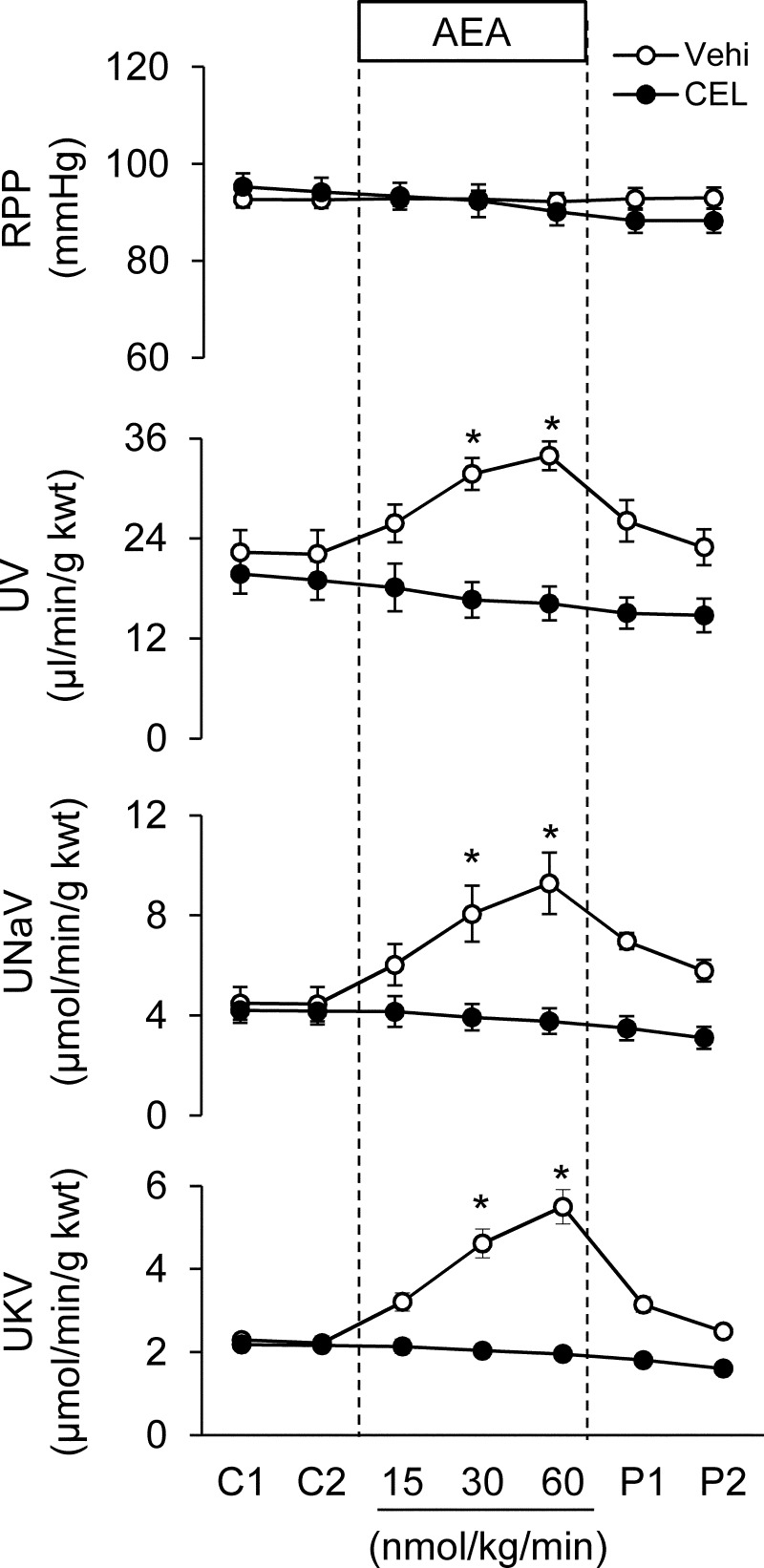
An acute renal function study was performed by using anesthetized mice with surgically implanted catheters for measuring renal perfusion pressure and urine formation. These results are presented in Fig. 4. After a 1.5-h equilibration period and two 15-min control periods (labeled C1 and C2 in Fig. 4), addition of AEA to the medullary infusion solution was tested. Medullary infusion of AEA had little apparent effect on blood pressure, but increased urine flow and sodium and potassium excretion adjusted for kidney weight. The effects of AEA were dose-dependent between 15 and 60 nmol/kg/min. Removal of the AEA from the medullary infusion solution resulted in the urine flow and sodium excretion values returning toward the control level. Pretreatment of the mice with an intravenous infusion of celecoxib, a COX-2-selective inhibitor, at a rate of 0.1 μg/kg/min for 30 min completely blocked the effects of AEA on both urine flow and excretion of sodium and potassium. This observation suggests that COX-2 metabolites of AEA mediate the diuretic and natriuretic effects of AEA during its intramedullary infusion in the mouse kidney.
Fig. 4.
Effect of intramedullary interstitial infusion of AEA on mean arterial pressure and renal water and sodium and potassium excretion in the absence or presence of a selective COX-2 inhibitor. RPP, renal perfusion pressure; UNaV, urinary sodium excretion; UKV, urinary potassium excretion. C indicates control infusion period; 15, 30, and 60 indicate treatments with increasing AEA infusion rate (15, 30 or 60 nmol/kg/min), and P indicates control infusion of post-treatment. One group of animals was infused intravenously with celecoxib (CEL; 0.1 μmol/min/kg) beginning with the control infusion period. Vehi, vehicle. *, a significant difference versus respective control group (p < 0.05) (n = 6 per group).
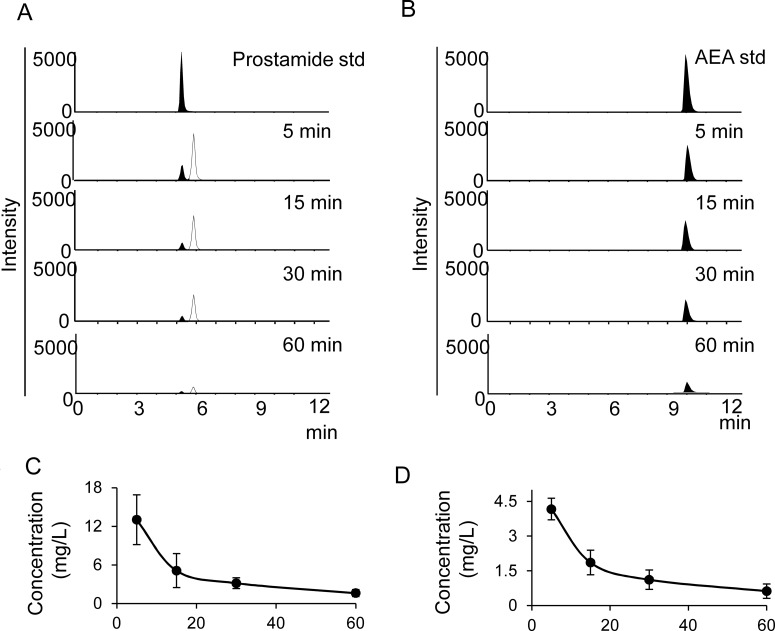
Kinetics of AEA and Prostamide E2 after Intravenous Bolus Injection.
Plasma samples from control C57BL6 mice before and after intravenous bolus doses of AEA or prostamide E were analyzed for AEA and prostamide E2 levels (Fig. 5). Basal levels of AEA (3–8 nM) but not prostamide E2 (data not shown) were detectable in plasma collected immediately before the bolus intravenous injection (3–8 nM; 1–3 ng/ml). After an intravenous AEA bolus (3.6 mg/kg; Fig. 5A), AEA exhibited a peak plasma level by 5 min after administration (489 ng/ml), which declined subsequently with a half-life of 20 to 30 min. No prostamide E2 was detected in the plasma of the AEA-injected mice. Intravenous injection of a prostamide E2 bolus resulted in a maximal plasma concentration of prostamide E2 by 5 min after injection (141 ng/ml; Fig. 5B). There was no apparent change in plasma AEA concentration after prostamide E2 dosing, which was consistent with the COX-2 metabolite being resistant to FAAH-mediated hydrolysis (J. K. Ritter and P.-L. Li, unpublished data). Prostamide E2 exhibited an elimination half-life of 21 min. An unidentified prostamide E2 metabolite having identical parent ion ([MH]+ 396) and product ion ([MH]+ 378) as prostamide E2 was detected in the prostamide E2 multiple reaction monitoring chromatograms. The signal for this metabolite was detected only in mice injected with prostamide E2 and became more intense than prostamide E2 by 30 min and later times after injection, suggesting higher abundance. The elimination of this unknown isomeric metabolite paralleled that of prostamide E2 (half-life of 26 min).
Fig. 5.
Plasma clearance of AEA and prostamide E2. Intravenous bolus doses of AEA or prostamide E2 were administrated into mice, and plasma samples (50 μl) were then collected in EDTA tubes at timed postdose intervals and analyzed for the indicated lipids by LC-MS/MS. A, representative chromatograms for prostamide E2 monitored by using the 396/378 multiple reaction monitoring show prostamide E2 (in black) with accompanying formation of an unidentified isomeric metabolite, which elutes slightly later (6.14 min) than prostamide E2 (5.51 min). B, representative chromatograms for AEA. C, plasma prostamide E2 levels after a prostamide E2 bolus (3.6 μmol/kg body weight). D, plasma AEA concentrations after an AEA bolus (3.6 μmol/kg body weight). Values represent the mean ± S.E.M of the lipid concentration in units of ng/mL. (n = 4 per group).
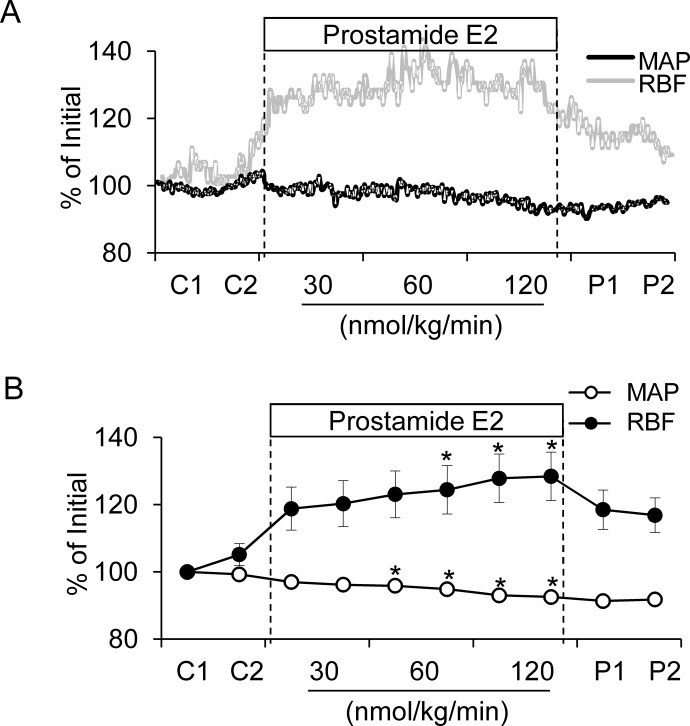
Effect of Intravenous Prostamide E2 Infusion on Blood Pressure and RBF.
The influence of intravenously infused prostamide E2 on MAP and RBF was investigated in anesthetized mice. Representative blood pressure and RBF tracings from a single animal showing the effects of prostamide E2 on these parameters are shown in Fig. 6A. Prostamide E2 had modest, but statistically significant, effects on MAP and RBF under control conditions. At the highest dose tested (120 nmol/kg/min) prostamide E2 decreased MAP by 7% from 99.4 to 92.6 mm Hg and increased RBF by 23% (4.7 to 5.8 ml/min/g kidney weight) (Fig. 6B).
Fig. 6.
Effect of prostamide E2 infusion on mean arterial pressure and renal blood flow. A, representative tracings from a mouse given prostamide E2 by continuous intravenous infusion are shown. Effects on MAP (black tracing) and RBF (gray tracing) are shown. The prostamide E2 infusion rate was 30, 60, or 120 nmol/kg/min. B, summarized data for the average effect of prostamide E2 for each of the two 10-min treatment periods at each infusion rate. *, a significant difference versus control group (p < 0.05) (n = 7.)
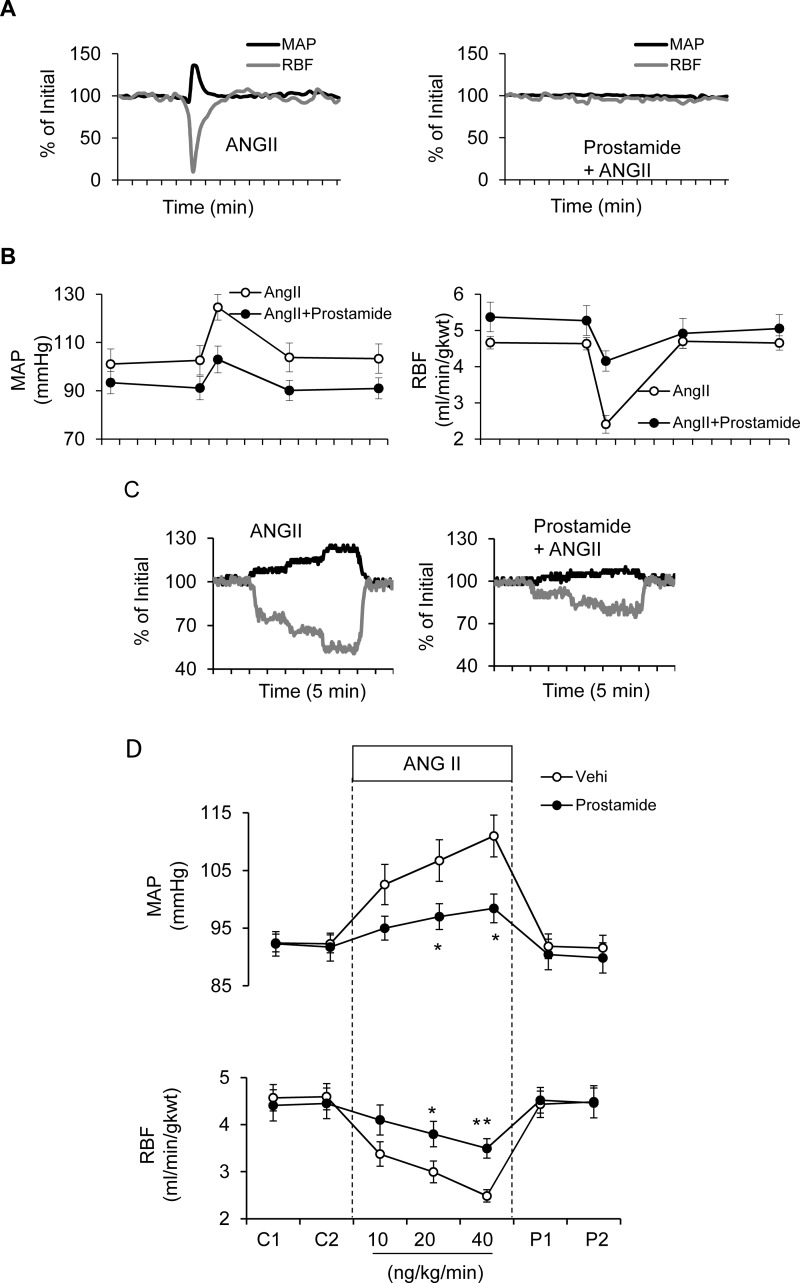
Inhibition of Ang II-Mediated Increases in MAP and Reductions of RBF by Prostamide E2.
The influence of prostamide E2 was also evaluated on Ang II-induced elevation in MAP and reduction of RBF. Administration of the Ang II bolus transiently elevated MAP and decreased RBF, but both of these effects were inhibited by prostamide E2 (see Fig. 7A for representative tracings). The summarized data in Fig. 7B show that whereas the Ang II bolus alone elevated MAP by an average 22 mm Hg, this response was reduced 46% to 12 mm Hg by prostamide E2 (Fig. 7B, left). Likewise, Ang II reduced RBF by 48% (4.6 to 2.4 ml/min/g kidney weight) in the presence of vehicle and by 23% (5.3 to 4.2 ml/min/g kidney weight) in the presence of infused prostamide E2 (Fig. 7B, right). Similar antagonistic effects of prostamide E2 were observed when Ang II was administered by continuous intravenous infusion. In tracings from a representative animal (Fig. 7C), infusion of Ang II at increasing concentrations (5, 10, and 20 ng/kg/min) elevated MAP and decreased RBF in a concentration-dependent manner (Fig. 7C, left), and these effects were inhibited by the presence of prostamide E2 (Fig. 7C, right). Summarized data showing significant inhibition of Ang II effects at the intermediate and high Ang II concentrations are shown in Fig. 7D.
Fig. 7.
Angiotensin II intravenous dose response of mean arterial pressure and renal blood flow with and without prostamide E2 pretreatment in mice. A, representative tracings show the effect of an intravenous bolus injection of Ang II (50 ng/kg) and Ang II given after prostamide E2 pretreatment (120 nmol/kg/min) on MAP (black lines) and RBF (gray lines). B, summarized data for acute bolus intravenous injection of Ang II in the absence and presence of prostamide E2 on MAP and RBF. The data are presented as the mean ± S.E.M. expressed as the percentage of change from the basal level. C, representative tracings show the dose response of a continuous intravenous infusion of Ang II (5–20 ng/kg/min) and the effect of prostamide E2. D, summarized data for the effect of continuous intravenous infusion of Ang II with or without prostamide E2 (n = 7). *, p < 0.05; **, p < 0.01, versus control group.
Discussion
The purpose of this study was to evaluate AEA and its prostamide metabolites for their possible relationship to the renomedullary neutral antihypertensive lipid medullipin (Muirhead, 1993). The observation that AEA is enriched in the mouse renal medulla relative to cortex (Fig. 1A) has not been described previously. The AEA enrichment in the renal medulla is not clearly understood but may be caused by relatively low levels of FAAH in medullary cells. The presence of AEA in neurons is well known, but it is also present in cells that are non-neuronal in origin (Liu et al., 2006). In the kidney, AEA has been identified in cultured renal microvascular endothelial cells and glomerular mesangial cells (Deutsch et al., 1997). Based on the hypothesis that AEA or an AEA metabolite represents medullipin, it may be speculated that AEA is present in the lipid-rich granules of renomedullary interstitial cells, the proposed source of medullipin, but further studies are needed to confirm this possibility.
It was also of interest to evaluate the distribution in the kidney of two known AEA-metabolizing enzymes, FAAH and COX-2, in relation to that of AEA. Multiple lines of evidence support opposing patterns of FAAH and COX-2 expression in the renal cortex and medulla. FAAH protein (Fig. 2) and a measure of its activity, hydrolysis of AEA to arachidonic acid (Fig. 3), were higher in the cortex than medulla, whereas the pattern for COX-2 was the opposite. Although the kidney has been reported to have high FAAH (Long et al., 2011), its localization in the kidney has never been studied to our knowledge. FAAH immunostaining was strong in various cortical tubular segments including proximal convoluted tubule, thick ascending limbs of the Loop of Henle, and cortical distal and connecting tubules. Some positive staining of FAAH was evident in glomeruli, but the intensity was lower than that in tubules. These immunohistochemical-based observations agree with the higher FAAH levels observed by Western blotting and enzymatic analysis. Increased FAAH activity in the renal cortex could at least in part explain the relatively low levels of AEA there and suggests the physiologic importance for maintaining low AEA concentrations in the renal cortex. Alternatively, AEA in conjunction with high FAAH in the renal cortex could serve as a source of arachidonic acid for prostaglandin synthesis in the tubuloglomerular feedback mechanism (Araujo and Welch, 2009). In the renal medulla, the inner medullary collecting ducts exhibited intense positive FAAH immunostaining. Further research is necessary to understand the role of FAAH and the regulation of AEA synthesis and release in the cortical and medullary regions of kidney.
In contrast, the COX-2 protein and an associated enzymatic activity (conversion of AEA to prostamide E2) both were higher in the renal medulla compared with the cortex (Figs. 2 and 3). This differential expression of COX-2 in the renal medulla was supported by >10-fold higher COX-2 mRNA expression in the renal medulla compared with the cortex (data not shown). The immunohistochemical analysis with COX-2 was less convincing, because positive COX-2 immunostained cells were clearly visible only in the renal cortex. The pattern of COX-2 immunostaining observed in our study is consistent with other reports (Harris et al., 1994). In the renal cortex, positive COX-2 staining was observed in isolated cells or clusters of cells in distal tubules adjacent to glomeruli, consistent with expression in cortical thick ascending limb cells and cells of the macula densa (Harris et al., 1994). The low COX-2 immunostaining in the mouse kidney medulla is in agreement with a previous report (Câmpean et al., 2003). Yet, it contrasts with the higher levels in the medulla suggested by Western and enzymatic activity analyses. Because prostamide formation from AEA is known at present only to be catalyzed by COX-2 (Yu et al., 1997), the failure to detect COX-2 in the renal medulla by immunohistochemistry may be caused by COX-2 expression being too broad and diffuse to permit visualization of positive staining above background.
In the acute renal function studies, a link between AEA and COX-2 is suggested by the data (Fig. 4). When AEA was infused into the renal medulla, urine flow and sodium excretion were increased. However, this effect was completely prevented if the mice were pretreated with a selective COX-2 inhibitor. This finding indicates that a COX-2 metabolite of AEA indirectly mediates the renal excretory effects of AEA. Using a similar experimental approach in rats, Li and Wang (2006) showed that the intramedullary infusion of the methyl analog of AEA, methanandamide, stimulated urine volume and reduced MAP (Li and Wang, 2006). Methandamide has similar cannabinoid receptor-stimulating specificity to AEA but is resistant to FAAH-mediated hydrolysis (Abadji et al., 1994). The effect of intramedullarily infused methanandamide was not blocked by pretreatment with either a cannabinoid receptor-1 or vanilloid receptor antagonist, indicating a unique mechanism. It is plausible that this cannabinoid-independent mechanism for methanandamide also involves its conversion to COX-2 metabolites, because methanandamide is also an efficient COX-2 substrate (Kozak et al., 2003). Further studies are needed to determine which COX-2 metabolites of AEA underlie the mechanism of its diuresis and natriuresis after its infusion into the renal medulla.
According to the hypothesis of Muirhead et al. (1991), medullipin is secreted from the renal medulla into the blood to elicit antihypertensive effects through its properties as a vasodepressor and inhibitor of sympathetic activity. Prostaglandin E2, the major renal vasodilatory factor, was excluded as a candidate for this mechanism, based in part on its ultra-short duration of activity in plasma, on the order of seconds (Ferreira and Vane, 1967; Hamberg and Samuelsson, 1973). In this study, the plasma kinetics of AEA and its major renal prostamide metabolite, prostamide E2, were evaluated in mice (3.6 μmol/kg). In contrast to prostaglandin E2, which disappears completely from the plasma within 4 to 6 min (Hamberg and Samuelsson, 1971), both AEA and prostamide E2 remained detectable in plasma for at least 1 h after a bolus injection (Fig. 5). Estimates of the plasma half-lives for AEA and prostamide E2 were 21 and 27 min, respectively. The half-life of prostamide E2 measured in normal mice is more than 4-fold longer than the ∼6-min half-life in rats given a comparable dose (2 mg/kg) (Kozak et al., 2002). The relative resistance of prostamide E2 to elimination is consistent with the idea that prostamides are released into the blood to exert effects at sites distant from their tissue of origin (Kozak et al., 2001; Weber et al., 2004). In this study, a metabolite of prostamide E2 was observed that has an identical [M+H]+ mass-to-charge ratio and slightly higher lipophilicity. Given that the two main pathways of prostaglandin metabolism are dehydrogenation of the 15-hydroxyl group and reduction of the 13,14-double bond (Dunn and Hood, 1977), the observed metabolite could be explained by similar reactions acting in sequence. The extent to which this pathway contributes to the plasma clearance of prostamide E2 and its potential contribution to the biological activity of prostamides remains open to investigation.
Muirhead et al. (1991) proposed that medullipin and Ang II were physiological counterparts having opposite effects on vasomotor tone, sympathetic activity, and salt and water retention by the kidney. AEA given by intravenous bolus (4 mg/kg) to anesthetized rats elicits a triphasic response, including a prolonged depressor third phase that was attributed to cannabinoid receptor-1-mediated inhibition of sympathetic outflow (Varga et al., 1995). In addition, multiple studies have reported vasorelaxant effects of AEA in various arteriolar resistance beds (Mair et al., 2010). In this study, we showed that prostamide E2 given in a continuous infusion modestly decreased MAP and increased RBF (Fig. 6), which was opposite to those of Ang II. Further study is needed to determine whether the mechanism of this novel observation is caused by direct vasorelaxation or inhibition of sympathetic tone and to determine the receptors that mediate the effect of prostamide E2, possibly prostaglandin E2 receptors (Ross et al., 2002). Additional data conclusively demonstrate that prostamide E2 can directly reverse Ang II-induced pressor response and reduction of RBF (Fig. 7). Although further studies are needed to explore the molecular mechanisms, the counteracting actions of prostomide E2 on Ang II-induced hemodynamic response are consistent with the nature of medullipin as proposed by Muirhead et al. (1991).
In summary, the results of the present study demonstrate that AEA or one of its COX-2-metabolites, prostamide E2, shares several properties in common with those proposed for medullipin, the antihypertensive lipid with vasodilator, sympatholytic, and diuretic/natriuretic actions. In addition to its neutral lipid character, its close relationship to arachidonic acid, and an apparent role for metabolism in mediating its antihypertensive effects, AEA infused in the renal medulla is diuretic and natriuretic, and this effect is mediated by COX-2. Thus, we propose that AEA or prostamide E2 may represent medullipin. A key to this hypothesis is that AEA or its COX-2 metabolites are synthesized in the renal medulla and released in response to increases in renal medullary blood flow. Studies in our laboratory are ongoing to address this key issue.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK54927]; the National Institutes of Health National Institute on Drug Abuse [Grant DA009789]; the Thomas and Kate Jeffress Memorial Foundation [Grant J963]; a Virginia Commonwealth University Clinical and Translational Science Award with support from the National Institutes of Health National Center for Research Resources; and the A.D. Williams' Fund of the Virginia Commonwealth University [Grant UL1RR031990].
Portions of this work were presented previously: Ritter JK, Li C, Xia M, Li C, Lichtman A, and Li P-L (2012) Anandamide and its metabolites as novel natriuretic and antihypertensive lipids from the renal medulla, at the Federation of American Societies for Experimental Biology Meeting; 2012 April 21–25; San Diego, CA. Federation of American Societies for Experimental Biology, Bethesda, MD.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- RBF
- renal blood flow
- AEA
- anandamide
- AA
- arachidonic acid
- Ang II
- angiotensin II
- COX-2
- cyclooxygenase 2
- FAAH
- fatty acid amide hydrolase
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MAP
- mean arterial pressure
- 2-AG
- 2-arachidonoyl glycerol
- PEA
- palmitoyl ethanolamine
- OEA
- oleoyl ethanolamine
- UV
- urine flow rate.
Authorship Contributions
Participated in research design: Ritter, C. Li, Xia, Poklis, Lichtman, Dewey, and P.-L. Li.
Conducted experiments: Ritter, C. Li, Xia, Poklis, and Abdullah.
Performed data analysis: Ritter, C. Li, Xia, Poklis, Lichtman, Abdullah, Dewey, and P-L. Li.
Wrote or contributed to the writing of the manuscript: Ritter, C. Li, Xia, Poklis, Lichtman, Abdullah, Dewey, and P.-L. Li.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (1994) (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem 37:1889–1893 [DOI] [PubMed] [Google Scholar]
- Araujo M, Welch WJ. (2009) Cyclooxygenase 2 inhibition suppresses tubuloglomerular feedback: roles of thromboxane receptors and nitric oxide. Am J Physiol Renal Physiol 296:F790–F794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Câmpean V, Theilig F, Paliege A, Breyer M, Bachmann S. (2003) Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Renal Physiol 285:F19–F32 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 98:9371–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, et al. (1997) Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 100:1538–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949 [DOI] [PubMed] [Google Scholar]
- Dunn MJ, Hood VL. (1977) Prostaglandins and the kidney. Am J Physiol Renal Physiol 233:F169–F184 [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Vane JR. (1967) Prostaglandins: their disappearance from and release into the circulation. Nature 216:868–873 [DOI] [PubMed] [Google Scholar]
- Hamberg M, Samuelsson B. (1971) On the metabolism of prostaglandins E 1 and E 2 in man. J Biol Chem 246:6713–6721 [PubMed] [Google Scholar]
- Hamberg M, Samuelsson B. (1973) Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc Natl Acad Sci U S A 70:899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. (1994) Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94:2504–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga D, Santa T, Fukushima T, Homma H, Imai K. (1997) Liquid chromatographic-atmospheric pressure chemical ionization mass spectrometric determination of anandamide and its analogs in rat brain and peripheral tissues. J Chromatogr B Biomed Sci Appl 690:7–13 [DOI] [PubMed] [Google Scholar]
- Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, Hayashi M, Saruta T. (2004) Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol 15:1488–1494 [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. (2002) Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem 277:44877–44885 [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Ray JL, Tai HH, Morrow JD, Marnett LJ. (2001) Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamides in vitro and in vivo. J Biol Chem 276:36993–36998 [DOI] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JJ, Rowlinson SW, Prudhomme DR, Marnett LJ. (2003) Amino acid determinants in cyclooxygenase-2 oxygenation of the endocannabinoid anandamide. Biochemistry 42:9041–9049 [DOI] [PubMed] [Google Scholar]
- Li J, Wang DH. (2006) Differential mechanisms mediating depressor and diuretic effects of anandamide. J Hypertens 24:2271–2276 [DOI] [PubMed] [Google Scholar]
- Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. (2007) Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol 292:F207–F216 [DOI] [PubMed] [Google Scholar]
- Li N, Zhang G, Yi FX, Zou AP, Li PL. (2005) Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289:F1048–F1056 [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, et al. (2006) A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A 103:13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, LaCava M, Jin X, Cravatt BF. (2011) An anatomical and temporal portrait of physiological substrates for fatty acid amide hydrolase. J Lipid Res 52:337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Roman RJ, Mattson DL, Cowley AW., Jr (1992) Renal medullary interstitial infusion of diltiazem alters sodium and water excretion in rats. Am J Physiol Regul Integr Comp Physiol 263:R1064–R1070 [DOI] [PubMed] [Google Scholar]
- Mair KM, Robinson E, Kane KA, Pyne S, Brett RR, Pyne NJ, Kennedy S. (2010) Interaction between anandamide and sphingosine-1-phosphate in mediating vasorelaxation in rat coronary artery. Br J Pharmacol 161:176–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson DL. (2003) Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 284:R13–R27 [DOI] [PubMed] [Google Scholar]
- Muirhead EE. (1993) Renal vasodepressor mechanisms: the medullipin system. J Hypertens Suppl 11:S53–S58 [PubMed] [Google Scholar]
- Muirhead EE, Brooks B, Byers LW, Brown P, Pitcock JA. (1991) Medullipin system. Generation of medullipin II by isolated kidney-liver perfusion. Hypertension 18: III158–163 [DOI] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, et al. (2011) Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther 339:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, Ellington HC. (2002) Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J Pharmacol Exp Ther 301:900–907 [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. (2009a) Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J 11:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Lichtman AH. (2009b) Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J 11:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G. (1995) Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol 278:279–283 [DOI] [PubMed] [Google Scholar]
- Weber A, Ni J, Ling KH, Acheampong A, Tang-Liu DD, Burk R, Cravatt BF, Woodward D. (2004) Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J Lipid Res 45:757–763 [DOI] [PubMed] [Google Scholar]
- Yang T. (2003) Regulation of cyclooxygenase-2 in renal medulla. Acta Physiol Scand 177:417–421 [DOI] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. (1997) Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem 272:21181–21186 [DOI] [PubMed] [Google Scholar]
- Zhu Q, Xia M, Wang Z, Li PL, Li N. (2011) A novel lipid natriuretic factor in the renal medulla: sphingosine-1-phosphate. Am J Physiol Renal Physiol 301:F35–F41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou AP, Yang ZZ, Li PL, Cowley AW., JR (2001) Oxygen-dependent expression of hypoxia-inducible factor-1α in renal medullary cells of rats. Physiol Genomics 6:159–168 [DOI] [PubMed] [Google Scholar]