Abstract
Neurodegenerative diseases are a large group of disabling disorders of the nervous system, characterized by the relative selective death of neuronal subtypes. In most cases, there is overwhelming evidence of impaired mitochondrial function as a causative factor in these diseases. More recently, evidence has emerged for impaired mitochondrial dynamics (shape, size, fission-fusion, distribution, movement etc.) in neurodegenerative diseases such as Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, and Alzheimer's disease. Here, we provide a concise overview of the major findings in recent years highlighting the importance of healthy mitochondria for a healthy neuron.
Introduction
Mitochondria, aptly termed “powerhouses of the cell,” are responsible for production of most of the cell's “energy currency,” in the form of ATP. ATP is the end product of a series of pathways involving oxidation of substrates, mainly carbohydrates and fat, in cytosol (glycolysis) and mitochondria [pyruvate decarboxylation, tricarboxylic acid cycle (Krebs cycle), and oxidative phosphorylation (OXPHOS)/respiratory chain complex)]. The respiratory chain complex consists of four distinct multisubunit complexes (I-IV) and two electron carriers that generate a proton gradient across the mitochondrial inner membrane, which in turn drives ATP synthase (complex V) to generate ATP. Mitochondria are also the seat of many of the cell's housekeeping functions, including the biosynthesis of amino acids and steroids, β-oxidation of fatty acids, maintenance of cytosolic calcium homeostasis, buffering of calcium fluctuations, and production and modulation of reactive oxygen species (ROS). Mitochondria also play a central role in apoptosis (Davis and Williams, 2012). Considering the intense energy demands and limited regenerative capacity of neurons, improper functioning of mitochondria can have devastating effects on neuronal survival. There is ample evidence of impaired mitochondrial function as a cause rather than consequence of neurodegeneration.
Mitochondrial Dynamics
Mitochondria are dynamic organelles that are transported on cytoskeletal proteins (mitochondrial trafficking). They fuse and divide (fusion is mediated by OPA1, Mfn1, and Mfn2, and fission is mediated by the proteins fission 1 and Drp1), fragment, swell, extend, and are recycled (mitophagy or vesicle formation) constantly and in a regulated fashion. Unbalanced fusion leads to mitochondrial elongation, and unbalanced fission leads to excessive mitochondrial fragmentation and small mitochondria, both of which impair the function of mitochondria. It has been shown that exchange of mitochondrial contents is important for mitochondrial function as well as organelle distribution in neurons. Mitochondrial fusion, in particular that mediated by Mfn2, is required for proper development and maintenance of the cerebellum (Chen et al., 2007). Mutations in the Mfn2 gene cause the neurodegenerative disease Charcot-Marie-Tooth type 2A, and mutations in OPA1 cause dominantly inherited optic atrophy. There is now increasing evidence of altered mitochondrial trafficking and fusion-fission dynamics in Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS).
Mitochondrial Dysfunction, Altered Mitochondrial Dynamics, and Neurodegeneration
AD.
AD is defined by progressive impairments in memory and cognition and the presence of extracellular neuritic plaques and intracellular neurofibrillary tangles. β-Amyloid peptide (Aβ) is the major component of the plaque, and the tangles are composed of hyperphosphorylated tau proteins. The molecular events leading to the development of sporadic late-onset AD have not been defined. Advanced age is the greatest risk factor for AD, and glucose/energy metabolism is diminished in AD. It has been proposed that in sporadic AD mitochondrial dysfunction is the primary event that causes Aβ deposition, synaptic degeneration, and formation of neurofibrillary tangles (Swerdlow et al., 2010). Energy deficiency is a fundamental characteristic feature of both AD brains and peripheral cells derived from patients with AD (Gibson et al., 1998; Manczak et al., 2004; reviewed in Beal, 2005). Activities of the three key tricarboxylic acid cycle enzyme complexes, pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, are impaired in postmortem AD brain and fibroblasts from patients with AD (Bubber et al., 2005). Reduced OXPHOS complex I, III, and IV activities have been reported in platelets and lymphocytes from patients with AD and in postmortem brain tissue (Kish et al., 1992; Bosetti et al., 2002) (summarized in Table 1). Several studies have reported impaired mitochondrial dynamics that involve the abnormal expression of Drp1 in postmortem brains from patients with AD, AD mouse models, and APP cell lines (Cho et al., 2009; reviewed in Reddy et al., 2011). There is also impaired mitochondrial biogenesis (Sheng et al., 2012).
TABLE 1.
Known OXPHOS complex deficiencies in major neurodegenerative disorders
| OXPHOS Complex | Huntington's Disease | Alzheimer's Disease | Parkinson's Disease | Amyotrophic Lateral Sclerosis |
|---|---|---|---|---|
| Complex I (NADH dehydrogenase or NADH:ubiquinone oxidoreductase) | Unaltered in brain of patients with HD, but reduced in skeletal muscle | Reduced in mitochondria, platelets, and lymphocytes from patients with AD and postmortem brain tissue | Impaired complex I activity is seen in substantia nigra, platelets; and skeletal muscle of patients with; complex I inhibitors are used to model PD | Increased in patients with familial ALS with SOD1 mutations, reduced in skeletal muscle samples from patients with sporadic ALS |
| Complex II (succinate dehydrogenase) | Reduced enzyme activity in brain of patients with advanced-stage HD and in muscle of transgenic mice; complex II inhibitors produce a HD phenotype in mice and primates | Reduced in mitochondria from patients with AD | Complex II + III activities are reduced in spinal cords of patients with ALS | |
| Complex III (CoQ-cytochrome c reductase) | Reduced enzyme activity in patients with advanced-stage HD | Core 1 protein is significantly reduced in temporal cortex of patients with AD; also reduced in mitochondria, platelets, and lymphocytes from patients with AD and postmortem brain tissue | Complex I + III activities reduced in spinal cords of patients with ALS | |
| Complex IV (cytochrome c oxidase) | Reduced COX activity in myoblasts and brain samples from patients with HD | Decreased activity and mRNA levels in brains, platelets and lymphocytes from AD patients | Reduced complex IV activity was reported in platelet mitochondria and skeletal muscle from patients with PD | Decreased COX activity in individual motor neurons, spinal cords and skeletal, muscle of patients with sporadic ALS; decreased COX subunits in G93A ALS mice |
| Complex V (ATP synthase) | ATP production is impaired in striatal cells from mutant htt mice | Reduced in mitochondria from patients with AD | Reduced enzyme activity for complex V in skin fibroblast cultures from patients with PD | Levels of different subunits (D, α, and β) are decreased in G93A ALS mice |
PD.
PD is the second most common neurodegenerative disorder. Clinically, PD is characterized by the triad of resting tremor, bradykinesia, and rigidity. These symptoms are considered to be a direct consequence of neurodegeneration and loss of dopaminergic (DA) neurons. Pathologically, the hallmark feature of PD is loss of pigmented dopaminergic neurons in the substantia nigra and the presence of abnormal protein aggregates called Lewy bodies, which are cytoplasmic eosinophilic inclusions composed of the presynaptic protein α-synuclein. Over the last several decades, evidence has accumulated that mitochondrial dysfunction is strongly associated with PD. A mild deficiency in mitochondrial electron transport chain NADH dehydrogenase (complex I) activity was first found in the substantia nigra of patients with PD, followed by studies identifying a similar complex I deficit in platelets, lymphocytes, and, less consistently, in muscle tissue from patients with PD (reviewed in Beal, 2007). Consistent with this, inhibitors of OXPHOS complex I, such as rotenone and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), in animal models, produce neuropathologic and behavioral symptoms similar to human PD. A number of proteins that are genetically linked to familial PD, phosphatase and tensin homolog-induced putative kinase 1 (PINK1), DJ-1, α-synuclein, leucine-rich repeat kinase 2, and parkin, are either mitochondrial proteins or are associated with mitochondria (Table 2). There is evidence to suggest that parkin and PINK1 have a direct role in the cell's mitochondrial quality-control pathways, identifying impaired mitochondria with reduced membrane potential and selectively eliminating them from the mitochondrial network by mitophagy. This implicates a failure of mitophagy as playing a role in the pathogenesis of PD (Narendra and Youle, 2011). Moreover, there is evidence for a direct involvement of PINK1 and parkin in abnormal mitochondrial dynamics in fly, rat, and mouse models of PD (Wang et al., 2011; reviewed in Reddy et al., 2011).
TABLE 2.
Proteins implicated in the pathogenesis of major neurodegenerative disorders and their association/interaction with mitochondria
| Protein | Disease | Mitochondrial Association |
|---|---|---|
| htt | HD |
|
| APP | AD |
|
| Presenilins, PS1 and PS2 | AD |
|
| Aβ | AD |
|
| Tau | AD/PD |
|
| α-Synuclein | PD |
|
| Parkin | PD |
|
| PINK1 | PD |
|
| DJ-1 | PD |
|
| LRRK2 | PD |
|
| SOD1 | ALS |
|
The “MitoPark” mice are an excellent example of the “mitochondrial hypothesis” of PD. In MitoPark mice mitochondrial function is selectively disrupted in DA neurons by elimination of the mitochondrial transcription factor A (Tfam) gene (Ekstrand et al., 2007). The Tfam gene is encoded in the nuclear genome, and the Tfam protein is subsequently imported into mitochondria, where it acts as a DNA binding protein, essential for both transcription and maintenance of mitochondrial DNA (mtDNA) in mammals. It stabilizes mtDNA, regulates mtDNA copy number in vivo, and is essential for mitochondrial biogenesis (Larsson et al., 1998). The MitoPark mice survive to adulthood and then slowly develop a Parkinsonian phenotype (Ekstrand et al., 2007; Galter et al., 2010). Furthermore, cellular changes similar to those seen in idiopathic PD are observed, such as intracellular inclusions in DA neurons, degeneration of DA pathways, and loss of striatal dopamine. Also similar to PD, the substantia nigra pars compacta DA neurons of MitoPark mice degenerate before those in the ventral tegmental area.
HD.
HD is a dominantly inherited progressive neurodegenerative disease, caused by an abnormal CAG repeat expansion in the huntingtin (htt) gene. The disease is characterized by progressive motor impairment, personality changes, psychiatric illness, and gradual intellectual decline. Pathologically, there is a preferential and progressive loss of the medium spiny neurons in the striatum, as well as cortical atrophy, and degeneration of other brain regions later in the disease. There is extensive evidence for bioenergetic deficits and mitochondrial dysfunction in HD, such as a pronounced weight loss despite sustained caloric intake, NMR spectroscopy showing increased lactate in the cerebral cortex and basal ganglia, decreased activities of OXPHOS complexes II and III, reduced aconitase activity in the basal ganglia, abnormal mitochondrial membrane depolarization in patient lymphoblasts, abnormal ultrastructure of mitochondria in cortical biopsies obtained from patients with both juvenile and adult-onset HD, and pathologic-grade dependent reductions in numbers of mitochondria in HD postmortem brain tissue and in striatal cells from mutant htt (mhtt)-knock-in mice; both mitochondrial respiration and ATP production are significantly impaired (reviewed in Browne and Beal, 2004) (Table 1). We have shown that the phenotypic and neuropathologic features of HD can be modeled in rodents and primates with the mitochondrial toxin 3-nitropropionic acid (3-NP) (Beal et al., 1993). We and others have shown impaired brain creatine kinase activity and significant alterations in levels of high-energy phosphate intermediates in transgenic mouse models of HD (Zhang et al., 2011; Mochel et al., 2012). We also found a reduction in numbers and size of mitochondria, identified by a reduction in immunohistochemical markers for cytochrome c oxidase 2 (COX2), superoxide dismutase (SOD) 2, and cytochrome c, that worsened with increasing disease severity (Kim et al., 2010). In addition, both the Tfam, a regulator of mtDNA transcription and replication, and peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α), a key transcriptional regulator of energy metabolism and mitochondrial biogenesis, are significantly reduced as disease severity increases. Abnormalities in mitochondrial dynamics were observed: Drp1 was found to be significantly increased, and Mfn1 was significantly decreased (Kim et al., 2010). In addition, a direct interaction of mhtt with mitochondria or various protein complexes has been proposed to play an important role in disease pathogenesis, by regulating mitochondrial fission-fusion events and mitochondrial trafficking along axons and dendrites (reviewed in Bossy-Wetzel et al., 2008; Reddy et al., 2009; Johri et al., 2012) (Table 2).
ALS.
ALS is a progressive neurodegenerative disease that targets motor neurons in the brain and spinal cord, resulting in muscle weakness, atrophy, and eventual death. Although there have been extensive research efforts investigating the pathogenesis of ALS, its etiology is still largely unknown. In approximately 20% of the familial ALS cases, the disease is associated with one or more mutations in the gene that encodes copper-zinc SOD1. Dominant mutations in two DNA/RNA binding proteins, TDP-43 (TAR DNA-binding protein 43) and FUS/TLS (fused/translocated in liposarcoma), have also been reported and account for ∼5 and 4% of ALS cases, respectively (Da Cruz and Cleveland, 2011). Mutations in the valosin-containing protein (also known as transitional endoplasmic reticulum ATPase) gene were recently reported to be the cause of 1 to 2% of familial ALS cases, and they have effects on the regulation of mitochondrial calcium (De Vos et al., 2012). The ultimate cause of neuronal death and the debilitating phenotypes in ALS are currently unknown; however, several studies have reported mitochondrial damage and dysfunction in patients with ALS and SOD1 mutant transgenic mice (reviewed in Beal, 2005). Furthermore, degenerating mitochondria in the perinuclear region have been reported in ALS transgenic mice with TDP-43 or FUS mutations. Mitochondrial abnormalities (e.g., swelling, deformed cristae, defects in respiratory chain activity, and a decrease in mtDNA copy number) are among the earliest signs of disease onset in the transgenic mouse models with SOD1 mutations. Disruption of axonal transport was shown in both patients with ALS and mutant SOD1 transgenic mice (reviewed in Magrané and Manfredi, 2009). A proportion of SOD1 mutant protein is misfolded onto the cytoplasmic surface of mitochondria, and the axonal mitochondria of motor neurons are the primary in vivo targets for misfolded SOD1 (Vande Velde et al., 2011). Mutant SOD1 alters axonal mitochondrial morphology and distribution, with dismutase active SOD1 causing mitochondrial clustering at the proximal side of Schmidt-Lanterman incisures within motor axons, and dismutase inactive SOD1 producing aberrantly elongated axonal mitochondria beginning presymptomatically and increasing in severity as the disease progresses. Somal mitochondria are altered by mutant SOD1, with loss of the characteristic cylindrical, networked morphology and its replacement by a less elongated, more spherical shape (Vande Velde et al., 2011). Recently, Magrané et al. (2012) showed that mutant SOD1 motor neurons have impaired mitochondrial fusion in both axons and cell bodies. There is selective impairment of retrograde axonal transport, smaller mitochondrial size, decreased mitochondrial density, and defective mitochondrial membrane potential. Furthermore, mislocalization of mitochondria at synapses among motor neurons in vitro correlates with abnormal synaptic number, structure, and function (Magrané et al., 2012). Expressing mutant SOD1 confined to mitochondria is sufficient to produce loss of motor neurons and an ALS phenotype (Igoudjil et al., 2011).
Mitochondrial DNA Mutations and Neurodegeneration
Mitochondria have their own DNA (mtDNA) that encodes 13 of the 92 polypeptides of the OXPHOS system; the remaining structural polypeptides and assembly factors are encoded by nuclear DNA. Mutations in either mtDNA or nuclear DNA, resulting in OXPHOS dysfunction, are particularly known to affect tissues with high energy demands such as the central nervous system, skeletal muscle, and heart. Mitochondria are thought to contribute to aging through the accumulation of mtDNA mutations and net production of ROS. Mitochondrial DNA mutations, mitochondrial abnormalities, and mitochondrial respiratory chain-deficient cells are also present in age-related neurodegenerative diseases such as PD and AD (De Coo et al., 1999; Coskun et al., 2004; Smigrodzki et al., 2004; Parker and Parks, 2005; Bender et al., 2006, 2008; Reeve et al., 2008). The types of mtDNA deletions in the substantia nigra neurons from patients with PD and age-matched controls were found to be similar to those that occur in patients with Kearns-Sayre, Twinkle, or multiple-deletion disorder (Reeve et al., 2008).
Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α in Mitochondrial Dysfunction and Neurodegeneration
Peroxisome proliferator-activated receptor (PPAR)-γ coactivator (PGC)-1α is a transcriptional coactivator that interacts with a broad range of transcription factors involved in a wide variety of biological processes and/or responses including mitochondrial biogenesis, OXPHOS, antioxidant defense, adaptive thermogenesis, glucose/fatty acid metabolism, fiber type switching in skeletal muscle, and heart development (Puigserver and Spiegelman, 2003; Liang and Ward, 2006; St-Pierre et al., 2006). PGC-1α does not bind to DNA directly, but forms heteromeric complexes with transcription factors, including nuclear respiratory factors, NRF-1 and NRF-2, and the nuclear receptors, PPARα, PPARδ, PPARγ, and estrogen-related receptor α (Lin et al., 2005). These transcription factors, in turn, regulate the expression of many nuclear-encoded mitochondrial genes, such as cytochrome c, complexes I to V, and Tfam (Kelly and Scarpulla, 2004; Handschin and Spiegelman, 2006). In recent years, impaired PGC-1α expression and/or function has emerged as a common underlying cause of mitochondrial dysfunction in neurodegenerative diseases such as HD, PD, and AD.
There is substantial evidence for the impairment of PGC-1α levels and activity in HD (Cui et al., 2006; Weydt et al., 2006; Chaturvedi et al., 2009, 2010; Johri et al., 2011b). The involvement of PGC-1α in HD was first indicated by the findings that PGC-1α knockout mice exhibited mitochondrial dysfunction, defective bioenergetics, a hyperkinetic movement disorder, and striatal degeneration, which are features also observed in HD (Lin et al., 2004; Leone et al., 2005). Selective ablation of PGC-1α leads to increased striatal neuron degeneration and increased susceptibility to the mitochondrial toxin 3-NP in HD transgenic mice (Cui et al., 2006). Furthermore, impaired PGC-1α function and levels occur in striatal cell lines, transgenic mouse models of HD, and postmortem brain tissue from patients with HD (Cui et al., 2006; Weydt et al., 2006). We showed a pathologic grade-dependent significant reduction in numbers of mitochondria in striatal spiny neurons, which correlated with reductions in PGC-1α and Tfam (Kim et al., 2010). Sequence variation in the PGC-1α gene modifies the age of onset of HD (Taherzadeh-Fard et al., 2009; Weydt et al., 2009). Recent studies showed that expression of mhtt in primary oligodendrocytes results in decreased expression of PGC-1α, and decreased expression of myelin basic protein and deficient myelination were found in the R6/2 mouse model of HD (Xiang et al., 2011). A decrease in myelin basic protein and deficient postnatal myelination also occurs in the striatum of PGC-1α knockout mice (Xiang et al., 2011).
A meta-analysis of 17 independent genomewide gene expression microarray studies revealed the strongest association between PD and nuclear genes encoding for OXPHOS subunits in mitochondria and enzymes involved in glucose metabolism, all of which are regulated by PGC-1α (Zheng et al., 2010). These genes showed decreased expression in (laser microdissected) substantia nigra dopaminergic neurons even in the earliest stages of PD. Activation of PGC-1α results in increased expression of OXPHOS subunits and blocks the dopaminergic neuron loss induced by mutant α-synuclein, or the pesticide rotenone, in cultured dopaminergic neurons from embryonic rat midbrain and human catecholaminergic SH-SY5Y cells (Zheng et al., 2010). Transgenic mice overexpressing PGC-1α in dopaminergic neurons are resistant against cell degeneration induced by the neurotoxin MPTP (Mudò et al., 2012). Earlier, it was noted that genetic ablation of the PGC-1α gene markedly enhances MPTP-induced loss of tyrosine hydroxylase-positive neurons in the substantia nigra (St-Pierre et al., 2006). Recently, a parkin-interacting substrate was identified that accumulates in models of parkin inactivation and human PD brain, and it was shown to repress the expression of PGC-1α and its target gene, NRF-1, by binding to insulin response sequences in the PGC-1α promoter (Shin et al., 2011). Clark et al. (2011) provided limited evidence of an association of certain PGC-1α single-nucleotide polymorphisms with the risk or age of onset of PD.
Using genomewide complementary DNA microarray analysis, Qin et al. (2009) showed that PGC-1α expression is decreased in the brain of patients with AD as a function of dementia severity. PGC-1α protein content was negatively associated with both AD-type neuritic plaque pathology and β-amyloid contents. Qin et al. also showed that adenoviral-mediated exogenous PGC-1α expression in Tg2576 neurons attenuated hyperglycemic-mediated β-amyloidogenesis.
Recently, Liang et al. (2011) showed an age-dependent decrease in PGC-1α in SOD1-G93A mice. Moreover, they showed that overexpression of PGC-1α slowed the progression of ALS, moderately extended lifespan, and improved motor (rotarod) performance. These improvements were associated with a significant decrease in motor neuron cell death and less neuromuscular junction damage in the G93A mice that overexpressed PGC-1α (Liang et al., 2011). In the same year, the Pasinetti laboratory also showed that PGC-1α overexpression significantly improved motor function and survival of SOD1-G93A mice (Zhao et al., 2011). The behavioral improvements were accompanied by reduced blood glucose levels and protection against motor neuron loss, restoration of mitochondrial electron transport chain activities, and inhibition of stress signaling in the spinal cord (Zhao et al., 2011).
Transcriptional Approaches to Improve Mitochondrial Function
PGC-1α and PPARs.
PGC-1α is now increasingly being recognized as an important therapeutic target for neurodegenerative disorders. As discussed above, PGC-1α expression and/or function is impaired in all major neurodegenerative diseases; therefore, pharmacologic/transcriptional activation of the PGC-1α pathway is expected to have neuroprotective effects. Indeed, overexpression of PGC-1α was shown to reduce Aβ plaque in an in vitro model of AD, produce neuroprotective effects in a transgenic mouse model of ALS, and enhance the mitochondrial membrane potential and reduce mitochondrial toxicity in in vitro models of HD (Weydt et al., 2006; Qin et al., 2009; Liang et al., 2011; Zhao et al., 2011). Lentiviral delivery of PGC-1α to the striatum of R6/2 HD mice prevented striatal atrophy at the site of PGC-1α injections (Cui et al., 2006). Recently, Da Cruz et al. (2012) showed that increasing PGC-1α activity in muscle in a transgenic mouse model of ALS caused by a mutation in SOD1 is able to sustain muscle function throughout the disease course, although survival was not extended. Another potential approach to activating the PGC-1α pathway, and thereby improving mitochondrial function, is via activation of PPARs. The PPARs are a subfamily of nuclear receptors that are ligand-modulated transcription factors that regulate gene-expression programs of metabolic pathways. PPAR agonists increase oxidative phosphorylation capacity in mouse and human cells and enhance mitochondrial biogenesis. Administration of a PPARγ agonist, thiazolidinedione, was shown to produce beneficial effects on weight loss, mhtt aggregates, and global ubiquitination profiles in R6/2 mice (Chiang et al., 2010). Earlier, it was shown in STHdhQ111 cells that PPARγ activation by rosiglitazone prevents mitochondrial dysfunction and oxidative stress that occurs when mutant striatal cells are challenged with pathological increases in calcium (Quintanilla et al., 2008). We recently showed that bezafibrate, which is a pan-PPAR agonist, improved the expression of PGC-1α and downstream target genes, improved behavioral deficits, survival, and striatal atrophy, and reduced oxidative damage in the R6/2 transgenic mouse model of HD (Johri et al., 2012). Both pioglitazone and rosiglitazone, which are PPAR-γ agonists, were shown to exert beneficial effects in in vitro and in vivo models of PD and AD (reviewed in Chaturvedi and Beal, 2008; Mandrekar-Colucci and Landreth, 2011).
PGC-1α, SIRT1, and AMP-Activated Protein Kinase.
Sirtuins (silent information regulators) are members of the NAD+-dependent histone deacetylase family of proteins in yeast, and its homologs in mice and humans participate in a variety of cellular processes, including mitochondrial functions, cellular metabolism, energy metabolism, gluconeogenesis, cell survival, and aging. Although the role of sirtuins in promoting lifespan extension in lower organisms has been contested recently, there is strong evidence to suggest that sirtuins are an integrative link between metabolic control and transcriptional regulation, and the role of SIRT1 in activating the master regulator PGC-1α remains uncontested. Increased intracellular NAD+ concentrations activate SIRT1 in brain after caloric restriction, resulting in a reduction in amyloid pathology in a mouse model of AD; increased SIRT1 protects against hippocampal degeneration in a mouse model of AD, and direct injection of SIRT1 lentivirus in the hippocampus of AD transgenic mice produces significant neuroprotection (reviewed in Chaturvedi and Beal, 2008). A SIRT1 activator, resveratrol, increases the activity of PGC-1α and improves mitochondrial activity as a consequence of SIRT1-mediated deacetylation of PGC-1α, which increases its effects on liver, fat, and muscle metabolism. SIRT1 activation by resveratrol increases the survival of motor neurons in transgenic ALS mice and reduces learning impairments and neurodegeneration in AD mouse models, by decreasing the acetylation of the SIRT1 substrates PGC-1α and p53 (Kim et al., 2007). Resveratrol also protects against 3-NP-induced motor and behavioral deficits. We showed that resveratrol treatment of the N171-82Q HD transgenic mice produced increased PGC-1α and reduced the apparent vacuolization in brown adipose tissue and reduced glucose levels, but there were no beneficial effects in the striatum, probably because of poor brain penetration (Ho et al., 2010). Overexpression of SIRT1 improves motor function, reduces brain atrophy, and attenuates mhtt-mediated metabolic abnormalities in three different transgenic mouse models of HD (Jeong et al., 2012; Jiang et al., 2012). Recently, it was shown that SIRT1 protects against α-synuclein aggregation by activating molecular chaperones, heat shock factor 1 and Hsp70, in the brains of mice with the A53T α-synuclein mutation (Donmez et al., 2012). Another sirtuin, which is of particular interest as a target for therapeutic intervention, is SIRT3. It is one of the three sirtuins that are located in mitochondria, where it interacts with complex I of the respiratory chain and deacetylates several proteins in complex I. It also increases fatty acid oxidation, SOD2 activity, and levels of glutathione and inhibits activation of the mitochondrial permeability transition.
AMP-activated protein kinase (AMPK) is a Ser/Thr kinase that is activated as a consequence of increased AMP levels, reflecting low ATP availability and low energy reserve. AMPK activation results in a cascade of phosphorylation-dependent adaptive modifications of several factors, including PGC-1α, to switch on the catabolic pathways (such as fatty acid oxidation and mitochondrial respiratory chain activity) to produce ATP, while simultaneously shutting down energy-consuming anabolic processes. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) is a compound that has been used to activate PGC-1α through AMPK. It does so by generating inosine monophosphate, which acts as an AMPK agonist by mimicking AMP. AICAR was shown to inhibit tau phosphorylation in an in vitro model of AD; however, AMPK activation by AICAR was shown to produce adverse effects in R6/2 HD mice in that it enhanced brain atrophy, neuronal loss, and aggregate formation in the striatum. Recently, it was shown that AMPK activity is increased in spinal cord cultures expressing mutant SOD1, as well as in spinal cord lysates from mutant SOD1 mice (Lim et al., 2012). Reducing AMPK activity either pharmacologically or genetically prevented mutant SOD1-induced motor neuron death in vitro (Lim et al., 2012). Metformin is another AMPK activator that was shown to be effective in male HD transgenic mice in that it prolonged survival and decreased hind limb clasping (Ma et al., 2007).
Although the effects of resveratrol and SIRT1 on PGC-1α are well established, lately there has been a great deal of controversy about the mechanism by which this regulation is achieved. The ability of resveratrol to elicit cellular changes in a SIRT1-independent manner, combined with the observations that AMPK-deficient mice exhibit a blunted response to resveratrol treatment, pointed toward AMPK as a critical mediator of resveratrol action (Um et al., 2010). Studies by Park et al. (2012) suggested that resveratrol does not target SIRT1 directly but instead stimulates the AMPK pathway by inhibiting cAMP-degrading phosphodiesterases (mainly phosphodiesterase 4), resulting in increased cAMP levels, which in turn increases cellular calcium levels, thereby stimulating the phosphorylation of AMPK (Park et al., 2012). It was proposed that AMPK then activates SIRT1 indirectly by elevating intracellular levels of its cosubstrate, NAD+ (Fulco et al., 2008; Canto et al., 2009). Recently, this issue was addressed in an elegant manner in a study by Sinclair's group, which used a conditional SIRT1 knockout mouse to show that with moderate doses of resveratrol in vitro or in vivo the increases in phosphorylated AMPK, NAD+, liver kinase B1 acetylation, and mitochondrial function entirely depended on SIRT1 (Price et al., 2012). Using this adult-inducible SIRT1 knockout mouse strain, Price et al. showed that the beneficial effects of resveratrol treatment on muscle mitochondrial function depend on SIRT1 in vivo. They also showed that overexpression of SIRT1 in a transgenic mouse strain mimicked the effects of resveratrol treatment in skeletal muscle. These studies support a model in which lower doses of resveratrol stimulate SIRT1 upstream of AMPK via deacetylation of liver kinase B1, one of the activating protein kinases of AMPK (Price et al., 2012). At present, the entire resveratrol/sirtuin field seems to be changing at daunting speed, and much remains to be proven/disproven about the elusive mechanisms of action of resveratrol and sirtuins and their potential role in neurodegeneration and life extension. However, the beneficial effects of resveratrol and SIRT1 that were observed in several mouse models of neurodegenerative diseases cannot be overlooked, and the quest for small-molecule activators of sirtuins with potential neuroprotective effects should continue.
Nrf2/Antioxidant Response Element Pathway.
ROS damage to mitochondria is well known in all of the major neurodegenerative disorders; therefore, therapies targeting the Nrf2/antioxidant response element (ARE) pathway are of particular interest. Synthetic triterpenoids (TPs) are analogs of oleonolic acid and powerful inhibitors of oxidative stress and cellular inflammatory processes. Synthetic TP compounds are potent inducers of the ARE/Nrf2/Keap1 signaling pathway. After activation by TP, Nrf2 dissociates from Keap1, translocates to the nucleus, and binds to the ARE promoter sequences, leading to coordinated induction of a battery of cytoprotective genes, including antioxidants and anti-inflammatory genes. Neuronal cultures derived from Nrf2 knockout mice show increased susceptibility to oxidative damage, as well as damage produced by mitochondrial electron transport gene complex inhibitors such as MPP+ and rotenone. Nrf2-deficient mice show increased susceptibility to the mitochondrial toxins MPTP and 3-NP. Recently, we tested neuroprotective effects of the synthetic triterpenoid CDDOmethylamide (CDDO-MA), which is a potent activator of the Nrf2/ARE signaling pathway (Yang et al., 2009a). CDDO-MA produced marked protection in the 3-NP rat model and both the acute and chronic MPTP mouse models. CDDO-MA exerted significant protection against tertbutylhydroperoxide-induced ROS in vitro. It increased the expression of genes involved in mitochondrial biogenesis, as well as those involved in glutathione synthesis and in the expression of antioxidant enzymes (Yang et al., 2009a). Triterpenoids also protect in transgenic mouse models of ALS, HD, and AD.
Several bioenergetic agents have efficacy in improving mitochondrial function including creatine, coenzyme Q10 (CoQ10), nicotinamide, riboflavin, and lipoic acid (reviewed in Beal, 2009). CoQ10 is an essential biologic factor of electron transport chain where it accepts electrons from complexes I and II. It also serves as an important antioxidant in mitochondrial lipid membranes. We showed that oral administration of CoQ10 protects against lesions produced by amino-oxyacetic acid and the mitochondrial toxins malonate and 3-NP. We also found modest neuroprotective effects of COQ10 in a transgenic mouse model of ALS and more marked neuroprotective effects in transgenic mouse models of HD. We recently found that CoQ10 treatment decreases brain oxidative stress, Aβ42 levels, and β-amyloid plaque area and number, and improves cognition in a transgenic mouse model of AD (Dumont et al., 2011). High doses of CoQ10 significantly extend survival and improve motor performance, grip strength, and brain atrophy in R6/2 HD mice in a dose-dependent manner. Furthermore, we found that the combination of creatine and CoQ10 exerts additive neuroprotective effects in the MPTP model of PD, the 3-NP model of HD, and a transgenic mouse model of HD (Yang et al., 2009b). These compounds, therefore, show neuroprotective effects, which may be a useful target for treating neurodegenerative diseases.
A new concept emerging in the field of neurodegenerative diseases, and which opens up new avenues for understanding disease progression and developing novel therapeutics, is that of the prion-like spread of misfolded proteins. The prion concept was developed by Prusiner (1998) and involves proteinaceous particles, the prions, which are the infectious agents in Jacob-Creutzfeld and other neurological disorders. This concept may also be applicable to neurodegenerative diseases with genetic experiments providing convincing evidence that mutant Aβ, α-synuclein, and tau can be transferred between neighboring neurons, and then induce pathological changes in these neurons (Harris et al., 2010; Luk et al., 2012; de Calignon et al., 2012).
Conclusion and Future Perspectives
As discussed above, there is strong evidence implicating the role of mitochondrial dysfunction in the pathogenesis of neurodegenerative diseases. In a number of instances, there is direct involvement of the genetic defect with mitochondria such as in Friedreich's ataxia. In a number of neurodegenerative diseases, the genetic evidence is more indirect. This is the case in AD and HD. In AD, the mitochondrial dysfunction may be a consequence of the accumulation of Aβ within mitochondria. Likewise, in PD α-synuclein has been demonstrated to associate with mitochondria, and recent studies have shown that LRRK2, which is localized on the outer surface of mitochondria, may increase mitochondrial fission (Niu et al., 2012). The autosomal recessive genes involved in PD all are linked to mitochondrial dysfunction. For instance, both parkin and PINK1 play a role in mitophagy, and DJ-1 modulates oxidative damage within mitochondria. In HD, the mitochondrial impairment may be caused by an impairment of the activity of PGC-1α, although there is other evidence that mutant htt can directly associate with mitochondria and may increase mitochondrial fission (Costa et al., 2010; Johri et al., 2011a; Shirendeb et al., 2011; Song et al., 2011). There is recent evidence showing an impairment of PGC-1α in PD based on findings in microarray studies and transgenic mouse models in which a parkin deficiency leads to impaired PGC-1α transcription, which is caused by an accumulation of parkin-interacting substrate (Shin et al., 2011).
The field of mitochondrial dynamics, which is involved in the trafficking and turnover of mitochondria, is another area in which there is increasing evidence for a critical role in neurodegenerative diseases. Mutations in the mitochondrial fusion proteins, mitofusin 2 and OPA1, are responsible for Charcot-Marie tooth disease and autosomal dominant optic atrophy, respectively. Mutant htt binds to DRP1 and increases its GTPase activity, and similar effects have been reported in AD (Manczak et al., 2011). There is evidence that mutant SOD1, a cause of autosomal dominant ALS, forms aggregates, which then bind to the outer mitochondrial membrane impairing the activity of the voltage-dependent anion channel, Bcl-2, as well as impairing protein uptake (Israelson et al., 2010).
The field of mitochondrial-targeted therapeutics is one that is growing and is of great importance. The development of transgenic mouse models of neurodegenerative diseases has been valuable in providing ways of testing and developing new therapies. Creatine is involved in buffering energy metabolism, produces neuroprotective effects in transgenic mouse models of HD and ALS, and is protective in the MPTP model of PD (Beal, 2011). It is presently being tested in phase III clinical trials in both PD and HD. Likewise, coenzyme Q is a component of the electron transport chain and is also an important antioxidant, and it is effective in transgenic mouse models of neurodegenerative diseases as well as MPTP and 3-NP. A recent phase III clinical trial in PD was unsuccessful (unpublished work); however, trials in HD and Friedreich's ataxia are continuing. Several compounds have been developed that can specifically target mitochondria. These include compounds such as mitoQ, a form of coenzyme Q linked to triphosphonium ions, which results in selective accumulation within mitochondria. There are also novel peptide antioxidants, termed SS31 and SS20, which bind to the inner mitochondrial membrane, and are neuroprotective in transgenic mouse models of ALS as well as in neurotoxin models (Petri et al., 2006). Dexpramipexole, an isomer of the dopamine agonist pramipexole, accumulates in mitochondria, where it exerts antioxidant effects, and inhibits the activation of the mitochondrial permeability transition. Dexpramipexole has shown efficacy in a phase II clinical trial in ALS, where it produced improvement on the ALS functional rating scale and mortality (Cudkowicz et al., 2011).
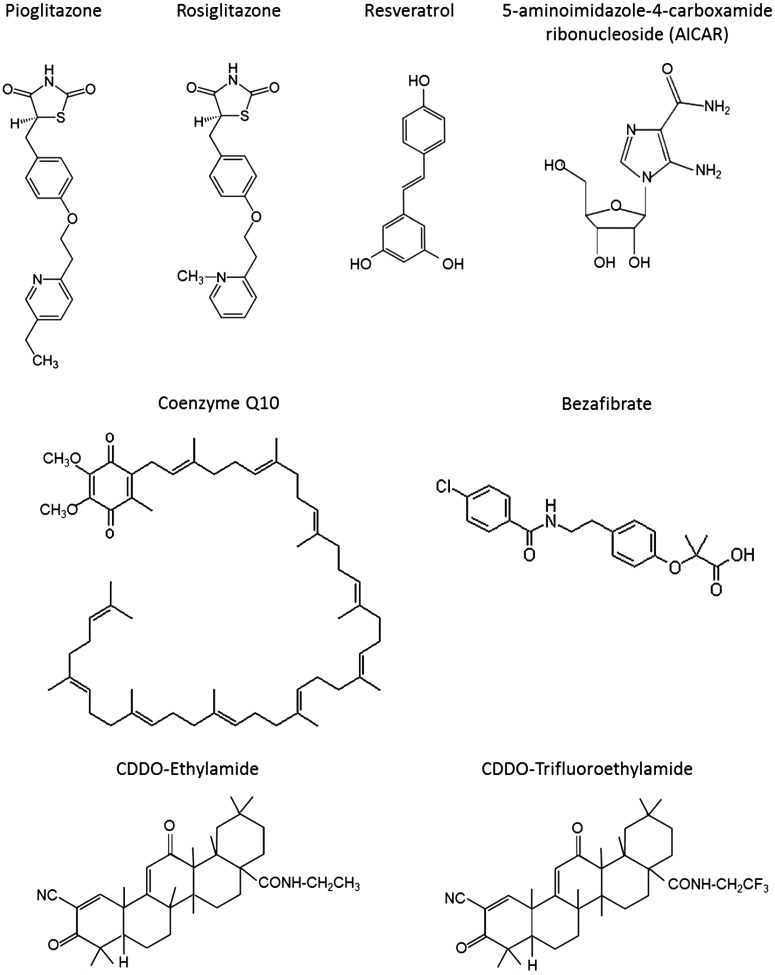
Other approaches are to modulate transcription, such as the Nrf2/ARE pathway, which when activated by triterpenoids exerts neuroprotective effects in transgenic mouse models of AD, HD, and ALS, and against MPTP. Dimethylfumarate, another agent that activates this pathway, is protective in multiple sclerosis (Gold et al., 2012). PGC-1α induces mitochondrial biogenesis and expression of antioxidant enzymes, and we and others have shown that pharmacologic agents that activate PGC-1α are protective in transgenic mouse models of neurodegenerative diseases including HD, ALS, and Aβ toxicity (Weydt et al., 2006; Qin et al., 2009; Liang et al., 2011; Zhao et al., 2011; Johri et al., 2012). PGC-1α activity is modulated by SIRT1, an NAD-dependent deacetylase, and activation of SIRT1 is protective in models of HD (Jeong et al., 2012; Jiang et al., 2012). There are therefore a number of promising new compounds and therapeutic targets that modulate mitochondria and produce neuroprotective effects (Fig. 1). These compounds show great promise for treating patients who suffer from neurodegenerative diseases, for which there is as yet no effective treatment to slow or halt the underlying disease processes.
Fig. 1.
Structures of the compounds that produce beneficial effects in mice against mitochondrial dysfunction.
This work was supported by the National Institutes of Health National Institute on Aging [Grant P01AG14930] and the Huntington Disease Society of America Coalition for the Cure (M.F.B.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- OXPHOS
- oxidative phosphorylation
- Aβ
- β-amyloid peptide
- AD
- Alzheimer's disease
- AICAR
- 5-aminoimidazole-4-carboxamide ribonucleoside
- ALS
- amyotrophic lateral sclerosis
- AMPK
- AMP-activated protein kinase
- APP
- amyloid precursor protein
- ARE
- antioxidant response element
- CDDO-MA
- CDDOmethylamide
- CoQ10
- coenzyme Q10
- COX
- cytochrome c oxidase
- DA
- dopaminergic
- Drp1
- dynamin related-protein 1
- HD
- Huntington's disease
- Hsp
- heat shock protein
- htt
- huntingtin
- mhtt
- mutant htt
- LRRK2
- leucine-rich repeat kinase 2
- Mfn
- mitofusin
- MMP
- mitochondrial membrane potential
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mtDNA
- mitochondrial DNA
- NRF
- nuclear respiratory factor
- 3-NP
- 3-nitropropionic acid
- OMM
- outer mitochondrial membrane
- OPA1
- optic atrophy 1
- PD
- Parkinson's disease
- PGC-1α
- peroxisome proliferator-activated receptor-γ coactivator-1α
- PINK1
- phosphatase and tensin homolog-induced putative kinase 1
- PPAR
- peroxisome proliferator-activated receptor
- PS
- presenilin
- ROS
- reactive oxygen species
- SIRT
- silent mating type information regulation 2 homolog
- SOD
- superoxide dismutase
- Tfam
- mitochondrial transcription factor A
- TP
- triterpenoid.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Johri and Beal.
References
- Beal MF. (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58:495–505 [DOI] [PubMed] [Google Scholar]
- Beal MF. (2007) Mitochondria and neurodegeneration. Novartis Found Symp 287:zpg183–192; discussion 192–186 [DOI] [PubMed] [Google Scholar]
- Beal MF. (2009) Therapeutic approaches to mitochondrial dysfunction in Parkinson's disease. Parkinsonism Relat Disord 15 (Suppl 3):S189–S194 [DOI] [PubMed] [Google Scholar]
- Beal MF. (2011) Neuroprotective effects of creatine. Amino Acids 40:1305–1313 [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. (1993) Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci 13:4181–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38:515–517 [DOI] [PubMed] [Google Scholar]
- Bender A, Schwarzkopf RM, McMillan A, Krishnan KJ, Rieder G, Neumann M, Elstner M, Turnbull DM, Klopstock T. (2008) Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. J Neurol 255:1231–1235 [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. (2002) Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging 23:371–376 [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Petrilli A, Knott AB. (2008) Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci 31:609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Beal MF. (2004) The energetics of Huntington's disease. Neurochem Res 29:531–546 [DOI] [PubMed] [Google Scholar]
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. (2005) Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol 57:695–703 [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi RK, Adhihetty P, Shukla S, Hennessy T, Calingasan N, Yang L, Starkov A, Kiaei M, Cannella M, Sassone J, et al. (2009) Impaired PGC-1α function in muscle in Huntington's disease. Hum Mol Genet 18:3048–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi RK, Beal MF. (2008) PPAR: a therapeutic target in Parkinson's disease. J Neurochem 106:506–518 [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Calingasan NY, Yang L, Hennessey T, Johri A, Beal MF. (2010) Impairment of PGC-1α expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington's disease following chronic energy deprivation. Hum Mol Genet 19:3190–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC. (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130:548–562 [DOI] [PubMed] [Google Scholar]
- Chiang MC, Chen CM, Lee MR, Chen HW, Chen HM, Wu YS, Hung CH, Kang JJ, Chang CP, Chang C, et al. (2010) Modulation of energy deficiency in Huntington's disease via activation of the peroxisome proliferator-activated receptor γ. Hum Mol Genet 19:4043–4058 [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. (2009) S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science 324:102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J, Reddy S, Zheng K, Betensky RA, Simon DK. (2011) Association of PGC-1α polymorphisms with age of onset and risk of Parkinson's disease. BMC Med Genet 12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun PE, Beal MF, Wallace DC. (2004) Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A 101:10726–10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, Malorni W, Davies KJ, Carafoli E, Scorrano L. (2010) Mitochondrial fission and cristae disruption increase the response of cell models of Huntington's disease to apoptotic stimuli. EMBO Mol Med 2:490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz M, Bozik ME, Ingersoll EW, Miller R, Mitsumoto H, Shefner J, Moore DH, Schoenfeld D, Mather JL, Archibald D, et al. (2011) The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med 17:1652–1656 [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. (2006) Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127:59–69 [DOI] [PubMed] [Google Scholar]
- Da Cruz S, Cleveland DW. (2011) Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr Opin Neurobiol 21:904–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cruz S, Parone PA, Lopes VS, Lillo C, McAlonis-Downes M, Lee SK, Vetto AP, Petrosyan S, Marsala M, Murphy AN, et al. (2012) Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab 15:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Williams M. (2012) Mitochondrial function and dysfunction: an update. J Pharmacol Exp Ther 342:598–607 [DOI] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, et al. (2012) Propagation of τ pathology in a model of early Alzheimer's disease. Neuron 73:685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coo IF, Renier WO, Ruitenbeek W, Ter Laak HJ, Bakker M, Schägger H, Van Oost BA, Smeets HJ. (1999) A 4-base pair deletion in the mitochondrial cytochrome b gene associated with parkinsonism/MELAS overlap syndrome. Ann Neurol 45:130–133 [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Mórotz GM, Stoica R, Tudor EL, Lau KF, Ackerley S, Warley A, Shaw CE, Miller CC. (2012) VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum Mol Genet 21:1299–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. (2012) SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J Neurosci 32:124–132 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dumont M, Kipiani K, Yu F, Wille E, Katz M, Calingasan NY, Gouras GK, Lin MT, Beal MF. (2011) Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 27:211–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, et al. (2007) Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A 104:1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14:661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter D, Pernold K, Yoshitake T, Lindqvist E, Hoffer B, Kehr J, Larsson NG, Olson L. (2010) MitoPark mice mirror the slow progression of key symptoms and l-DOPA response in Parkinson's disease. Genes Brain Behav 9:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP. (1998) Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm 105:855–870 [DOI] [PubMed] [Google Scholar]
- Gold R, Linker RA, Stangel M. (2012) Fumaric acid and its esters: an emerging treatment for multiple sclerosis with antioxidative mechanism of action. Clin Immunol 142:44–48 [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. (2006) Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27:728–735 [DOI] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, Kim D, Hamto P, Lo I, Yu GQ, et al. (2010) Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron 68:428–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. (2010) Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Exp Neurol 225:74–84 [DOI] [PubMed] [Google Scholar]
- Igoudjil A, Magrané J, Fischer LR, Kim HJ, Hervias I, Dumont M, Cortez C, Glass JD, Starkov AA, Manfredi G. (2011) In vivo pathogenic role of mutant SOD1 localized in the mitochondrial intermembrane space. J Neurosci 31:15826–15837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelson A, Arbel N, Da Cruz S, Ilieva H, Yamanaka K, Shoshan-Barmatz V, Cleveland DW. (2010) Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron 67:575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR, 3rd, Bordone L, Guarente L, et al. (2012) Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med 18:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, et al. (2012) Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat Med 18:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Calingasan NY, Hennessey TM, Sharma A, Yang L, Wille E, Chandra A, Beal MF. (2012) Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington's disease. Hum Mol Genet 21:1124–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Chaturvedi RK, Beal MF. (2011a) Hugging tight in Huntington's. Nat Med 17:245–246 [DOI] [PubMed] [Google Scholar]
- Johri A, Starkov AA, Chandra A, Hennessey T, Sharma A, Orobello S, Squitieri F, Yang L, Beal MF. (2011b) Truncated peroxisome proliferator-activated receptor-γ coactivator 1α splice variant is severely altered in Huntington's disease. Neurodegener Dis 8:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18:357–368 [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Moody JP, Edgerly CK, Bordiuk OL, Cormier K, Smith K, Beal MF, Ferrante RJ. (2010) Mitochondrial loss, dysfunction and altered dynamics in Huntington's disease. Hum Mol Genet 19:3919–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN. (1992) Brain cytochrome oxidase in Alzheimer's disease. J Neurochem 59:776–779 [DOI] [PubMed] [Google Scholar]
- Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236 [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, et al. (2005) PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Ward WF. (2006) PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 30:145–151 [DOI] [PubMed] [Google Scholar]
- Liang H, Ward WF, Jang YC, Bhattacharya A, Bokov AF, Li Y, Jernigan A, Richardson A, Van Remmen H. (2011) PGC-1α protects neurons and alters disease progression in an amyotrophic lateral sclerosis mouse model. Muscle Nerve 44:947–956 [DOI] [PubMed] [Google Scholar]
- Lim MA, Selak MA, Xiang Z, Krainc D, Neve RL, Kraemer BC, Watts JL, Kalb RG. (2012) Reduced activity of AMP-activated protein kinase protects against genetic models of motor neuron disease. J Neurosci 32:1123–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370 [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, et al. (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119:121–135 [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. (2012) Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med 209:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR. (2007) Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci Lett 411:98–103 [DOI] [PubMed] [Google Scholar]
- Magrané J, Manfredi G. (2009) Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxid Redox Signal 11:1615–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrané J, Sahawneh MA, Przedborski S, Estévez ÁG, Manfredi G. (2012) Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. J Neurosci 32:229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid β with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet 20:2495–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromol Med 5:147–162 [DOI] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Landreth GE. (2011) Nuclear receptors as therapeutic targets for Alzheimer's disease. Expert Opin Ther Targets 15:1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochel F, Durant B, Meng X, O'Callaghan J, Yu H, Brouillet E, Wheeler VC, Humbert S, Schiffmann R, Durr A. (2012) Early alterations of brain cellular energy homeostasis in Huntington disease models. J Biol Chem 287:1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudò G, Mäkelä J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Mälkiä A, Bonomo A, Kairisalo M, et al. (2012) Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol Life Sci 69:1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Youle RJ. (2011) Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal 14:1929–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Yu M, Wang C, Xu Z. (2012) Leucine-rich repeat kinase 2 (LRRK2) disturbs mitochondrial dynamics via dynamin-Like protein (DLP1). J Neurochem http://dx.doi.org/10.1111/j.1471–4159.2012.07809.x [DOI] [PubMed]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD, Jr, Parks JK. (2005) Mitochondrial ND5 mutations in idiopathic Parkinson's disease. Biochem Biophys Res Commun 326:667–669 [DOI] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. (2006) Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem 98:1141–1148 [DOI] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, et al. (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. (1998) Prions. Proc Nat Acad Sci U S A 95:13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. (2003) Peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90 [DOI] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. (2009) PGC-1α expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol 66:352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. (2008) Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-γ (PPARγ) in the pathogenesis of Huntington disease. J Biol Chem 283:25628–25637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Mao P, Manczak M. (2009) Mitochondrial structural and functional dynamics in Huntington's disease. Brain Res Rev 61:33–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67:103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AK, Krishnan KJ, Elson JL, Morris CM, Bender A, Lightowlers RN, Turnbull DM. (2008) Nature of mitochondrial DNA deletions in substantia nigra neurons. Am J Hum Genet 82:228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. (2012) Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem 120:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. (2011) PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson's disease. Cell 144:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. (2011) Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage. Hum Mol Genet 20:1438–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigrodzki R, Parks J, Parker WD. (2004) High frequency of mitochondrial complex I mutations in Parkinson's disease and aging. Neurobiol Aging 25:1273–1281 [DOI] [PubMed] [Google Scholar]
- Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, et al. (2011) Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med 17:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, et al. (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408 [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. (2010) The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis 20 (Suppl 2):S265–S279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherzadeh-Fard E, Saft C, Andrich J, Wieczorek S, Arning L. (2009) PGC-1α as modifier of onset age in Huntington disease. Mol Neurodegener 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. (2010) AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59:554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Velde C, McDonald KK, Boukhedimi Y, McAlonis-Downes M, Lobsiger CS, Bel Hadj S, Zandona A, Julien JP, Shah SB, Cleveland DW. (2011) Misfolded SOD1 associated with motor neuron mitochondria alters mitochondrial shape and distribution prior to clinical onset. PLoS One 6:e22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147:893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, et al. (2006) Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1α in Huntington's disease neurodegeneration. Cell Metab 4:349–362 [DOI] [PubMed] [Google Scholar]
- Weydt P, Soyal SM, Gellera C, Didonato S, Weidinger C, Oberkofler H, Landwehrmeyer GB, Patsch W. (2009) The gene coding for PGC-1α modifies age at onset in Huntington's Disease. Mol Neurodegener 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Valenza M, Cui L, Leoni V, Jeong HK, Brilli E, Zhang J, Peng Q, Duan W, Reeves SA, et al. (2011) Peroxisome-proliferator-activated receptor γ coactivator 1α contributes to dysmyelination in experimental models of Huntington's disease. J Neurosci 31:9544–9553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, et al. (2009a) Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One 4:e5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF. (2009b) Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem 109:1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SF, Hennessey T, Yang L, Starkova NN, Beal MF, Starkov AA. (2011) Impaired brain creatine kinase activity in Huntington's disease. Neurodegener Dis 8:194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Varghese M, Yemul S, Pan Y, Cheng A, Marano P, Hassan S, Vempati P, Chen F, Qian X, et al. (2011) Peroxisome proliferator activator receptor γ coactivator-1α (PGC-1α) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener 6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, et al. (2010) PGC-1α, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med 2:52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]