Abstract
Nicotinic agonists display a wide-range profile of antinociceptive activity in acute, tonic, and chronic pain models. However, their effectiveness is limited by their unacceptable side effects. We investigated the antinociceptive effects of two new α4β2* nicotinic partial agonists, varenicline and sazetidine-A, in acute thermal and tonic pain mouse models. Both drugs failed to induce significant effects in the tail-flick and hot-plate tests after subcutaneous administration. However, they blocked nicotine's effects in these tests at very low doses. In contrast to acute pain tests, varenicline and sazetidine-A dose-dependently induced an analgesic effect in the mouse formalin test after systemic administration. Their antinociceptive effects were mediated, however, by different nicotinic acetylcholine receptor (nAChR) subtypes. Sazetidine-A effects were mediated by β2* nAChR subtypes, whereas varenicline actions were attributed to α3β4 nAChRs. Moreover, low inactive doses of varenicline blocked nicotine's actions in phase II of the formalin test. Overall, our results suggest that the antagonistic actions of varenicline at low doses are mediated by β2*-nAChRs and at higher doses as an agonist by α3β4*-nAChRs. In contrast, both actions of sazetidine-A are mediated by β2*-nAChR subtypes. These results suggest that nicotinic partial agonists possess analgesic effects in a rodent tonic pain model and may provide a potential treatment for the treatment of chronic pain disorders.
Introduction
The current therapies for chronic pain have limited efficacy and are associated with dose-limiting side effects (Rau et al., 1993; Perkins et al., 1994). Compounds that act at nAChR s in the CNS and periphery have been reported to show antinociceptive activity in several rodent acute and chronic pain models (Decker et al., 2001). nAChRs are ligand-gated ion channels composed of α and β subunits that assemble to form heteropentomers or homopentomers (Corringer et al., 2000), which are widely distributed in the peripheral and central nervous system. These nAChRs are expressed in the CNS, including many areas contributing to pain such as the midbrain (Mattila et al., 1968), medulla (Iwamoto and Marion, 1993), nucleus raphe magnus (Iwamoto, 1991), thalamus, pedunculopontine tegmental nucleus (Iwamoto, 1989), and spinal cord (Aceto et al., 1986; Christensen and Smith, 1990; Khan et al., 1997; Damaj et al., 1998). The most common CNS subtype, α4β2* (asterisk indicates assembly with other nAChR subunits), is found in the thalamus, dorsal raphe nucleus, and spinal cord (Gillberg et al., 1988; Cucchiaro et al., 2005). Although α3β4* is the major subtype expressed in peripheral ganglia (Mao et al., 2006), it is also expressed in the medial habenula, cerebellum, and spinal cord (Turner and Kellar, 2005). Over the last several years, α4β2* nicotinic full agonists were reported to display a wide-range profile of antinociceptive activity in acute models (such as tail-flick and hot-plate tests), tonic or persistent models (such as the formalin test), and chronic pain models (Bitner et al., 1998; Damaj et al., 1998, 2007; Flores et al., 1999; Marubio et al., 1999). We reported previously that the α5 nicotinic subunit, which coassembles with α4β2* and α3β4* nAChRs in the CNS (Mao et al., 2007), mediates nicotine-induced antinociception in both the hot-plate and tail-flick tests (Jackson et al., 2010). Although nicotinic full agonists were reported to be effective in these acute pain tests, partial agonists such as cytisine (Damaj et al., 1998) and varenicline (Carroll et al., 2008) were not. In fact, varenicline blocked nicotine's effects in these pain models (Carroll et al., 2008). In contrast, varenicline and sazetidine-A both were reported to be possess antinociceptive activity in tonic pain models such as the formalin test in rodents (Cucchiaro et al., 2008; Gao et al., 2010). However, despite the in vitro binding data indicating that varenicline and sazetidine-A have higher affinity than nicotine to α4β2* nAChRs (more than 10-fold), they possessed lower potencies and efficacies than nicotine in the formalin test (Cucchiaro et al., 2008; Gao et al., 2010). It is unclear whether these in vivo discrepancies are the result of differences in receptor efficacy and/or desensitization at various nAChR subtypes.
We therefore investigated the nAChR receptor mechanisms of the antinociceptive effects of these two α4β2* nicotinic partial agonists in acute and persistent pain models. Varenicline, an approved antismoking drug (Chantix, Pfizer, New York, NY), is a potent partial agonist for the α4β2* nAChRs with 40 to 60% of the agonist efficacy of nicotine (Rollema et al., 2007). In addition, it is a full agonist at α3β4 nAChRs and the homopentameric α7 receptors (Mihalak et al., 2006; Rollema et al., 2007, 2009). Sazetidine-A is a newly developed and highly selective α4β2* partial agonist with very low affinities for all other nAChR subtypes (Xiao et al., 2006). It is a full agonist on the high-affinity α4(2)β2(3) nAChRs, whereas it had a very low efficacy on the low-affinity α4(3)β2(2) nAChRs in expressed oocytes (Zwart et al., 2008).
The present study was designed to characterize the antinociceptive effects of varenicline and sazetidine-A in acute thermal pain tests (hot plate and tail-flick tests) and the formalin test after acute administration in mice. The formalin test is commonly used as a model for tonic pain (Tjølsen et al., 1992; Abbott et al., 1995; Watson et al., 1997), and subcutaneous formalin injection into one hind paw in the conscious mouse produces biphasic nociceptive behaviors characterized by a brief initial phase (first phase) and a prolonged later phase (second phase), each consisting of elevation, licking, flinching, and even biting of the injected hind paw. Traditionally, the first phase of formalin test has been viewed as being caused by an acute activation of nociceptors in the periphery, whereas the second phase is caused by the ensuing inflammatory response or a central sensitization. We first studied the activity and potency of varenicline and sazetidine-A in the above-mentioned pain tests and examined the role of the main nAChR subtypes, β2*-containing receptors and α3β4* and α7 subtypes, in mediating their antinociceptive responses.
Materials and Methods
Animals
Male ICR mice obtained from Harlan (Indianapolis, IN) and male C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME) were used throughout the study. Mice null for the α5 subunit (The Jackson Laboratory) and β2 subunit (Institut Pasteur, Paris, France) and their wild-type littermates were bred in an animal care facility at Virginia Commonwealth University. For all experiments, mice were backcrossed at least 8 to 10 generations. Mutant and wild types were obtained from crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Mice were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility. They were housed in groups of six and had free access to food and water. The rooms were on a 12-h light/dark cycle (lights on at 7:00 AM). Mice were 8 to 10 weeks of age and weighed approximately 20 to 25 g at the start of the experiments. All experiments were performed during the light cycle (between 7:00 AM and 7:00 PM), and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drugs
(−)-Nicotine hydrogen tartrate salt and mecamylamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Methyllycaconitine (MLA) citrate and dihydro-β-erythroidine (DHβE) were purchased from Sigma/RBI (Natick, MA). All drugs were dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously at a total volume of 1 ml/100 g of body weight unless noted otherwise. α-Conotoxin AuIB was synthesized as described previously (Luo et al., 1998) and was given intrathecally. Varenicline [7,8,9,10-tetrahydro- 6,10-methano- 6H-pyrazino (2,3-h)(3) benzazepine] and sazetidine-A [6-[5-[(2S)-2-azetidinylmethoxy]-3-pyridinyl]-5-hexyn-1-ol] were supplied by the National Institute of Drug Abuse Drug Supply Program (Bethesda, MD). All doses are expressed as the free base of the drug.
Intrathecal Injections
Intrathecal injections were performed free-hand between the L5 and L6 lumbar spaces in unanesthetized male mice according to the method of Hylden and Wilcox (1980). The injection was performed by using a 30-gauge needle attached to a glass microsyringe. The injection volume in all cases was 5 μl. The accurate placement of the needle was evidenced by a quick “flick” of the mouse's tail. Thus, the accurate placement of all injections could be assured by watching the tail motion of the mouse.
Antinociceptive Tests
Tail-Flick Test.
The antinociceptive effect of drugs was assessed by the tail-flick method of D'Amour and Smith (1941), as modified by Dewey et al. (1970). A control response (2- to 4-s latency) was determined for each mouse before treatment, and test latency was determined after drug administration. To minimize tissue damage, a maximum latency of 10 s was imposed. Antinociceptive response was calculated as percentage of maximum possible effect (%MPE), where %MPE = [(test value − control value)/(cutoff (10 s) − control value)] × 100. Groups of six to eight animals were used for each dose and each treatment. The mice were tested 5, 15, and 30 min after subcutaneous injections of nicotinic partial agonists for the dose-response evaluation. Antagonism studies were carried out by pretreating the mice with either saline or nicotinic antagonists 15 min before nicotinic agonists. The animals were tested 5 min after administration of the agonist.
Hot-Plate Test.
Mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus, Columbus, OH) as a measure of antinociception. The hot plate was a rectangular heated surface surrounded by Plexiglas and maintained at 55°C. The device was connected to a manually operated timer that recorded the amount of time the mouse spent on the heated surface before showing signs of nociception (e.g., jumping, paw licks). Two control latencies at least 10 min apart were determined for each mouse. The normal latency (reaction time) of 8 to 12 s was assessed with a saline injection. To avoid tissue damage, the hot plate automatically disengaged after 40 s. Groups of 8 to 12 mice were used for each dose and treatment condition. Antinociceptive response was calculated as %MPE, where %MPE = [(test value − control)/(cutoff time (40 s) − control) × 100]. The reaction time was scored when the animal jumped or licked its paws. Eight mice per dose were injected subcutaneously with the partial agonists and tested at various times thereafter to establish a time course. The mice were tested 5, 15, and 30 min subsequent to subcutaneous injection of nicotinic partial agonists for the dose-response evaluation. The antagonism studies were carried out by pretreating the mice with either saline or various antagonists 15 min before the injection of the nicotine. All animals were tested 5 min after the final injection of the nicotine.
Formalin Test.
The formalin test was carried out in an open Plexiglas cage with a mirror placed at a 45° angle behind the cage to allow an unobstructed view of the paws. Mice were allowed to acclimate for 15 min in the test cage before injection. Either nicotinic analogs or control solution were injected subcutaneously at varying time points before the formalin injection. Each animal was injected with 20 μl of 2.5% formalin in the intraplantar region of the right hindpaw. Each mouse was then immediately placed in a Plexiglas box. Up to two mice at one time were observed from 0 to 5 min (phase 1) and 20 to 45 min (phase 2) postformalin injection. The period between the two phases of nociceptive responding is generally considered to be a phase of weak activity. The amount of time spent licking the injected paw was recorded with a digital stopwatch.
Motor Coordination
To measure motor coordination, we used a rotarod (IITC Inc. Life Science, Woodland Hills, CA). The animals were placed on textured drums (1¼-inch diameter) to avoid slipping. When an animal dropped onto the individual sensing platforms, test results were recorded. Five mice tested at a rate of 4 rpm. Naive mice were trained until they could remain on the rotarod for 5 min. Animals that failed to meet this criterion within three trials were discarded. Fifteen minutes after the injection of vehicle or drugs mice were placed on the rotarod for 3 min. If a mouse fell from the rotarod during this time period, it was scored as motor impaired. Percentage of impairment was calculated as: % impairment = [(180 − test time)/(180 × 100)]. Mice were pretreated with saline, varenicline (0.5, 1, and 3 mg/kg), and sazetidine-A (0.1, 0.5, and 1.5 mg/kg) subcutaneously 15 min before the test.
Statistical Analysis
Data were expressed as mean ± S.E.M. of licking time. Statistical analysis was done by analysis of variance followed by post hoc Tukey test. p < 0.05 was considered to be statistically significant. ED50 (effective dose 50%) and AD50 (antagonist dose 50%) values with 95% confidence limits for behavioral data were calculated by unweighted least-squares linear regression as described by Tallarida and Murray (1987).
Results
Effects of Varenicline and Sazetidine-A in the Tail-Flick and Hot-Plate Tests.
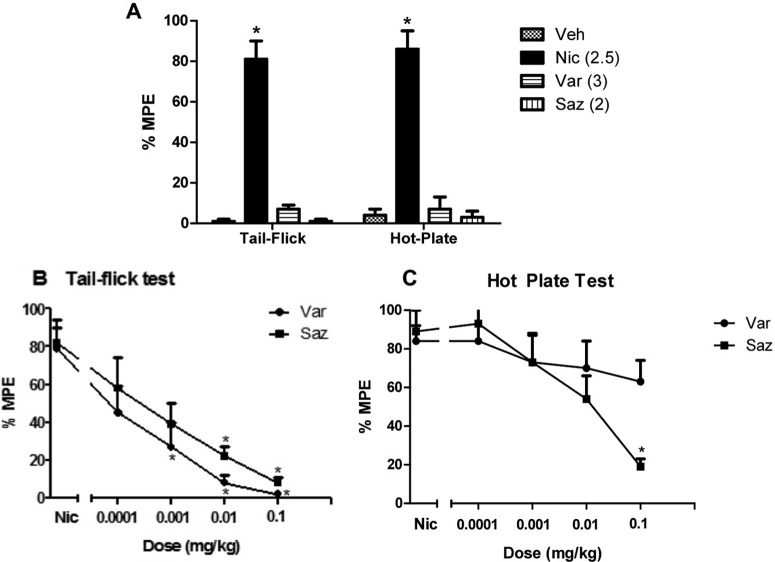
Various doses of varenicline and sazetidine-A were tested in the tail-flick and hot-plate tests after subcutaneous injection the drugs. As expected, nicotine induced significant antinociceptive effects in both tests at a dose of 2.5 mg/kg when tested 5 min after injection (Fig. 1A). In contrast, neither varenicline nor sazetidine-A showed significant antinociceptive effects at any of the doses tested. Figure 1A shows the lack of antinociceptive activity 15 min after administration of the highest doses of varenicline (3 mg/kg) and sazetidine-A (2 mg/kg). A similar lack of effect was observed at lower doses of the drug and different pretreatment times (data not shown).
Fig. 1.
Effects of varenicline (Var) and sazetidine-A (Saz) in the tail-flick and hot-plate tests. A, effects of varenicline (3 mg/kg s.c.), sazetidine-A (2 mg/kg s.c.), and nicotine (Nic) (2.5 mg/kg, s.c.) in the tail-flick and hot-plate tests in mice are shown. Mice were tested 5 and 15 min after nicotine and varenicline/sazetidine-A, respectively. Each group represents the mean ± S.E. of 8 to 12 mice. *, p < 0.05 versus vehicle (Veh). B and C, the ability of varenicline and sazetidine-A to antagonize a 2.5 mg/kg dose of nicotine in the tail-flick (B) and hot-plate (C) tests was also determined. The two drugs were given subcutaneously 15 min before nicotine, and mice were tested 5 min later. Each group represents the mean ± S.E. of 8 to 12 mice. *, p < 0.05 versus nicotine.
Varenicline and sazetidine-A were then evaluated for their ability to antagonize a 2.5 mg/kg dose of nicotine in the tail-flick and hot-plate procedures. As shown in Fig. 1B, both varenicline and sazetidine-A dose-dependently blocked nicotine-induced antinociception with an AD50 of 0.0002 (0.0001–0.0005) and 0.00085 (0.00065–0.0011) mg/kg, respectively when given subcutaneously 15 min before nicotine in the tail-flick test (F4,25 = 9.707, p < 0.05; F4,25 = 7.609, p < 0.05, respectively). In contrast, only sazetidine-A blocked nicotine-induced antinociception in the hot-plate test with an AD50 of 0.0055 (0.003–0.009) mg/kg (F4,25 = 7.831; p < 0.05) (Fig. 1C). Varenicline at the highest dose of 0.1 mg/kg failed to significantly reduce nicotine-induced antinociception in the hot-plate test. There were no observed significant effects of sazetidine-A and varenicline in the hot-plate and tail-flick tests at any of the doses used on their own (data not shown).
Effects of Varenicline and Sazetidine-A in the Formalin Test.
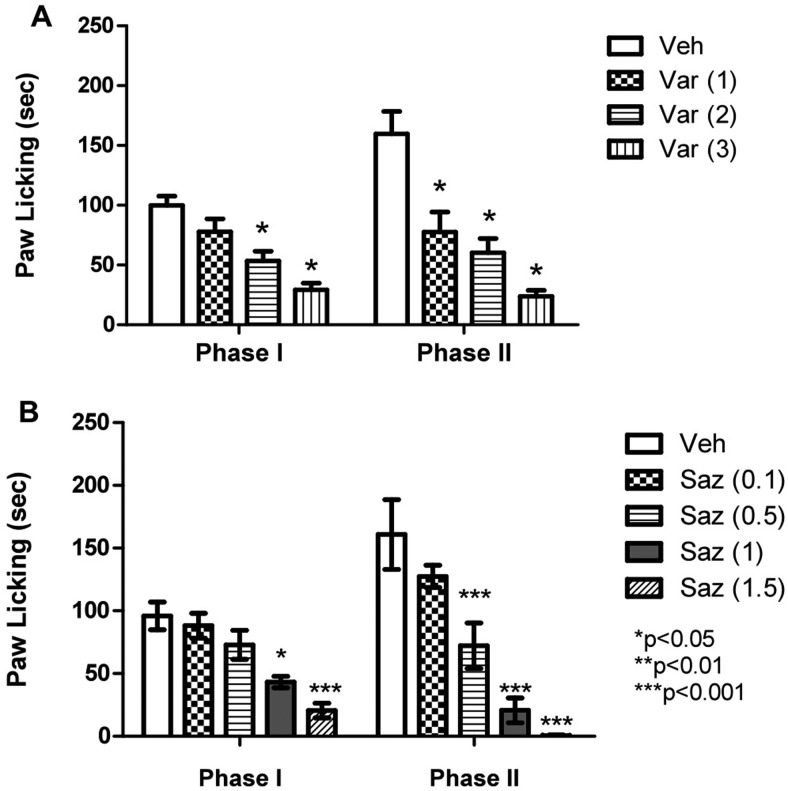
The effect of systemic (subcutaneous) varenicline and sazetidine-A treatment on both phase 1 (0–5 min) and phase 2 (20–45 min) of the formalin test was investigated 15 min after injection of the drugs. In both phases (Fig. 2A), varenicline dose-dependently attenuated nocifensive responding as indicated by an overall significant effect of treatment (early phase, F3,20 = 13.53, p < 0.05; late phase, F3,20 = 16.51, p < 0.05). However, varenicline was less potent than sazetidine-A in both phases as determined by ED50 values of 0.70 (0.49–1.0) and 1.9 (1.5–2.4) mg/kg for phase 1 and 0.3 (0.24–0.38) and 1.1 (0.78–1.5) mg/kg for phase 2, respectively. Both drugs were more potent in reducing the nociceptive behavior in phase II compared with phase I (Fig. 2).
Fig. 2.
Effects of varenicline and sazetidine-A in the mouse formalin test. The effects of various doses of varenicline (1, 2, and 3 mg/kg) (A) and sazetidine-A (0.1, 0.5, 1, and 1.5 mg/kg) (B) after subcutaneous administration on formalin-induced pain behavior in the mouse are shown. Mice were treated subcutaneously with varenicline and sazetidine-A 15 min before formalin (2.5%; 20 μl) injection into the plantar region of the right hind paw. The cumulative pain response of time of licking was measured for 0 to 5 min (first phase) and 20 to 40 min (second phase). Data are expressed as mean ± S.E.M. of licking time. Each group represents the mean ± S.E. of 8 to 12 mice.
Role of Various nAChR Subtypes in Varenicline-Induced Antinociception in the Formalin Test.
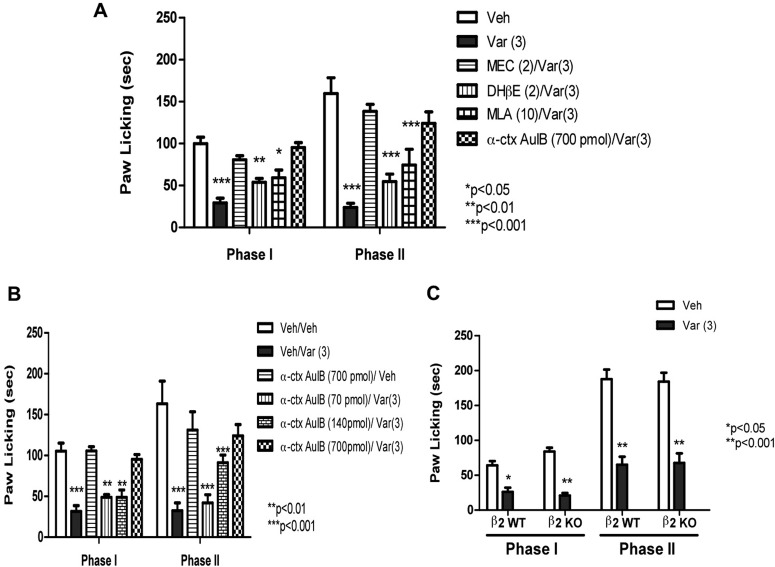
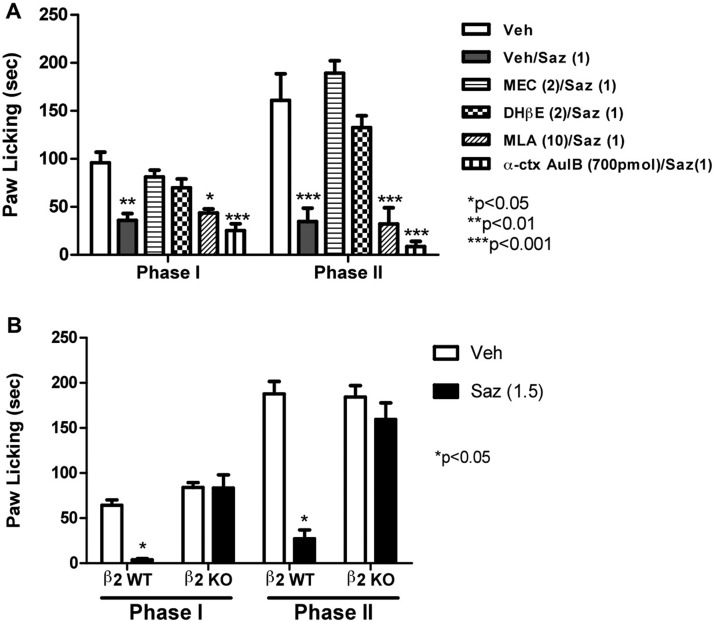
Using antagonists for various nAChR subtypes, we examined the role of β2*, α3β4*, and α7 nAChR subtypes in mediating the antinociceptive effect of varenicline (3 mg/kg s.c.). As predicted, mecamylamine (2 mg/kg s.c.), a noncompetitive and nonselective nicotinic antagonist, blocked varenicline's effects in both phase I and II (Fig. 3A). In contrast, DHβE (2 mg/kg s.c.), a β2-containing selective antagonist, failed to block varenicline's actions in the formalin test. A similar lack of antagonism was also seen with MLA (10 mg/kg s.c.), a relatively selective α7 antagonist (Fig. 3A). We were surprised to find that intrathecal pretreatment with conotoxin AulB (700 pmol/mouse), an α3β4 nicotinic antagonist, completely blocked varenicline's effects in both phase I and II (Fig. 3, A and B). The dose of α-conotoxin AuIB used in this assessment did not significantly alter nociceptive behavior in the formalin test on its own (Fig. 3B). Likewise, mecamylamine, MLA, and DHβE failed to have significant effects in the formalin test on their own (data not shown). Furthermore, the blockade of varenicline-induced antinociception by intrathecal α-conotoxin AuIB was dose-dependent, with an AD50 of 167 (108–260) pmol/mouse (Fig. 3B) in phase II. The lower dose of α-conotoxin AulB (70 pmol/mouse i.t.) did not significantly block the antinociceptive effect of varenicline (3 mg/kg s.c.) in both phases; however, increasing the dose to 140 pmol/mouse blocked only the second phase, whereas the higher dose of 700 pmol/mouse totally reversed varenicline-induced antinociception in both phases of the formalin test (Fig. 3B).
Fig. 3.
Nicotinic receptors subtypes involved in varenicline-induced antinociception in the formalin test. A, blockade of the antinociceptive effect of varenicline in the formalin test by different nicotinic antagonists is shown. Mice were pretreated with various nicotinic antagonists [MEC, 2 mg/kg s.c.; DHβE, 2 mg/kg s.c.; MLA, 10 mg/kg s.c.; α-conotoxin AuIB (α-ctx AuIB), 700 pmol/mouse i.t.] 15 min (5 min for α-conotoxin AuIB) before an active dose of 3 mg/kg varenicline. Fifteen minutes later, mice were injected with formalin (2.5% intraplantary; 20 μl) and then observed for pain behaviors. B, effects of different doses of the α3β4* antagonist α-conotoxin AuIB on varenicline-induced antinociception in the formalin test are shown. Mice were injected intrathecally with different doses of α-conotoxin AuIB (70, 140, and 700 pmol/mouse), and 5 min later they received a dose of 3 mg/kg varenicline. Fifteen minutes later, mice were injected with formalin (2.5% intraplantary; 20 μl) and then observed for pain behaviors. C, antinociceptive effects of vareniciline in the β2 WT and KO mice are shown. Mice received a dose of 3 mg/kg varenicline and 15 min later were tested in the formalin test. Data are expressed as mean ± S.E.M. of licking time. Each group represents the mean ± S.E. of 8 to 12 mice. *, p < 0.05 versus vehicle.
That the β2 subunit is not required for varenicline's action was confirmed by using the β2 KO mice. As shown in Fig. 3C, varenicline-induced antinociception in both phase I and II was preserved. No difference in the baselines of nociceptive behaviors was observed between the β2 KO and WT mice in the vehicle-treated group (Fig. 3C).
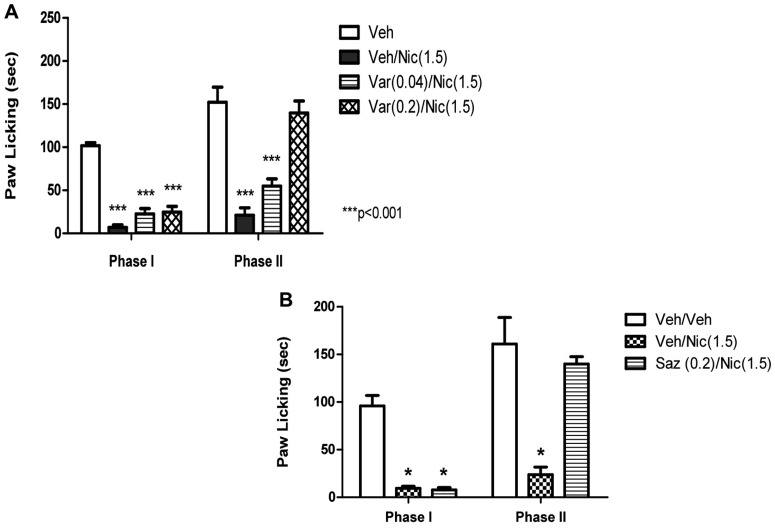
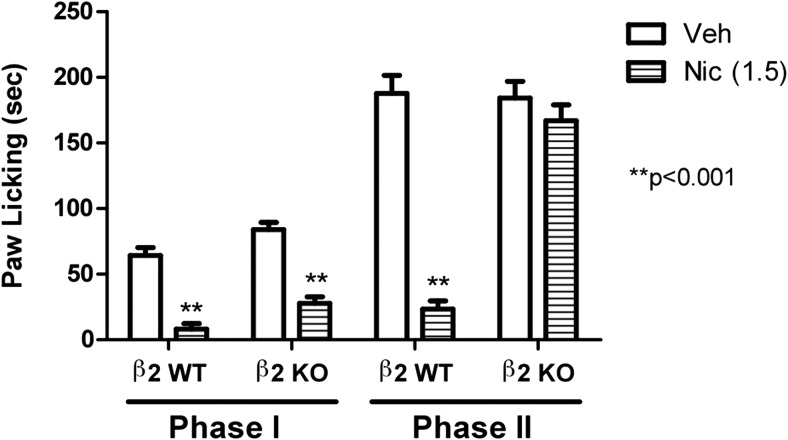
It is noteworthy that low inactive doses of veranicline (0.04 and 0.2 mg/kg) blocked nicotine's effects in the formalin test (Fig. 4A) only in the second phase (F2,22 = 32.405; p < 0.0001). This effect of low doses of varenicline in phase II seems to be mediated by β2-containing nAChR subtypes, because nicotine's effects in this phase were totally eliminated in β2 KO mice (Fig. 5).
Fig. 4.
Blockade of nicotine-induced antinociception by varenicline and sazetidine-A in the formalin test. Effects of varenicline (A) and sazetidine-A (B) on the antinociceptive effect of nicotine in the formalin test are shown. Mice were pretreated with varenicline (0.04 and 0.2 mg/kg s.c.) or sazetidine-A (0.2 mg/kg s.c.) 15 min before an active dose of nicotine (1.5 mg/kg s.c.). Five minutes later, mice were injected with formalin (2.5% intraplantary; 20 μl) and then observed for pain behaviors. Data are expressed as mean ± S.E.M. of licking time. Each group represents the mean ± S.E. of 8 to 12 mice. *, p < 0.05 versus vehicle.
Fig. 5.
Effects of nicotine in β2 KO mice using the formalin test. Antinociceptive effects of nicotine in the β2 WT and KO mice are shown. Mice received a dose of 1.5 mg/kg s.c. nicotine and 5 min later were tested in the formalin test. Data are expressed as mean ± S.E.M. of licking time. Each group represents the mean ± S.E. of 8 to 12 mice.
Role of Various nAChR Subtypes in Sazetidine-A-Induced Antinociception in the Formalin Test.
Similar to varenicline, we examined the role of β2*, α3β4*, and α7 nAChR subtypes in mediating the antinociceptive effect of sazetidine-A (1 mg/kg s.c.). As predicted, mecamylamine (2 mg/kg s.c.) completely blocked sazetidine-A's effects in both phase I and II (Fig. 6A). Likewise, DHβE (2 mg/kg s.c.) blocked sazetidine-A's actions in the formalin test. In contrast, MLA (10 mg/kg s.c.) and α-conotoxin AuIB (700 pmol/mouse i.t.) failed to significantly block sazetidine-A's effects in both phases (Fig. 6A).
Fig. 6.
Nicotinic receptor subtypes involved in sazetidine-A-induced antinociception in the formalin test. A, blockade of the antinociceptive effect of sazetidine-A in the formalin test by different effects of nicotinic antagonists is shown. Mice were pretreated with various nicotinic antagonists (MEC, 2 mg/kg s.c.; DHβE, 2 mg/kg s.c.; MLA, 10 mg/kg, s.c.; α-conotoxin AuIB, 700 pmol/mouse i.t.) 15 min (5 min for α-conotoxin AuIB) before a dose of 1.5 mg/kg sazetidine-A. Fifteen minutes later, mice were injected with formalin (2.5% intraplantary; 20 μl) and then observed for pain behaviors. B, antinociceptive effects of sazetidine-A in the β2 WT and KO mice are shown. Mice received an active dose of sazetidine-A (1.5 mg/kg s.c.), and 15 min later they were tested in the formalin test. Data are expressed as mean ± S.E.M. of licking time. Each group represents the mean ± S.E. of 8 to 12 mice. *, p < 0.05 versus vehicle.
The blockade of sazetidine-A's effects in the formalin test being mediated through a β2* nAChR was confirmed by using the β2 KO mice. As shown in Fig. 6B, sazetidine-A-induced antinociception in both phase I and II was lost in the β2 KO mice compared with their WT littermates.
As observed with varenicline, a low inactive dose of sazetidine-A (0.2 mg/kg) blocked nicotine's effects in the formalin test (Fig. 4B) only in the second phase.
Effects of Varenicline and Sazetidine-A on Mouse Locomotor Coordination.
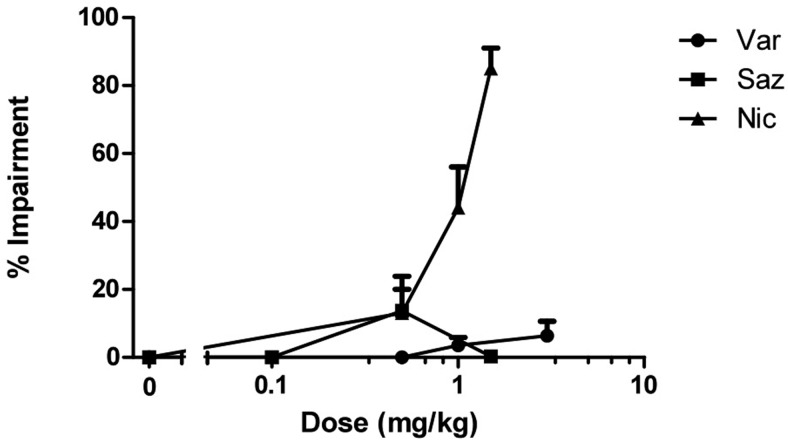
Various doses of varenicline and sazetidine-A were tested in the rotarod test after subcutaneous injection. As expected, nicotine induced significant impairment of the animals' motor coordination in a dose-related manner when tested 5 min after injection (Fig. 7). In contrast, varenicline and sazetidine-A failed to significantly alter the animals' motor coordination 15 min after subcutaneous injection of various doses of the drugs. Doses of varenicline (0.5, 1, and 3 mg/kg) and sazetidine-A (0.1, 0.5, and 1.5 mg/kg) that were found active in the formalin test did not significantly induce locomotor incoordination compared with the vehicle group (Fig. 5).
Fig. 7.
Effects of nicotinic partial agonists on motor coordination. Dose-response curves of nicotine, varenicline, and sazetidine-A in the rotarod test after subcutaneous administration in mice are shown. Mice were tested 5 and 15 min after nicotine and varenicline/sazetidine-A injections, respectively, for 5 min on the rotarod. Each point represents the mean ± S.E. of 8 to 12 mice.
Discussion
In the present study, we examined the antinociceptive effects of two nicotinic partial agonists with differing activity profiles, varenicline and sazetidine-A, in acute and tonic mouse pain models. Unlike nicotine, both of the nicotinic partial agonists tested lacked significant antinociceptive effect in the acute thermal models (the tail-flick and hot-plate tests) at the doses and times tested. In contrast, both drugs were active in the formalin test, a persistent and tonic pain model. These results suggest that efficacy of nicotinic agonists at nicotinic receptors, in particular β2* nAChRs, plays an important role for their effects in acute thermal pain models and both varenicline and sazetidine-A do not activate β2*-nAChRs sufficiently to produce measurable effects in the tail-flick and hot-plate tests. It is noteworthy, however, that both drugs blocked nicotine-induced antinociception in the tail-flick test at very low doses. Indeed, the results shown in Fig. 1B demonstrate that varenicline inhibits the effects of a 2.5 mg/kg dose of nicotine with an ED50 value of 0.0002 mg/kg (0.94 nmol/kg). Sazetidine-A was 2.5 times less potent than varenicline in blocking nicotine's effects in the tail-flick test with an ED50 value of 0.00085 mg/kg (2.56 nmol/kg). These two partial agonists were at least 50- to 100-fold more potent than mecamylamine in blocking nicotine's effects in the tail-flick test (Damaj et al., 1995). However, only sazetidine-A blocked nicotine's effects in the hot-plate test with a 11-fold lower potency than in the tail-flick test (Fig. 1C). These results support the idea that varenicline and sazetidine-A block nicotine's effects by either competing with nicotine and/or desensitizing the α4β2* nAChRs for several reasons. Nicotine-induced antinociception in the tail-flick and hot-plate tests were largely mediated by α4β2* nAChRs in spinal and supraspinal sites (see Introduction). In addition, the potency of varenicline and sazetidine-A in blocking nicotine's effects was in line with their binding affinity to α4β2* nAChRs (Ki values for sazetidine-A and varenicline were 0.4 and 0.2 nM, respectively) (Xiao et al., 2006; Rollema et al., 2007). Finally, in vitro experiments have demonstrated that exposure of α4β2* nAChRs to low concentrations of varenicline effectively blocks subsequent activation by acetylcholine in the low nanomolar range, similar to binding Ki values (Mihalak et al., 2006; Rollema et al., 2010; Papke et al., 2011). Our results with nicotine-induced antinociception were similar to those recently reported with varenicline blocking the effects of nicotine-induced hypothermia and locomotor depression through a β2* nAChR mechanism (Ortiz et al., 2012).
The fact that sazetidine-A, but not varenicline, blocked nicotine's effects in the hot-plate test was surprising. It is possible that differences in α4β2* nAChR subtype distributions or forms mediating nicotine's effects in the two pain tests could explain sazetidine-A's effects. It has been recognized that α4β2 nAChR subtypes are present in two main forms: the high-affinity α4(2)β2(3) or the low-affinity α4(3)β2(2). It is conceivable that nicotine responses in the tail-flick engage both low- and high-affinity α4β2* nAChR subtypes, whereas the effects in the hot-plate test were mediated mainly by the high-affinity α4β2* nAChR form. Sazetidine-A might then mediate its functional antagonistic effects in the hot-plate test by acting on α4(2)β2(3) high-affinity subtypes, because it was reported to be a full agonist on these high-affinity subtypes, whereas it had a very low efficacy on the low-affinity α4(3)β2(2) nAChRs (Zwart et al., 2008).
Acute administration of relatively high doses of varenicline and sazetidine-A elicited antinociception actions in both phase I and II of the formalin test without impairing the motor coordination of the animals (Fig. 7). The potency of both drugs in the mouse formalin test was higher than the one reported in the rat formalin model (Cucchiaro et al., 2008; Gao et al., 2010), suggesting possible species differences in drug pharmacokinetics and/or dynamics. Although both partial agonists were active in the formalin test, their antinociceptive effects were mediated by different nAChR subtypes as shown by the use of various nicotinic antagonists and nicotinic KO mice. Sazetidine-A elicits its nociceptive effects in the formalin test by activation of β2* nAChRs in (Fig. 6) as shown in the β2 KO mice and after pretreatment with DHβE, a selective β2* nAChR antagonist. Neither α7 nor α3β4* nAChR subtypes were shown to mediate the effects of sazetidine-A in the formalin test. In contrast, β2* nAChR subtypes do not mediate the effects of varenicline in the same test. Varenicline's antinociceptive effects were not significantly reduced in the β2 KO mice or after pretreatment with DHβE (Fig. 3). A similar lack of involvement of α7 nAChR subtypes was also observed, because MLA, a relatively selective α7 antagonist, failed to reduce varenicline's effects in the formalin test. However, α3β4* nAChR subtypes in the spinal cord seem to play a predominant role in varenicline effects. Indeed, pretreatment with intrathecal α-conotoxin AuIB, a selective α3β4 antagonist (Luo et al., 1998), dose-dependently blocked varenicline-induced antinociception (Fig. 3). This is in line with the recent study of Ortiz et al. (2012), which reported that the agonist effects of varenicline in inducing hypothermia and hypomotility in the mouse are mediated by α3β4* nAChR subtypes. These results are consistent with in vitro assays showing that varenicline is a full agonist at α3β4* nAChR subtypes (Mihalak et al., 2006; Rollema et al., 2010). Although varenicline is also a full agonist at the α7 nAChRs (Mihalak et al., 2006) in in vitro functional assays, this receptor subtype does not play an important role in varenicline's in vivo effects because no loss of effectiveness of the drug was seen after pretreatment with the α7 antagonist MLA (Fig. 3). It is noteworthy that lower doses of varenicline (0.04 and 0.2 mg/kg) blocked the effects of nicotine only in phase II of the formalin test (Fig. 4), probably indicating an antagonist effect at β2* nAChRs. This is consistent with the fact that nicotine's effects in phase II, but not phase I, of the formalin were eliminated in the β2 KO mice (Fig. 4). Overall, the actions of varenicline at low doses, as an antagonist of β2* nAChRs, and at higher doses, as an agonist of α3β4* nAChRs as seen in the formalin test, seem consistent with varenicline's effects on body temperature, locomotion (Ortiz et al., 2012), and responding for food in mice (Cunningham and McMahon, 2011). In contrast, the agonist effects of sazetidine-A in the formalin test seems to be mediated largely by β2* nAChR subtypes. As observed with varenicline, low doses of sazetidine-A blocked nicotine's effects in the phase II of the formalin test. Taken together, the data of acute and persistent pain models suggest that the actions of sazetidine-A at low doses, as an antagonist, and at higher doses, as an agonist, are mediated by β2* nAChR subtypes.
In more complex tests for cognition and attention in rats, low doses of sazetidine-A (as low as 0.01 mg/kg) reversed dizocilpine-induced impairments in performance (Rezvani et al., 2011), suggesting that it is the functional “antagonist” effect of sazetidine-A that may mediate changes in these behaviors. However, in mouse tests for anxiety and depression relatively high doses of sazetidine-A similar in range to our formalin test results (0.5–1.5 mg/kg) (Turner et al., 2010) were shown to be active in these models, supporting a role for “agonist” effects of the drug in these behaviors.
In summary, these studies demonstrated the efficacy of the nicotinic partial agonists sazetidine-A and varenicline in tonic, but not acute, pain models. The efficacy and potency of sazetidine-A in the formalin test suggest that its agonist effects at α4β2* nAChR subtypes may provide a potential treatment for chronic pain disorders.
Acknowledgments
We thank Tie Han for technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA-019377 (to M.I.D.), DA12001 (to F.I.C.)]; the National Institutes of Health National Institute of Mental Health [Grant MH53631] (to J.M.M.); and the National Institutes of Health National Institute of General Medical Sciences [Grant GM48677] (to J.M.M.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- nAChR
- nicotinic acetylcholine receptor
- DHβE
- dihydro-β-erythroidine
- WT
- wild type
- KO
- knockout
- MEC
- mecamylamine
- MLA
- methyllycaconitine
- CNS
- central nervous system
- Var
- varenicline
- Saz
- sazetidine-A
- Veh
- vehicle
- Nic
- nicotine
- MPE
- maximum possible effect
- AD50
- antagonist dose at 50%
- α-ctx AuIB
- α-conotoxin AuIB.
Authorship Contributions
Participated in research design: AlSharari and Damaj.
Conducted experiments: AlSharari.
Contributed new reagents or analytic tools: Carroll and McIntosh.
Performed data analysis: AlSharari and Damaj.
Wrote or contributed to the writing of the manuscript: AlSharari, Carroll, McIntosh, and Damaj.
References
- Abbott FV, Franklin KB, Westbrook RF. (1995) The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain 60:91–102 [DOI] [PubMed] [Google Scholar]
- Aceto MD, Bagley RS, Dewey WL, Fu TC, Martin BR. (1986) The spinal cord as a major site for the antinociceptive action of nicotine in the rat. Neuropharmacology 25:1031–1036 [DOI] [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL, Curzon P, Arneric SP, Bannon AW, Decker MW. (1998) Role of the nucleus raphe magnus in antinociception produced by ABT-594: immediate early gene responses possibly linked to neuronal nicotinic acetylcholine receptors on serotonergic neurons. J Neurosci 18:5426–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Yokota Y, Ma W, Lee JR, Brieaddy LE, Burgess JP, Navarro HA, Damaj MI, Martin BR. (2008) Synthesis, nicotinic acetylcholine receptor binding, and pharmacological properties of 3′-(substituted phenyl)deschloroepibatidine analogs. Bioorg Med Chem 16:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MK, Smith DF. (1990) Antinociceptive effects of the stereoisomers of nicotine given intrathecally in spinal rats. J Neural Transm Gen Sect 80:189–194 [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Le Novère N, Changeux JP. (2000) Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol 40:431–458 [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Chaijale N, Commons KG. (2005) The dorsal raphe nucleus as a site of action of the antinociceptive and behavioral effects of the α4 nicotinic receptor agonist epibatidine. J Pharmacol Exp Ther 313:389–394 [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Xiao Y, Gonzalez-Sulser A, Kellar KJ. (2008) Analgesic effects of Sazetidine-A, a new nicotinic cholinergic drug. Anesthesiology 109:512–519 [DOI] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. (2011) The effects of nicotine, varenicline, and cytisine on schedule-controlled responding in mice: differences in α4β2 nicotinic receptor activation. Eur J Pharmacol 654:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. (1998) Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther 284:1058–1065 [PubMed] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, Collins AC, Martin BR. (2007) Genetic approaches identify differential roles for α4β2* nicotinic receptors in acute models of antinociception in mice. J Pharmacol Exp Ther 321:1161–1169 [DOI] [PubMed] [Google Scholar]
- Damaj MI, Welch SP, Martin BR. (1995) In vivo pharmacological effects of dihydro-β-erythroidine, a nicotinic antagonist, in mice. Psychopharmacology (Berl) 117:67–73 [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79 [Google Scholar]
- Decker MW, Meyer MD, Sullivan JP. (2001) The therapeutic potential of nicotinic acetylcholine receptor agonists for pain control. Expert Opin Investig Drugs 10:1819–1830 [DOI] [PubMed] [Google Scholar]
- Dewey WL, Harris LS, Howes JF, Nuite JA. (1970) The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J Pharmacol Exp Ther 175:435–442 [PubMed] [Google Scholar]
- Flores CM, Wilson SG, Mogil JS. (1999) Pharmacogenetic variability in neuronal nicotinic receptor-mediated antinociception. Pharmacogenetics 9:619–625 [PubMed] [Google Scholar]
- Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk M, Deng H, Guo W, Lehto SG, Matson D, et al. (2010) Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain 149:33–49 [DOI] [PubMed] [Google Scholar]
- Gillberg PG, d'Argy R, Aquilonius SM. (1988) Autoradiographic distribution of [3H]acetylcholine binding sites in the cervical spinal cord of man and some other species. Neurosci Lett 90:197–202 [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. (1980) Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67:313–316 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Iwamoto ET. (1989) Antinociception after nicotine administration into the mesopontine tegmentum of rats: evidence for muscarinic actions. J Pharmacol Exp Ther 251:412–421 [PubMed] [Google Scholar]
- Iwamoto ET. (1991) Characterization of the antinociception induced by nicotine in the pedunculopontine tegmental nucleus and the nucleus raphe magnus. J Pharmacol Exp Ther 257:120–133 [PubMed] [Google Scholar]
- Iwamoto ET, Marion L. (1993) Adrenergic, serotonergic and cholinergic components of nicotinic antinociception in rats. J Pharmacol Exp Ther 265:777–789 [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj MI. (2010) Role of α5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther 334:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IM, Buerkle H, Taylor P, Yaksh TL. (1998) Nociceptive and antinociceptive responses to intrathecally administered nicotinic agonists. Neuropharmacology 37:1515–1525 [DOI] [PubMed] [Google Scholar]
- Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM. (1998) α-Conotoxin AuIB selectively blocks α3β4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci 18:8571–8579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. (2007) The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem 104:446–456 [DOI] [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. (2006) Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose ganglia. Mol Pharmacol 70:1693–1699 [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, Changeux JP. (1999) Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398:805–810 [DOI] [PubMed] [Google Scholar]
- Mattila MJ, Ahtee L, Saarnivaara L. (1968) The analgesic and sedative effects of nicotine in white mice, rabbits and golden hamsters. Ann Med Exp Biol Fenn 46:78–84 [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. (2006) Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805 [DOI] [PubMed] [Google Scholar]
- Ortiz NC, O'Neill HC, Marks MJ, Grady SR. (2012) Varenicline blocks β2*-nAChR-mediated response and activates β4*-nAChR-mediated responses in mice in vivo. Nicotine Tob Res 14:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Trocmé-Thibierge C, Guendisch D, Al Rubaiy SA, Bloom SA. (2011) Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther 337:367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Goettler J, Caggiula AR, Reynolds WA, Stiller RL, Scierka A, Jacob RG. (1994) Chronic and acute tolerance to subjective, behavioral and cardiovascular effects of nicotine in humans. J Pharmacol Exp Ther 270:628–638 [PubMed] [Google Scholar]
- Rau H, Schweizer R, Zhuang P, Pauli P, Brody S, Larbig W, Heinle H, Müller M, Elbert T, Dworkin B. (1993) Cigarette smoking, blood lipids, and baroreceptor-modulated nociception. Psychopharmacology (Berl) 110:337–341 [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Sexton H, Xiao Y, Brown ML, Paige MA, McDowell BE, Kellar KJ, Levin ED. (2011) Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor ligand: effects on dizocilpine and scopolamine-induced attentional impairments in female Sprague-Dawley rats. Psychopharmacology (Berl) 215:621–630 [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, et al. (2007) Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994 [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajós M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, et al. (2009) Preclinical pharmacology of the α4β2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol 78:813–824 [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O'Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, et al. (2010) Pre-clinical properties of the α4β2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol 160:334–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. (1987) Manual of Pharmacological Calculations with Computer Programs, Springer-Verlag, New York [Google Scholar]
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. (1992) The formalin test: an evaluation of the method. Pain 51:5–17 [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. (2010) Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther 334:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Kellar KJ. (2005) Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci 25:9258–9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Sufka KJ, Coderre TJ. (1997) Optimal scoring strategies and weights for the formalin test in rats. Pain 70:53–58 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. (2006) Sazetidine-A, a novel ligand that desensitizes α4β2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 70:1454–1460 [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, et al. (2008) Sazetidine-A is a potent and selective agonist at native and recombinant α4β2 nicotinic acetylcholine receptors. Mol Pharmacol 73:1838–1843 [DOI] [PubMed] [Google Scholar]