Abstract
5-Diethylaminoethylamino-8-hydroxyimidazoacridinone, C-1311 (NSC-645809), is an antitumor agent shown to be effective against breast cancer in phase II clinical trials. A similar compound, 5-dimethylaminopropylamino-8-hydroxytriazoloacridinone, C-1305, shows high activity against experimental tumors and is expected to have even more beneficial pharmacological properties than C-1311. Previously published studies showed that these compounds are not substrates for cytochrome P450s; however, they do contain functional groups that are common targets for glucuronidation. Therefore, the aim of this work was to identify the human UDP-glucuronosyltransferases (UGTs) able to glucuronidate these two compounds. High-performance liquid chromatography analysis was used to examine the activities of human recombinant UGT1A and UGT2B isoforms and microsomes from human liver [human liver microsomes (HLM)], whole human intestinal mucosa [human intestinal microsomes (HIM)], and seven isolated segments of human gastrointestinal tract. Recombinant extrahepatic UGT1A10 glucuronidated 8-hydroxyl groups with the highest catalytic efficiency compared with other recombinant UGTs, Vmax/Km = 27.2 and 8.8 μl · min−1 · mg protein−1, for C-1305 and C-1311, respectively. In human hepatic and intestinal microsomes (HLM and HIM, respectively), high variability in UGT activities was observed among donors and for different regions of intestinal tract. However, both compounds underwent UGT-mediated metabolism to 8-O-glucuronides by microsomes from both sources with comparable efficiency; Vmax/Km values were from 4.0 to 5.5 μl · min−1 · mg protein−1. In summary, these studies suggest that imid azoacridinone and triazoloacridinone drugs are glucuronidated in human liver and intestine in vivo and may form the basis for future translational studies of the potential role of UGTs in resistance to these drugs.
Introduction
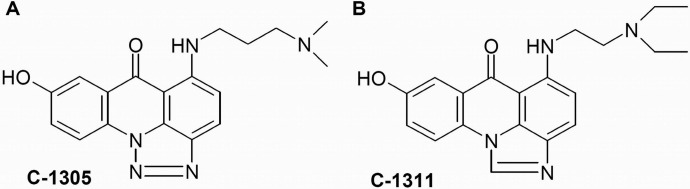
Among several groups of diaminoalkyloacridines with heterocyclic aromatic rings condensed with an acridine core, triazoloacridinones and imidazoacridinones exhibited the most potent antineoplastic properties toward a wide spectrum of transplantable tumors (Konopa, 2001) (Fig. 1). Triazoloacridinones exhibited significant and clearly differentiated cytotoxic activity in vitro toward 64 human tumor cell lines in the National Cancer Institute screening system (Bethesda, MD) and also displayed high antitumor activity against several experimental tumors in mice, particularly leukemias and colon carcinomas (Cholody et al., 1990a; Kuśnierczyk et al., 1994). One highly active and promising derivative of this group, C-1305 (5-dimethylaminopropylamino-8-hydroxytriazoloacridinone), has been selected for extended preclinical trials. The best known imidazoacridinone analog, C-1311 (5-diethylaminoethylamino-8-hydroxyimidazoacridinone, NSC-645) (Cholody et al., 1990b), has shown potent activity against experimental models of murine and human colorectal cancer in vitro and in animals (Burger et al., 1996). It was evaluated in phase I clinical trials in patients with advanced solid tumors (Thomas et al., 2008; Isambert et al., 2010) and was effective in a phase II clinical trial in women with metastatic breast cancer (Capizzi et al., 2008). Other studies have shown that C-1311 in combination with paclitaxel was effective against human bladder cancer in the in vivo hollow fiber assay (Smith et al., 2011).
Fig. 1.
Structures of the triazoloacridinone, C-1305, and the imidazoacridinone, C-1311.
The biological and biochemical mechanisms of C-1305 and C-1311 action are under investigation (Mazerska et al., 2001, 2003; Augustin et al., 2006; Skwarska et al., 2007). It has been shown that both compounds intercalate to DNA; however, physicochemical DNA binding is not crucial for the observed antitumor activity of these compounds (Dziegielewski et al., 2002; Koba and Konopa, 2007). It is postulated that the anticancer properties of these compounds are related to their inhibition of topoisomerase II activity (Skladanowski et al., 1996; Lemke et al., 2004) and interstrand covalent DNA cross-linking in tumor cells by metabolically activated compounds (Dziegielewski and Konopa, 1996; Koba and Konopa, 2007).
Taking into account the suggested role of metabolism in the ability of these compounds to covalently bind DNA (Mazerska et al., 2001; Koba and Konopa, 2007), this work attempts to elucidate the metabolic pathways of these agents. It has been shown previously that although cytochrome P450s are not involved in C-1305 or C-1311 activation, both compounds are selective irreversible inhibitors of CYP1A2 and CYP3A4 but not CYP2 isoforms (Fedejko-Kap et al., 2011; Potega et al., 2011). Each compound was also found to be metabolized by rat liver microsomes (RLM) and human liver microsomes (HLM) to Nω-oxide derivatives in the aminoalkyl side chain. Identical metabolites were found to be products in reactions with the human recombinant flavin-containing monooxygenases FMO1 and FMO3. Therefore, Nω-oxide derivatives of C-1305 and C-1311 are believed to be the FMO-mediated products of RLM and HLM (Fedejko-Kap et al., 2011; Potega et al., 2011).
Conjugative metabolism has also been investigated, and UDP-glucuronosyltransferases (UGTs) have been identified as being involved in the biotransformation of C-1311. These studies revealed that C-1311 undergoes O-glucuronidation by RLM and recombinant UGT1A1 but not UGT2B7 (Potega et al., 2011). Glucuronidation of C-1311 has been shown to also occur in mice, with high levels of C-1311 glucuronide found in both plasma and liver (Calabrese et al., 1999). Therefore, the present work evaluates the role of human UGTs in the metabolism of C-1311 and C-1305 using a set of recombinant UGTs, HLM, and HIM. UGTs exhibit tissue-specific expression patterns. As such, the UGT enzymes selected for screening will shed light on the glucuronidation in extrahepatic tissues.
This work is expected to result in the generation of information concerning how minor structural differences between triazoloacridinone and imidazoacridinone moieties of these compounds will affect their glucuronidation. In addition, the results obtained will also provide information on the pharmacokinetics of C-1305 and C-1311 in human liver and intestine. The identification of specific human UGT isoforms will allow us to focus on their role in drug resistance and/or regulation of toxicity in cancer cells.
Materials and Methods
Chemicals.
C-1305 and C-1311 and their 8-methoxy derivatives were synthesized in the Department of Pharmaceutical Technology and Biochemistry, Gdansk University of Technology, as described previously (Cholody et al., 1990a,b, 1992). All chemicals used were of at least reagent grade. Ethyl alcohol (95%) was purchased from Aaper Alcohol and Chemical (Shelbyville, KY). Ammonium formate and formic acid were from Thermo Fisher Scientific (Waltham, MA). UDP-GlcUA Na3 salt, β-glucuronidase (GUS), and all other chemicals and reagents, unless otherwise specified, were purchased from Sigma-Aldrich (St. Louis, MO).
Enzymes.
Recombinant human UGT1A1, UGT1A3, UGT1A4, and UGT1A6–1A10 were produced in baculovirus-infected insect cells as described previously (Kurkela et al., 2007). The expression level of individual recombinant UGTs was estimated by Western blot analysis using monoclonal antibodies (Tetra-His antibodies; QIAGEN GmbH, Hilden, Germany) against the His tag that all of them carry (Kurkela et al., 2007). For activity comparison between individual UGTs, the enzyme levels were normalized as described previously (Kurkela et al., 2007). Human UGT2B4, UGT2B7, UGT2B15, and UGT2B17 (5 mg of protein per ml), expressed in baculovirus-insect cells (Supersomes), were purchased from BD Biosciences (Woburn, MA). Each enzyme tested in this study is known to be active toward substrates specific for that isoform. HLM and human intestinal microsomes (HIM) were obtained as described previously (Antonio et al., 2003; Sabolovic et al., 2006). Human intestinal and liver tissues were obtained from organ donors by transplant surgeons at University Hospital (Little Rock, AR) according to a protocol approved by the Human Research Advisory Committee of the University of Arkansas for Medical Science and were prepared as described previously (Antonio et al., 2003; Sabolovic et al., 2006).
Screening of Human Liver and Intestinal Microsomes and Recombinant UGT Isoforms.
HLM and HIM (20 μg of protein), each from a single donor, or recombinant UGT isoform protein (5 μg of protein) was assayed for activity toward C-1305 and C-1311 as follows. The proteins were incubated in a buffer containing 100 μM Tris-HCl (pH 7.4), 5 mM MgCl2, 5 mM saccharolactone, and 2% dimethyl sulfoxide supplemented with either 100 or 200 μM substrate in a total volume of 30 μl (2% dimethyl sulfoxide was added to permeabilize the membrane; Zielinska et al., 2008). Substrates were added in water. Reactions were started by the addition of UDP-GlcUA (3 mM) and were incubated for 60 min at 37°C. The reactions were stopped by the addition of 30 μl of ethanol, followed by centrifugation at 12,000g for 8 min to pellet the denatured protein. The supernatant fractions were used for high-performance liquid chromatography (HPLC) analysis. Control reactions omitting substrate were run with each assay. All incubations were performed in triplicate. Hydrolysis with GUS was used to identify the glucuronide peaks. For this purpose, after incubation of the compound with enzymatic proteins and UDP-GlcUA, 1000 U of GUS (type VII-A from Escherichia coli) was added, and samples were incubated for an additional 60 min. The reaction was stopped, mixture was centrifuged to pellet protein, and supernatant was analyzed by HPLC.
HPLC-UV-Visible Analysis.
HPLC analyses of the supernatants were performed using an HP1050 HPLC system and the Agilent ChemStation software package (Agilent Technologies, Santa Clara, CA). Samples were separated using a reversed-phase 5 μm Suplex pKb-100 analytical column (0.46 × 25 cm, C18) (Supelco, Bellefonte, PA) warmed to 25°C. The analyses were performed at a flow rate of 1 ml/min with the following mobile-phase system: a linear gradient from 15 to 80% methanol in ammonium formate buffer (0.05 M, pH 3.4) for 25 min, followed by a linear gradient from 80 to 100% methanol in ammonium formate for 3 min. The column was then re-equilibrated at initial conditions for 10 min between runs. The elution of each metabolite was monitored at 420 nm. Primary standards for the glucuronidated metabolites of C-1305 and C-1311 are not available; however, it was demonstrated that the GlcUA moiety does not alter the absorption maximum for C-1305 at 420 nm, as has been shown previously for C-1311 (Potega et al., 2011). Therefore, product concentrations were calculated using the response for substrate. The calculated limits of detection were equal to 0.504 and 0.357 μM for C-1305 and C-1311, respectively. The reproducibilities (percentage of relative S.D.s) were 1.2 for C-1305 and 1.0% for C-1311.
Liquid Chromatography-Tandem Mass Spectrometry Analysis.
HPLC-tandem mass spectrometry analyses of the products were performed by electrospray ionization (ESI) with positive ion detection and an Agilent 1100 LS-MSD mass spectrometer (Agilent Technologies). Samples were separated according to the procedure described under HPLC-UV-Visible Analysis.
Steady-State Enzyme Kinetics Assays.
Kinetic parameters were determined by incubation of recombinant UGT membrane protein (5 μg) or microsomes (20 μg) in the presence of varying concentrations of the substrate (5–1000 μM) at a fixed concentration of UDP-GlcUA (3 mM) for 60 min. All other conditions were identical to those of the screening experiments. Kinetic data for the glucuronidation of C-1305 and C-1311 by UGTs were estimated by plotting the measured initial reaction velocity values as a function of substrate concentration and fitting these to the Michaelis-Menten (eq. 1), the Hill (eq. 2), or the uncompetitive substrate inhibition model (eq. 3), using Prism 4 software (GraphPad Software Inc., San Diego, CA):
where Km is a Michaelis-Menten constant, Vmax is the maximal velocity, S50 is the substrate concentration at 50% Vmax (analogous to Km in Michaelis-Menten kinetics), n is the Hill coefficient, which can be considered to be a measure of autoactivation and reflects the extent of cooperativity among multiple binding sites, and Ksi is the inhibition constant.
Results
Glucuronidation of C-1305 and C-1311 by Human Recombinant UGTs.
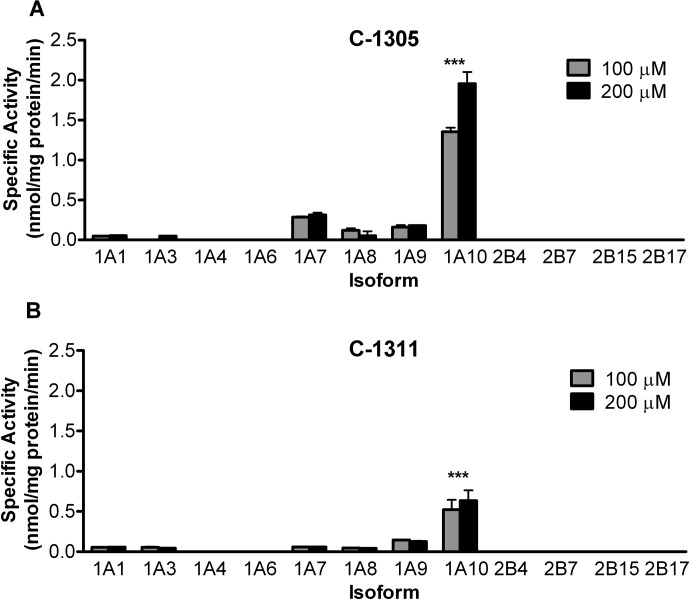
Recombinant human UGT1A isoforms expressed in Sf9 cells as His-tag proteins and four human recombinant UGT2B isoforms (Supersomes) were screened for their ability to glucuronidate C-1305 and C-1311. For these preliminary screenings, two concentrations (100 and 200 μM) of each drug were used (Fig. 2), and the formation of a single metabolite of each was shown (Fig. 3). The purpose of these experiments was to identify which human isoforms are involved in the conjugation of these two drugs, as well as to determine what range of substrate concentrations should be used for kinetic analysis. Screening data indicated that both compounds were glucuronidated by UGT1A1 and UGT1A3 at very low levels (<0.1 nmol/mg protein/min) and by UGT1A7–1A9 isoforms to a greater extent (0.2–0.3 nmol/mg protein/min), and the glucuronidation rate with UGT1A10 was the highest for both compounds (>0.5 nmol/mg protein/min). UGT1A7 was moderately active toward C-1305 but showed only a minor activity toward C-1311. UGT1A10 glucuronidated C-1305 at a rate nearly 3 times higher than with C-1311. No UGT2B isoforms had measurable activity with either of the substrates tested.
Fig. 2.
Glucuronidation of C-1305 (A) and C-1311 (B) by human recombinant UGTs. Glucuronidation activities of human recombinant UGTs (5 μg protein) UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A7–1A10, UGT2B4, UGT2B7, UGT2B15, and UGT2B17 were measured. The substrate and cosubstrate (UDP-GlcUA) concentrations were 0.1, 0.2, and 3 mM for C1305, C1211, and UDP-GlcUA, respectively, and the reactions were incubated for 60 min. Activities are expressed in nmol/mg protein/min and are shown with S.D. based on three experiments. ***, p < 0.001, indicates the significant difference of UGT1A10 activities in comparison to all other UGTs (one-way analysis of variance).
Fig. 3.
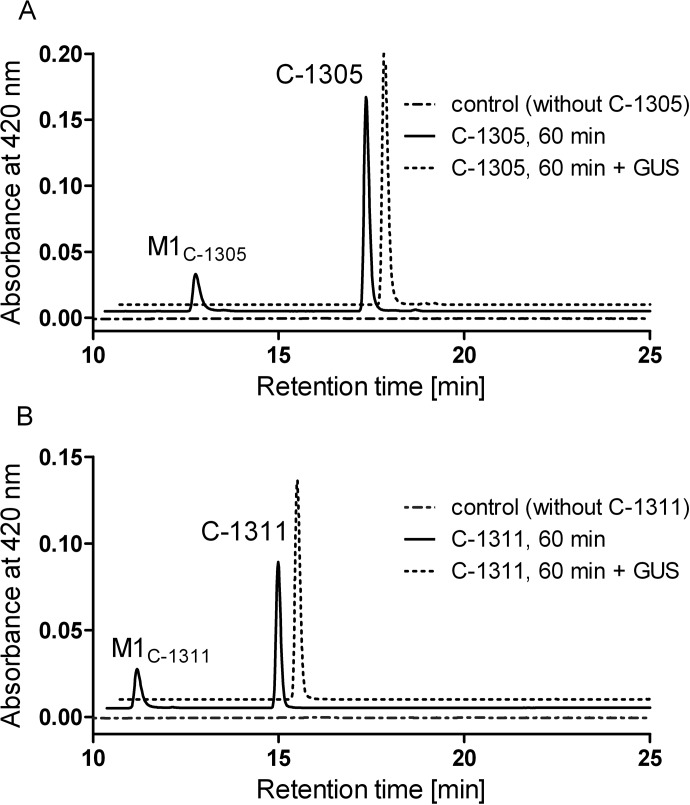
Metabolism of C-1305 (A) and C-1311 (B) by human recombinant UGT1A10. Representative HPLC chromatograms are shown from 60-min incubations of 5 μg of UGT1A10 (solid line), 5 μg of UGT1A10, and 1000 U of GUS, GUS (dotted line) with 0.2 mM substrate and 3 mM UDP-GlcUA, and control with enzymes and cofactors but without C-1305 or C-1311, respectively (dashed-dotted line).
Glucuronidation of C-1305 and C-1311 by HLM and HIM.
Because both drugs were found to be glucuronidated by isoforms known to be expressed in both hepatic (UGT1A1 and UGT1A9) and extrahepatic tissues (UGT1A7 and UGT1A10) (Harbourt et al., 2012), screening experiments for glucuronidation activity were also done in human hepatic and intestinal microsomes. The activities of pooled HLM from seven donors and intestinal microsomes isolated from 10 donors were assessed for their activity toward C-1305 and C-1311 (Fig. 4). ESI-mass spectrometry (MS) analysis confirmed that only one metabolite was formed from each compound by HIM and HLM with the retention time identical to that of the product formed with recombinant UGT1A10 (Fig. 3).
Fig. 4.
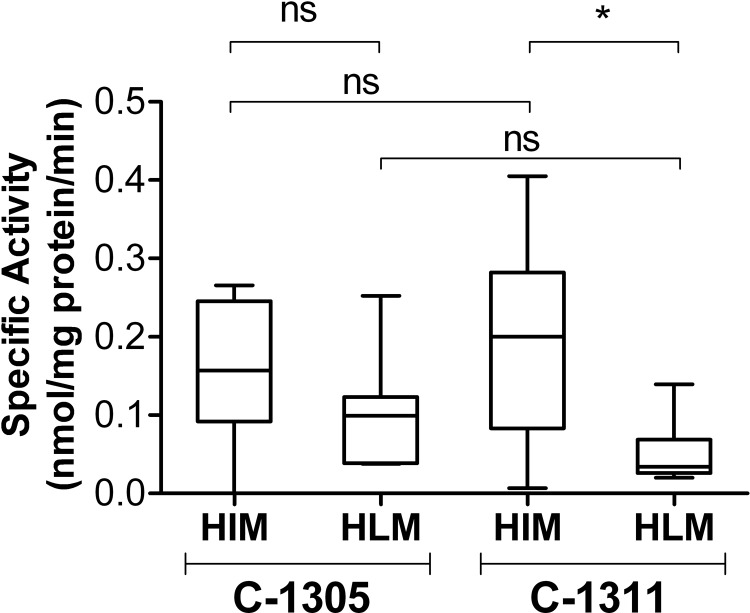
Glucuronidation of C-1305 and C-1311 by human intestinal and liver microsomes. Glucuronidation activities of HIM and HLM were measured using microsomes isolated from different donors (20 μg of total protein). The substrate concentration was 0.2 mM, the UDP-GlcUA concentration was 3 mM, and the reactions were incubated for 60 min. For each box and whisker plot, the line bisecting the box is the median value, the upper and lower limits of the box are the 25th and 75th percentile values, respectively, and the error bars (whiskers) extend to the lowest and highest values. Statistical comparisons, performed using the Kruskal-Wallis nonparametric analysis of variance followed by Dunn's multiple comparison test, are indicated by the lines above the plots. *, p < 0.01; ns, not significant.
The box and whisker plots presented in Fig. 4 showed high interindividual differences among the donors in the activity of both HIM and HLM toward C-1305 and C-1311. The glucuronidation potency expressed as median HIM- and HLM-specific activities toward C-1305 were not significantly different, whereas median HIM activity toward of C-1311 was significantly higher than that of HLM. However, statistical comparison of the data indicates no significant differences in either median HIM or HLM activity toward C-1305 and C-1311.
Kinetic Analysis of Selected Recombinant UGTs, HLM, and HIM with C-1305 and C-1311.
On the basis of activity screening data, recombinant UGT1A1, UGT1A7, UGT1A9, and UGT1A10 and the microsomal preparations with the highest activity, HIM34 and HLM114, were subjected to kinetic analysis. The apparent kinetic constants are presented in Table 1. As indicated by screening assays, steady-state kinetics confirmed that UGT1A10 was the most active recombinant UGT isoform with Vmax values of 2.56 and 0.63 nmol · min−1 · mg protein−1 for C-1305 and C-1311, respectively. Although UGT1A10 activity (Vmax) toward C-1305 was 4 times greater than that toward C-1311, it showed a similar affinity for C-1305 and C-1311 with apparent Km values of 94 and 71 μM, respectively. Accordingly, UGT1A10 catalytic efficiencies, measured as the Vmax/Km ratios, were higher than those observed for other recombinant UGTs. The ratios were 27 for C-1305 and 9 μl · min−1 · mg−1 for C-1311.
TABLE 1.
Steady-state parameters for glucuronidation of C-1305 and C-1311 by UGT isoforms, HIM, and HLM
Parameters were determined from the fit of initial velocities to a Michaelis-Menten kinetic scheme or substrate inhibition equations using GraphPad Prism 4. Data are presented as mean ± S.E.M. of three determinations.
| Enzyme | Kinetic Model | Substrate |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| C-1305 |
C-1311 |
||||||||
| Km | Vmax | Ksi | V/K | Km | Vmax | Ksi | V/K | ||
| μM | nmol · min−1 · mg protein−1 | μM | μl · min−1 · mg−1 | μM | nmol · min−1 · mg protein−1 | μM | μl · min−1 · mg−1 | ||
| UGT1A1 | M-M | 149 ± 29 | 0.262 ± 0.017 | 1.8 | 28.9 ± 9.0 | 0.115 ± 0.008 | 4 | ||
| UGT1A7 | M-M | 29.3 ± 3.2 | 0.306 ± 0.008 | 11 | N.D. | N.D. | |||
| UGT1A9 | SI | 54.6 ± 22.4 | 0.201 ± 0.038 | 1651 ± 1106 | 3.7 | 63.3 ± 22.6 | 0.206 ± 0.037 | 958 ± 452 | 3.3 |
| UGT1A10 | M-M | 94.1 ± 10.3 | 2.557 ± 0.082 | 27 | 70.6 ± 6.01 | 0.624 ± 0.015 | 8.8 | ||
| HIM 34 | M-M/Hill | 76.9 ± 11.1 (N.S.) | 0.413 ± 0.015 (N.S.) | 5.4 | 158.6 ± 5.2*** | 0.823 ± 0.013a,*** | 5.2 | ||
| HLM 114 | M-M | 71.3 ± 6.0 (N.S.) | 0.386 ± 0.009 (N.S.) | 5.4 | 39.3 ± 6.1*** | 0.156 ± 0.006*** | 4 | ||
M-M, Michaelis-Menten; SI, substrate inhibition; Hill, Hill equation; N.S., not significant (p > 0.05); N.D., not determined.
p < 0.001. Values between HIM 34 and HLM 114 were compared with Student's unpaired t test.
Hill equation; n = 1.65.
The lowest Km values found were 29 μM for glucuronidation of C-1305 by UGT1A7 and of C-1311 by UGT1A1, indicating that these enzymes have the highest affinity for the glucuronidation of acridinone derivatives. Both compounds were metabolized by UGT1A9 to a moderate degree (Vmax ∼ 0.2 nmol · min−1 · mg protein−1) and with similar affinities (Km, 60 μM for both compounds). In addition, with UGT1A9, initial velocities of both compounds increased as a function of substrate concentration to 0.15 nmol/mg protein/min at 300 μM C-1305 and to 0.14 nmol/mg protein/min at 235 μM C-1311 and then decreased (data not shown), indicating that there was substrate inhibition with high Ksi value of 1700 μM (Table 1).
Two representative microsomal fractions from liver (HLM114) and intestine (HIM34) were selected for evaluation of kinetic parameters, for comparison with the data obtained with the recombinant enzymes. In the case of C-1305, parameters Km and Vmax were close for intestine and liver microsomes. However, those parameters were strongly different for C-1311. For instance, glucuronidation of this compound with HIM34 gave Vmax significantly higher than with HLM14. On the other hand, Vmax/Km values obtained for HIM and HLM for both compounds were very similar.
Glucuronidation of C-1305 and C-1311 throughout the Human Gastrointestinal Tract.
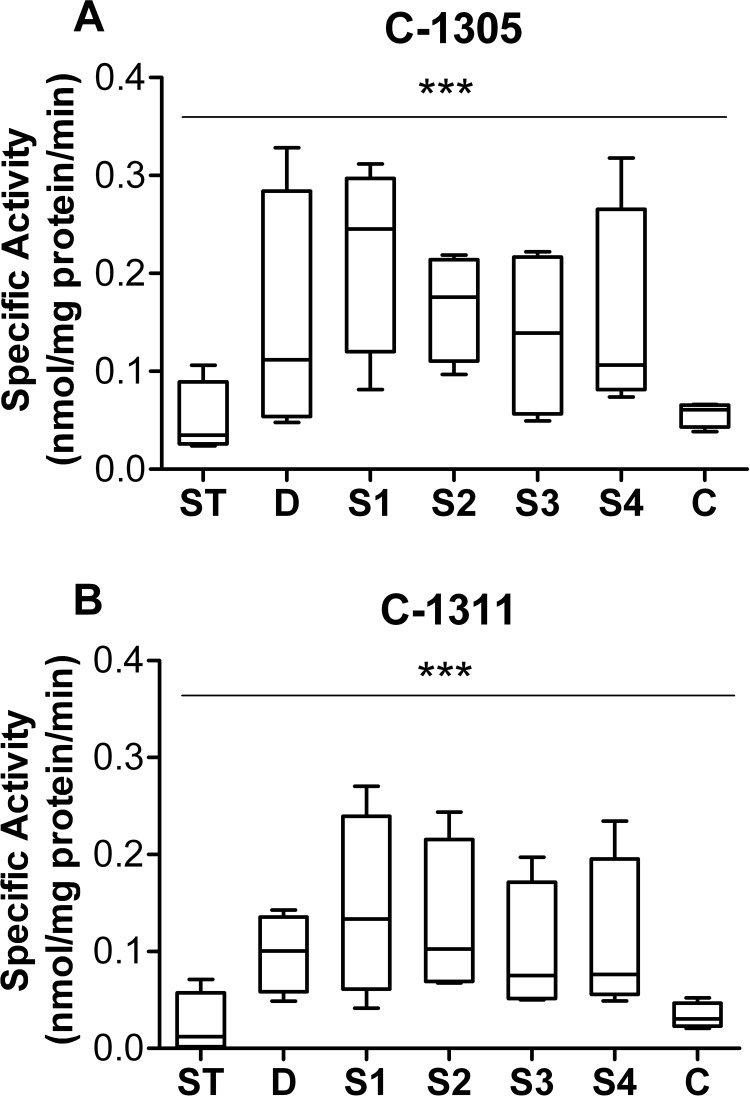
To extend our knowledge about the role of the gastrointestinal (GI) tract in glucuronidation of C-1305 and C-1311, substrates were incubated with HIM prepared from stomach (ST), duodenum (D), jejunum (S1), intermediate small intestine (S2 and S3), ileum (S4), and colon (C) from four donors. The results in Fig. 5 show that the two compounds were glucuronidated to different degrees by different intestinal segments and that C-1305 seemed to be a better substrate than C-1311 for UGTs distributed throughout the GI tract. Strong interindividual variations were observed in microsomes isolated from tissues throughout the length of the digestive tract. Activity was generally low in stomach and colon, compared with the duodenum and small intestine segments.
Fig. 5.
Glucuronidation of C-1305 (A) and C-1311 (B) by human stomach and intestine microsomes. Microsomes (20 μg) were prepared from the mucosa of stomach (ST), duodenum (D), four segments of small intestine (S1–S4, proximal to distal), and colon (C) from four different donors. The substrate and UDP-GlcUA concentrations were 0.1 and 3 mM, respectively, and the reactions were incubated for 60 min. For each box and whisker plot, the line bisecting the box is the median value, the upper and lower limits of the box are the 25th and 75th percentile values, respectively, and the error bars (whiskers) extend to the lowest and highest values. ***, p < 0.001, indicates a significant effect of donor and fragment with significant interaction (two-way analysis of variance with Bonferroni posttest).
Identification of Glucuronide Products.
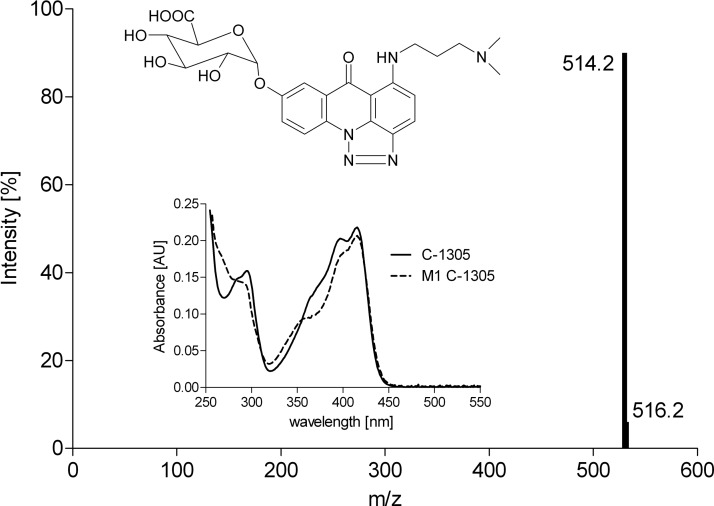
Structural assignments for microsomal metabolites were made by comparison of ESI-MS and UV/visible (UV-Vis) spectra of metabolites with those obtained with recombinant UGT1A10. The spectral data and the proposed structure of the C-1305 metabolite are presented in Fig. 6. The metabolite of C-1305 with m/z 514.2 [mass ion, 338.1 (C-1305) + 175 (free glucuronic acid residue) + 1] had a UV/Vis spectrum slightly changed from that of the substrate. Analogous observations were noticed previously with the C-1311 metabolite with m/z 526.2 [mass ion, 350.1 (C-1311) + 175 (free glucuronic acid residue) + 1] (Potega et al., 2011). Metabolites of both C-1305 and C-1311 were sensitive to GUS treatment (Fig. 3). To determine the functional group in C-1305 and C-1311 that is prone to glucuronidation, the glucuronidation activity of the recombinant UGTs and selected HIM and HLM with 8-methoxy derivatives of C-1305 and C-1311 was assayed. No glucuronidation activity was observed when 8-methoxy substrates (Cholody et al., 1990a, 1992) were incubated instead of 8-hydroxy. These results strongly support the proposed metabolite structures as C-1305/C-1311 8-O-glucuronides (Fig. 6).
Fig. 6.
Structure analysis of metabolite M1 of C-1305 from incubations of microsomal proteins and UGT1A10. ESI-MS and UV/Vis spectra of metabolite and parent compound were extracted from the appropriate HPLC samples.
Glucuronidation of C-1305 and C-1311 by UGT1A10 Mutants and Comparison to Wild-Type UGT1A10.
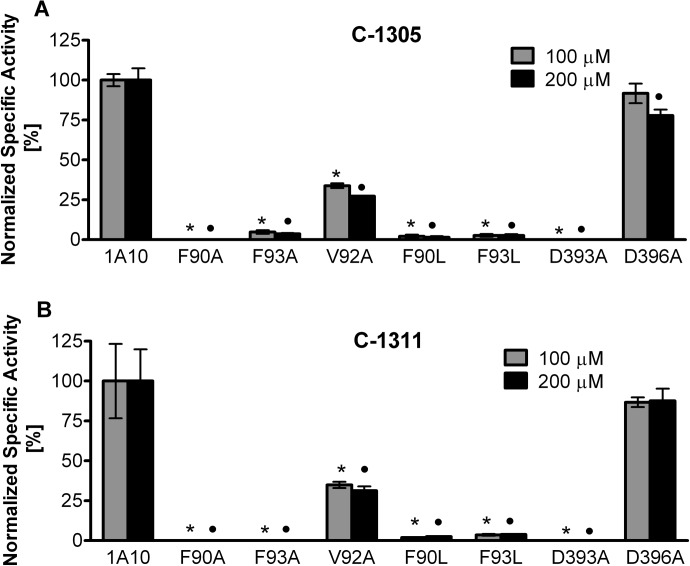
To investigate the structure-function relationships of UGT1A10 in relation to the glucuronidation of C-1305 and C-1311, five recombinant proteins with point mutations of specific amino acids previously identified as being crucial for the binding of phenol substrates or the nucleotide sugar of UDP-GlcUA were evaluated for their activity toward these substrates (Fig. 7). Changing amino acids Phe90 and Phe93 of the side-chain aromatic ring to either A or L totally abolished the activity of the enzyme toward both substrates. The only substrate binding site mutant of UGT1A10 that retained activity toward the substrates was V92A; however, its specific activity was less than 35% of wild-type (WT) UGT1A10 with either substrate. The mutation at Asp393 in the N-terminal cosubstrate binding domain of UGT1A10 caused total inactivation of this isoform. In contrast, in the D396A mutant, activity toward C-1305 and C-1311 was preserved at a level close to that of WT UGT1A10.
Fig. 7.
Glucuronidation activities of human recombinant WT UGT1A10 and its mutants toward C-1305 (A) and C-1311 (B). UGT1A10 and its mutants were assayed by incubating membrane fractions of recombinant UGTs with substrate (0.1 and 0.2 mM for C-1305 and C-1311, respectively) and UDP-GlcUA (3 mM) for 60 min at 37°C. Activities are expressed in nmol/mg total protein/min and are shown as mean ± S.D. for three determinations. Levels were considered significant at p < 0.001. *, significance of results with respect to WT UGT1A10 at the concentration of 0.1 mM; ●, significance of results with respect to WT UGT1A10 at the concentration of 0.2 mM. Analysis of variance (two-way analysis of variance with Bonferroni post hoc analysis) was performed using GraphPad Prism (GraphPad Software Inc.).
Kinetic Analysis of WT and Mutant UGT1A10 with C-1305 and C-1311.
The kinetic parameters of the two active UGT1A10 mutants, V92A and D396A, were determined and compared with the apparent kinetic constants of the WT isoform (Fig. 8). The activity of the V92A mutant toward C-1305 and C-1311 was decreased by 72 and 60%, respectively, relative to WT UGT1A10. However, increased affinity of this mutant for C-1305 (Km 94.1 and 64.7 μM for WT and V92A, respectively) and C-1311 (Km 70.6 and 40.7 μM for WT and V92A, respectively) was observed. The change of Asp396 to A produced an enzyme with a 23% decrease in the catalytic activity toward C-1305, but a 30% increase in the catalytic activity toward C-1311. For C-1305, the affinity of D396A was 2-fold higher than that of WT, whereas for C-1311, it remained almost unchanged compared with WT.
Fig. 8.
Kinetic constants for C-1305 (A) and C-1311 (B) glucuronidation by recombinant WT UGT1A10 and mutants. Glucuronidation activities of recombinant WT UGT1A10 (●) and the D396A (▴) and V92A (■) mutants were measured by incubating membrane fractions containing recombinant UGT1A10 and its mutants (5 μg) with increasing concentrations (shown in the figure) of the substrates at a constant concentration of UDP-GlcUA (3 mM) for 60 min at 37°C. Curve fits and kinetic constants were determined using GraphPad Prism 4 software (GraphPad Software Inc.). The graphical fits of the data from each of the analyses with each substrate (mean ± S.D. of three determinations) are shown. Km values are given in μM, and Vmax values are given in nmol/mg protein/min.
Discussion
Previous studies on the in vitro metabolism of the antitumor acridinone derivatives, C-1305 and C-1311, demonstrated that human recombinant cytochrome P450 isoforms do not catalyze the metabolism of these compounds. In contrast, both compounds were shown to inhibit the activity of human recombinant and microsomal CYP1A2 and CYP3A4 (Fedejko-Kap et al., 2011; Potega et al., 2011), which suggested the formation of some reactive intermediates, followed by covalent binding to the active center of the enzyme. Furthermore, the 8-hydroxyl group of C-1311 participated in peroxidase-mediated metabolism, which produced reactive intermediates susceptible to substitution in the presence of cellular nucleophiles (Mazerska et al., 2003).
Structural analysis of the two compounds indicated that there were two potential targets for glucuronidation, the 8-hydroxyl group and the two amino groups (Fig. 1). Therefore, the current studies were undertaken to determine whether human UGTs were active toward C-1305 and C-1311 and to rigorously characterize any glucuronides formed. HPLC-MS spectra of UGT-mediated metabolic products from glucuronidation assays revealed that each acridinone was metabolized by recombinant and microsomal UGTs to a single glucuronide derivative (Fig. 3). The biosynthetic products from these experiments were treated with GUS to verify that the products were indeed glucuronides. Protection of the 8-hydroxyl group by the addition of a methyl group eliminated glucuronidation of these compounds, confirming that these hydroxyl groups were the sole targets of the reaction and not N-glucuronidation of the two amino groups.
Several recombinant human UGTs were shown to have activity toward C-1305 and C-1311 (Fig. 2). These studies demonstrated the involvement of UGT1A isoform; however, no activity was seen with any of the UGT2B family isoforms tested. Recombinant UGT1A1, UGT1A7, UGT1A9, and UGT1A10 were found to have activity toward these compounds. UGT1A10 expressed the highest activity, especially toward C-1305 (Vmax values 10 times higher than those for UGT1A1, UGT1A7, and UGT1A9) (Table 1). This indicated that small differences in the structure between the two compounds (Fig. 1) resulted in different interactions with UGT1A10. The compounds differ in the electron density of the triazole and imidazole rings, which has been shown to influence specific interactions of C-1305 with DNA (Lemke et al., 2004). Therefore, one can suspect that changes in the electron density of the heterocyclic ring supported by the longer distance between the amino groups in the side chain as well as by the presence of more easily dealkylated methyl group are responsible for C-1305 being a better substrate for UGTs.
For this work, we took advantage of the availability of a unique collection of hepatic and intestinal microsomes. The intestinal samples are especially interesting because of the availability of not only microsomes derived from the full length of the small intestine, but also samples isolated from the individual segments of the GI tract. Glucuronidation activity in these human microsomes was assayed to confirm that the activities identified using recombinant UGTs were present in native human organs.
The graphs in Figs. 4 and 5 representing the glucuronidation activity of microsomes derived from different human donors clearly show strong interindividual variation, particularly when microsomes from the individual segments of GI tract are assayed. This pattern is similar to that reported previously for other endogenous and exogenous compounds (Antonio et al., 2003; Sabolovic et al., 2006). UGT-mediated activity toward C-1305 and C-1311 was seen in human liver and intestinal microsomes (Table 1). The results presented in Fig. 4 demonstrated that glucuronidation of C-1311 was generally higher by HIM than by HLM (p < 0.01), what is not observed in the case of C-1305.
Drawing clear conclusions from the kinetic analysis of the microsomal studies is nearly impossible because an unknown number of individual UGT isoforms are present in these preparations. However, the results do indicate that metabolism of C-1305 and C-1311 in both the liver and the intestine must be considered when evaluating the first-pass metabolism of these drugs. The involvement of UGT1A9, which is the most highly expressed in human kidney (Harbourt et al., 2012), and UGT1A7, which is expressed in the aerodigestive tract (Zheng et al., 2001, 2002) in the metabolism of these compounds necessitates future investigation of the glucuronidation of these compounds in these tissues as well.
Although our main original aim was to examine the possible contribution of glucuronidation to the metabolism of these two compounds, the results steered the focus of this research to a new aspect. C-1311 and C-1305 turned out to be useful probe substrates for UGT1A10. These compounds largely follow the two criteria for a good probe compound, namely that it is selective for one isoform and the individual enzyme exhibits a similar affinity for the substrate, as do human tissues or microsomes (Court, 2005). The availability of several recombinant enzymes containing point mutations in the substrate and cosubstrate binding sites of this isoform allowed for additional structure function relationship studies using these compounds. Therefore, we were able to test the hypothesis that the Phe90, Phe93, Val92, and Asp393, Asp396 amino acids of UGT1A10 are important for proper recognition and conjugation of C-1311 and C-1305.
These studies demonstrated that Phe90 is critical and Phe93 is significant for glucuronidation of both compounds (Fig. 7). The importance of Phe90 and Phe93 has also been shown to be crucial for the glucuronidation of p-nitrophenol and 4-methylumbelliferone (Xiong et al., 2006), dopamine (Itäaho et al., 2009), estrogen (Starlard-Davenport et al., 2007; Höglund et al., 2011), and warfarin (Miller et al., 2008). As with the hydroxylated estrogens, 6- and 7-hydroxylated warfarin, and dopamine glucuronidation, the mutation of Phe90 to A totally abolished UGT1A10 activity toward C-1305 and C-1311. Furthermore, in contrast to other reports, the F90L mutation was also destructive for UGT1A10 activity. The Vmax values found with the V92A mutation and C-1305 and C-1311 were reduced 3.5- to 2.5-fold, respectively, whereas this mutation had preserved or even increased activity of UGT1A10 toward estrogens and warfarin metabolites (Miller et al., 2008; Höglund et al., 2011).
Additional experiments were done to clarify the effect of the Asp393 and Asp396 mutations localized in the GlcUA binding site on the catalytic activity of UGT1A10. Of the two mutated residues, Asp393, is apparently essential for binding UDP-GlcUA because of the complete loss of activity in the D393A mutant. In contrast, Asp396 plays a minor role in substrate turnover. Therefore, our results have confirmed the crucial role of Asp393 for UGT catalysis and indicated a slight influence of the D396A mutation on the UDP-GlcUA association for C-1305 and C-1311 in the catalytic center of UGT1A10. The novelty of the role of Asp393 in UGT1A10 activity has broader implications because of the conservation of this residue in all human UGTs (Xiong et al., 2008).
It is increasingly recognized that UGTs are involved in cancer cell drug resistance (Cummings et al., 2003; Kostrubsky et al., 2005; Gagnon et al., 2006; Tallman et al., 2007; Lazarus and Sun, 2010; Starlard-Davenport et al., 2010; de Almagro et al., 2011). It is also recognized that glucuronidation does not always result in inactivation of biologically active compounds but can produce products that retain or have significantly increased biological activity (Ritter, 2000; Sallustio et al., 2006). The best examples of this are morphine (Osborne et al., 1992; Klepstad et al., 2000; Murthy et al., 2002) as well as retinoids, and estrogens, metabolites of which all appear to exhibit biological effects distinct from the parent compounds (Ritter, 2000). In parallel studies (M. Pawlowska, R. Chu, B. Fedejko-Kap, E. Augustin, Z. Mazerska, A. Radominska-Pandya, and T. Chambers, manuscript submitted for publication), we have been able to show that C-1305 represents another example of this phenomenon. We have been able to show that in cancer cell models, the C-1305 glucuronide has cytotoxic properties that are greater than those for the native compound. The present studies are essential also because they identify the UGT isoforms involved in this substrate activation.
In summary, the present studies have demonstrated that both the drugs investigated here are glucuronidated by several UGTs expressed in the liver, intestine, kidney, and the aerodigestive tract. The information on which UGT isoforms are involved in the glucuronidation of C-1305 and C-1311 is very important for the design of new drugs and the management of existing ones. In addition, the knowledge gained from these structure function experiments could be exploited for the design of analogs of clinical and pharmacological importance with the aim of increasing and/or decreasing the biological response of UGTs and/or eliminating any undesired side effects of glucuronidation. The question of whether the glucuronidation contributes to overall drug toxicity or drug resistance in certain tissue-specific cancers has yet to be answered.
Acknowledgments
We thank Agata Kot-Wasik for help with mass spectrometry analysis and Anna Gallus-Zawada for skillful technical assistance. We also thank Joanna M. Little for careful editing of this article.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM075893]; Ministry of Science and Higher Education (Poland) [Grant N401 159 32/3045]; a fellowship grant from the European Union within European Social Foundation, project “Development of Interdisciplinary Doctoral Studies” (to B.F.-K.); and the Sigrid Juselius Foundation.
Portions of this work were presented as a poster as follows: Fedejko-Kap B, Bratton SM, Finel M, Mazerska Z, and Radominska-Pandya A (2011) The role of intestine and liver in imidazo- and triazoloacridinone antitumor agents, C-1311 and C-1305 glucuronidation: high affinity substrates for UGT1A10. 17th North American Regional ISSX Meeting; 2011 Oct 16–20; Atlanta, GA. International Society for the Study of Xenobiotics, Washington, DC.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- C-1305
- 5-dimethylaminopropylamino-8-hydroxytriazoloacridinone
- C-1311
- 5-diethylaminoethylamino-8-hydroxyimidazoacridinone
- RLM
- rat liver microsomes
- HLM
- human liver microsomes
- HIM
- human intestinal microsomes
- FMO
- flavin-containing monooxygenase
- GI
- gastrointestinal
- UGT
- UDP-glucuronosyltransferase
- GUS
- β-glucuronidase
- HPLC
- high-performance liquid chromatography
- ESI
- electrospray ionization
- MS
- mass spectrometry
- UV/Vis
- UV-visible
- WT
- wild type.
Authorship Contributions
Participated in research design: Fedejko-Kap, Radominska-Pandya, and Mazerska.
Conducted experiments: Fedejko-Kap and Bratton.
Contributed new reagents or analytic tools: Finel and Radominska-Pandya.
Performed data analysis: Fedejko-Kap, Bratton, Radominska-Pandya, and Mazerska.
Wrote or contributed to the writing of the manuscript: Fedejko-Kap, Bratton, Radominska-Pandya, and Mazerska.
References
- Antonio L, Xu J, Little JM, Burchell B, Magdalou J, Radominska-Pandya A. (2003) Glucuronidation of catechols by human hepatic, gastric, and intestinal microsomal UDP-glucuronosyltransferases (UGT) and recombinant UGT1A6, UGT1A9, and UGT2B7. Arch Biochem Biophys 411:251–261 [DOI] [PubMed] [Google Scholar]
- Augustin E, Moś-Rompa A, Skwarska A, Witkowski JM, Konopa J. (2006) Induction of G2/M phase arrest and apoptosis of human leukemia cells by potent antitumor triazoloacridinone C-1305. Biochem Pharmacol 72:1668–1679 [DOI] [PubMed] [Google Scholar]
- Burger AM, Double JA, Konopa J, Bibby MC. (1996) Preclinical evaluation of novel imidazoacridinone derivatives with potent activity against experimental colorectal cancer. Br J Cancer 74:1369–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese CR, Loadman PM, Lim LS, Bibby MC, Double JA, Brown JE, Lamb JH. (1999) In vivo metabolism of the antitumor imidazoacridinone C1311 in the mouse and in vitro comparison with humans. Drug Metab Dispos 27:240–245 [PubMed] [Google Scholar]
- Capizzi RL, Roman LA, Tjulandin S, Smirnova I, Manikhas A, Paterson JS, Major A, Lundberg AS, Fumoleau P. (2008) Phase II trial of C1311, a novel inhibitor of topoisomerase II in advanced breast cancer. J Clin Oncol 26 (May 20 Suppl):abstr 1055 [Google Scholar]
- Cholody WM, Martelli S, Konopa J. (1990a) 8-Substituted 5-[(aminoalkyl)amino]-6H-v-triazolo[4,5,1-de]acridin-6-ones as potential antineoplastic agents. Synthesis and biological activity. J Med Chem 33:2852–2856 [DOI] [PubMed] [Google Scholar]
- Cholody WM, Martelli S, Paradziej-Lukowicz J, Konopa J. (1990b) 5-[(Aminoalkyl)amino]imidazo[4,5,1-de]acridin-6-ones as a novel class of antineoplastic agents. Synthesis and biological activity. J Med Chem 33:49–52 [DOI] [PubMed] [Google Scholar]
- Cholody WM, Martelli S, Konopa J. (1992) Chromophore-modified antineoplastic imidazoacridinones. Synthesis and activity against murine leukemias. J Med Chem 35:378–382 [DOI] [PubMed] [Google Scholar]
- Court MH. (2005) Isoform-selective probe substrates for in vitro studies of human UDP-glucuronosyltransferases. Meth Enzymol 400:104–116 [DOI] [PubMed] [Google Scholar]
- Cummings J, Ethell BT, Jardine L, Boyd G, Macpherson JS, Burchell B, Smyth JF, Jodrell DI. (2003) Glucuronidation as a mechanism of intrinsic drug resistance in human colon cancer: reversal of resistance by food additives. Cancer Res 63:8443–8450 [PubMed] [Google Scholar]
- de Almagro MC, Selga E, Thibaut R, Porte C, Noé V, Ciudad CJ. (2011) UDP-glucuronosyltransferase 1A6 overexpression in breast cancer cells resistant to methotrexate. Biochem Pharmacol 81:60–70 [DOI] [PubMed] [Google Scholar]
- Dziegielewski J, Konopa J. (1996) Interstrand crosslinking of DNA induced in tumor cells by a new group of antitumor imidazoacridinones. Proc Am Assoc Cancer Res 37:410 [Google Scholar]
- Dziegielewski J, Slusarski B, Konitz A, Skladanowski A, Konopa J. (2002) Intercalation of imidazoacridinones to DNA and its relevance to cytotoxic and antitumor activity. Biochem Pharmacol 63:1653–1662 [DOI] [PubMed] [Google Scholar]
- Fedejko-Kap B, Niemira M, Radominska-Pandya A, Mazerska Z. (2011) Flavin monooxygenases, FMO1 and FMO3, not cytochrome P450 isoenzymes, contribute to metabolism of anti-tumour triazoloacridinone, C-1305, in liver microsomes and HepG2 cells. Xenobiotica 41:1044–1055 [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Bernard O, Villeneuve L, Têtu B, Guillemette C. (2006) Irinotecan inactivation is modulated by epigenetic silencing of UGT1A1 in colon cancer. Clin Cancer Res 12:1850–1858 [DOI] [PubMed] [Google Scholar]
- Harbourt DE, Fallon JK, Ito S, Baba T, Ritter JK, Glish GL, Smith PC. (2012) Quantification of human uridine-diphosphate glucuronosyl transferase 1A isoforms in liver, intestine, and kidney using nanobore liquid chromatography-tandem mass spectrometry. Anal Chem 84:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund C, Sneitz N, Radominska-Pandya A, Laakonen L, Finel M. (2011) Phenylalanine 93 of the human UGT1A10 plays a major role in the interactions of the enzyme with estrogens. Steroids 76:1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isambert N, Campone M, Bourbouloux E, Drouin M, Major A, Yin W, Loadman P, Capizzi R, Grieshaber C, Fumoleau P. (2010) Evaluation of the safety of C-1311 (SYMADEX) administered in a phase 1 dose escalation trial as a weekly infusion for 3 consecutive weeks in patients with advanced solid tumours. Eur J Cancer 46:729–734 [DOI] [PubMed] [Google Scholar]
- Itäaho K, Court MH, Uutela P, Kostiainen R, Radominska-Pandya A, Finel M. (2009) Dopamine is a low-affinity and high-specificity substrate for the human UDP-glucuronosyltransferase 1A10. Drug Metab Dispos 37:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepstad P, Kaasa S, Borchgrevink PC. (2000) Start of oral morphine to cancer patients: effective serum morphine concentrations and contribution from morphine-6-glucuronide to the analgesia produced by morphine. Eur J Clin Pharmacol 55:713–719 [DOI] [PubMed] [Google Scholar]
- Koba M, Konopa J. (2007) Interactions of antitumor triazoloacridinones with DNA. Acta Biochim Pol 54:297–306 [PubMed] [Google Scholar]
- Konopa J. (2001) Antitumor acridines with diaminoalkylo pharmacophoric group. Pure Appl Chem 73:1421–1428 [Google Scholar]
- Kostrubsky SE, Sinclair JF, Strom SC, Wood S, Urda E, Stolz DB, Wen YH, Kulkarni S, Mutlib A. (2005) Phenobarbital and phenytoin increased acetaminophen hepatotoxicity due to inhibition of UDP-glucuronosyltransferases in cultured human hepatocytes. Toxicol Sci 87:146–155 [DOI] [PubMed] [Google Scholar]
- Kurkela M, Patana AS, Mackenzie PI, Court MH, Tate CG, Hirvonen J, Goldman A, Finel M. (2007) Interactions with other human UDP-glucuronosyltransferases attenuate the consequences of the Y485D mutation on the activity and substrate affinity of UGT1A6. Pharmacogenet Genomics 17:115–126 [DOI] [PubMed] [Google Scholar]
- Kuśnierczyk H, Chołody WM, Paradziej-Lukowicz J, Radzikowski C, Konopa J. (1994) Experimental antitumor activity and toxicity of the selected triazolo- and imidazoacridinones. Arch Immunol Ther Exp 42:415–423 [PubMed] [Google Scholar]
- Lazarus P, Sun D. (2010) Potential role of UGT pharmacogenetics in cancer treatment and prevention: focus on tamoxifen and aromatase inhibitors. Drug Metab Rev 42:182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke K, Poindessous V, Skladanowski A, Larsen AK. (2004) The antitumor triazoloacridone C-1305 is a topoisomerase II poison with unusual properties. Mol Pharmacol 66:1035–1042 [DOI] [PubMed] [Google Scholar]
- Mazerska Z, Dziegielewski J, Konopa J. (2001) Enzymatic activation of a new antitumour drug, 5-diethylaminoethylamino-8-hydroxyimidazoacridinone, C-1311, observed after its intercalation into DNA. Biochem Pharmacol 61:685–694 [DOI] [PubMed] [Google Scholar]
- Mazerska Z, Sowiński P, Konopa J. (2003) Molecular mechanism of the enzymatic oxidation investigated for imidazoacridinone antitumor drug, C-1311. Biochem Pharmacol 66:1727–1736 [DOI] [PubMed] [Google Scholar]
- Miller GP, Lichti CF, Zielinska AK, Mazur A, Bratton SM, Gallus-Zawada A, Finel M, Moran JH, Radominska-Pandya A. (2008) Identification of hydroxywarfarin binding site in human UDP glucuronosyltransferase 1a10: phenylalanine90 is crucial for the glucuronidation of 6- and 7-hydroxywarfarin but not 8-hydroxywarfarin. Drug Metab Dispos 36:2211–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy BR, Pollack GM, Brouwer KL. (2002) Contribution of morphine-6-glucuronide to antinociception following intravenous administration of morphine to healthy volunteers. J Clin Pharmacol 42:569–576 [DOI] [PubMed] [Google Scholar]
- Osborne R, Thompson P, Joel S, Trew D, Patel N, Slevin M. (1992) The analgesic activity of morphine-6-glucuronide. Br J Clin Pharmacol 34:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potega A, Dabrowska E, Niemira M, Kot-Wasik A, Ronseaux S, Henderson CJ, Wolf CR, Mazerska Z. (2011) The imidazoacridinone antitumor drug, C-1311, is metabolized by flavin monooxygenases but not by cytochrome P450s. Drug Metab Dispos 39:1423–1432 [DOI] [PubMed] [Google Scholar]
- Ritter JK. (2000) Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem Biol Interact 129:171–193 [DOI] [PubMed] [Google Scholar]
- Sallustio BC, Degraaf YC, Weekley JS, Burcham PC. (2006) Bioactivation of carboxylic acid compounds by UDP-Glucuronosyltransferases to DNA-damaging intermediates: role of glycoxidation and oxidative stress in genotoxicity. Chem Res Toxicol 19:683–691 [DOI] [PubMed] [Google Scholar]
- Sabolovic N, Humbert AC, Radominska-Pandya A, Magdalou J. (2006) Resveratrol is efficiently glucuronidated by UDP-glucuronosyltransferases in the human gastrointestinal tract and in Caco-2 cells. Biopharm Drug Dispos 27:181–189 [DOI] [PubMed] [Google Scholar]
- Skladanowski A, Plisov SY, Konopa J, Larsen AK. (1996) Inhibition of DNA topoisomerase II by imidazoacridinones, new antineoplastic agents with strong activity against solid tumors. Mol Pharmacol 49:772–780 [PubMed] [Google Scholar]
- Skwarska A, Augustin E, Konopa J. (2007) Sequential induction of mitotic catastrophe followed by apoptosis in human leukemia MOLT4 cells by imidazoacridinone C-1311. Apoptosis 12:2245–2257 [DOI] [PubMed] [Google Scholar]
- Smith SC, Havaleshko DM, Moon K, Baras AS, Lee J, Bekiranov S, Burke DJ, Theodorescu D. (2011) Use of yeast chemigenomics and COXEN informatics in preclinical evaluation of anticancer agents. Neoplasia 13:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starlard-Davenport A, Xiong Y, Bratton S, Gallus-Zawada A, Finel M, Radominska-Pandya A. (2007) Phenylalanine(90) and phenylalanine(93) are crucial amino acids within the estrogen binding site of the human UDP-glucuronosyltransferase 1A10. Steroids 72:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starlard-Davenport A, Lyn-Cook B, Beland FA, Pogribny IP. (2010) The role of UDP-glucuronosyltransferases and drug transporters in breast cancer drug resistance. Exp Oncol. 32:172–180 [PubMed] [Google Scholar]
- Tallman MN, Miles KK, Kessler FK, Nielsen JN, Tian X, Ritter JK, Smith PC. (2007) The contribution of intestinal UDP-glucuronosyltransferases in modulating 7-ethyl-10-hydroxy-camptothecin (SN-38)-induced gastrointestinal toxicity in rats. J Pharmacol Exp Ther 320:29–37 [DOI] [PubMed] [Google Scholar]
- Thomas AL, Anthoney A, Scott E, Ahmed S, Lundberg AS, Major A, Capizzi RL, Twelves CJ. (2008) C-1311, a novel inhibitor of FLT3 and topoisomerase II: a phase 1 trial of a once every three week schedule in patients with advanced solid tumors (Abstract). J Clin Oncol 26 (Suppl):2576 [Google Scholar]
- Xiong Y, Bernardi D, Bratton S, Ward MD, Battaglia E, Finel M, Drake RR, Radominska-Pandya A. (2006) Phenylalanine 90 and 93 are localized within the phenol binding site of human UDP-glucuronosyltransferase 1A10 as determined by photoaffinity labeling, mass spectrometry, and site-directed mutagenesis. Biochemistry 45:2322–2332 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Patana AS, Miley MJ, Zielinska AK, Bratton SM, Miller GP, Goldman A, Finel M, Redinbo MR, Radominska-Pandya A. (2008) The first aspartic acid of the DQxD motif for human UDP-glucuronosyltransferase 1A10 interacts with UDP-glucuronic acid during catalysis. Drug Metab Dispos 36:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Park JY, Guillemette C, Schantz SP, Lazarus P. (2001) Tobacco carcinogen-detoxifying enzyme UGT1A7 and its association with orolaryngeal cancer risk. J Natl Cancer Inst 93:1411–1418 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Fang JL, Lazarus P. (2002) Glucuronidation: an important mechanism for detoxification of benzo[a]pyrene metabolites in aerodigestive tract tissues. Drug Metab Dispos 30:397–403 [DOI] [PubMed] [Google Scholar]
- Zielinska A, Lichti CF, Bratton S, Mitchell NC, Gallus-Zawada A, Le VH, Finel M, Miller GP, Radominska-Pandya A, Moran JH. (2008) Glucuronidation of monohydroxylated warfarin metabolites by human liver microsomes and human recombinant UDP-glucuronosyltransferases. J Pharmacol Exp Ther 324:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]