Abstract
Previous studies on the prevalence of mucopolysaccharidoses (MPS) in different populations have shown considerable variations. There are, however, few data with regard to the prevalence of MPSs in Fenno–Ugric populations or in north-eastern Europe, except for a report about Scandinavian countries. A retrospective epidemiological study of MPSs in Estonia was undertaken, and live-birth prevalence of MPS patients born between 1985 and 2006 was estimated. The live-birth prevalence for all MPS subtypes was found to be 4.05 per 100,000 live births, which is consistent with most other European studies. MPS II had the highest calculated incidence, with 2.16 per 100,000 live births (4.2 per 100,000 male live births), forming 53% of all diagnosed MPS cases, and was twice as high as in other studied European populations. The second most common subtype was MPS IIIA, with a live-birth prevalence of 1.62 in 100,000 live births. With 0.27 out of 100,000 live births, MPS VI had the third-highest live-birth prevalence. No cases of MPS I were diagnosed in Estonia, making the prevalence of MPS I in Estonia much lower than in other European populations. MPSs are the third most frequent inborn error of metabolism in Estonia after phenylketonuria and galactosemia.
Introduction
The mucopolysaccharidoses (MPSs) are inborn errors of lysosomal glycosaminoglycan (GAG) degradation. The un-degraded material is stored in the lysosomes of all cells of the body (except erythrocytes) and excreted in urine in increased amounts. There are 10 known enzyme defects that cause seven distinct MPS disorders (I–IV, VI, and VII). (OMIM #252800, #309900, #252900, #252920, #252930, #252940, #253000, #253010, #253200, and #253220). All, except type II (X-linked recessive), are inherited in an autosomal recessive manner. All MPSs represent chronic progressive disorders, usually exhibiting a wide variety of clinical manifestations (Neufeld and Muenzer, 1995).
Earlier studies on the prevalence of MPSs in different European populations offer incidence rates between 1.75 (Denmark) and 4.8 (Northern Portugal) per 100,000 live births. Nevertheless, the average prevalence rate is around 4 in 100,000 live births in most populations (Nelson, 1997; Poorthuis et al., 1999; Pinto et al., 2004; Baehner et al., 2005; Malm et al., 2008; Poupetová et al., 2010).
Since there are few data regarding MPS prevalence in north-eastern Europe and especially in Fenno–Ugric populations, we conducted a retrospective epidemiological study of MPS patients diagnosed in Estonia since 1990, and calculated the live-birth prevalence for diagnosed MPS subtypes. We compare our data with previous reports from different European populations.
Patients and Methods
Data pertaining to MPS patients in Estonia are available since 1990, when a medical genetics service was set up at the Children's Hospital in Tartu. There are currently two centralized hospitals with a genetics service: Children's Hospital in Tallinn and the Department of Genetics of Tartu University Hospital. The following data were collected from case histories recorded at both centers: date of birth, sex, ethnic origin, family history, age of diagnosis, and the results of biochemical and enzymatic analyses.
Selective screening for MPS was performed using a qualitative toluidine blue spot test (Berry test), followed by quantitative analysis of GAGs in urine (heparan-, dermatan-, keratan-, and chondroitinsulfate) in patients with clinical suspicion of MPS. Approximately 2% of children born during a certain year (regardless of age at testing) have been tested with the Berry spot test due to clinical suspicion of MPS. When urinary levels of GAGs were elevated, enzyme analysis was performed from lymphocytes or skin fibroblasts. Quantitative GAGs and enzymatic analyses were performed at the Rotterdam Erasmus University in The Netherlands. Since 2008, parallel quantitative urinary GAGs analyses have also been performed in Estonia, at the Chemistry Laboratory of the Central Laboratory of the Health Board, using quantitative dimethylene blue GAG analysis (de Jong et al., 1989; de Jong et al., 1992), followed by two-dimensional electrophoresis of urinary GAGs (Whiteman and Henderson, 1977).
The live-birth prevalence of MPS was defined as the total number of cases with a particular type of MPS born within a certain period of time, divided by the total number of live births in the same period. Annual live-birth data were obtained from the database of Statistics Estonia of the Estonian Ministry of Finance (www.stat.ee). Familial cases were included. This study was approved by the Research Ethics Committee of the University of Tartu.
Results
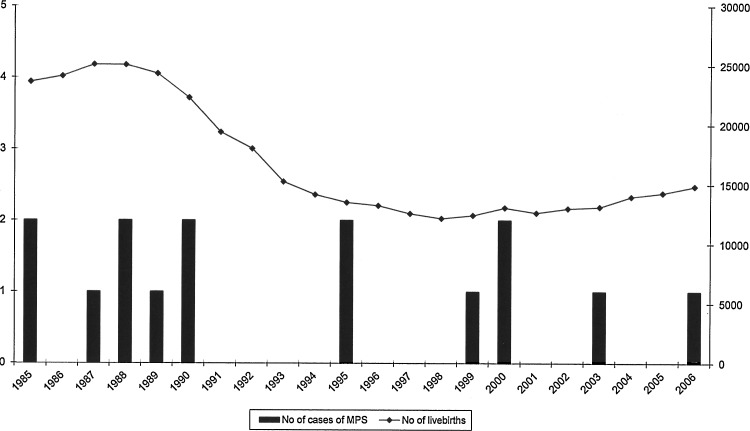
Since 1990, the diagnosis of MPS has been confirmed in 16 patients from 14 families. All, except one woman with MPS VI born in 1977, were born in the years 1985–2006. Therefore, we decided to calculate the live-birth prevalence over the period just mentioned (21 years) and exclude the data of the patients born in 1977 (Fig. 1).
FIG. 1.
Diagnosed cases of mucopolysaccharidoses and number of live births between 1985 and 2006.
The medium age at diagnosis was 5 years and 3 months (from 3 years to 6 years and 11 months). Ten families were Estonians, and three were of Slavic origin. This distribution is quite similar compared with the Estonian population as a whole (one third of the population is of Slavic origin).
During the study period (1985–2006), there were 370,298 live births in Estonia, varying from 12,167 to 25,086 per year (Fig. 1). The live-birth prevalence for all MPS subtypes was 4.05 per 100,000 live births (Table 1). MPS II (Hunter syndrome) had the highest calculated incidence, with 2.16 per 100,000 live births (4.2 per 100,000 male live births), representing 53% of all MPS cases diagnosed. The distribution of all MPS subtypes is shown in Table 1.
Table 1.
Prevalence of Mucopolysaccharidoses in Estonia During 1985–2006
| Disease | Number of patients | Live-birth prevalence | Prevalence per 105 live births | Proportion of the subtype |
|---|---|---|---|---|
| MPS I | 0 | 0 | 0 | 0 |
| MPS II | 8 | 1:46,287 (1:23,825)a | 2.16 (4.2)a | 53% |
| MPS IIIAb | 6 | 1:61,716 | 1.62 | 40% |
| MPS IV | 0 | 0 | 0 | 0 |
| MPS VI | 1 | 1:370, 298 | 0.27 | 7% |
| MPS VII | 0 | 0 | 0 | 0 |
| Total | 15 | 1:24, 687 | 4.05 | 0 |
Based on male live births.
No cases of MPS III subtypes B-D were found.
MPS, mucopolysaccharidoses.
Discussion
The overall prevalence of all MPSs as a group is estimated at 4.05 per 100,000 live births (1:24,687) in Estonia, which is similar to previous studies in The Netherlands and Germany, that is, 4.5 and 3.53 per 100,000 live births, respectively (Poorthuis et al., 1999; Baehner et al., 2005). For details of previously published prevalence of MPSs in Europe, we can take a look at Tables 2 and 3. Therefore, we can assume that we have not missed any cases over 21 years, which, in our opinion, is quite a prolonged period for calculating the prevalence of a rare disorder in a small population such as Estonia.
Table 2.
Prevalence of Mucopolysaccharidoses in European Countries: A Review of the Literature
| |
|
|
Estimated incidence (per 105 live births) |
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Years | No of cases | MPSI | MPSII | MPSIII | MPSIV | MPSVI | MPSVII | MSD | All types | References |
| Ireland (Northern) | 1958–1985 (27 years) | 34 | 1.67 | 0.71 (1.39)a | 0.36 | 1.30 | 0 | 0 | 4.00 | Nelson [1997] | |
| Netherlands | 1970–1996 (27 years) | 331 | 1.19 | 0.67 (1.30) | 1.89 | 0.36 | 0.15 | 0.24 | 0.05 | 4.50 | Poorthuis et al. [1999] |
| Portugal (northern) | 1982–2001 (20 years) | 62 | 2.66 | 1.09 | 0.84 | 0.60 | 0.42 | 0 | 0.48 | 4.80 | Pinto et al. [2004] |
| Germany | 1980–1995 (16y) | 474 | 0.69 | 0.64 (1.30) | 1.57 | 0.38 | 0.23 | 0 | 3.53 | Baehner et al. [2005] | |
| Sweden | 1975–2004 (30 years) | 52 | 0.67 | 0.27 | 0.67 | 0.07 | 0.07 | 0 | 1.75 | Malm et al. [2008] | |
| Norway | 1975–2004 (30 years) | 45 | 1.85 | 0.13 | 0.27 | 0.76 | 0.07 | 0 | 3.08 | Malm et al. [2008] | |
| Denmark | 1979–2004 (26 years) | 33 | 0.54 | 0.27 | 0.43 | 0.48 | 0.05 | 0 | 1.77 | Malm et al. [2008] | |
| Czech Republic | 1975–2008 (34 years) | 119 | 0.72 | 0.43 (0.83) | 0.91 | 0.73 | 0.05 | 0.02 | 0.26 | 3.72 | Poupetová et al. [2010] |
| Estonia | 1985–2006 (21years) | 15 | 0 | 2.16 (4.20) | 1.62 | 0 | 0.27 | 0 | 4.05 | Present study | |
Based on male live births.
Table 3.
Comparison of Published Proportions of Various Forms of Mucopolysaccharidoses in European Countries: A Review of the Literature
| |
|
|
Proportion of subtypes in% |
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Years | No of cases | MPSI | MPSII | MPSIII | MPSIV | MPSVI | MPSVII | MSD | Unspecified | References |
| Ireland (Northern) | 1958–1985 (27 years) | 34 | 41 | 18 | 9 | 32 | 0 | 0 | n.i. | Nelson [1997] | |
| Netherlands | 1970–1996 (27 years) | 331 | 25 | 16 | 47 | 8 | 2 | 2 | 1 | Poorthuis et al. [1999] | |
| Portugal (northern) | 1982–2001 (20 years) | 62 | 13 | 34 | 23 | 10 | 16 | 0 | 4.8 | Pinto et al. [2004] | |
| Germany | 1980–1995 (16 years) | 474 | 20 | 18 | 45 | 11 | 7 | 0 | n.i. | Baehner et al. [2005] | |
| Sweden | 1975–2004 (30 years) | 52 | 38 | 15 | 38 | 4 | 4 | 0 | n.i. | Malm et al. [2008] | |
| Norway | 1975–2004 (30 years) | 45 | 60 | 4 | 9 | 24 | 2 | 0 | n.i. | Malm et al. [2008] | |
| Denmark | 1979–2004 (26 years) | 33 | 30 | 15 | 24 | 27 | 3 | 0 | n.i. | Malm et al. [2008] | |
| Czech Republic | 1975–2008 (34 years) | 119 | 17 | 18 | 20 | 13 | 2 | 1 | 3 | 27 | Poupetová et al. [2010] |
| Estonia | 1985–2006 (21 years) | 15 | 0 | 53 | 40 | 0 | 7 | 0 | n.i. | Present study | |
n.i., not investigated.
MPSs account for more than one tenth of all diagnosed patients with inborn errors of metabolism (IEM) in Estonia (data not shown), and MPS is the third most frequent inherited metabolic disease after phenylketonuria–1:6010 (Ounap et al., 1998) and galactosemia–1:19,700 (Õunap et al., 2010).
Our study showed the highest prevalence for MPS type II in Estonia (2.16 in 100,000 live births; 4.2 in 100,000 male live births), which is twice higher than in other studied European populations (Table 2). The highest prevalence in Europe was reported by Pinto et al. (2004) in northern Portugal (1.09 in 100,000 live births), and outside Europe in Israel, where it was estimated to be about 1.48 in 100,000 (Schaap and Bach, 1980). This may be caused by a founder effect of “milder” mutations that may have a selection advantage (e.g., a higher resistance to tuberculosis) (Baehner et al., 2005).
The second most common type of MPS in Estonia is MPS IIIA (1.62 per 100,000), which was the only observed subtype of MPS III. This is similar to most Western communities (Poorthuis et al., 1999; Baehner et al., 2005; Poupetová et al., 2010), except northern Portugal (Pinto et al., 2004).
During our observation period, only one patient was diagnosed with MPS VI. Therefore, it is difficult to draw any significant conclusions. In addition, MPS VI has been diagnosed in one girl born before our observation period. MPS VI belongs to the less frequent MPSs in most populations with prevalence rates from 0 in Northern Ireland (Nelson, 1997) to 0.42 in northern Portugal (Pinto et al., 2004). The prevalence of MPS VI in Estonia falls between these two rates, with 0.27 in 100,000.
Only three MPS subtypes were found in Estonia, and no cases of MPS I, IV, or VII were determined. The rare occurrence of MPS VII is usually common in other European countries as well (Tables 2 and 3).
No cases of MPS I were diagnosed in Estonia, making the prevalence of MPS I in Estonia much lower than in other European populations (Malm et al., 2008; Nelson, 1997; Pinto et al., 2004; Poorthuis et al., 1999; Baehner et al., 2005; Poupetová et al., 2010). Only in Taiwan was the prevalence of MPS I found to be very low–0.11 in 100,000 live births (Lin et al., 2009). It is unlikely that any cases were missed due to the lack of ascertainment, as the phenotype of MPS I, and especially the Hurler syndrome, is very apparent compared with other types of MPSs. Even in closely related countries such as Norway and Sweden, the incidence and pattern of subgroups differ remarkably (Malm et al., 2008).
In our survey, MPS II was more prevalent than MPS I, paralleling that reported in Israel (Schaap and Bach, 1980) and also in Taiwan (Lin et al., 2009), but contrasting most European studies, which showed a contrary tendency (Table 3).
In addition, no cases of MPS IV, a relatively frequent subtype in Denmark, Norway, and Northern Ireland, were diagnosed in Estonia (Tables 2 and 3).
Only three subtypes of MPS have been diagnosed in Estonia—MPS II, IIIA, and VI. We acknowledge that milder phenotypes may be overlooked. However, since the Estonian population is small and MPSs are also very rare IEMs, it is possible that other MPS subtypes are very uncommon in Estonia, and simply have not yet occurred.
Another problem that arose during this study was that the comparison of estimated prevalence was difficult, as the estimates in different studies are based on varying population sizes and study designs. Standardized rate estimates would be advisable from an epidemiological point of view.
We could not find any reports of MPS prevalence in Fenno–Ugric populations or in neighboring countries, such as Latvia, Lithuania, and Western Russia, except for the report of MPS incidence and prevalence in Scandinavian countries (Malm et al., 2008).
In conclusion, MPSs are rare genetic disorders, but as a group are relatively common, being the third most frequent IEM in Estonia after phenylketonuria and galactosemia, and they represent an economic burden for the health system. New therapeutic options are available, and knowledge of prevalence is important from the health economics point of view.
Acknowledgment
This research was supported by the GARLA 8175 grant from the Estonian Science Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- Baehner F. Schmiedeskamp C. Krummenauer F, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- de Jong JG. Wevers RA. Laarakkers C, et al. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- de Jong JG. Wevers RA. Liebrand-van Sambeek R. Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin Chem. 1992;38:803–807. [PubMed] [Google Scholar]
- Lin HY. Lin SP. Chuang CK, et al. Incidence of the mucopolysaccharidoses in Taiwan: 1984–2004. Am J Med Genet A. 2009;149A:960–964. doi: 10.1002/ajmg.a.32781. [DOI] [PubMed] [Google Scholar]
- Malm G. Lund AM. Mansson JE, et al. Mucopolysaccharidoses in the Scandinavian countries: incidence and prevalence. Acta Paediatr. 2008;97:1577–81. doi: 10.1111/j.1651-2227.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- Neufeld EF. Muenzer J. Valle D. The mucopolysaccharidoses. In: Scriver CR, editor; Beaudet AL, editor; Sly WS, editor; The metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 1995. pp. 2465–2494. [Google Scholar]
- Õunap K. Joost K. Temberg T, et al. Classical galactosemia in Estonia: selective neonatal screening, incidence, and genotype/phenotype data of diagnosed patients. J Inherit Metab Dis. 2010;33:175–176. doi: 10.1007/s10545-010-9045-2. [DOI] [PubMed] [Google Scholar]
- Ounap K. Lillevali H. Metspalu A, et al. Development of the phenylketonuria screening programme in Estonia. J Med Screen. 1998;5:22–23. doi: 10.1136/jms.5.1.22. [DOI] [PubMed] [Google Scholar]
- Pinto R. Caseiro C. Lemos M, et al. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004;12:87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- Poorthuis BJ. Wevers RA. Kleijer WJ, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- Poupetová H. Ledvinova J. Berna L, et al. The birth prevalence of lysosomal storage disorders in the Czech Republic: comparison with data in different populations. J Inherit Metab Dis. 2010;33:387–396. doi: 10.1007/s10545-010-9093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap T. Bach G. Incidence of mucopolysaccharidoses in Israel: is Hunter disease a “Jewish disease”? Hum Genet. 1980;56:221–223. doi: 10.1007/BF00295699. [DOI] [PubMed] [Google Scholar]
- Whiteman P. Henderson H. A method for the determination of amniotic-fluid glycosaminoglycans and its application to the prenatal diagnosis of Hurler and Sanfilippo diseases. Clin Chim Acta. 1977;79:99–105. doi: 10.1016/0009-8981(77)90466-1. [DOI] [PubMed] [Google Scholar]