Abstract
This study examined the role of endocannabinoid signaling in stress-induced reinstatement of cocaine seeking and explored the interaction between noradrenergic and endocannabinergic systems in the process. A well-validated preclinical model for human relapse, the rodent conditioned place preference assay was used. Cocaine-induced place preference was established in C57BL/6 mice using injections of 15 mg/kg cocaine. Following extinction of preference for the cocaine-paired environment, reinstatement of place preference was determined following 6 min of swim stress, or cocaine injection (15 mg/kg, i.p.). The role of endocannabinoid signaling was studied using the cannabinoid antagonist AM-251 (3 mg/kg, i.p.). Another cohort of mice was tested for reinstatement following administration of the cannabinoid agonist CP 55,940 (10, 20 or 40 μg/kg, i.p.). The alpha-2 adrenergic antagonist BRL-44408 (5 mg/kg, i.p.) with or without CP 55,940 (20 μg/kg) was administered to a third group of mice. We found that: 1) AM-251 blocked forced swim-induced but not cocaine-induced reinstatement of cocaine-seeking behavior; 2) the cannabinoid agonist CP 55,940 did not reinstate cocaine-seeking behavior when administered alone but did synergize with a non-reinstating dose of the alpha-2 adrenergic antagonist BRL-44408 to cause reinstatement. These results are consistent with the hypothesis that stress exposure triggers the endogenous activation of CB1 receptors and that activation of the endocannabinoid system is required for the stress-induced relapse of the mice to cocaine seeking. Further, the data suggests that the endocannabinoid system interacts with noradrenergic mechanisms to influence stress-induced reinstatement of cocaine-seeking behavior.
Keywords: cannabinoids, cocaine, norepinephrine, relapse, stress, conditioned place preference
Cocaine abuse and dependence are important problems with significant psychosocial, medical and financial implications. One of the more troubling aspects of addiction is the persistent vulnerability to relapse to drug use that continues even after long periods of abstinence. Although relapse can be induced by re-exposure to cocaine, drug-associated cues or stress (Childress et al., 1992; See et al., 1999; Shaham et al., 2000; Stewart, 2000; Kalivas and McFarland, 2003), exposure to stress is particularly injurious given its inescapability. The investigation of neurobiological mechanisms at the intersection of motivated behavior and stress could prove fruitful in the search for potential treatments for stress-induced drug relapse. The endocannabinoid system (ECS), including the cannabinoid receptors (CB1 and CB2), endocannabinoids (N-arachidonylethanolamine and 2-arachidonoylglycerol) and enzymes and transporters which control synaptic concentrations of endocannabinoids, are attractive targets in this regard.
The preclinical investigation of relapse involves the use of reinstatement protocols in which various stimuli are tested for their ability to re-establish extinguished drug-seeking behavior (Shaham 2003). Conditioned place preference is commonly used to study reinstatement in mice. Using this method, cocaine-induced conditioned place preference becomes extinguished with repeated drug-free exposure to the cocaine-paired environment. The ability of various stimuli to re-establish the cocaine preference is then tested (Tzschentke, 2007). Using this protocol, priming injections of cocaine and a variety of stressors have been shown to produce reinstatement (Itzhak and Martin, 2002; Kreibich and Blendy, 2004; Ribeiro Do Couto et al., 2006; Tzchentke, 2007; Orsini, et al., 2008; Redila and Chavkin, 2008; Mantsch et al., 2010).
There is strong evidence that the ECS is activated in multiple brain regions involved in addiction by stress exposure (Di et al, 2005; Gorzalka et al., 2008; Patel and Hillard, 2008; Rademacher et al, 2008; Hill et al., 2009). It is hypothesized that the result of this activation in most brain regions is to oppose the effects of stress (Cota 2008; Gorzalka et al., 2008; Hill et al., 2010). For example, CB1 receptor knock out animals have increased basal and stress-induced HPA activity, (Barna et al., 2004; Cota et al., 2007), CB1 antagonists increase basal and stress induced glucocorticoid secretion (Manzanares et al., 1999; Patel et al., 2004; Wade et al., 2006), and CB1 agonists and clearance inhibitors reduce stress-induced glucocorticoid secretion (Patel et al., 2004).
Central noradrenergic systems also play an important role in the physiological response to stress (Abercrombie et al., 1988; Tanaka et al., 1991; Finlay et al., 1995) and interactions between the noradrenergic system and cocaine are well documented (Sofuoglu and Sewell, 2009). Repeated cocaine exposure produces long-lasting adaptations within central noradrenergic systems that can lead to enhanced noradrenergic responsiveness during stress (Belej, et al 1996; Macey, et al 2003; Baumann, et al 2004; Beveridge, et al 2005; Lanteri, et al 2008) and norepinephrine (NE) release is required for stress-induced drug reinstatement using both rat self-administration models (Erb et al., 2000; Leri et al., 2002) and mouse conditioned place preference protocols (Mantsch et al., 2010).
The ECS has a well established role in enhancing the motivational effects and promoting drug seeking and relapse behavior of alcohol, nicotine, opioids and marijuana (Tanda and Goldberg, 2003; Lupica et al., 2004; Gardner, 2005; Cohen et al., 2005). Studies of the role of the ECS in the rewarding aspects of cocaine have shown inconsistent effects (Fattore et al., 1999; Soria et al., 2005; Xi et al., 2008; Wiskerke et al., 2008; Orio et al., 2009) but the ECS seems to play an important role in relapse behavior. DeVries et al., (2001) found that a cannabinoid agonist reinstated cocaine seeking in rats that had been conditioned and extinguished in a self-administration paradigm and that a cannabinoid antagonist prevented cocaine- and cue- but not stress- induced reinstatement of cocaine seeking. Cannabinoid mechanisms were also implicated in cocaine-primed reinstatement in rats (Xi et al., 2006; Filip et al., 2006). In mice, however, cue-induced but not cocaine-induced reinstatement was blocked by a cannabinoid antagonist (Ward et al., 2009). Surprisingly, given the well-established link between the ECS and stress, there has been only one investigation of the role of stress-induced cocaine reinstatement (De Vries et al., 2001) and there have been no studies on the interaction between the endocannabinoid and noradrenergic systems in stress-induced cocaine relapse.
The purpose of this study was to examine the role of the ECS in cocaine- and stress-induced relapse of cocaine seeking behavior and its interaction with noradrenergic mechanisms in mice using a conditioned place preference reinstatement approach.
Experimental Procedures
Animals
Male C57BL/6 mice, 8-9 weeks old, were purchased from Harlan (Indianapolis, IN). The mice were housed singly in a temperature- and humidity-controlled, AAALAC- accredited animal facility under a 12 h/12 h light/dark cycle (lights on at 7:00 AM) and had access to food and water at all times, except when in the experimental chambers. The animals were acclimatized for one week in the animal care facility before use in the studies. All experiments had been reviewed and approved by the Institutional Animal Care and Use Committee and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals.
Drugs
Cocaine HCl was acquired from the National Institute on Drug Abuse (Bethesda, MD) through the NIDA Drug Supply Program. CP 55,940 and AM-251 were purchased from Sigma-Aldrich (St. Louis, MO) and BRL-44408 maleate was acquired from Tocris Bioscience (Minneapolis, MN). Cocaine and BRL-44408 were dissolved in saline (0.9% NaCl solution) and CP 55,940 and AM-251 were dissolved in a 1:1:18 ratio of ethanol, Cremophor EL (Sigma-Aldrich), and saline. Cocaine and BRL-44408 solutions were administered i.p. in a volume of 0.1 ml/25 g body weight. CP 55,940 and AM-251 were administered i.p. in a volume of 0.1 ml/10 g body weight. Pretreatment times for CP 55,940, BRL-44408 and AM-251 were 30 min.
Equipment
Behavioral testing was conducted using six ENV-3013 three-chambered mouse place preference chambers from Med-Associates, Inc (St. Albans, VT). Two 46.5 × 12.7 × 12.7 cm side compartments were each connected to a 7.2 cm × 12.7 cm gray-colored center compartment. One side compartment had white walls and a stainless steel mesh floor and the other side compartment had black walls and a stainless steel grid rod floor. The clear tops of the compartments were hinged to permit placement and removal of the mice. Room ceiling lights were turned on and, to balance unconditioned side preference, the black compartments were illuminated with additional suspended lights, providing a total illumination of approximately 300 lux. Automated data collection was accomplished using photobeams (6 beams for the white and black test areas and 2 beams for the center gray area) which were evenly spaced across the length of the chamber and interfaced with a computer containing Med-PC software.
Cocaine-Induced Conditioned Place Preference
Cocaine-induced conditioned place preference was established using an unbiased design in which one of the side compartments was randomly designated as the cocaine compartment and the other as the saline compartment. On the first day of the procedure, mice were placed into the center compartment of the chamber and provided free access to both side compartments for 30 minutes in the absence of saline or cocaine pretreatment to determine pre-conditioning preference. During the 8-day conditioning phase of the experiment, mice received cocaine (15 mg/kg, ip) and saline injections on alternating days after which time they were confined to the randomly designated treatment-appropriate compartment for 30 minutes. After the final conditioning session, mice were tested for the expression cocaine-induced conditioned place preference by once again placing them into the center compartment of the chamber and providing them with free access to the side compartments for 30 minutes. Conditioned place preference was defined as the change in time spent in the cocaine-paired compartment after conditioning compared to the initial pre-conditioning session. The 15 mg/kg cocaine dose was selected based on previous results (Mantsch et al., 2010).
Extinction
Following conditioning, daily extinction training was conducted. During the extinction sessions, mice were placed into the center compartment and allowed free access to the side compartments for 30 min. Mice underwent daily extinction training until the conditioned place preference for the cocaine-paired compartment during the session was reduced by at least 50%.
Reinstatement Testing
The reinstatement sessions were identical to the extinction sessions except that mice received exposure to stressors and/or drug injections prior to the session. Reinstatement was defined as the increase in time spent in the cocaine-paired compartment on the test day (reinstatement day) compared to the time spent on the cocaine-paired compartment on the previous day (extinction day). Mice were tested more than once for reinstatement, receiving all treatments within a given experiment. The stressors and/or drugs were given in counter-balanced order to avoid potential sequence effects. Reinstatement sessions were separated by additional extinction sessions. Mice were required to reach the extinction criterion once again before the next reinstatement test was conducted.
Forced Swim Stress-Induced Reinstatement Testing
Forced swim stress (FS)-induced reinstatement was examined by placing the mice into a 30 cm h × 20 cm d cylindrical polypropylene container filled with water (20-25° C) for 6 min, 24 min after pretreatment with drug or respective vehicles. Following FS, mice were placed back into their home cages for 1-2 min prior to introduction into the center compartment of the place conditioning chamber for reinstatement testing.
Cocaine-Induced Reinstatement Testing
Reinstatement induced by a cocaine priming injection was tested in mice by injection of 15 mg/kg dose of cocaine i.p. immediately before placement into the center compartment of the place conditioning chamber for reinstatement testing.
Effect of the CB1 Receptor Antagonist, AM-251, on Stress- and Cocaine-Induced Reinstatement
In order to determine if CB1 receptor activation plays a role in stress- or cocaine-induced reinstatement, 24 min prior to forced swim testing or 30 minutes prior to cocaine injection, the mice were injected with AM-251 or vehicle. The mice were also injected with AM-251 or vehicle 30 minutes before testing in order to determine whether the AM-251 or vehicle injection could cause reinstatement. All mice received six treatments (FS+ vehicle, FS+AM-251, cocaine+vehicle, cocaine+AM-251, AM-251 + vehicle, and vehicle+vehicle) in counterbalanced order. Mice reached extinction criterion before retesting.
Reinstatement by the CB1 Receptor Agonist CP 55,940
In order to determine if CB1 receptor activation alone was sufficient to induced reinstatement, mice were tested for reinstatement in response to the synthetic CB1 receptor agoninst CP 55,940. Thirty minutes prior to testing, mice were injected with vehicle or 10, 20 or 40 μg/kg doses of CP 55,940. All mice received all 4 treatments in counterbalanced order. Mice reached extinction criterion before retesting.
Reinstatement by CP 55,940 in Combination with BRL-44408
Another group of mice were tested for reinstatement by CP 55,940 in combination with a subthreshold dose of the selective alpha-2 adrenergic receptor antagonist and pharmacological stressor, BRL-44408, in order to determine if cannabinoid receptor activation could potentiate a pharmacological stressor-induced reinstatement and whether cannabinoid receptor activation interacted with noradrenergic mechanisms to induce reinstatement. It has been shown previously that a sufficient dose of BRL-44408 can induce reinstatement (Mantsch, et al., 2010). We have found that 5 mg/kg BRL-44408 is a subthreshold dose. Therefore, 20 μg/kg CP 55,940 and 5 mg/kg BRL-44408 were injected i.p. 30 minutes prior to reinstatement testing.
Statistical Data Analyses
Statistical analysis was carried out using SigmaPlot 11 statistical software (Systat Software, San Jose, CA). Data are expressed as mean +/- standard error of the mean. The ability of a drug and/or stress procedure to elicit reinstatement was determined by the difference between the time spent in the cocaine-paired compartment on the day of testing (reinstatement day) and the preceding extinction session (extinction day). Comparisons between effects of different drugs and/or stress on reinstatement were also determined. Statistical significance was determined by two-way repeated measures (RM) ANOVA with post-hoc multiple comparisons using the Holm-Sidak method.
Results
Establishment of cocaine-induced conditioned place preference
Previous studies had determined that 15 mg/kg cocaine dose was optimal for producing conditioned place preference (Mantsch et al., 2010). We found significant cocaine-induced conditioned place preference, defined as an increase in time spent in the cocaine-paired compartment, was observed in each of the experiments (p≤0.001 for each experiment). The average number of extinction sessions prior to the first reinstatement test session was 5.9. Nineteen percent of mice were not tested for reinstatement since they did not display cocaine-induced conditioned place preference or failed to reach the extinction criterion.
The effect of AM-251 on stress- and cocaine-induced reinstatement
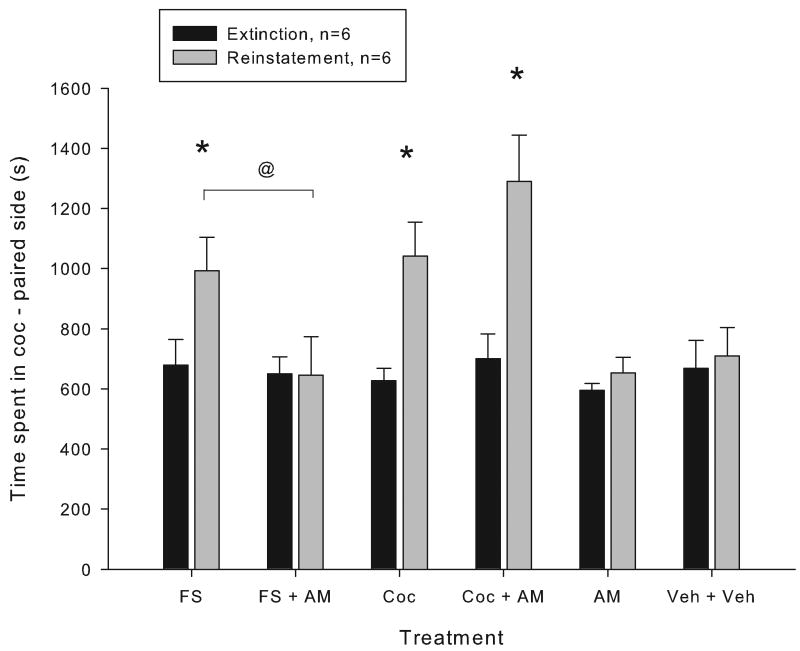
Reinstatement was tested in mice which had been conditioned to cocaine and whose place preference had been extinguished. Both FS (6 minutes) and cocaine (15 mg/kg, i.p.) produced reinstatement, as indicated by significant increases in time spent on the cocaine-paired side compared to the preceding extinction day (p<0.05 Holm-Sidak following a significant interaction between time (extinction and reinstatement day) and treatment (F(5,25)=8.142, p<0.001, two-way RM ANOVA, Figure 1). To determine whether endocannabinoid signaling was involved in either type of reinstatement, the CB1 receptor antagonist AM-251 was injected before FS and cocaine. AM-251 treatment completely blocked the forced swim-induced reinstatement (no significant difference in the time spent in the cocaine-paired environment between the extinction and reinstatement day within the AM+FS group and a significant effect of treatment within reinstatement days between FS and AM+FS, p<0.05, Holm Sidak). AM-251 treatment did not block cocaine-induced reinstatement (significant effect of time in both Coc and Coc+AM, p<0.05, Holm-Sidak, no significant difference between reinstatement days with Coc vs Coc+AM-251). Neither AM-251 nor vehicle produced any reinstatement when given alone.
Figure 1.
The effect of the cannabinoid antagonist AM-251 on forced swim- and cocaine-induced reinstatement. Data represent the time spent in the cocaine-paired compartment (mean +/- SEM) during the extinction session prior to reinstatement testing and after reinstatement testing with forced swim (6 minutes) + vehicle (FS), FS + AM-251(3 mg/kg, i.p.) (FS+AM), cocaine (15 mg/kg, i.p) + vehicle (Coc), cocaine + AM-251 (Coc+AM), vehicle, or AM-251. Vehicle and AM-251 were injected 30 minutes prior to reinstatement testing. Coc and FS were administered immediately prior to reinstatement testing. A significant increase in the time spent in the cocaine-paired compartment during reinstatement testing compared to the preceding extinction session was seen after both FS and cocaine (* =p<0.05, Holm-Sidak multiple comparison test) but not after AM-251 or vehicle alone. A significant difference between the time spent in the cocaine-paired compartment on reinstatement days was seen between the FS and FS+AM251 treatment groups (@ = p<0.05, Holm-Sidak multiple comparison test) but not between the Coc and the Coc + AM-251 groups.
The effect of CP 55,940 on reinstatement of place preference for cocaine
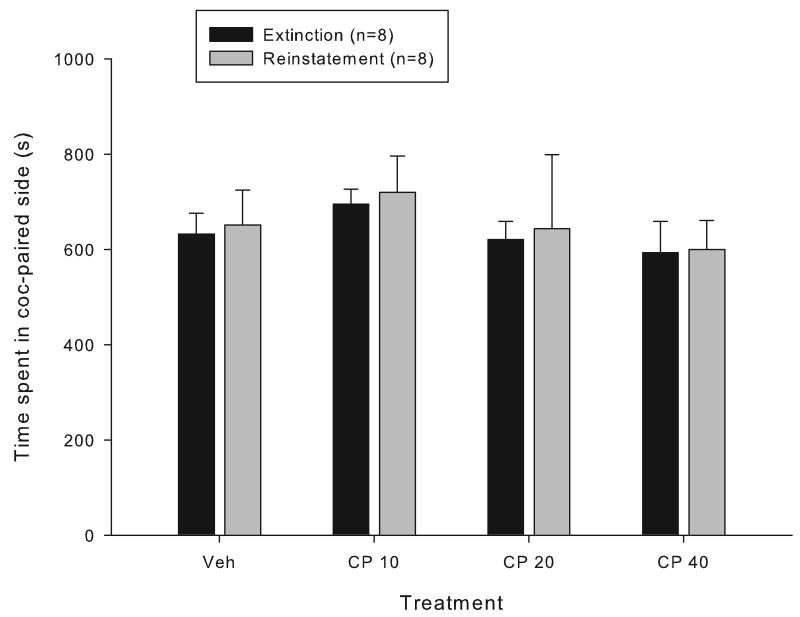
Mice which had been conditioned to cocaine and whose place preference had been extinguished were tested with vehicle and three doses of the CP 55,940 to determine if administration of a cannabinoid agonist was sufficient to induce reinstatement of place preference (Figure 2). No reinstatement was observed following 10, 20 or 40 μg/kg administration of CP 55,940 (F(1,21)=0.0875, p=0.776, main effect of time (extinction vs. reinstatement day), two-way RM ANOVA).
Figure 2.
The effect of the cannabinoid agonist CP 55,940 on reinstatement of extinguished cocaine-induced conditioned place preference. Data represent the time spent in the cocaine-paired compartment (mean +/- SEM) during the extinction session prior to reinstatement testing and after reinstatement testing with vehicle (Veh) or 10, 20 or 40 μg/kg, i.p. CP-55,940, 30 min prior to testing. There were no significant differences (two way RM ANOVA).
The involvement of noradrenergic mechanisms in cannabinoid mediation of cocaine place preference
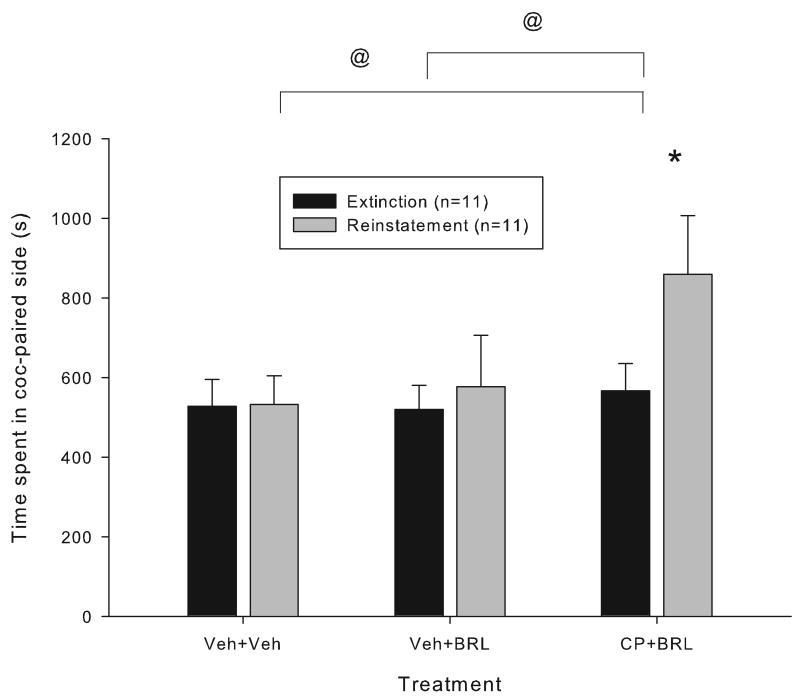
In order to examine the interaction between cannabinergic and noradrenergic systems in cocaine reinstatement, another group of mice which had been conditioned to cocaine and whose place preference had been extinguished were tested with either 1) vehicle, 2) the alpha-2 adrenergic antagonist BRL-44408 (5 mg/kg, i.p.) or 3) BRL-44408 (5 mg/kg, i.p.) and CP 55,940 (20 μg/kg). BRL-44408 alone did not induce reinstatement but when combined with the non-reinstating dose of CP 55,940, reinstatement was induced (F(2,20)=3.499, p<0.05, main effect of treatment, two-way RM ANOVA followed by Holm-Sidak multiple comparisons, P<0.05, Figure 3).
Figure 3.
The effect of combined administration of subthreshold doses of the cannabinoid agonist CP-55,940 and the alpha-2 adrenergic antagonist BRL-44408 on reinstatement of extinguished cocaine-induced conditioned place preference. Data represent the time spent in the cocaine-paired compartment (mean +/- SEM) during the extinction session prior to reinstatement testing and after reinstatement testing with vehicles (Veh + Veh), BRL-44408 (5 mg/kg, i.p.) and vehicle (BRL+ Veh), and BRL-44408 and CP 55,940 (20 μg/kg, i.p.) (CP+BRL). All injections were administered 30 min prior to reinstatement testing. A significant increase in the time spent in the cocaine-paired compartment during reinstatement testing compared to the preceding extinction session was seen after administration of CP and BRL (* =p<0.05, Holm-Sidak multiple comparison test) but not after BRL or vehicle alone. A significant difference between the time spent in the cocaine-paired compartment on reinstatement days was seen between the CP+BRL and the BRL+Veh and the Veh+Veh treatment groups (@ = p<0.05, Holm-Sidak multiple comparison test).
Discussion
We found that systemic administration of a cannabinoid antagonist can reverse stress- but not cocaine-induced reinstatement of cocaine seeking behavior in male C57Bl/6 mice. Cannabinoid receptors are located in many brain areas involved in stress and addiction (Herkenham et al., 1990; Mailleux and Vanderhaeghen, 1992). They influence the release of multiple neurotransmitters, including GAB A, glutamate, norepinephrine and dopamine (Lopez-Moreno et al., 2008). Given the widespread distribution of cannabinoid receptors and the multiplicity of neurotransmitter systems with which the ECS interacts, it is not surprising that the effects of systemic administration of cannabinoid agonists and antagonists have complex and even contradictory effects on addictive behavior. In particular, several studies which used rats to examine the effect of cannabinoids on cocaine reinstatement using a cocaine self-administration model found that cannabinoid antagonists block cocaine-induced reinstatement (De Vries et al., 2001; Filip et al., 2006 ; Xi et al., 2006) but not stress-induced reinstatement of cocaine seeking behavior (De Vries et al., 2001). In contrast, Ward et al., (2009) found that a cannabinoid antagonist did not block cocaine-induced reinstatement in mice. It is possible that different aspects of endocannabinoid effects on addiction are being revealed in studies that use different species, different tests to measure relapse, different drugs and dosages, and different stress paradigms.
The neurochemical pathways that mediate cocaine- and stress-induced reinstatement are not identical. There is evidence that stress-induced but not cocaine-induced reinstatement involves brain noradrenergic pathways, in particular noradrenergic systems that terminate in the bed nucleus of the stria terminalis (BNST) and central nucleus of the amygdala (Leri et al., 2002). In rat self administration models of cocaine reinstatement, treatments that reduce noradrenergic signaling, either by blocking beta adrenergic receptors (Leri et al., 2002) or suppressing the release of NE by alpha-2 adrenergic receptor activation (Erb et al 2000), reduce stress-induced but not cocaine-induced reinstatement of cocaine seeking. In addition, Mantsch et al., (2010) reported that beta adrenergic receptor antagonists or drugs that decrease NE release block stress-induced reinstatement but not cocaine-induced reinstatement using the mouse conditioned place preference methodology.
It is possible that the ECS plays a role in stress-induced reinstatement by enhancing the release of NE. We found that systemic injection of the cannabinoid agonist, CP 55,940 could not induce reinstatement when injected alone, but did cause reinstatement when combined with a non-reinstating dose of BRL-44408, an alpha-2A adrenergic antagonist that causes increased release of NE. Stress has been shown to alter endocannabinoid concentrations (Patel and Hillard, 2008; Cota, 2008; Hill et al., 2010) and there is significant interaction between the endocannabinoid and noradrenergic systems. Cannabinoid receptors are present in the locus coeruleus (LC) and nucleus of the solitary tract, noradrenergic brainstem nuclei that project to forebrain areas including the BNST (Herkenham et al., 1991) and electrophysiological studies have demonstrated that cannabinoid agonists affect firing rates of neurons in the LC (Mendiguren and Pineda, 2006; Muntoni et al., 2006). Further, the cannabinoid agonist WIN 55,212-2 increases c-fos expression in the LC (Patel and Hillard, 2003) and increases forebrain NE release as well as increase indices of noradrenergic activity (Oropeza et al., 2005; Page et al., 2007; Page et al., 2008). In addition to possibly acting on brain stem noradrenergic projection neurons, endocannabinoids could also be acting within the BNST. CB1 receptors have been localized on presynaptic excitatory and inhibitory synaptic terminals within the BNST (Puente et al., 2010) and mRNA for CB1 receptors has been found in both projection neurons to the BNST as well as in intrinsic inhibitory neurons (Matsuda et al., 1993). The CB1 receptors within the BNST have been linked anatomically and functionally to reward pathways (Massi et al., 2008; Grueter et al., 2006).
Alternative hypotheses regarding the interaction of cannabinergic and noradrenergic systems are also possible. For example, activation of CB1 receptors on GABAergic terminals could suppress inhibition of neurons downstream from NE release in the BNST.
The hypothesis that the ECS may serve as a link between stress and noradrenergic dependent processes has also been proposed for the consolidation of aversive memories. It is known that noradrenergic transmission within the basolateral amygdala is critical for stress-induced enhancement of memory consolidation (Roozendaal et al., 2002; Roozendaal et al., 2004; Roozendaal et al., 2006). Campolongo et al., (2009) have found that cannabinoid antagonists impair and cannabinoid agonists enhance stress-induced memory consolidation and have proposed that stress may act to enhance memory, at least in part, by stimulating the release of endocannabinoids which then lead to the release of NE.
Although the results of this study support a possible interaction between the ECS and noradrenergic systems in stress-induced reinstatement of cocaine seeking behavior, blockade of swim stress-induced reinstatement by AM-251 may also reflect the interaction of the ECS with other brain pathways and neurotransmitters. For example, the ECS has been implicated in the regulation of stress response by the hypothalamic-pituitary-adrenal axis, as well as the release of numerous neurotransmitter systems involved in addiction, including GABA, glutamate, dopamine, serotonin, acetylcholine and neuropeptides (Lopez-Moreno). The interaction of the ECS with these systems in stress-induced reinstatement remains to be investigated.
We found that a cannabinoid antagonist blocks stress- but not cocaine-induced reinstatement of cocaine seeking behavior in mice. Further, we have found that non-reinstating dose of a cannabinoid agonist and a non-reinstating dose of an alpha-2 adrenergic autoreceptor antagonist synergize to produce reinstatement. These results are consistent with the hypothesis that stress exposure but not cocaine triggers the endogenous activation of CB1 receptors and that the ECS interacts with noradrenergic mechanisms to influence stress-induced reinstatement of cocaine-seeking behavior.
Highlights.
Endocannabinoids and stress-induced cocaine reinstatement
Cannabinoid antagonist AM-251 blocks stress- induced cocaine reinstatement
Cannabinoid antagonist AM-251 does not block cocaine-induced cocaine reinstatement
Cannabinoid agonist does not induce cocaine reinstatement
Cannabinoid and noradrenergic agents synergize to induce cocaine reinstatement
Acknowledgments
This work was supported by National Institute on Drug Abuse (NIDA) grant number DA15758 to JRM, and DA09155 to CJH, and a Marquette University COR RRG to LKV.
Abbreviations
- NE
norepinephrine
- LC
locus coeruleus
- BNST
bed nucleus of the stria terminalis
- RM
repeated measures
- FS
forced swim
- ECS
endocannabinoid system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John R. Mantsch, Email: John.Mantsch@mu.edu.
Cecilia J. Hillard, Email: Chillard@mcw.edu.
References
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Barna I, Zelena D, Arszovszki AC, Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci. 2004;75:2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Milchanowski AB, Rothman RB. Evidence for alterations in alpha2-adrenergic receptor sensitivity in rats exposed to repeated cocaine administration. Neuroscience. 2004;125:683–90. doi: 10.1016/j.neuroscience.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Belej T, Manji D, Sioutis S, Barros HM, Nobrega JN. Changes in serotonin and norepinephrine uptake sites after chronic cocaine: pre- vs. post-withdrawal effects. Brain Res. 1996;736:287–96. doi: 10.1016/0006-8993(96)00713-5. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Effects of chronic cocaine self administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology. 2005;180:781–788. doi: 10.1007/s00213-005-2162-1. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdale enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O'Brien CP. Classically conditioned factors in drug dependence. In: Lowinson JH, Ruiz P, Millman RB, editors. Substance abuse: a comprehensive textbook. Baltimore: Williams and Wilkins; 1992. pp. 56–69. [Google Scholar]
- Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005;81:387–395. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cota D. The role of the endocannabinoid system in the regulation of hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol. 2008;20(1):35–38. doi: 10.1111/j.1365-2826.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grubler Y, Stalla J, Pasquali R, Lutz B, Stalla GK, Pagotto U. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary adrenal axis function. Endocrinology. 2007;148:1574–1581. doi: 10.1210/en.2005-1649. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and GABA inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self administration in rats. Behav Brain Res. 1999;104:141–146. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Filip M, Golda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegalinski E. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–819. [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in the medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hillard CJ, Hill MM. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–60. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–45. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology. 2002;26:130–134. doi: 10.1016/S0893-133X(01)00303-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;224:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–34. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Moreno JA, González-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addict Biol. 2008;13:160–87. doi: 10.1111/j.1369-1600.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, Porrino LJ. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Neuropsychopharmacol. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Fuentes JA. Opioid and cannabinoid receptor mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of delta (9)-tetrahydrocannabinol in rats. Brain Res. 1999;839:173–179. doi: 10.1016/s0006-8993(99)01756-4. [DOI] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitatioin of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–10508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur J Pharmacol. 2006;534:83–88. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–94. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Genetic liability increases propensity to prime-induced reinstatement of conditioned place preference in mice exposed to low cocaine. Psychopharmacology. 2008;198:287–296. doi: 10.1007/s00213-008-1137-4. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci Lett. 2008;431:1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid-induced Fos expression within A10 dopaminergic neurons. Brain Res. 2003;963:15–25. doi: 10.1016/s0006-8993(02)03797-6. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: A novel mechanism for stress habituation. Eur J Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Puente N, Elezgarai I, Lafourcade M, Reguero L, Marsicano G, Georges F, Manzoni OJ, Grandes P. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS One. 2010;5:e8869. doi: 10.1371/journal.pone.0008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–16. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Armario A, Miñarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004;81:150–154. doi: 10.1016/j.nlm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor–cAMP/PKA system in influencing memory consolidation. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- See RE, Grimm JW, Kruzich PJ, Rustay N. The importance of a compound stimulus in conditioned drug-seeking behavior following one week of extinction from self-administered cocaine in rats. Drug Alcohol Depend. 1999;57:41–49. doi: 10.1016/s0376-8716(99)00043-5. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology, and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Mendiza′bal V, Tourin∼o C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- andstress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Yokoo H, Mizoguchi K, Yoshida M, Tsuda A, Tanaka M. Noradrenaline release in the rat amygdala is increased by stress: studies with intracerebral microdialysis. Brain Res. 1991;544:174–176. doi: 10.1016/0006-8993(91)90902-8. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids reward, dependence, and underlying neurochemical mechanisms-a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Wade MR, Degroot A, Nomikos GG. Cannabinoid CB1 receptor antagonism modulates plasma corticosterone in rodents. Eur J Pharmacol. 2006;551:162–167. doi: 10.1016/j.ejphar.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–255. doi: 10.1016/j.drugalcdep.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. The role of CB1 receptors in psychostimulant addiction. Addict Biol. 2008;13:225–238. doi: 10.1111/j.1369-1600.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng XQ, Gardner EL. Cannabinoid CB1 receptor antagonists attenuate cocaine's rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]