Abstract
Preclinical research has suggested that the endocannabinoid system may be involved in the etiology and/or treatment of depression; however, there are no detailed studies examining endocannabinoid content in patients with clinical depression. This study examined the endocannabinoids (anandamide; AEA) and 2-arachidonylglycerol (2-AG) in serum from ambulatory, medication-free female patients diagnosed with minor or major depression, and in controls matched for demographic characteristics. Serum 2-AG content was significantly decreased in patients diagnosed with major depression, and this decrease was correlated significantly and negatively with duration of the depressive episode, such that 2-AG content was progressively lower the longer the depressive episode. While AEA was not associated with major depression per se, a strong negative correlation was found between serum AEA content and Hamilton ratings for cognitive and somatic anxiety, suggesting that AEA content may relate to the anxiety dimension of affective disorders. In subjects with minor depression, serum AEA was significantly elevated, with 2-AG content demonstrating a similar, but statistically insignificant trend. These are the first clinical data to indicate that the endocannabinoid system may be disturbed in affective disease, and suggest that future research is required to determine the relevance of these changes with respect to disease manifestation and pharmacotherapy.
Keywords: affective disease, CB1 cannabinoid receptor, anxiety, antidepressant
Introduction
The traditional theory, that major depression is a consequence of deficient monoamine activity has been revised by the addition of novel theories suggesting that disturbances in other systems are important for the pathophysiology of depression [16]. In particular, there is evidence that glucocorticoids, cytokines and neurotrophins are involved in the manifestation and treatment of this disease [17, 22, 34, 37, 40]. Mounting preclinical evidence implicates the endocannabinoid system in the pathophysiology of depression. For example, chronic unpredictable stress, which often elicits biochemical changes reminiscent of those seen in depressed populations [23], has been shown to both reduce the tissue content of the endocannabinoid ligand 2-arachidonylglycerol (2-AG) and down-regulate the central cannabinoid receptor (CB1) in the hippocampus of rats [14]. These data suggest that endocannabinoid activity could be compromised in depression, a suggestion that is supported by findings that pharmacological activation of the endocannabinoid system elicits an antidepressant-like response in both the forced swim test and tail suspension test in rodents [8, 12]. Furthermore, mice that are deficient in the CB1 receptor exhibit a constellation of behavioral changes that are reminiscent of symptoms of depression such as anhedonia, anxiety, inability to extinguish aversive memories, heightened stress responsiveness and disturbances in neurovegetative functions such as feeding behavior and weight regulation [2, 3, 24, 25, 32; reviewed in 13]. Together, these data imply that deficient endocannabinoid activity could be associated with the manifestation and progression of depression [13].
However, there is also evidence suggesting that up-regulation of the endocannabinoid system contributes to the symptoms of depression. Specifically, antagonism of the endocannabinoid system also produces antidepressant-like effects in preclinical animal models of antidepressant efficacy [10, 33, 36]. Post-mortem studies have revealed that the CB1 receptor is up-regulated in the prefrontal cortex of depressed, suicide victims [18]. While this would appear to suggest that the endocannabinoid system is up-regulated in depression, it should be noted that no endocannabinoid ligand measurements were done on this population, so the functional consequences of this change are not clear. The only published study to date of endocannabinoids in a population with affective disorders examined cerebrospinal fluid (CSF) content of N-arachidonylethanolamine (anandamide; AEA) in patients with affective disorders that were being used as a psychiatric control group; notably, these subjects were not a homogeneous population of depressed patients but included bipolar patients currently in a manic phase [6]. This study failed to find differences in the CSF AEA between control subjects and those with affective disorders, which could have been due, in part, to the diagnostic heterogeneity of the participants. This study also did not examine 2-AG, which is the endocannabinoid that has been shown to be altered in an animal model of depression [14]. The current state of knowledge would suggest that the endocannabinoid system could be involved in depression; however, due to a paucity of data from clinical populations, hypotheses concerning the nature of this involvement are premature.
Serum endocannabinoids present an interesting variable to examine in depressed populations for two reasons. First, changes in serum endocannabinoids could be representative of changes in CNS endocannabinoid content. It is known that endocannabinoids cross the blood brain barrier [7, 27, 39]; however, it should be noted that no direct correlation has been found between serum AEA and CSF AEA [6]. Regardless, recent clinical work has demonstrated that increases in serum endocannabinoid content following osteopathic treatment are correlated to specific subjective and behavioral measures suggestive of a cannabimimetic response, indicating that serum endocannabinoids could be reflective of changes in central endocannabinoid activity [26]. Second, peripheral actions of endocannabinoids could also be relevant in the context of depression. Endocannabinoids are known to potently modulate immunomodulatory processes and cardiovascular function [20, 21], both of which have been associated with morbidity and illness associated with depression. Thus, the peripheral actions of endocannabinoids themselves should not be discounted in this scenario.
The goal of the present study was to examine serum endocannabinoid content in clinically depressed individuals. We have quantified the endocannabinoids AEA and 2-AG in the serum of ambulatory, medication free, female subjects suffering from minor or major depression and compared them to psychiatrically healthy controls matched for demographic characteristics.
Methods
Patients
A total of 56 adult women from Saint Louis, MO USA participated in the study. Half of them (n= 28) met DSM-IV diagnostic criteria for clinical depression [1]; the other half (n=28) had no lifetime history of psychiatric illness. The depressed and control subjects were matched on a case-by-case basis with respect to age and ethnicity. All subjects were in good health, defined as having (a) no history of chronic medical illness, (b) no indications of acute infectious disease at study entry, as evidenced by self-report of symptoms and a normal complete blood count, and (c) no prescribed medication regimen, other than oral contraceptives, in the past six months including anti-depressants. Candidates were excluded if they were older than 55; had been pregnant in the past year; were menopausal, postmenopausal, or had irregular menses; were undernourished as evidenced by serum albumin ≤3.3 g/dL; or reported abusing illicit substances including cannabis, cocaine, and heroin.
Depressed patients were recruited through advertisements in local newspapers seeking individuals “feeling down and depressed, losing interest in enjoyable activities, or having trouble with eating, sleeping, or concentration.” To qualify for the study, depressed patients had to meet criteria for a current Major Depressive Episode (N=16) or Minor Depressive Episode (N=12) according to DSM-IV [1]. Specifically, the diagnosis of major depression requires the presence of at least five symptoms, from those detailed by the DSM-IV [1], for a duration of two weeks; whereas, the diagnosis of minor depression requires the presence of only two symptoms for a duration of two weeks [1]. Diagnoses were made by trained interviewers utilizing the Depression Interview and Structured Hamilton [4]. This instrument combines the probes needed to diagnose clinical depression according to DSM-IV with those needed to judge symptom severity on the 17-item Hamilton Rating Scale for Depression [37]. Based on criteria detailed in the DSM-IV [1], patients with comorbid psychotic, eating, alcohol, substance (other than nicotine dependence), or anxiety disorders (other than generalized anxiety disorder) were excluded using modules from the Diagnostic Interview Schedule [31] and the Primary Care Evaluation of Mental Disorders [35]. Control subjects were also recruited through newspaper advertisements; these postings sought “medically healthy adults for a study of mood and health.” To qualify for the study, control subjects had to match a depressed subject in terms of age and ethnicity, and have a lifetime history free of psychiatric illness, as documented in structured interviews using the Depression Interview and Structured Hamilton, and modules from the Diagnostic Interview Schedule and the Primary Care Evaluation of Mental Disorders. They also needed to score < 5 on the 10-item Center for Epidemiologic Studies Depression Scale [30].
Procedures
During an initial session at the laboratory, research assistants explained study procedures, and subjects provided written informed consent. A battery of structured psychiatric interviews was then administered to determine eligibility, namely the Depression Interview and Structured Hamilton, and modules from the Diagnostic Interview Schedule and the Primary Care Evaluation of Mental Disorders. Eligible subjects were interviewed regarding their medical history, completed a battery of questionnaires about their health practices, and underwent a series of anthropometric and cardiovascular assessments (data not shown). Next, subjects were seated in a comfortable chair and had 35-ml of blood drawn through antecubital venipuncture in serum separating tubes. The blood was subsequently centrifuged for 15 min at 1000 × g, and the serum was aspirated, divided into aliquots, and frozen at −70° C until the end of the study. All serum samples were frozen by 120 minutes following venipuncture. Thawed serum was later used to assess contents of 2-AG and AEA. All blood draws were performed between 0900h and 1200h to control for diurnal variation. Upon completion of the study, participants were compensated $150. These procedures were approved by the Institutional Review Board of Washington University, USA.
Serum endocannabinoids extraction and measurement
All extractions were performed using Bond Elut C18 solid-phase extraction columns (1 ml; Varian Inc, Lake Forest, CA). Serum samples (0.5 ml each) were thawed and made up to 15% ethanol, to which the internal standards [2H8]-AEA (16.9 pmol) and [2H8]-2-AG (46.5 pmol) (Cayman Chemicals, Ann Arbor, MI) were added. Samples were then vortexed and centrifuged at 1000 × g for 4 min. The supernatant was loaded on C18 columns, which have been conditioned with 1 ml redistilled ethanol and 3 ml of double distilled water (ddH2O). The remaining pellet was washed with 100 μl of 15% ethanol and centrifuged again for 3 min. The resulting supernatant was also loaded onto the C18 column. Columns were washed with 5 ml ddH2O and eluted with 1 ml of ethyl acetate. The ethyl acetate layer in the resulting elute was removed and dried under N2. Lipids in the residual ddH2O phase were extracted by mixing with an additional 1 ml of ethyl acetate, which was added to the original ethyl acetate solution. Once dried, samples were resuspended in 20 μl of methanol and stored at −80°C. AEA and 2-AG were quantified using isotope-dilution, atmospheric pressure, chemical ionization liquid chromatography/mass spectrometry (LC-APCI-MS) as described previously [28].
Statistics
Comparisons between individuals with depression and matched controls on serum endocannabinoid contents were performed using independent samples t-tests. To verify that observed associations were not inflated by potential confounders, a series of univariate analyses of variance were then performed. These analyses involved comparing endocannabinoid contents across depressed and control groups, while covarying for factors that differed across diagnostic group, which were body mass index (BMI) and percentage of daily smokers. Bivariate correlations were also performed to examine the relationships between serum endocannabinoids and the clinical profiles of patients, including the duration of their current episode, its severity, and the intensity of particular symptom clusters.
Results
The demographic profile of the population that was used in this analysis can be seen in Table 1. For the sample of individuals with major depression and their matched controls, there were no differences in age (p = 0.93), race (p = 1.00) or years of education (p = .15). Individuals with major depression exhibited a significantly higher body mass index (BMI; p = 0.001) compared with their matched controls, and they were also more likely to be daily smokers (p = 0.02). The number of alcoholic drinks consumed in a week was higher, but not significantly, in individuals with major depression (p = 0.07). For the sample of individuals with minor depression and their matched controls, there were no differences between the two groups on age (p = 0.80), race (p = 0.84), percentage of daily smokers (p = 0.17) or alcoholic drinks consumed per week (p = 0.62), however years of total education was non-significantly lower in individuals with minor depression (p = 0.06).
Table 1.
Demographic characteristics of sample population (mean variable +/− SD)
| Major Depression (n=16) | Control (n=16) | Minor Depression (n=12) | Control (n=11) | |
|---|---|---|---|---|
| Age | 27.6 +/− 9.7 | 27.9 +/− 9.2 | 31.0+/− 8.0 | 30.2 +/− 6.9 |
| BMI | 31.2 +/− 8.1* | 23.8 +/− 2.4 | 31.8 +/− 10.9 | 27.6 +/− 6.7 |
| Education (years) | 14.4 +/− 1.8 | 15.4 +/− 1.8 | 14.6 +/− 2.0 | 16.2 +/− 1.9 |
| % daily smokers | 31.3 %+/− 47.9* | 0 +/− 0 | 16.7 % +/− 38.9 | 0 +/− 0 |
| Alcoholic drinks / week | 3.4 +/− 5.7 | 0.6 +/− 1.3 | 1.5 +/− 3.1 | 2.4 +/− 5.6 |
| % recurrent depression | 75% | N/A | 75% | N/A |
| Total HAM-D Score | 20 +/− 4.0 | N/A | 15.3 +/− 4.7 | N/A |
| Length of Current | ||||
| Episode (weeks) | 35.4 +/− 30.1 | N/A | 41.3 +/− 51.2 | N/A |
| % currently in therapy | 12.5 % +/− 34.2 | N/A | 0 % +/− 0 | N/A |
| Race‡ | 6C; 8AA; 1H; 1A | 6C; 8AA; 1H; 1A | 6C; 6AA | 6C; 5AA |
C=Caucasian; AA=African American; H=Hispanic; A=Asian
= significantly different from their matched control group, p < 0.05.
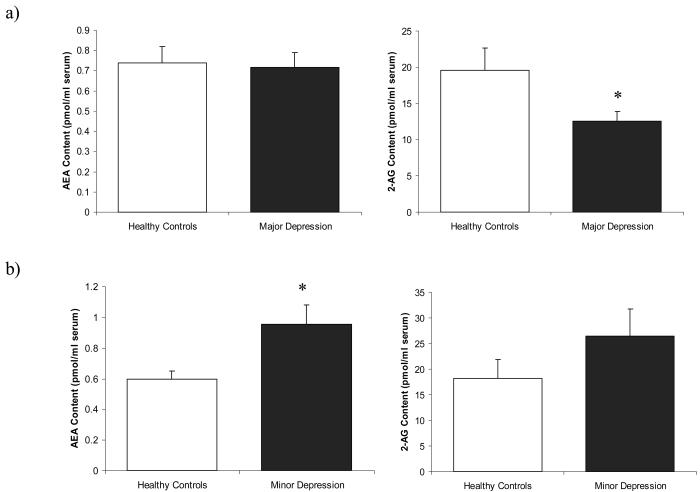
In women with major depression, serum 2-AG was significantly reduced [t (30) = 2.098, p = 0.04; Fig. 1a] (serum 2-AG for major depression: 12.5 +/− 5.6 pmol/ml serum vs. matched controls: 19.6 +/− 12.5 pmol/ml serum), with an effect size of 0.57 standard deviations. This effect was marginally reduced by controlling for tobacco use (p = 0.08), and marginally enhanced by controlling for BMI (p = 0.03).
Figure 1.
Serum content of the endocannabinoids anandamide (AEA) and 2-arachidonylglycerol (2-AG) in women with: a) major depression and their matched controls (n=16/group); or b) women with minor depression and their matched controls (n=12 for minor depression; n=11 for controls). Values are denoted in pmol/ml of serum AEA or 2-AG content. * Significantly different from control (p < .05).
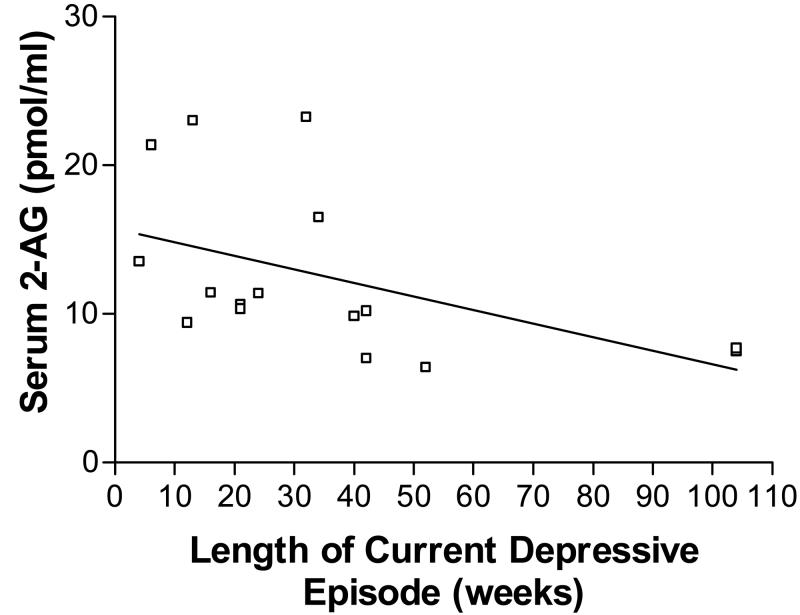
To examine if this reduction in serum 2-AG was related to severity, chronicity or symptom profile of major depression, we examined the correlation of serum 2-AG to each of these variables in the major depression cohort. Serum 2-AG content was found to exhibit a significant negative correlation with duration of current depressive episode (r = −.492, p = 0.05; Fig. 2). Within the sample of subjects with major depression, there was no difference in serum 2-AG between those with recurrent major depression and those in their first episode [t (14) = 0.133, p = 0.90]. Serum 2-AG did not significantly correlate with total Hamilton score (r = −.09, p = 0.74), suggesting that this reduction of 2-AG was not directly related to depression severity.
Figure 2.
Serum content of the endocannabinoid 2-arachidonlyglycerol (2-AG) exhibited a significant negative correlation (r = −.492) with the duration of the current depressive episode (in weeks).
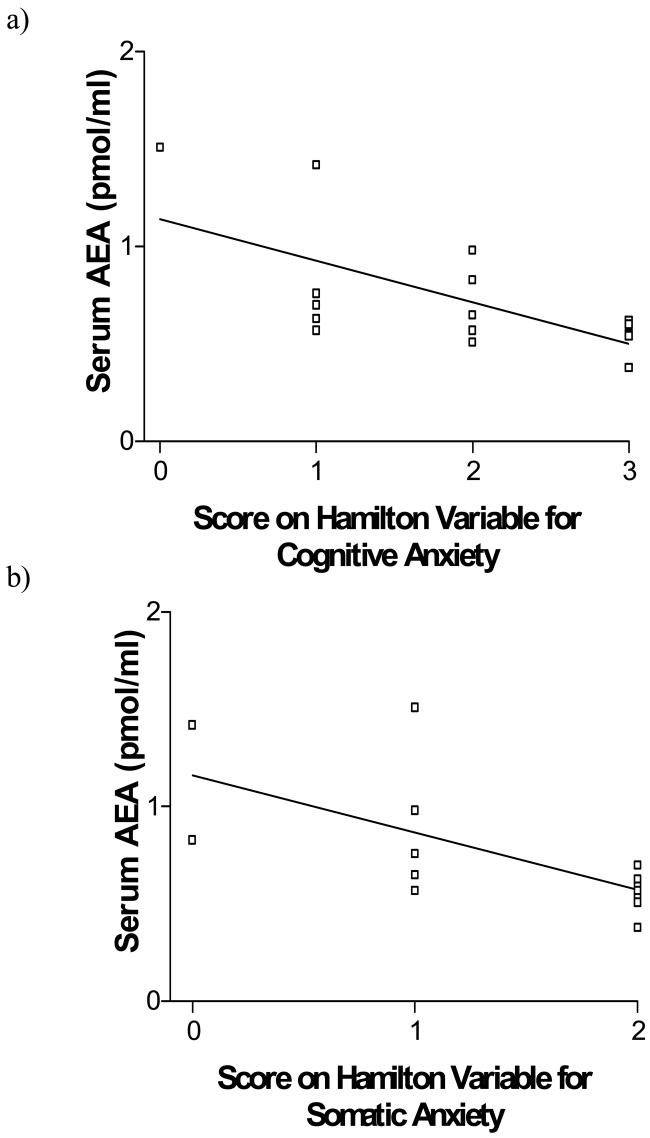
Serum AEA content did not differ between subjects with major depression and their matched controls [t (30) = 0.205, p = 0.84; Fig. 1a] (serum AEA for major depression: 0.74 +/− 0.32 pmol/ml serum vs. matched controls: 0.72 +/− 0.29 pmol/ml serum). While serum AEA was not correlated with total Hamilton score (r = −.24, p = 0.38), serum AEA exhibited a highly significant, negative correlation with scores on both the Hamilton variable for cognitive anxiety (r = −.647, p < 0.01; Fig. 3a) and somatic anxiety (r = −.674, p < 0.01; Fig. 3b), such that individuals who demonstrated higher measures of anxiety, exhibited lower serum AEA content. Serum AEA content did not correlate with any other Hamilton rating. Serum AEA did not correlate with duration of current depressive episode (r = .30, p = 0.25), nor did serum AEA content differ between subjects who experienced recurrent depression as opposed to those who were experiencing their first episode [t (14) = 1.12, p = 0.28].
Figure 3.
(a) Serum content of the endocannabinoid N-arachidonylethanolamine (anandamide; AEA) exhibited a significant negative correlation (r = −.647) with Hamilton ratings for cognitive anxiety.
(b) Serum content of the endocannabinoid AEA exhibited a significant negative correlation (r = −.674) with Hamilton ratings for somatic anxiety.
Serum AEA content was significantly increased in patients with minor depression compared to their matched controls [t (21) = 2.48, p = 0.02; Fig. 1b] (serum AEA for minor depression: 0.95 +/− 0.44 pmol/ml serum vs. matched controls: 0.60 +/− 0.18 pmol/ml serum), with a robust effect size of 1.96 standard deviations. Serum 2-AG was higher in patients with minor depression; however, this was not significant [t (21) = 1.26, p = 0.22; Fig. 1b] (2-AG in minor depression: 26.4 +/− 18.32 pmol/ml serum vs matched controls: 18.18 +/− 12.37 pmol/ml serum, effect size = 0.67 standard deviations). The length of the current depressive episode appeared to correlate with both serum AEA (r = .51, p = .09) and serum 2-AG (r = .50, p = .10), such that serum contents of both these molecules were higher the longer the duration of the episode. However, because of the small number of patients in these analyses, these effects were not statistically significant. There were no significant differences in serum AEA [t (10) = −.169, p = .12] or serum 2-AG [t (10) = 1.76, p = .11] between those experiencing a first episode of depression versus recurrent depression. There was no significant correlation between total Hamilton score and serum AEA (r = −.19, p = .55). The correlation between total Hamilton scores and serum 2-AG content was positive (r = .51, p = .10), but nonsignificant because of the sample size of 12 subjects. It should be noted that one sample from the control group to the minor depression patients was compromised during the extraction procedure and thus data from this individual is not included in the data set.
Discussion
The major finding of this study was that in a population of ambulatory, medication free females diagnosed with major depression, serum content of the endocannabinoid 2-AG was significantly decreased. The magnitude of this decrease was significantly related to the duration of the current depressive episode, such that as an episode progressed, 2-AG content decreased more substantially. These data provide the first demonstration that major depression could be associated with a hypoactive endocannabinoid system.
While serum AEA content was not significantly altered in major depression, amounts of this molecule were strongly and negatively correlated with somatic and cognitive anxiety. That is, patients with high anxiety also exhibited low serum AEA. This finding suggests that while AEA may not be related to depression per se, it could be inversely related to the extent of anxiety present. Consistent with this, animal research has demonstrated that selective inhibition of AEA metabolism elicits robust anxiolytic effects [15, 19, 32]. Together, these data support the idea that low AEA may be associated with the manifestation of anxiety and that inhibition of AEA metabolism is a logical target for the development of anxiolytic drugs [5, 15, 19, 29].
The relationship between serum endocannabinoid content and central endocannabinoid content is not known. Since endocannabinoids possess the ability to cross the blood brain barrier [7, 27, 39], it is certainly plausible that significant changes in central endocannabinoid content would result in spillover into the serum. Alternatively, it is also possible that endocannabinoid synthesis in the periphery may regulate to some degree endocannabinoid levels in the central nervous system. The finding that significant correlations were found in this study between serum endocannabinoids and specific clinical profiles on the Hamilton scale is circumstantial evidence that serum endocannabinoids reflect central endocannabinoid content to some degree. This hypothesis is substantiated by recent work demonstrating that cannabimimetic-like responses to osteopathic manipulations are associated with increases in serum endocannabinoid content [26].
An unexpected finding in this study was that the changes in serum endocannabinoids observed in minor depression were the opposite of those seen in major depression. Specifically, AEA was significantly higher whereas 2-AG was appreciably, but not signficantly, elevated among women with minor depression compared to their matched controls. Given that minor depression is only a less severe variant of major depression it is surprising that these opposite effects were found. One possible interpretation of this finding is that endocannabinoids may act as a protective buffer against the progression of affective disease; however, the current data require replication and extension before this hypothesis can be further substantiated.
There are several limitations to this research that require attention. For example, the effect that has been documented here is exclusively in a population of women, thus a determination of whether this effect is present in both genders or is limited to women is critical to the advancement of theories concerning the role of endocannabinoids in depression. In line with this limitation, menstrual cycle was not controlled for in this study; however, fluctuations in serum endocannabinoid content throughout the menstrual cycle would likely introduce increased variability that would occlude detection of a significant finding. Furthermore, analysis of CSF endocannabinoids, especially 2-AG, in depressive disorders is essential to determine if a deficiency is consistently observed in the central nervous system. Another limitation with these findings is that these data are cross-sectional and thus do not give a direct understanding of the direction of causality, or insights into how these changes in endocannabinoid serum content progress throughout remission and relapse within a given individual. Finally, the possibility does exist that an undetermined third variable could mediate the effects documented here. Analysis of covariance determined that the deficit in 2-AG seen in major depression was somewhat reduced when tobacco use was covaried; however, this covariance accounted for only a small proportion of the documented effect. We view this as an unlikely explanation for our findings, however, as it cannot parsimoniously account for why the direction of change of serum endocannabinoids was opposite for minor and major depression when both groups possessed more smokers than their matched controls. Additionally, while exclusion criteria for this study included cannabis dependence, it is unknown whether patients occasionally consumed cannabis in the weeks leading up to assessment. Some depressed patients do use cannabis as self medication [11], and it is possible that such use could disrupt endogenous production of 2-AG or AEA. However, animal research has suggested that repeated administration of exogenous cannabinoid ligands does not reliably reduce brain endocannabinoid content [9], and, as with smoking, this effect could not explain the bidirectional alterations seen in the two depressed populations.
These data are the first demonstration of an alteration in the endocannabinoid system in clinical depression. These data demonstrate for the first time, that serum levels of the endocannabinoid 2-AG are significantly reduced in major depression, where as serum levels of the endocannabinoid AEA are significantly increased in minor depression. Furthermore, these data also demonstrate that serum AEA levels exhibited a strong, negative correlation with anxiety symptoms in the major depression population. While the current data cannot explain the nature of the relationship between these changes in endocannabinoid levels and the clinical manifestation of depressive disorders, they provide the first step to which this question can begin to be explored, and possibly the first clinical evidence supporting the development of agents which non-selectively inhibit uptake or metabolism of AEA and 2-AG as an effective form of treatment for comorbid depression-anxiety disorders.
Acknowledgements
This research was supported by operating grants from the American Heart Association and the Canadian Institute of Health Research (CIHR) to GEM; a Young Investigator Award from NARSAD and a Michael Smith Foundation for Health Research (MSFHR) Scholar Award to GEM; an Independent Investigator NARSAD Award and a NIH grant DA16967 to CJH; a Natural Sciences and Engineering Research Council of Canada (NSERC) operating grant to BBG; and a MSFHR postgraduate trainee award and a NSERC Canadian Graduate Scholarship to MNH. The authors would like to thank Craig Roelke for his technical assistance.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- 2.Barna I, Zelena D, Arszovszki AC, Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci. 2004;75:2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedland KE, Skala JA, Carney RM, Raczynski JM, Taylor CB, Mendes de Leon CF. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosom Med. 2002;64:897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 5.Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends Mol Med. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 7.Glaser ST, Gatley SJ, Gifford AN. Ex vivo imaging of fatty acid amide hydrolase activity and its inhibition in the mouse brain. J Pharmacol Exp Ther. 2006;316:1088–1097. doi: 10.1124/jpet.105.094748. [DOI] [PubMed] [Google Scholar]
- 8.Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez S, Fernandez-Ruiz J, Di Marzo V, Hernandez M, Arevalo C, Nicanor C, et al. Behavioral and molecular changes elicited by acute administration of SR141716 to Delta9-tetrahydrocannabinol-tolerant rats: an experimental model of cannabinoid abstinence. Drug Alcohol Depend. 2004;74:159–170. doi: 10.1016/j.drugalcdep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Gruber AJ, Pope HG, Jr, Brown ME. Do patients use marijuana as an antidepressant? Depression. 1996;4:77–80. doi: 10.1002/(SICI)1522-7162(1996)4:2<77::AID-DEPR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Hill MN, Gorzalka BB. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav Pharmacol. 2005;16:333–352. doi: 10.1097/00008877-200509000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- 15.Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Hindmarch I. Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur Psychiatry. 2002;17(Suppl 3):294–299. doi: 10.1016/s0924-9338(02)00653-3. [DOI] [PubMed] [Google Scholar]
- 17.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 18.Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- 19.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 20.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 21.Kunos G, Jarai Z, Batkai S, Goparaju SK, Ishac EJ, Liu J, et al. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- 22.Leonard BE, Song C. Stress and the immune system in the etiology of anxiety and depression. Pharmacol Biochem Behav. 1996;54:299–303. doi: 10.1016/0091-3057(95)02158-2. [DOI] [PubMed] [Google Scholar]
- 23.Lopez JF, Chalmers DT, Little KY, Watson SJ. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 24.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 25.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 26.McPartland JM, Giuffrida A, King J, Skinner E, Scotter J, Musty RE. Cannabimimetic effects of osteopathic manipulative treatment. J Am Osteopath Assoc. 2005;105:283–291. [PubMed] [Google Scholar]
- 27.Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 28.Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. J App Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Robins LN, Helzer JE, Croughan J, Ratcliff K. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 32.Sanchis-Segura C, Cline BH, Marsicano G, Lutz B, Spanagel R. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology. 2004;176:223–232. doi: 10.1007/s00213-004-1877-8. [DOI] [PubMed] [Google Scholar]
- 33.Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, et al. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Song C, Horrobin DF, Leonard BE. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry. 2006;39:88–99. doi: 10.1055/s-2006-941557. [DOI] [PubMed] [Google Scholar]
- 35.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- 36.Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaidya VA, Duman RS. Depresssion--emerging insights from neurobiology. Br Med Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- 38.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 39.Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther. 1997;282:243–247. [PubMed] [Google Scholar]
- 40.Yukimasa T, Yoshimura R, Tamagawa A, Uozumi T, Shinkai K, Ueda N, et al. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39:52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]