Abstract
Aims
Multidrug resistance (MDR) mediated by overexpression of drug efflux transporters such as P-glycoprotein (P-gp), is a major problem, limiting successful chemotherapy of breast cancer. The use of siRNA to inhibit P-gp expression in MDR tumors is an attractive strategy to improve the effectiveness of anticancer drugs.
Method
We have synthesized a novel conjugate between a phospholipid (dioleoylphosphatidylethanolamine) and polyethylenimine (PEI) for siRNA delivery, for the purpose of silencing P-gp to overcome doxorubicin resistance in MCF-7 human breast cancer cells.
Results
The dioleoylphosphatidylethanolamine-PEI conjugate enhanced the transfection efficacy of low-molecular-weight PEI, which was otherwise totally ineffective. In addition, the polyethylene glycol/lipid coating of the new complexes gave rise to small micelle-like nanoparticles with improved biocompatibility properties. Both coated and noncoated formulations delivered P-gp-specific siRNA to MDR cells.
Discussion
The combination of doxorubicin and P-gp silencing formulations led to a twofold increase of doxorubicin uptake and a significant improvement of the therapeutic effect of doxorubicin in resistant cells.
Keywords: MCF-7 breast cancer cell, micelle-like nanoparticle, multidrug resistance, P-glycoprotein, polyethylenimine, siRNA delivery
Multidrug resistance (MDR) is a major problem, limiting the treatment of breast cancer. Several molecular mechanisms are involved in the development of a drug-resistant phenotype [1]. One of the most studied resistance mechanisms is the reduction of intracellular drug concentration by transporter proteins that pump drugs out of cells before they reach the site of action. Many of these transporters are members of the ATP-binding cassette transmembrane protein super-family, including P-glycoprotein (P-gp), MDR protein-1 (MRP-1) and breast cancer resistant protein. P-gp was the first described and is the best characterized to date.
P-gp encoded by the MDR-1 gene is a 170 kDa plasma membrane protein with 12 transmembrane domains and two ATP-binding domains. P-gp uses ATP energy to transport drugs and other xenobiotics from the intracellular to the extracellular compartment, although it may also interact with transmembrane substrates [2]. The localization of P-gp in normal tissues suggests that this transporter has the physiological function of detoxification and excretion of xenobiotics [3,4]. In tumor tissue, intrinsic or induced overexpression of P-gp after exposure to chemotherapy agents has been extensively investigated as one primary reason for chemotherapy failure in different MDR cancer types [5–8]. Regarding breast cancer, an induced expression of P-gp in cancer cells by exposure to anticancer drugs was detected in 52% of chemotherapy-treated patients [9]. P-gp expression has also been related to a significant increase in doxorubicin and taxol resistance of breast tumors, regardless of prior treatment [10].
Apart from chemical P-gp inhibitors [11,12], the silencing of the MDR-1 gene with siRNA could become a powerful tool to restore chemotherapy sensitivity in MDR cancer cells [13,14]. siRNA duplexes are short nucleotide molecules of approximately 21–25 base pairs that can trigger silencing of homologous gene expression by inducing degradation of the complementary mRNA [15]. Clear advantages of the siRNA approach compared with chemical inhibitors are its reduced toxicity towards nonspecific tissues and its high specificity. The main problems with siRNA include its poor in vivo stability (rapid degradation in plasma and cellular cytoplasm) and poor cellular uptake that have limited its clinical application to date.
Polyethylenimine (PEI) is widely used for nucleic acid delivery, including siRNA delivery [16–19]. The high charge density of PEI permits the condensation of nucleic acids by electrostatic interactions into dense particles (complexes) that protect the genetic material from enzymatic degradation and promote their cellular uptake by absorptive endocytosis. Additionally, PEI has an intrinsic endosomal escape mechanism known as the ‘proton sponge’ effect, which causes osmotic swelling and rupture of the endosome membrane, which then triggers the release of PEI complexes into the cytosol [20]. In general, high-molecular-weight PEIs provide high transfection efficiency but also have high toxicity due to nonspecific interactions with blood components and nontarget sites. By contrast, low-molecular-weight PEIs are more biocompatible but much less efficient. In order to achieve a more favorable balance between the efficacy and toxicity of PEI, different strategies have been suggested, including the conjugation with lipid or polyethylene glycol (PEG) moieties. Lipid substitution of cationic polymers, mainly grafting fatty acid or cholesterol, has been reported to improve the stability and uptake of the complexes, most probably because the lipid residues provide a better interaction with the cellular membrane [21–25]. Noncovalent association of PEI complexes with either PEGylated or non-PEGylated liposomal formulations has also led to nontoxic and efficient siRNA in vitro delivery [26,27].
In previous studies, we have demonstrated that phosphatidylcholine (PC)-modified PEI effectively condensed and protected siRNA or plasmid DNA from enzymatic degradation and improved its uptake by cancer cells. We have also reported on micelle-like nanoparticles (MNPs), that is, phospholipid–PEI complexes coated with a PEG–lipid/lipid mix [28,29]. MNPs showed similar transfection efficacy to that of PEI but with improved biocompatibility properties, such as the absence of in vitro or in vivo acute toxicity and prolonged blood circulation [28].
In this study, the positive results obtained with PC-modified PEI and MNPs have been further improved by grafting a different phospholipid, dioleoylphosphatidylethanolamine (DOPE), to the backbone of PEI. Additionally, the potential therapeutic application of this novel lipid-PEI carrier has been confirmed by combining P-gp silencing siRNA delivery with doxorubicin treatment to overcome the drug resistance in human breast cancer MCF-7 cells.
Materials & methods
Materials
All materials were purchased from Sigma-Aldrich (MO, USA), unless otherwise stated. Branched PEI with a molecular weight of 1.8 kDa was purchased from Polysciences, Inc. (PA, USA). 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1,2-disrearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (PEG)-2000] (PEG-PE), cholesterol, and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(glutaryl) were purchased from Avanti Polar Lipids (AL, USA). All siRNA duplexes were purchased from Dharmacon (CO, USA). Namely, siRNA targeting green fluorescent protein (GFP-siRNA): 5´-AUGAACUUCAGGGUCAGCUdTdT-3´ (sense) [30], siRNA targeting MDR-1 (siMDR-1): 5´-GGAAAAGAAACCAACUGUCdTdT-3´ (sense) [31], and a non-targeting control siRNA, (siNegative): 5´-AGUACUGCUUACGAUACGGdTdT-3´ (sense). Fluorescein isothiocyanate-labeled P-gp antibody (UIC2) was purchased from Abcam (MA, USA). Lipofectamine™ 2000 Reagent was purchased from Invitrogen (CA, USA). The CellTiter-Blue® Cell Viability Assay was purchased from Promega (WI, USA). Cells of the c166 cell line (mouse yolk sac embryo) stably transfected with a plasmid reporter vector, pEGFP-N1, encoding for the enhanced GFP, were obtained from the American Type Culture Collection (VA, USA). DMEM was supplemented with 10% fetal bovine serum from Atlanta Biologicals (GA, USA) and 0.2 mg/ml of Geneticin (G-418, Invitrogen). The wild-type (sensitive) and doxorubicin-resistant MCF-7 human breast adenocarcinoma cells were kindly provided by T Minko (The State University of New Jersey, NJ, USA). The resistant MCF-7 phenotype has been previously characterized as overexpressing the MDR-1 gene [32]. Both MCF-7 cell lines were grown at 37°C under 5% CO2 in DMEM supplemented with 10% fetal bovine serum and penicillin (100 units/ml) and streptomycin (100 µg/ml). DMEM and penicillin/streptomycin stock solutions were purchased from Cellgro (VA, USA). Heat-inactivated fetal bovine serum was purchased from Atlanta Biologicals (GA, USA). Nuclease-free water was purchased from Qiagen (MD, USA).
Methods
Synthesis of DOPE–PEI conjugates
The DOPE-PEI conjugate was synthesized from PEI 1.8 kDa and the glutaryl-modified phospholipid 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine. Briefly, the glutaryl-modified phospholipid 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine (5.5 µM) in chloroform was activated with N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide HCl and N-hydroxysuccinimide followed by addition of PEI 1.8 kDa (5.5 µM). The mixture was incubated with 4 µl of triethylamine at room temperature for 24 h with stirring. The chloroform was removed under nitrogen gas and the residue was suspended with 1 ml of distilled water. The product was purified by dialysis (molecular weight cut-off: 2000 Da) against distilled water and lyophilized. The solid product (5–10 mg) was dissolved in chloroform-d (1 ml) and characterized by 1H-nuclear magnetic resonance using Varian 500 mHz spectroscopy. Analysis of spectra from peak integration indicated the ratio of DOPE:PEI as 1:1.
Preparation of DOPE-PEI/siRNA complexes
Complexes were prepared by mixing a fixed amount of siRNA and varying amounts of DOPE-PEI that were separately diluted in equal volumes of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered glucose solution, (pH 7.4; nuclease-free). The siRNA solution was transferred to the polymer solution, mixed by pipetting and incubated for 15 min. The polymer/siRNA ratio was expressed as the PEI nitrogen/nucleic acid phosphate (N/P) ratio, and calculated assuming that each repeating unit of PEI containing one amine corresponds to 43 g/mol and that 316 g/mol corresponds to each repeating unit of siRNA containing one phosphate.
Gel retardation studies
For gel retardation studies, complexes containing 750 ng of siRNA with varying amounts of DOPE-PEI or PEI 1.8 kDa in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered glucose solution were subjected to electrophoresis on a 0.8% agarose gel, using an E-Gel electrophoresis system (Invitrogen Life Technologies) and evaluated under UV light.
Ethidium bromide exclusion assay
The binding of DOPE-PEI to siRNA was examined by the quenching method based on the use of ethidium bromide (EtBr). The experiments were carried out by measuring the fluorescence intensity of complexes prepared from siRNA (5 µg/ml) with EtBr intercalated at a molar ratio of 2:1 (siRNA:EtBr) as increasing amounts of DOPE-PEI or PEI 1.8 kDa were added. The fluorescence was measured using a 96-well plate reader (Multiscan MCC/340) at the excitation and emission wavelengths of 540 and 580 nm, respectively. The relative fluorescence values were determined as follows: Fr = (Fm–Fe) × 100/ (Fo–Fe), where Fr is the relative fluorescence, Fm is the measured fluorescence, Fe is the fluorescence of EtBr in the absence of siRNA and Fo is the initial fluorescence in the absence of the polycation.
Stability of siRNA in DOPE-PEI complexes against RNase digestion
Nuclease resistance of the siRNA in DOPE-PEI complexes was determined by the treatment of the samples with 1 U of RNase III/µg siRNA for 2 h at 37°C. Complexes were disassembled by adding heparin (40 U/µg siRNA) and analyzed by agarose gel electrophoresis. The integrity of siRNA in complexes was compared with that of naked siRNA.
Preparation of DOPE-PEI-based MNPs
The MNPs were assembled with DOPE-PEI:POPC:cholesterol:PEG-PE (4:3:3:0.3 mol/mol) and siRNA. First, 32 µg DOPE-PEI (21.7 µg as PEI) and 10 µg siRNA corresponding to an N/P ratio of 16 were diluted separately in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered glucose solution and mixed to a final volume of 200 µl. A dry lipid film was prepared from the mixture of POPC, cholesterol and PEG-PE (6.8, 3.5 and 2.5 µg; 3:3:0.3 mol/mol). The lipid film was hydrated with the preformed DOPE-PEI complexes and incubated for 1 h at room temperature.
Particle size & zeta-potential measurements
The particle size and zeta potential of the formulations were measured by quasi-electric light scattering using a zeta plus particle analyzer (Brookhaven Instruments Corp, CA, USA). Scattered light was detected at 25°C at an angle of 90°. Samples (100 µl) of complexes and MNPs were diluted in 1.7 ml of nuclease-free water and measured immediately after preparation.
GFP silencing
In vitro GFP silencing experiments were performed in stably transfected c166 GFP cells using GFP-siRNA. A nontargeting control duplex (negative-siRNA) was used as a non-specific control siRNA. Cells were seeded 24 h prior to transfection in 12-well plates at a density of 5 × 104 per well and complete medium was replaced with fresh serum-free medium. DOPE-PEI complexes at varying N/P ratios were added to cells to yield a final siRNA concentration of 100 nM. After 4 h of incubation, the complexes were removed and fresh complete media was added. The cells were further incubated for 48 h. Thereafter, the cells were washed, detached by trypsinization, and GFP downregulation was analyzed by flow cytometry. In the second set of experiments, c166 GFP cells were treated, under the above conditions, with free siRNA or siRNA formulated in PEI 1.8 kDa complexes, DOPE-PEI complexes or MNPs. All formulations were prepared at a N/P ratio of 16. Lipofectamine 2000 was used as a positive control according to the manufacturer’s protocol.
P-gp silencing
For P-gp silencing experiments, MCF-7 resistant cells were seeded in 12-well plates at a density of 5 × 104 per well. After 24 h, the cells were treated with DOPE-PEI complexes, MNPs or PEI 1.8 kDa complexes containing either siMDR-1 or a scramble siRNA (siNegative). All formulations were prepared at an N/P ratio of 16. Lipofectamine 2000 was used as a positive control. The final concentration of siRNA was 100 nM. After 4 h of incubation, the treatments were removed and cells were reincubated for 48 h. The cells were washed, detached by mechanical scrapping and resuspended in bovine serum albumin 0.5%. The cell suspensions were incubated with a fluorescein isothiocyanate-labeled antibody against P-gp at 4°C. After 40 min of incubation, the cells were washed with 0.5% bovine serum albumin and analyzed by flow cytometry.
Intracellular doxorubicin accumulation
MCF-7 resistant and sensitive cells were seeded in 12-well plates at a density of 5–8 × 104 per well. After 24 h, the cells were treated with DOPE-PEI complexes or MNPs containing either siMDR-1 or a scramble siRNA (siNegative). All formulations were prepared at an N/P of 16. The final concentration of siRNA was 100 nM. After 4 h of incubation, the treatments were removed and cells were reincubated for 48 h. Cells were then incubated for 1 h with doxorubicin (5 µg/ml). After incubation, suspended cells were washed, trypsinized and fixed in a 4% formalin–phosphate-buffered solution. Accumulation of intracellular doxorubicin was analyzed by flow cytometry.
Cytotoxicity assays
The effect of P-gp silencing on doxorubicin toxicity was studied in MCF-7 resistant and sensitive cells. Cells were seeded in 96-well plates at 3000 cells/well. After 24 h, the cells were treated with siRNA (siMDR-1 or siNegative) formulations for 4 h. Doxorubicin (1 µg/ml) was added 48 h post-siRNA. Control cells were treated with only doxorubicin. Cell viability was measured after 24, 48, 72 and 96 h of doxorubicin incubation with the CellTiter-Blue® (Promega). Briefly, 20 µl of CellTiter Blue was added to 100 µl each well, and the plates were reincubated for 2 h. The fluorescence was measured at the excitation and emission wavelength of 560 and 590 nm, respectively.
In a second set of experiments, the effect of the time lag between siRNA and doxorubicin treatments on the drug toxicity was investigated in MCF-7 resistant cells. The cells were seeded in 96-well plates at 3000 cells/well. After 24 h, the cells were treated with formulations prepared with siMDR-1 and combined with doxorubicin (1 µg/ml) after 0, 4, 8, 24 and 48 h. Control cells were treated only with doxorubicin. The cell viability was measured after 72 h of doxorubicin incubation with the CellTiter-Blue.
Statistical analysis
Results are presented as mean ± standard deviation, and statistical significance of differences was evaluated by variance analysis, ANOVA; p-values smaller than 0.05 were considered to indicate a significant difference.
Results
Intracellular delivery of siRNA with DOPE-PEI complexes
It is well-known that the capacity of PEI to condense and protect siRNA can diminish if the backbone of the polymer is grafted with lipid or PEG motifs [21,33,34]. Therefore, the first step for the characterization of these novel conjugates was to assess their ability to bind siRNA, form dense particles, and protect siRNA from enzymatic degradation (Supplementary Figure 1, see online www.futuremedicine.com/doi/suppl/10.2217/nnm.11.93). The DOPE-PEI conjugates showed siRNA complexation similar to nonmodified PEI. The biophysical characterization of DOPE-PEI complexes revealed particles ranging from 120 to 180 nm and with a positive surface charge at N/P ≥3. Only complexes formed at a N/P ratio of 3 led to bigger particles, less homogeneous in size and with negative zeta potential values. This increment in the particle size when electroneutrality is reached has been reported for low-molecular-weight PEI and other polymers [35,36]. DOPE-modified PEI protected siRNA from RNase III degradation for at least 2 h. The quantification of the intact siRNA (ImageJ, NIH) revealed that 92% of the condensed siRNA was recovered from DOPE-PEI complexes after the enzymatic treatment.
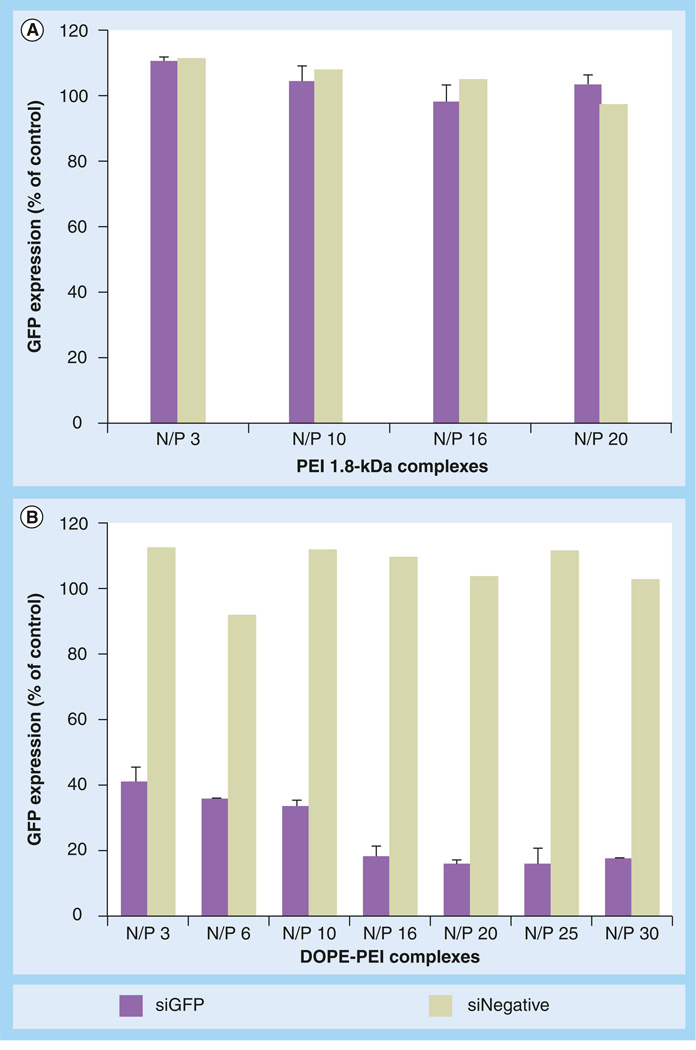
The gene silencing efficacy mediated by DOPE-PEI complexes was studied with stably GFP-expressing c166 cells (c166 GFP). In order to determine the optimal N/P ratio for the maximum silencing activity, DOPE-PEI/siRNA complexes were prepared at varying N/P ratios and evaluated for GFP downregulation. The silencing of GFP was measured by the decrease in the mean fluorescence of c166 GFP cells after the treatment with complexes prepared with GFP targeting siRNA. The lipid modification of PEI improved the silencing efficacy of the polymer. Nonmodified PEI (1.8 kDa) gave no detectable GFP supression regardless of the N/P employed (Figure 1A), whereas DOPE-PEI complexes showed a 2.5–4-fold fluorescence decrease compared with control cells depending on the N/P used (Figure 1B). Formulations prepared with a scrambled siRNA did not decrease the cell fluorescence, meaning that the decrease observed was due specifically to GFP downregulation. The highest downregulation, an almost 75% reduction of GFP fluorescence, was achieved with DOPE-PEI complexes prepared at a N/P ratio of 16. Silencing rate did not improve using higher N/P ratios and remained constant up to a N/P of 30. Therefore, a N/P ratio of 16 was selected for all further experiments.
Figure 1. Green fluorescent protein downregulation by siRNA formulations in c166-green fluorescent protein cells.
(A) With PEI 1.8-kDa complexes and (B) DOPE-PEI complexes. c166-GFP cells (stably expressing GFP) were treated with the formulations prepared with GFP-targeted siRNA (n = 3) or nontargeted siRNA (n = 1) at different N/P ratios. The siRNA concentration was 100 nM. After 4 h of incubation, complexes were removed and cells were incubated for 48 h. Cells were trypsinized and analyzed by flow cytometry. The downregulation of GFP was measured by the decrease in the mean fluorescence of the treated cells compared with nontreated and expressed as a percentage of nontreated control cells.
DOPE: Dioleoylphosphatidylethanolamine; GFP: Green fluorescent protein; N/P: Polyethylenimine nitrogen/nucleic acid phosphate; PEI: Polyethylenimine.
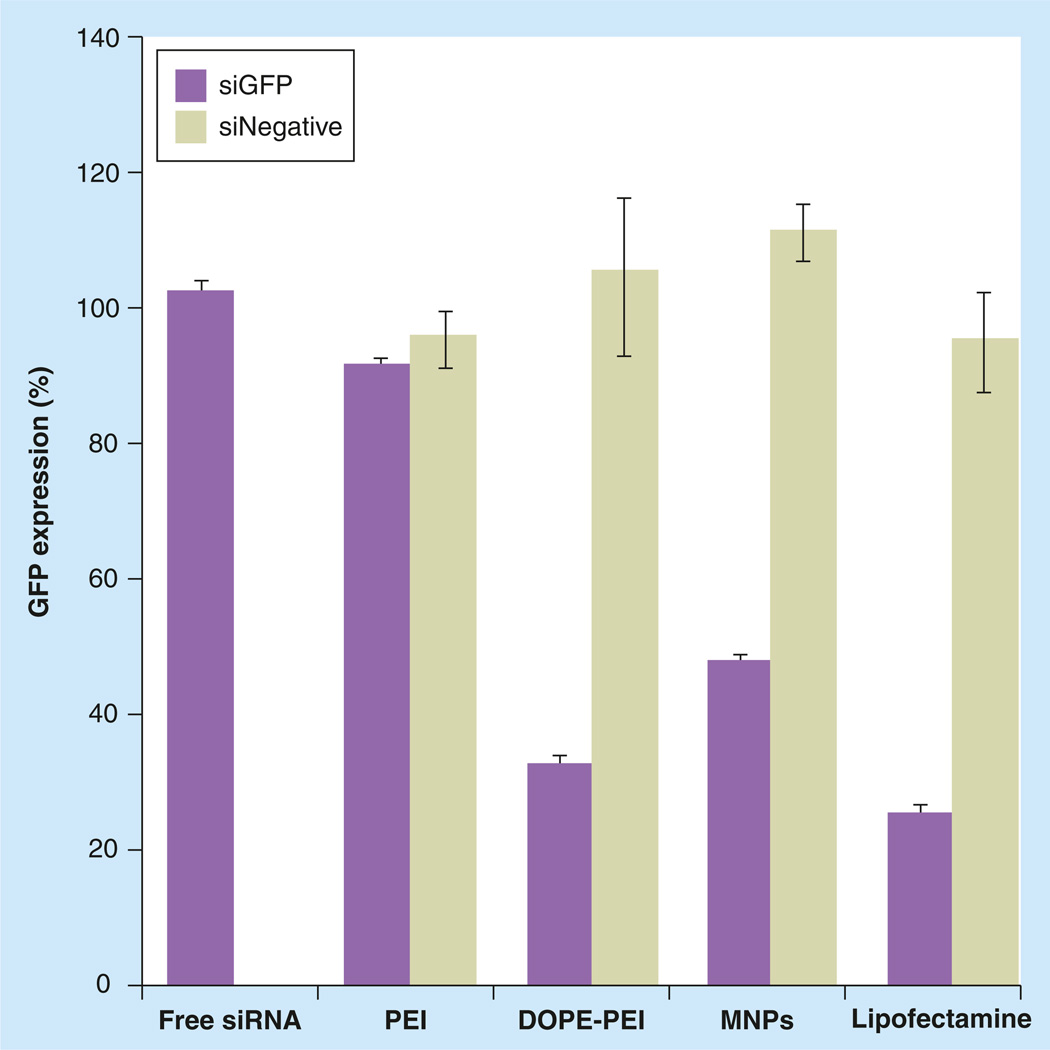
Finally, a PEG/lipid coating was incorporated in the complexes. The hydrophobic interactions between the lipid part of DOPE-PEI and the free lipids led to the formation of MNPs, with small size and a neutral zeta potential. The incorporation of PEG and lipids moderately increased the size of DOPE-PEI/siRNA complexes from 127 ± 2 to 178 ± 34 nm in MNPs, while the zeta potential decreased from 22 ± 5 mV to a near neutral surface charge (3 ± 1 mV). The effect of the PEG/lipid layer for MNPs silencing efficacy is shown in Figure 2. The presence of PEG and lipids surrounding the DOPE-PEI/siRNA complexes slightly decreased their silencing efficacy (Figure 2). Nevertheless, the ability of MNPs to deliver siGFP into cells and suppress GFP expression was high enough and comparable to that of Lipofectamine 2000 (positive control). The presence of a PEG coating, however, made the preparation even less toxic. The absence of MNP toxicity previously reported by [28,29] was confirmed for resistant and sensitive MCF-7 cells (Supplementary Figure 2, black bars). Long-term toxicity was measured after a 4-h formulation treatment. PEG/lipid coating of complexes improved long-term toxicity in MNPs and displayed approximately 100% cell viability compared with 50% viability for Lipofectamine complexes.
Figure 2. Effect of polyethylene glycol/lipid layer in micelle-like nanoparticles silencing efficacy.
c166-GFP cells (stably expressing GFP) were treated with the formulations prepared with GFP-targeted siRNA or nontargeted siRNA at PEI nitrogen/nucleic acid phosphate ratio of 16. The siRNA concentration was 100 nM. After 4 h of incubation, complexes were removed and cells were incubated for 48 h. Cells were trypsinized and analyzed by flow cytometry. The downregulation of GFP was measured by the decrease in the mean fluorescence of the treated cells compared with nontreated and expressed as a percentage of nontreated control cells. Data are expressed as the mean ± standard deviation (n = 3).
DOPE: Dioleoylphosphatidylethanolamine; GFP: Green fluorescent protein; MNP: Micelle-like nanoparticle; PEI: Polyethylenimine.
P-gp silencing with DOPE-PEI formulations
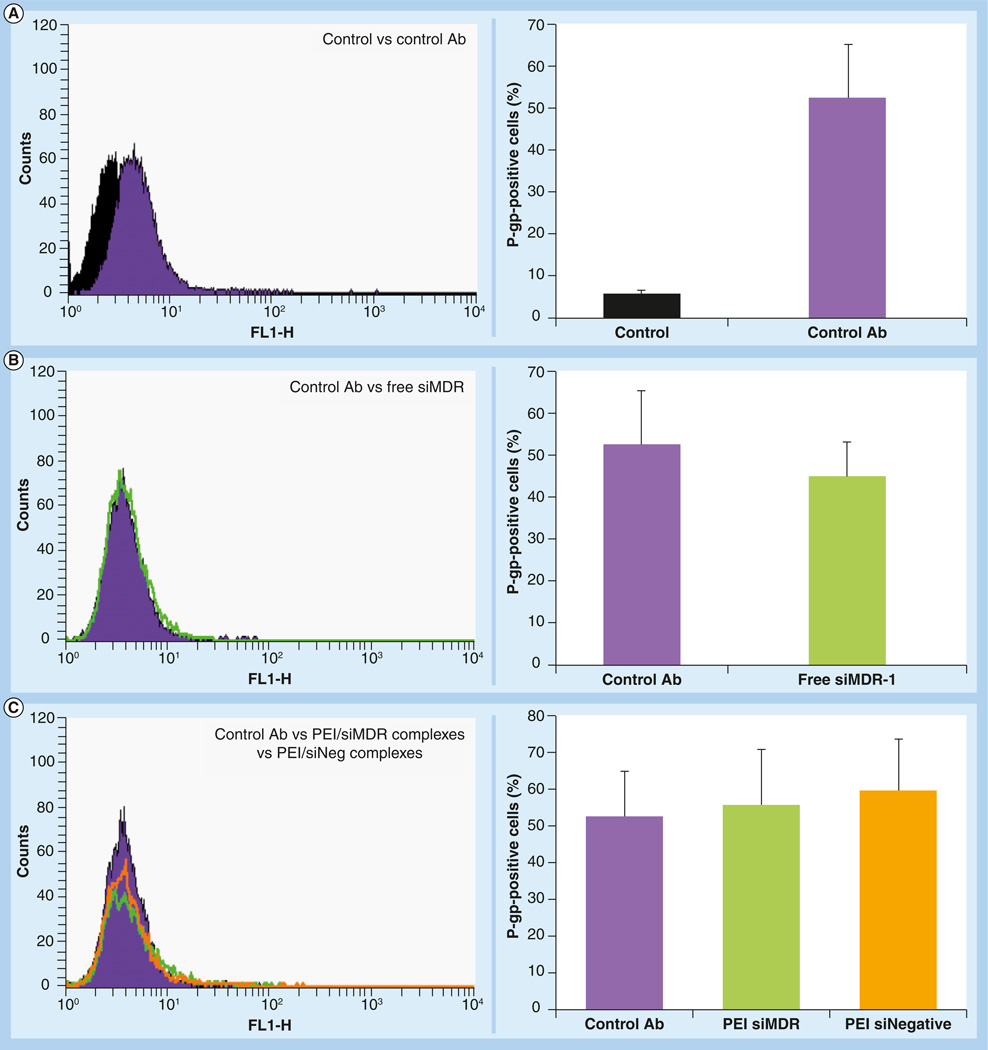
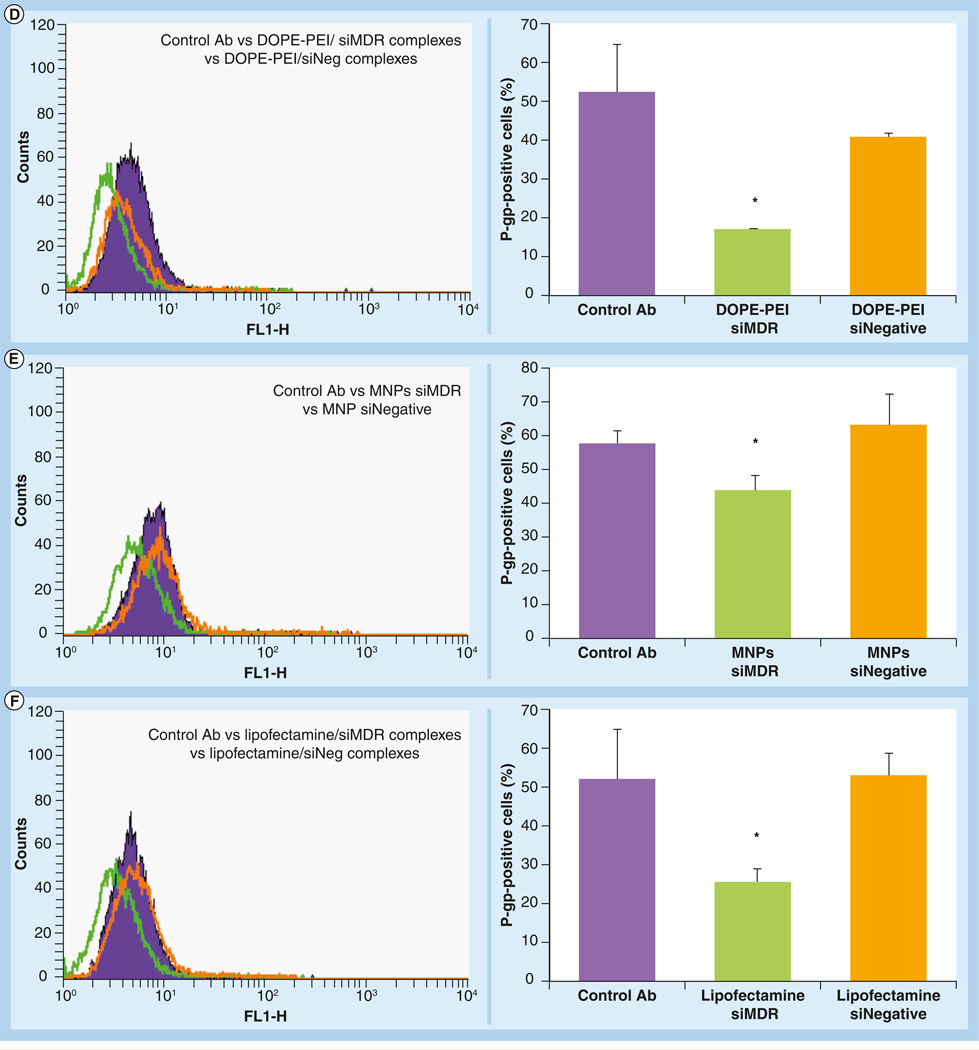
P-gp downregulation was evaluated in MCF-7-resistant cells using a siRNA targeting MDR-1 gene (siMDR-1). The changes in P-gp surface protein expression were measured by flow cytometry using a fluorescein isothiocyanate-labeled antibody, which recognizes an external epitope of the P-gp protein. The overexpression of P-gp in control resistant cells (nontreated with siMDR-1) was evident from a fluorescence increase in the presence of the antibody (Figure 3A). This shift was not observed when the experiment was performed in sensitive cells that have low basal levels of P-gp (Supplementary Figure 3).
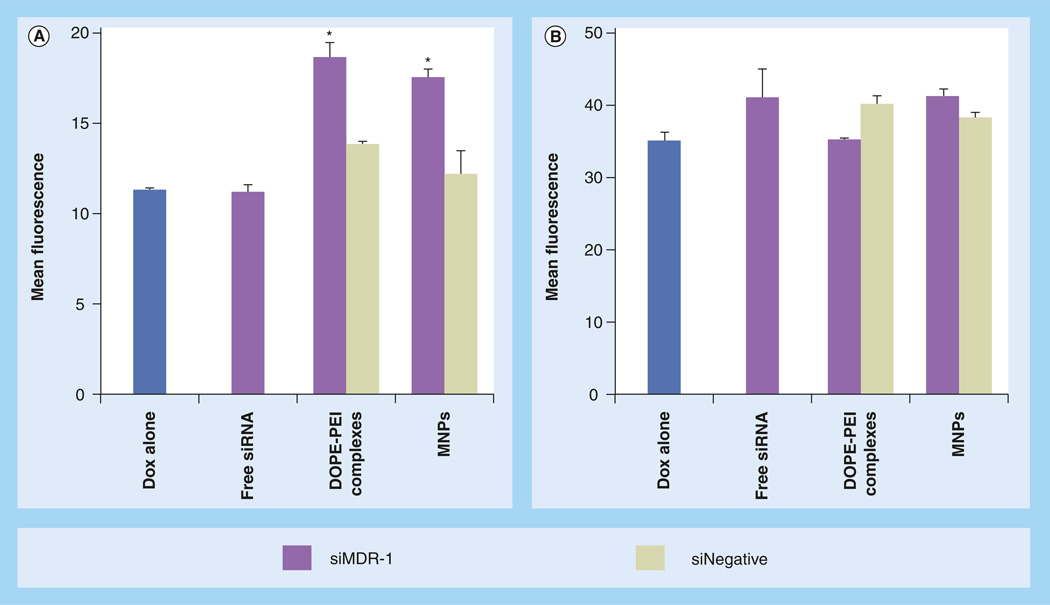
Figure 3. Decrease in the P-glycoprotein expression in resistant MCF-7 cells mediated by phosphoethanolamine-polyethylenimine complexes and micelle-like nanoparticles.
MCF-7 resistant cells were treated with different formulations prepared either with siMDR-1 or siNegative. After 4 h, the treatments were removed and fresh medium was added. The cells were reincubated for 48 h. The cells were detached by mechanical scrapping, resuspended in 0.5% bovine serum albumin, incubated with fluorescein isothiocyanate-labeled antibody against P-gp and analyzed by flow cytometry. Filled histograms correspond to nontreated cells (black, in the absence of antibody and blue in the presence of antibody), green histograms correspond to formulations prepared with siRNA targeting MDR-1 and orange histograms correspond to formulations prepared with scramble siRNA. A shift to the left is indicative of P-gp downregulation. The downregulation of P-gp was quantified by the decrease in mean fluorescence of the treated cells and expressed as a percentage of P-gp positive cells. Data are expressed as the mean ± standard deviation (n = 3).
*p < 0.05 versus control cells in the presence of antibody.
Ab: Antibody; MDR: Multiple drug resistance; MNP: Micelle-like nanoparticle; P-gp: P-glycoprotein; PEI: Polyethylenimine; siNegative: Scrambled siRNA; siMDR: siRNA targeting MDR-1.
DOPE-PEI complexes and MNPs effectively delivered siMDR-1 into in MCF-7 resistant cells and significantly downregulated P-gp expression (Figure 3D & 3E, respectively). siMDR-1 formulations (green histograms) showed less fluorescence than control cells (filled histograms), meaning that less P-gp was expressed and presented on the cell surface. Thus, less fluorescent antibody was attached. By contrast, those cells treated with siNegative formulations (orange histograms) showed fluorescence similar to that of control cells. The same results were obtained with Lipofectamine 2000 used as a positive control for siRNA transfection (Figure 3F). No changes in P-gp expression were observed when the cells were treated with free siMDR-1 or PEI complexes (Figure 3B & 3C, respectively).
Intracellular accumulation of doxorubicin
Intracellular doxorubicin was measured in MCF-7 cells to investigate whether the P-gp silencing achieved by DOPE-PEI formulations could inhibit the drug efflux activity of the transporter. Doxorubicin fluorescence increased approximately twofold in resistant cells treated with DOPE-PEI or MNPs (Figure 4A). Treatment with formulations loading nontargeted siRNA (siNegative) did not change the amount of drug inside the cells. This latter finding confirms the specificity of the silencing and does not support drug accumulation due to polymer permeabilization of cell membrane. As expected, the amount of drug in sensitive cells was considerably higher compared with resistant cells (note the different scales between Figure 4A & 4B). In sensitive cells, the intracellular doxorubicin was not affected by the siRNA, siMDR-1 or siNegative (Figure 4B). At these experimental conditions, the decrease of P-gp in the sensitive strain may not play a significant role in the accumulation of the drug.
Figure 4. Intracellular doxorubicin levels.
In (A) resistant and (B) sensitive MCF-7 cells mediated by DOPE-PEI complexes and MNPs. MCF-7 resistant and sensitive cells were treated with formulations prepared either with siRNA targeting MDR-1 or scramble siRNA. Control cells were treated only with medium. After 4 h, medium was exchanged. Cells were reincubated for 48 h. Cells were then treated for 1 h with doxorubicin (5 µg/ml), then washed, trypsinized and analyzed by flow cytometry. The accumulation of doxorubicin within the cells was measured by the increase in the mean fluorescence. Note ordinate scale differences. Data are expressed as the mean ± standard deviation (n = 3).
*p < 0.001 versus Doxalone.
DOPE: Dioleoylphosphatidylethanolamine; Dox: Doxorubicin; MNP: Micelle-like nanoparticle; PEI: Polyethylenimine; siNegative: Scrambled siRNA; siMDR: siRNA targeting MDR.
Cytotoxicity studies
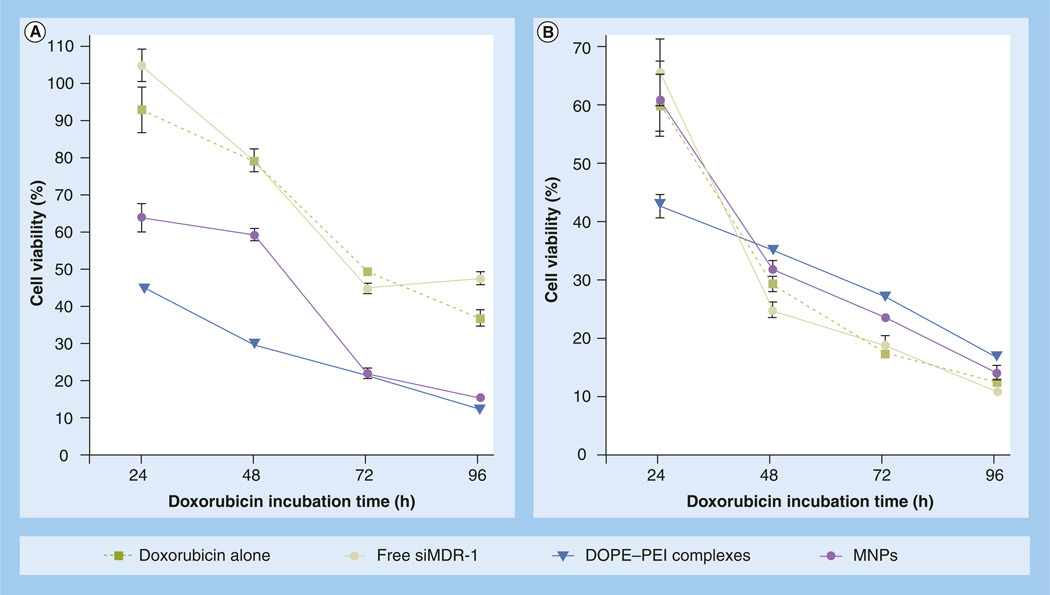
Cytotoxicity studies were performed to probe the potential therapeutic usefulness of the lipid–PEI system with different combinations of P-gp downregulation and doxorubicin to overcome the MDR in (breast) cancer cells. The experiments were designed to gain insight into how the P-gp downregulation and doxorubicin should be combined to exert their maximum effect. First, siRNA and doxorubicin were sequentially administered with a time lag of 48 h in order to maximize P-gp downregulation. Cell viability in resistant cells was measured after different drug incubation times and compared with that in sensitive cells (Figure 5). The pretreatment of resistant cells with either DOPE-PEI complexes or MNPs-loaded siMDR-1 improved doxorubicin cytotoxicity. Significant differences (p < 0.001) compared with drug-only treated cells were observed at all incubation time-points (Figure 5A). After 1 day of drug treatment, 95% of control resistant cells were viable. However, cells treated with siMDR-1 formulations had cell viability of 45 and 65%, respectively, for complexes and MNPs. After 2–3 additional days of drug treatment, the viability of siMDR-1-treated cells dropped to 16% compared with 40% for drug-only control cells.
Figure 5. Doxorubicin cytotoxicity.
In (A) resistant and (B) sensitive MCF-7 cells after treatment with DOPE-PEI complexes or MNPs. MCF-7 resistant and sensitive cells were treated with formulations prepared with siMDR-1. Control cells were treated with medium. After 4 h, the media was exchanged. Cells were reincubated for 48 h. Cells were treated with doxorubicin (1 µg/ml) for 24, 48, 72 and 96 h and viability was measured. Data are expressed as the mean ± standard deviation (n = 3).
p < 0.001 for DOPE-PEI treatments versus drug only treated cells for all time-points in resistant cells.
DOPE: Dioleoylphosphatidylethanolamine; MNP: Micelle-like nanoparticle; PEI: Polyethylenimine; siMDR-1: siRNA targeting MDR-1.
For sensitive cells, a significant improvement of drug efficacy was detected only for DOPE-based complexes after 24 h of doxorubicin treatment. The toxicity profiles of the treatments were similar and did not improve with longer incubation times (Figure 5B). The inherent sensitivity of the MCF-7 wild strain to doxorubicin may explain these results.
A comparison of doxorubicin toxicity towards resistant and sensitive cells indicated that the combination of P-gp downregulation and doxorubicin can improve the therapeutic efficacy of the drug against resistant cells to viability values similar to those for sensitive cells (Figure 5A & 5B). It is important to note that scrambled siRNA formulations, used as a control for P-gp silencing specificity, did not improve the drug efficacy and led to less drug toxicity than that of siMDR-1 formulations (white and hatched bars, Supplementary Figure 3).
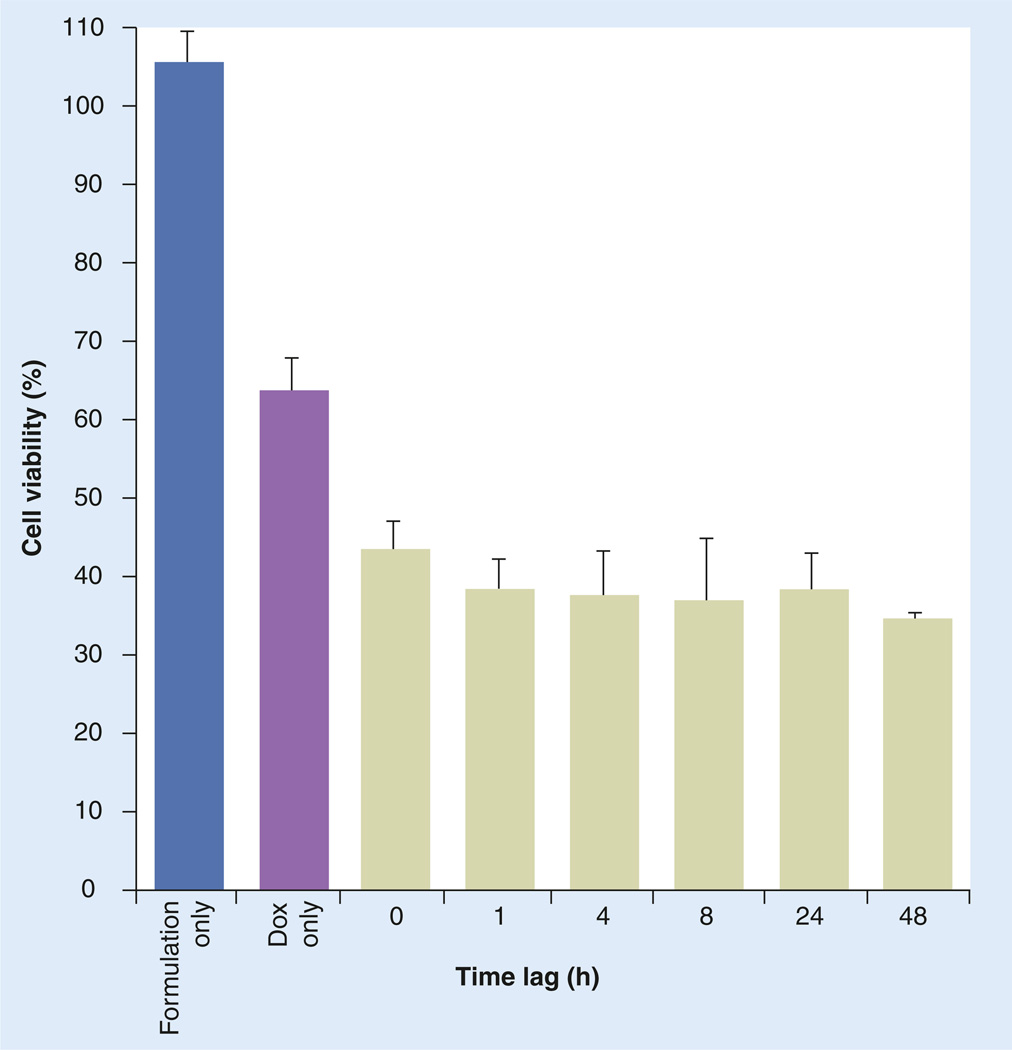
In the second set of experiments, the effect of different time lags between siRNA and drug administration on doxorubicin toxicity was studied. As shown in Figure 6, the effectiveness of doxorubicin was significantly improved by P-gp downregulation mediated by DOPE-PEI complexes regardless of the time lag, even including a simultaneous administration (time lag: 0 h; p < 0.001 vs doxorubicin only).
Figure 6. Doxorubicin cytotoxicity in resistant MCF-7 cells after treatment with phosphoethanolamine-polyethylenimine complexes at different time lags (h).
MCF-7 resistant and sensitive cells were treated with formulations prepared with siRNA targeting MDR-1 (siMDR-1). Control cells were treated with medium. At different time points post-siMDR-1, cells were treated with doxorubicin (1 µg/ml) for 72 h, and viability was measured. Data are expressed as the mean ± standard deviation (n = 3). p < 0.001 for dioleoylphosphatidylethanolamine-polyethylenimine treatments versus Dox-only treated cells, for all time-points.
Dox: Doxorubicin.
Discussion
Recent studies have shown that silencing of the MDR-1/P-gp gene using siRNA can improve the effectiveness of anticancer drugs on MDR tumors [22,25,37,38]. In the case of breast cancer, the wide availability of in vitro models and clinical data make this strategy even more attractive [39]. However, two main problems need to be resolved. The first is related to the inherent instability of siRNA that requires its association with viral or synthetic carriers. The development of an ‘ideal delivery system’, highly efficient, nontoxic and nonimmunogenic is still a major challenge. The second problem concerns the optimization of the combination of P-gp silencing and the anticancer drug administration for maximal therapeutic synergy. Some authors have shown that the sequential administration of siRNA/drug permits a sufficient time to achieve substantial downregulation of P-gp and, consequently, the maximum cell killing when the drug is administered to the cells [38,40]. By contrast, other authors suggested that the combination of drug and siRNA in a single carrier could promote the synergy of the treatments by temporally colocalizing them in the tumor cells [32,41,42]. In any case, it is desirable that a versatile carrier could fit different administration schedules.
With this in mind, we synthesized and evaluated a novel phospholipid-PEI conjugate for siRNA delivery and toxicity. These new conjugates were tested for P-gp silencing and combined sequentially or simultaneously with doxorubicin to overcome MDR in breast cancer cells.
The conjugate was prepared by coupling low-molecular-weight PEI (1.8 kDa) with DOPE phospholipid. PEI 1.8 kDa has a low molecular weight and a small number of superficial primary amines, which decreases its toxicity but also its transfection efficacy. The conjugation of DOPE did not diminish siRNA condensation capacity of PEI. Small complexes (<200 nm) were spontaneously formed when siRNA was mixed with DOPE-modified PEI. Importantly, however, the silencing efficacy of PEI was dramatically improved by DOPE conjugation. A wide range of N/P ratios (DOPE-PEI/siRNA or PEI/siRNA) was tested for GFP silencing and the benefits of DOPE modification were clearly shown for all (Figure 1). The absence of the GFP suppression observed for nonmodified PEI turned into a 40–75% GFP signal reduction for DOPE-modified PEI depending on the N/P ratio employed. This great improvement can be attributed to the DOPE acyl chains exposed in the outer-shell of complexes. DOPE is frequently used in gene delivery as a helper lipid to promote the fusion of the carrier with the cell membrane and facilitate the disruption of endosomes after the uptake of the complexes [43–45].
DOPE-PEI complexes were formulated in MNPs for improved biocompatibility. The lipid moiety of DOPE-PEI can hydrophobically interact with PEG-PE and free lipids to self-assemble into MNPs. We previously reported that MNPs had reduced cytotoxicity and improved in vivo stability compared with plain PEI complexes [28,29]. Here, the benefits of MNP formation were also demonstrated for DOPE-PEI complexes as MNP core. The positive surface charge of DOPE-PEI complexes (22 ± 5 mV) decreased in MNPs to an almost neutral and more biocompatible one (3 ± 1 mV). At the same time, the presence of PEG surrounding DOPE-PEI complexes did not significantly influence the silencing efficacy of MNPs but considerably decreased their cytotoxicity compared with Lipofectamine 2000 or noncoated complexes, especially in sensitive MCF-7 cells. MNPs should provide a useful platform for future in vivo targeted siRNA applications.
DOPE-PEI formulations were tested for P-gp silencing. The presence of P-gp on the surface of resistant cells decreased after treating cells with DOPE-PEI or MNPs loaded siMDR-1. This P-gp downregulation was translated into an effective inhibition of the drug efflux activity. The amount of doxorubicin inside MDR-1-treated cells doubled that for control cells. It is important to note that the reduction of P-gp expression and augmentation of drug accumulation do not necessarily correlate with a complete reversion of MDR phenotype [25,41]. In our case, P-gp downregulation mediated by DOPE-PEI and MNPs significantly improved doxorubicin toxicity in resistant cells (Figure 5). Pretreatment of resistant cells with siMDR-1 formulations before administration of doxorubicin, led to a twofold drop in cell viability, similar to values in sensitive cells. Interestingly, the combination of doxorubicin with DOPE-PEI complexes or MNPs led to somewhat different toxicity patterns in resistant cells depending on the drug incubation time. After 24–48 h of incubation with doxorubicin, MNPs showed lower cell kill values than DOPE-PEI complexes. At longer drug incubation times, the toxicity profiles of DOPE-PEI complexes and MNPs became similar. One possible explanation is that the PEG/lipid coating in MNPs may influence their uptake and intracellular stability and ultimately interfere with the siRNA release process in the cytoplasm. Differences in the silencing efficacy of PEG-coated (MNPs) and uncoated DOPE-PEI complexes were observed in the initial characterization of DOPE formulations using GFP silencing siRNA (Figure 2).
Most of the studies that focused on the combined use of P-gp siRNA and anticancer drugs (doxorubicin and paclitaxel) reported successful results using a time lag of 24–48 h between treatments. Yadav and collaborators studied the effect of separation duration (from 1 to 48 h) between siRNA and paclitaxel formulated in biodegradable nanoparticles. A significant enhancement in cytotoxicity was observed only in resistant ovarian cancer cells when paclitaxel was administered after 24 h of P-gp silencing [38]. Similarly, separated preparations of cationic liposomes of siRNA and doxorubicin showed a therapeutic improvement in mice but resulted in severe aggregation and doxorubicin leakage when coencapsulated [40]. In this sense, one of the advantages of DOPE-PEI is that the effectiveness of doxorubicin was improved regardless of the time lag between siRNA formulation and drug, including the case of simultaneous administration of the treatments (Figures 5 & 6).
Conclusion
We have demonstrated the feasibility and effectiveness of DOPE-PEI formulations to deliver siRNA and modulate the expression of P-gp. The downregulation of P-gp translated into an effective inhibition of the doxorubicin efflux activity, enhancement of intracellular doxorubicin accumulation and significant increase of drug toxicity in resistant human breast cancer cells.
Future perspective
The development of effective and safe siRNA carriers suitable for systemic application is a key step for future advances in cancer treatment with RNAi therapeutics. The phospholipid-PEI nanocarriers developed in this study have demonstrated an excellent performance in various cell lines (high silencing efficacy and low toxicity) and optimal biophysical properties for systemic application (small size, nuclease stability). All these features together with our previous results for DNA delivery in tumor-bearing mice suggests promising results for the in vivo systemic application of these novel siRNA carriers.
Additionally, the amphiphilic structure of the DOPE-PEI conjugates provides high flexibility to the system. The cationic polymeric part of the conjugate can condense and protect different types of negatively charged nucleotides (plasmid, siRNA, oligonucleotide), thereby opening new therapeutic opportunities. Similarly, the lipid graft permits the interaction with PEG and lipids, as in the case of MNPs, for prolonged blood circulation, but also permits the inclusion of other functional elements like cell-penetrating peptides or antibodies for enhanced and tumor-targeted delivery.
The use of a siRNA silencing approach to solve some of the limitations of traditional chemotherapy is gathering increased research interest. However, little is known about how the different biophysical properties and mechanisms of action of the anticancer drug and the siRNA will impact on their biodistribution and efficacy. In addition, the optimal dosage for a siRNA/drug combination to exert its maximal in vivo effect has not been fully elucidated. We have probed the potential therapeutic application of the lipid-PEI system with different combinations of P-gp silencing and anticancer drugs to overcome MDR in breast cancer cells. The in vivo evaluation of such systems after systemic administration using different dosage schedules should be a priority task for future advances.
Supplementary Material
Executive summary.
Background
-
▪
We have synthesized a novel conjugate between a phospholipid (dioleoylphosphatidylethanolamine [DOPE]) and polyethylenimine (PEI), for siRNA delivery for the purpose of silencing P-glycoprotein (P-gp) to overcome doxorubicin resistance in MCF-7 human breast cancer cells.
DOPE-PEI formulations are effective & feasible siRNA carriers
-
▪
The novel conjugates self-assembled in the presence of siRNA into 120-nm diameter particles with a positive zeta potential. DOPE-PEI complexes protected siRNA from enzymatic degradation.
-
▪
Phospholipid conjugation dramatically improved the silencing efficacy of PEI. The absence of green fluorescent protein suppression observed for nonmodified PEI complexes, resulted in a 75% green fluorescent protein signal reduction with DOPE-modified PEI complexes.
-
▪
Polyethylene glycol/lipid coating of the DOPE-PEI complexes gave rise to micelle-like nanoparticles with better biocompatibility properties, including a neutral surface charge and absence of cytotoxicity.
DOPE-PEI formulations mediate P-gp downregulation
-
▪
DOPE-PEI complexes and micelle-like nanoparticles effectively delivered specific siRNA into in MCF-7 resistant cells and downregulated P-gp expression.
DOPE-PEI formulations enhance intracellular doxorubicin accumulation
-
▪
The downregulation of P-gp translated into effective inhibition of the efflux activity of the transporter. The amount of drug inside the cells doubled that of non-siRNA-treated cells.
Combination of P-gp silencing by DOPE-PEI formulations & doxorubicin overcomes multidrug resistance in breast cancer cells
-
▪
P-gp silencing mediated by DOPE-PEI and micelle-like nanoparticles significantly increased the doxorubicin toxicity in resistant cells. This improvement of chemotherapeutic efficiency was observed when P-gp silencing siRNA and doxorubicin were administered separately or as cotherapy.
Acknowledgments
This work was supported by a fellowship from the Department of Education of the Navarra Regional Government (Spain) to GN and by the NHI RO1CA121838 and 1U54CA151881 to VP Torchilin.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
▪ of interest
- 1.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 2.Constantinides PP, Wasan KM. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/in vivo case studies. J. Pharm. Sci. 2007;96:235–248. doi: 10.1002/jps.20780. [DOI] [PubMed] [Google Scholar]
- 3. Staud F, Ceckova M, Micuda S, Pavek P. Expression and function of p-glycoprotein in normal tissues: effect on pharmacokinetics. Methods Mol. Biol. 2010;596:199–222. doi: 10.1007/978-1-60761-416-6_10. ▪ A comprehensive review of the physiological role of P-glycoprotein in normal tissues and its impact on the final fate of drugs.
- 4.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin. Pharmacol. Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Hao J, Wang L, Li Y. Coexpression of invasive markers (uPA, CD44) and multiple drug-resistance proteins (MDR1, MRP2) is correlated with epithelial ovarian cancer progression. Br. J. Cancer. 2009;101:432–440. doi: 10.1038/sj.bjc.6605185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TB, Park JH, Min YD, Kim KJ, Choi CH. Epigenetic mechanisms involved in differential MDR1 mRNA expression between gastric and colon cancer cell lines and rationales for clinical chemotherapy. BMC Gastroenterol. 2008;8:33. doi: 10.1186/1471-230X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahadevan D, List AF. Targeting the multidrug resistance-1 transporter in AML: molecular regulation and therapeutic strategies. Blood. 2004;104:1940–1951. doi: 10.1182/blood-2003-07-2490. [DOI] [PubMed] [Google Scholar]
- 8.Keshet GI, Goldstein I, Itzhaki O, et al. MDR1 expression identifies human melanoma stem cells. Biochem. Biophys. Res. Commun. 2008;368:930–936. doi: 10.1016/j.bbrc.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr. Relat. Cancer. 2003;10:43–73. doi: 10.1677/erc.0.0100043. [DOI] [PubMed] [Google Scholar]
- 10.Mechetner E, Kyshtoobayeva A, Zonis S, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin. Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 11.Colabufo NA, Berardi F, Cantore M, et al. Perspectives of P-glycoprotein modulating agents in oncology and neurodegenerative diseases: pharmaceutical, biological, and diagnostic potentials. J. Med. Chem. 2010;53:1883–1897. doi: 10.1021/jm900743c. [DOI] [PubMed] [Google Scholar]
- 12.Dantzig AH, de Alwis DP, Burgess M. Considerations in the design and development of transport inhibitors as adjuncts to drug therapy. Adv. Drug Deliv. Rev. 2003;55:133–150. doi: 10.1016/s0169-409x(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 13.Lage H. MDR1/P-glycoprotein (ABCB1) as target for RNA interference-mediated reversal of multidrug resistance. Curr. Drug Targets. 2006;7:813–821. doi: 10.2174/138945006777709566. [DOI] [PubMed] [Google Scholar]
- 14.Stege A, Kruhn A, Lage H. Overcoming multidrug resistance by RNA interference. Methods Mol. Biol. 2010;596:447–465. doi: 10.1007/978-1-60761-416-6_20. [DOI] [PubMed] [Google Scholar]
- 15. Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. ▪ First article describing how siRNA can block the expression of protein production by neutralizing specific mRNA molecules.
- 16.Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm. Res. 2006;23:1868–1876. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- 17.Hobel S, Aigner A. Polyethylenimine (PEI)/siRNA-mediated gene knockdown in vitro and in vivo. Methods Mol. Biol. 2010;623:283–297. doi: 10.1007/978-1-60761-588-0_18. [DOI] [PubMed] [Google Scholar]
- 18.Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 19.Ogris M, Steinlein P, Carotta S, Brunner S, Wagner E. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS PharmSci. 2001;3:E21. doi: 10.1208/ps030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshamsan A, Haddadi A, Incani V, et al. Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Mol. Pharm. 2009;6:121–133. doi: 10.1021/mp8000815. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Zhao G, Liu J, et al. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J. Control. Release. 2009;140:277–283. doi: 10.1016/j.jconrel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Gusachenko O, Kravchuk Y, Konevets D, et al. Transfection efficiency of 25-kDa PEI-cholesterol conjugates with different levels of modification. J. Biomater. Sci. Polym. Ed. 2009;20:1091–1110. doi: 10.1163/156856209X444448. [DOI] [PubMed] [Google Scholar]
- 24.Han S, Mahato RI, Kim SW. Water-soluble lipopolymer for gene delivery. Bioconjug. Chem. 2001;12:337–345. doi: 10.1021/bc000120w. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi M, Lavasanifar A, Berthiaume LG, Weinfeld M, Uludag H. Cationic polymer-mediated small interfering RNA delivery for P-glycoprotein downregulation in tumor cells. Cancer. 2010;116:5544–5554. doi: 10.1002/cncr.25321. [DOI] [PubMed] [Google Scholar]
- 26.Rothdiener M, Muller D, Castro PG, et al. Targeted delivery of SiRNA to CD33-positive tumor cells with liposomal carrier systems. J. Control. Release. 2010;144:251–258. doi: 10.1016/j.jconrel.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Schafer J, Hobel S, Bakowsky U, Aigner A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials. 2010;31:6892–6900. doi: 10.1016/j.biomaterials.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 28.Ko YT, Kale A, Hartner WC, Papahadjopoulos-Sternberg B, Torchilin VP. Self-assembling micelle-like nanoparticles based on phospholipid–polyethyleneimine conjugates for systemic gene delivery. J. Control. Release. 2009;133:132–138. doi: 10.1016/j.jconrel.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro G, Sawant R, Essex S, Tros de ILarduya C, Torchilin VP. Phospholipid–polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Deliv. Transl. Res. 2010;1:25–33. doi: 10.1007/s13346-010-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musacchio T, Vaze O, D’Souza G, Torchilin VP. Effective stabilization and delivery of siRNA: reversible siRNA-phospholipid conjugate in nanosized mixed polymeric micelles. Bioconjug. Chem. 2010;21:1530–1536. doi: 10.1021/bc100199c. [DOI] [PubMed] [Google Scholar]
- 31. Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515–1519. ▪ First article that demonstrated the feasibility of using siRNA to specifically and effectively modulate multidrug resistance.
- 32.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond.) 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao S, Neu M, Germershaus O, et al. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug. Chem. 2006;17:1209–1218. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- 34.Malek A, Czubayko F, Aigner A. PEG grafting of polyethylenimine (PEI) exerts different effects on DNA transfection and siRNA-induced gene targeting efficacy. J. Drug Target. 2008;16:124–139. doi: 10.1080/10611860701849058. [DOI] [PubMed] [Google Scholar]
- 35.Kabanov AV, Kabanov VA. DNA complexes with polycations for the delivery of genetic material into cells. Bioconjug. Chem. 1995;6:7–20. doi: 10.1021/bc00031a002. [DOI] [PubMed] [Google Scholar]
- 36.Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm. Res. 2006;23:1868–1876. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- 37.Peng Z, Xiao Z, Wang Y, et al. Reversal of P-glycoprotein-mediated multidrug resistance with small interference RNA (siRNA) in leukemia cells. Cancer Gene Ther. 2004;11:707–712. doi: 10.1038/sj.cgt.7700738. [DOI] [PubMed] [Google Scholar]
- 38.Yadav S, van Vlerken LE, Little SR, Amiji MM. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother. Pharmacol. 2009;63:711–722. doi: 10.1007/s00280-008-0790-y. [DOI] [PubMed] [Google Scholar]
- 39. Germano S, O’Driscoll L. Breast cancer: understanding sensitivity and resistance to chemotherapy and targeted therapies to aid in personalised medicine. Curr. Cancer Drug Targets. 2009;9:398–418. doi: 10.2174/156800909788166529. ▪ Exhaustive review on the mechanisms of drug resistance in breast cancer that gives an overview of the complexity of the multidrug resistance phenomena and the range of available therapies and predictive biomarkers.
- 40.Jiang J, Yang SJ, Wang JC, et al. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing siRNA or doxorubicin. Eur. J. Pharm. Biopharm. 2010;76:170–178. doi: 10.1016/j.ejpb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2009;31:358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun TM, Du JZ, Yao YD, et al. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano. 2011;5:1483–1494. doi: 10.1021/nn103349h. ▪ An interesting example of systemic delivery of siRNA and paclitaxel with a ‘two-in-one’ micelleplex system.
- 43.Philipp A, Zhao X, Tarcha P, Wagner E, Zintchenko A. Hydrophobically modified oligoethylenimines as highly efficient transfection agents for siRNA delivery. Bioconjug. Chem. 2009;20:2055–2061. doi: 10.1021/bc9001536. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki Y, Nango M, Matsuura M, et al. Polycation liposomes, a novel nonviral gene transfer system, constructed from cetylated polyethylenimine. Gene Ther. 2000;7:1148–1155. doi: 10.1038/sj.gt.3301217. [DOI] [PubMed] [Google Scholar]
- 45.Chen JL, Wang H, Gao JQ, Chen HL, Liang WQ. Liposomes modified with polycation used for gene delivery: preparation, characterization and transfection in vitro. Int. J. Pharm. 2007;343:255–261. doi: 10.1016/j.ijpharm.2007.05.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.