Abstract
Implants are predisposed to infection even years after implantation, despite ostensibly being surrounded by innumerable macrophages as part of the host foreign body response. The local implant environment could adversely influence the implant-associated macrophage phenotype, proliferative capacity, activation states, and ability to neutralize pathogens. This study monitored cultured macrophage proliferative states and phagocytotic competence on tissue culture plastic to address the hypothesis that extended contact with foreign materials alters macrophage phenotype. That such macrophage alterations might also occur around implants has significance to the foreign body response, infection, cancer, autoimmune and other diseases. Specifically, multiple indicators of macrophage proliferation in various culture conditions, including cell confluence, long-term culture (21 days), lipopolysaccharide (LPS) stimulation, passaging, and mitogenic stimulation are reported. Importantly, primary murine macrophages became quiescent at high confluence and senescent during long-term culture. Senescent macrophages significantly reduced their ability to phagocytose particles, while quiescent macrophages did not. Cell senescence and quiescence were not observed with repeated passaging. Primary macrophage stimulation with LPS delayed senescence but did not eliminate it. These results prompt the conclusion that both cell quiescence and senescence are observed under common macrophage culture conditions and could alter macrophage behavior and phenotypes in extended in vitro culture, such as the ability to phagocytose. Such macrophage transitions around foreign bodies in vivo are not documented: quiescence and senescence reported here in macrophage culture could be relevant to macrophage behavior both in vitro in bioassays and in vivo in the foreign body response and implant-centered infection.
INTRODUCTION
Macrophages play a primary role in modulating the foreign body response, immediately localizing to surfaces of every implanted material [1]. At the implant site, they are responsible for removing cell debris, foreign bodies and pathogens. After acute phase inflammation subsides, macrophages may reside at implant surfaces throughout the duration of the implantation, possibly for decades [2, 3], in some case producing multi-cellular macrophage layers around monolithic implants [1, 4], completely infiltrating porous implants [5], and fusing to form foreign body giant cells at these surfaces [1, 4, 6–8]. That any of these commonly observed chronic responses result from macrophage in situ proliferation versus continual new cell recruitment is not clear. However, a recent study found that during T helper 2 (Th2) inflammation, macrophages were capable of undergoing rapid proliferation in vivo [9]. Importantly, changes in their resident phenotypes, functional competence and capabilities to address infection risk over this implant duration, prompted by or correlated with their prolonged exposure and reaction to a foreign body (e.g., implant) are largely unknown.
Despite macrophage persistence at surfaces of implanted materials, implants retain substantial infection risk even years after implantation [10, 11]. This may be due to the fact that unlike host tissue that is continuously renewed, thereby limiting opportunities for bacterial colonization, tissue surrounding implanted materials remains relatively unchanged, encapsulated in fibrous scar tissue [1, 12–14]. This suggests that while abundant macrophages are present, they may be transformed by their chronic reactions to implants into states of relative inactivity, incapable of addressing microbial presence as effectively as during initial implant site recruitment.
Many cells in normal tissue are quiescent, a reversible, viable, non-dividing state-of-rest. Importantly, quiescent cells can be stimulated to divide [15, 16]. Cells can also become senescent, a viable but irreversible non-dividing state that cannot be overcome even with mitogenic stimuli [17]. Senescent and quiescent cells are distinguished by altered patterns of gene expression [18, 19]. Senescent and quiescent transitions in macrophages at implant surfaces could explain their inability to adequately address bacterial infection in vivo in this context.
Previous studies have demonstrated a decreased phagocytic ability in aged macrophages [20] and a susceptibility of cells under oxidative stress to senesce [20, 21]. That macrophages demonstrate increased intracellular reactive oxygen species with age [22] and reside in high oxidative stress environments surrounding foreign bodies [13] could indicate their propensity to senesce and their subsequent incompetence to phagocytose pathogens at implant surfaces over time. Interestingly, foreign body giant cells, the chronic multinucleated macrophage-derived phenotypic hallmark surrounding implanted materials, also display decreased phagocytic ability [23], and increased lysosomal activity [23, 24], consistent with senescent cells [25] also known to multinucleate [26]. Macrophages have also been purported to undergo frustrated phagocytosis, an exhausting metabolic phenomenon that could compel macrophages to senesce around implants [1, 4, 7, 8]. However, macrophage senescence and phagocytosis around chronically implanted foreign bodies or in long-term cultures on materials remains unaddressed in current literature.
Cultured macrophages are commonly employed in assays seeking information on aspects of their involvement in pathologies such as cancer, autoimmune diseases, and the foreign body response [27–32]. As an immunomodulatory cell, macrophages are highly susceptible to telomere attrition [22], increasing their potential to senesce. However, they are not commonly assayed for this phenotype. As both quiescence and senescence alter cell genetic profiles [18, 19], macrophage transitions to these states during in vitro culture likely influence assay outcomes, potentially leading to false conclusions, irreproducible results, and inconsistencies, especially when compared to in vivo phenotypes they intend to mimic. Maintenance of consistent macrophage phenotypes and activation states between in vivo and in vitro conditions is likely critical to ensuring proper in vitro model fidelity. Therefore, understanding the possible consequences of macrophage senescent and quiescent transitions has important implications both in vitro and in vivo.
This study identified proliferation states for both primary and secondary macrophages in several experimental culture conditions, including cell confluence, culture time, passage number, and biochemical stimulation. Cultured macrophage capacity to phagocytose in quiescent and senescent states raises important questions about macrophage phenotypic competence in extended contact with materials. Should this behavior also be observed in vivo, it has important implications for implanted biomaterials in the context of the foreign body response.
2. METHODS AND MATERIALS
2.1. Cell Culture
2.1.a. Immortalized RAW cell culture
Macrophage-like transformed murine cell line RAW 264.7 was purchased from the American Type Culture Collection (TIB-71, ATCC, Manassas, USA) and cultured in 96-well tissue culture-treated polystyrene plates (BD Falcon, San Jose, USA), unless otherwise specified, at 37°C with 5% supplemental CO2 according to the experiments detailed below. All RAW cells were used below passage 10 after purchase, unless passage number was explicitly specified. RAW cells were passaged by scraping with a rubber scraper (Starstedt, Newton, USA). Cells were always cultured in complete media (Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum (FBS), and 1% antibiotic/antimycotic, Invitrogen, Carlsbad, USA). Full media exchanges were performed every other day.
2.1.b. Murine primary cell sourcing
Specific pathogen-free, 2–3 month-old male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, USA). Animals were kept in the University of Utah animal facility, and provided water, mouse chow, bedding, and modes of enrichment ad libitum throughout this study. For primary macrophage harvests, animals were euthanized via CO2.
2.1.c. Primary macrophage cell culture
Bone marrow cells were collected from the femurs and tibias of 4–5 month-old euthanized male C57BL/6 mice and differentiated into bone marrow macrophages (BMMΦs) using a previously described method [33, 34]. On day 7, cells were removed from surfaces by rinsing cells 3X and incubating them in Ca+2/Mg+2-free phosphate buffered saline (PBS, Invitrogen) for 30 minutes at 37°C, and then rinsed from the surface using a 1 ml pipette tip, and collected. Cells were counted using a hemocytometer and cultured in 96-well tissue culture-treated polystyrene plates (BD Falcon, San Jose, USA), unless otherwise specified, at 37°C with 5% supplemental CO 2 according to the experiments outlined below. At least an equal volume of complete BMMΦ media (DMEM with 10% heat-inactivated FBS, 10% L929-conditioned media, 1% antibiotic/antimycotic, 1% MEM nonessential amino acids, 1% HEPES, and 1% sodium pyruvate, Invitrogen) was added to the suspended cells after plating, and fresh media was replaced every 2–3 days. Unless otherwise specified, all BMMΦs were passaged once after their differentiation for experimental use.
2.2. In vitro culture conditions for senescence examination
2.2.a. Cell confluence
Immortalized RAW macrophages were plated at densities of 5 ×103, 1 ×104, 2 ×104, 4 ×104, and 8 ×104 cells/well, and primary macrophages were plated at densities of 5 ×103, 1 ×104, 2 ×104, 4 ×104, 8 ×104, and 1.6 ×105 cells/well and cultured for 24 hours prior to fixing. To determine cell senescence versus quiescence, an equivalent cell density of 1.60 ×105 cells/well was cultured in a 30-mm Petri dish (BD Falcon) in parallel and passaged and plated at low confluence. These cells were then cultured for 7 days further (seen previously to be the time for maximum proliferation). Both control media and media with 50% serum (to encourage growth) were utilized to confirm macrophage proliferative capacity.
2.2.b. Comparisons of long-term macrophage cultures
Primary and immortalized macrophages were plated at 5 ×103 cells/well and cultured for 1, 2, 3, 5 and 7 days for secondary RAW 264.7 macrophages and 1, 2, 3, 5, 7, 10, 12, 14, 17, 19, and 21 days for primary BMMΦs prior to fixing. To determine senescence or quiescence in primary macrophages, a 30mm Petri dish (BD Falcon) with the same cell seeding density as the long-term experiment was passaged on Day 21 and plated at 5 x103 cells/well and analyzed for proliferation 5, 7, and 10 days later (i.e., the time at which the greatest proliferation during the initial 21 days was seen). Both control media and media with 50% serum (to encourage proliferation) were utilized to confirm macrophage proliferative capacity.
2.2.c. Lipopolysaccharide (LPS) treatment of cell cultures
For LPS-treated conditions, primary and immortalized macrophage cultures were treated with their respective media supplemented with 1 μg/ml LPS replaced every 2–3 days until the end of the experiment (i.e., 7 days for RAWs and 21 days for BMMΦs). This concentration was selected because it has been shown to effectively activate macrophages [35, 36]. RAW cells were also stimulated with LPS during studies of increasing confluence (details listed above).
2.2.d. Cell passaging
RAW 264.7 macrophages were cultured in 30-mm tissue culture-treated polystyrene Petri dishes (BD Falcon, San Jose, USA) and passaged 30 times and subsequently plated into 96-well plates and assayed for senescence and proliferation markers. Primary BMMΦs were cultured in 100-mm tissue culture-treated polystyrene Petri dishes (BD Falcon), passaged and plated into 96-well plates for subsequent characterization. This was repeated up to 10 passages, where passage 1 was the first passage after differentiation. Passages were fixed after 24 hours of culture for characterization. Both primary and secondary macrophage culture passages were performed every other day, to allow stock cultures sufficient time to properly adhere before serial passaging.
2.2.e. Cell culture biochemical stimulation
Primary BMMΦs were plated at 5 ×103 cells/well and cultured for 21 days and then treated for 48 hours with cytokines IFN-gamma, IL-6, MCP-1, TNF, GM-CSF, MIP-1β, MIP-1α, IL-4, RANTES, and IL-10, and mitogens TGF-β, IL-1β, MCP-1, and also 50% and 100% serum prior to fixing. This 48-hour incubation period was selected as the time reported for quiescent cells to reactivate [37].
2.2.f. Pre-differentiated BMMΦ cultures
Bone-marrow cells were plated into 96-well plates and characterized on Days 1, 3, 5, and 7 post-harvest for proliferation markers prior to full differentiation into macrophages.
2.3. Phagocytosis
Phagocytosis was measured in both senescent and quiescent cells. Blue-green fluorescent polystyrene beads (diameter 1 μm, Invitrogen) at a dilution of 0.5 μl stock to 100 μl media were added to macrophage cultures and allowed to incubate at 37°C with 5% supplemental CO 2 for 12 hours. After this incubation time, cells were washed to remove any beads not internalized and fixed with 4% paraformaldehyde (PFA, Sigma-Aldrich, St. Louis, USA), diluted in Ca+2/Mg+2-free PBS (Invitrogen) for 20 min at room temperature for subsequent imaging. Fixed cells were incubated with propidium iodide diluted 1:100 in PBS (Invitrogen) for 20 minutes which successfully stained the entire cell body. Using ImageJ (NIH freeware) the area of the cell occupied by beads was divided by the total cell area to determine the percentage of beads occupying the cells in each frame.
2.4. Cell labeling
2.4.a. Cell phenotypic makers
Mature macrophage-specific marker anti-F4/80 [38, 39] (clone BM8, rat anti-mouse IgG2a, pre-conjugated to phycoerythrin (PE), eBioscience, San Diego, USA) and M2 macrophage marker anti-CD206, macrophage mannose receptor [40] (clone MR5D3, rat anti-mouse IgG2a, preconjugated to fluorescein isothiocyanate (FITC), AbD Serotec, Raleigh, USA) were used to label macrophages on Days 1,7, and 21. Representative images from Day 21 are shown in Supplementary Figure 1.
2.4.b. Cell proliferation markers
Primary anti-mouse Ki-67 (IgG1, Novacastra, Buffalo Grove, USA) and phospho-histone H3 (Ser10) (Cell Signaling Technology, Danvers, USA) antibodies, both markers of actively proliferating cells [41, 42], were used at dilutions of 1:50 and 1:100, respectively. Secondary IgG1 goat-anti-mouse antibody conjugated to Alexa 488 (Invitrogen), used against both Ki-67 and phospho-histone H3, was diluted 1:500. Samples were rinsed 2X in PBS+Ca+2/Mg+2 (Invitrogen,) and fixed in 4% PFA (Sigma-Aldrich), diluted in Ca+2/Mg+2-free PBS for 20 min at room temperature. They were rinsed 2X in Ca+2/Mg+2-free PBS and then incubated in block solution (4% goat serum plus 0.1% triton-X 100, Invitrogen) for 1 hour at room temperature on a shaker plate. Each primary antibody, diluted in block solution, was added to the samples and the plate was sealed and placed at 4°C overnight. The primary antibody media was removed and the samples were washed 3X in Ca+2/Mg+2-free PBS. The secondary antibody, diluted in block solution, was added to the samples and incubated at room temperature for 1 hour on a shaker plate. The samples were washed 3X in Ca+2/Mg+2-free PBS and counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) according to manufacturer’s instructions prior to imaging.
2.4.c. Cell senescence assay
A colorimetric assay for senescence-associated beta-galactosidase (SA beta-gal) used as a labeling kit (Cell Signaling Technology, Danvers, USA) was used according to manufacturer’s instructions [21]. This commonly employed assay [17] labels fixed cells positive for SA beta-gal with a blue precipitate and allows for subsequent visualization of percent positive cells (imaging described below). A second quantitative fluorescence-based SA beta-gal assay, employed according to a previously established protocol [17] requires cell lysis and subsequently measures the relative fluorescence of total SA beta-gal in the culture well, rather than distinct SA-positive cells. Relative SA beta-gal fluorescence yield was analyzed using a plate reader (BioTek Synergy 2, Winooski, USA). All conditions were analyzed on the same plate at the same time, making comparison of RFUs meaningful between conditions. The quantitative fluorescent SA beta-gal lysate assay was normalized to cell density by dividing the relative fluorescence of the SA beta-gal assay by the BCA protein content from each culture well (described below). Both colorimetric and fluorescent senescence assays were conducted at pH 6, which suppresses the activity of native lysosomal beta-galactosidase that is only active at pH 4 [17]. Therefore, only SA beta-gal active at pH 6 could cleave the chromogenic substrate 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-gal), reducing non-senescent cell staining and increasing signal:noise ratios [17].
2.4.d. BCA cell protein assay
Cell density was approximated using cell-derived protein content from each culture well detected by the microBCA assay (Pierce Thermo Scientific, USA) used according to manufacturer’s instructions. Relative fluorescence from the assay was analyzed using a plate reader (BioTek Synergy 2, Winooski, USA). All conditions were analyzed on the same plate at the same time, making comparison of RFUs meaningful between conditions.
2.5. Cell imaging
Fluorescent false-color, brightfield, and true color microscopy images of cells in culture were acquired using a Nikon Eclipse TE2000-U microscope equipped with fluorescent optics, CCD camera, and Metamorph and Q Capture Pro software. Confocal images were captured using a FV1000 IX81 Olympus confocal microscope. Representative images were selected from 3 independent replicates.
2.6. Statistics
Statistics were performed using a one-way ANOVA with post-hoc Dunnett Multiple Comparisons Test or Student’s t-test, where specified. Cell counts were taken from 15X objective images in low confluency cultures and 40X images in high confluency cultures so that ~50–100 cells occupied each frame. For the colorimetric SA beta-gal assay, 3 frames per replicate were counted from 3 replicates from 3 different mice per condition. For the Ki-67 assay, 2 frames from 2 replicates were counted from 3 different mice per condition. For the fluorescent SA beta-gal assay, lysates from 3 replicates were combined from each of 3 mice. For RAW cells, 6 wells were counted in each condition for the colorimetric SA beta-gal and Ki-67 assays, and lysates from 3 wells were combined for each condition for the fluorescent SA beta-gal assay. For the colorimetric senescent assay, only dark blue cells were counted as they (and not any lighter blue cells) corresponded to cells that did not label for Ki-67. Ki-67-positive cells with a definite labeled nucleus were counted as positive. No values for primary cells were below the limit of detection for any of the assays. For bead quantification in phagocytosis assays, 2 frames from 2 replicates were counted from 3 different mice per condition.
3. RESULTS
3.1. Macrophage phenotype
Macrophages maintained strong F4/80 staining throughout 21 days of culture, supporting their mature macrophage phenotype [38, 39] (Supplementary Figure 1). Macrophages also maintained the strong M2 phenotypic marker, CD206 [40] (macrophage-mannose receptor), labeling to 21 days (Supplementary Figure 1), a macrophage polarization state shown capable of proliferation in vivo during inflammation [9]. Macrophage proliferation, peaked in culture near day 7 (Figure 1). Isotype controls for F4/80 and CD206 revealed some background staining; however, previous work also reported strong staining for these two markers after 21-day cultures (unpublished data, submitted 2012).
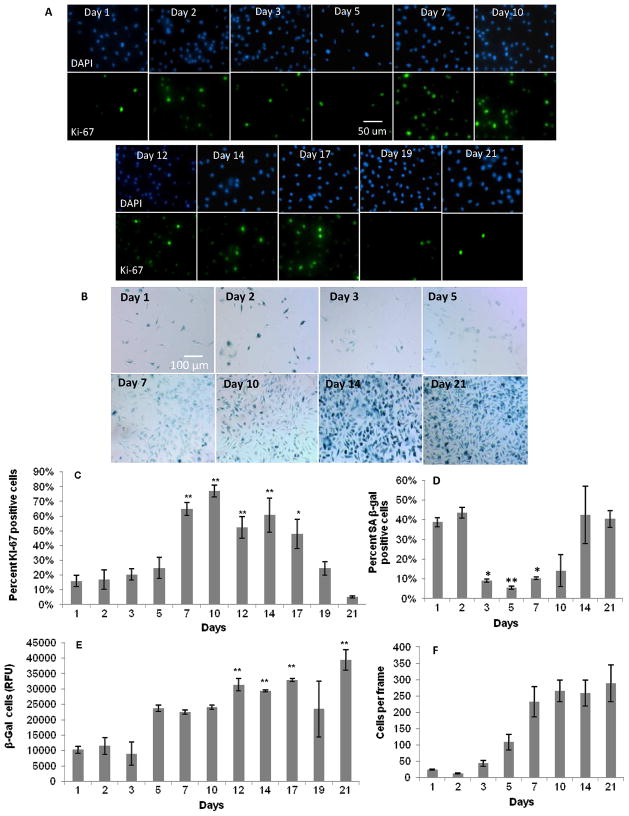
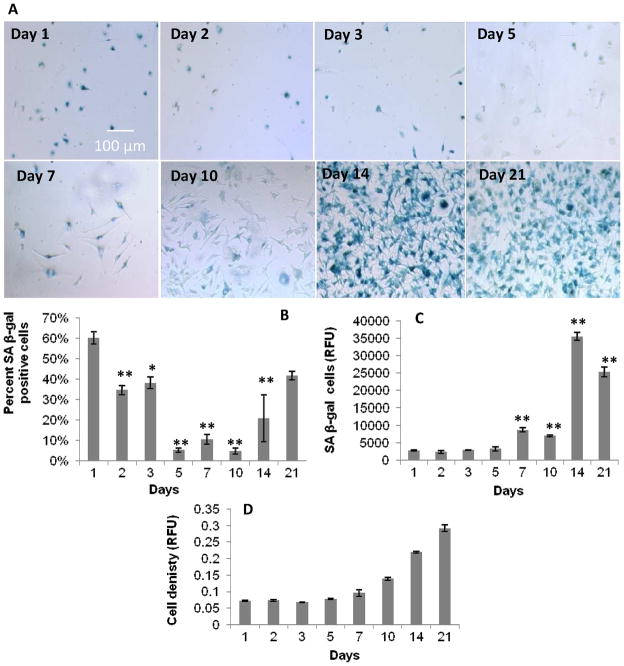
Figure 1.
Proliferative capacity of primary BMMΦ cultures over 21 days. Fluorescence and color images of: A) DAPI and Ki-67, and B) SA beta-gal staining, respectively, in primary BMMΦs over 21 days. Graphical representation of: C) percent positive Ki-67 cells; D) percent positive SA beta-gal cells (from fixed colorimetric beta-gal assay); E) relative fluorescence units (from lysed fluorescent SA beta-gal assay); and F) cells per frame, all over 21 days. These data show a decrease in Ki-67 staining and an increase in SA beta-gal staining after 21 days, suggesting an increase in macrophage senescence over 21 days. One-way ANOVA with post-hoc Dunnett Multiple Comparisons Test was applied, comparing all days to the day 1 time point. Significance is noted as p<.05 * and p<.001 **. No significant increase in Ki-67 expression after passaging (C) indicates macrophage senescence.
3.2. Culture Confluence
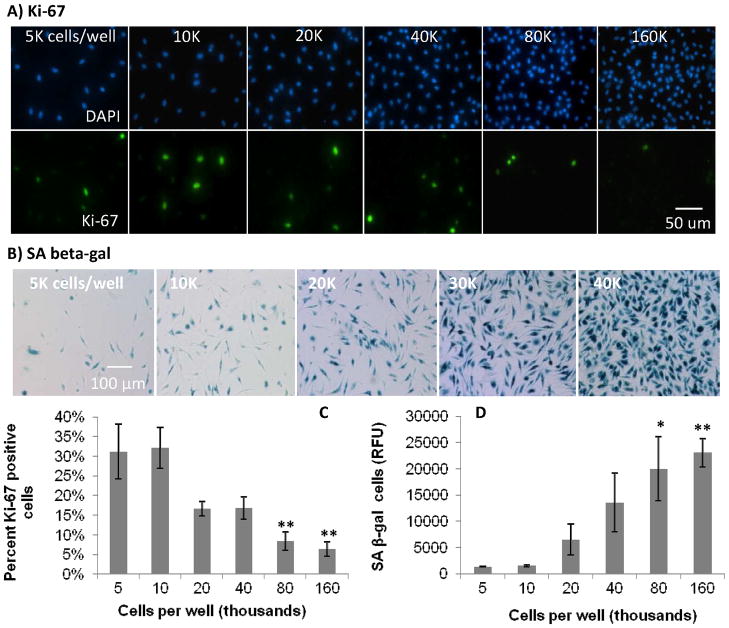
Increasing adherent macrophage confluence increased expression of senescence marker, SA beta-gal, and decreased expression of proliferation marker, Ki-67, in primary BMMΦ cells (Figure 2). Both the colorimetric qualitative and fluorescent quantitative senescence assays yielded the same trends. After confluence was reached, cells were passaged and re-plated at a low density and found capable of being restimulated to divide (Figure 3).
Figure 2.
Effect of increasing cell confluence on macrophage proliferation in culture. Fluorescence and color micrograph images of: A) DAPI and Ki-67, and B) SA beta-gal staining, respectively, in primary BMMΦs with increasing confluence on tissue culture plastic. Graphical representation of C) percent positive cells for Ki-67, and D) relative fluorescent signal, from lysed fluorescent SA beta-gal assay, showing increased SA beta-gal with increasing confluence in BMMΦ cultures. These data show decreased percent proliferative cells as seen by Ki-67 and an increase in SA beta-gal with increasing cell density. These data also confirm the ability of SA beta-gal to represent non-proliferative cells and the decrease in proliferative capacity of macrophages with increasing concentration. One-way ANOVA with post-hoc Dunnett Multiple Comparisons Test was applied, comparing all concentrations the 5K condition. Significance is noted as p<0.05 * and p<0.001 **.
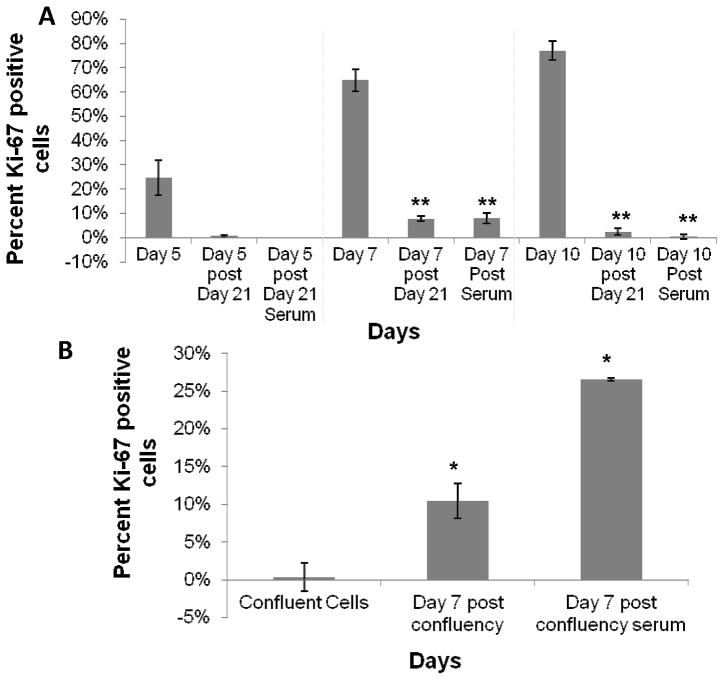
Figure 3.
Discrimination of senescent versus quiescent BMMΦs using Ki-67 staining of cultures. A) cells passaged after 21 days and allowed to grow for 5, 7, and 10 days. Passaged cells with and without 50% serum as a mitogenic stimulator were compared to their respective time point prior to day 21, indicating that after 21 days, cells decrease their ability to proliferate, reflecting senescence. B) proliferation of cells cultured for 1 day at high confluence and 7 days after those cells were passaged and plated at low confluence in the presence and absence of serum. These data indicate regained proliferative capacity of cells after being plated at sub-confluency, indicating confluent cells become quiescent and not senescent. One-way ANOVA with post-hoc Student’s t-test was applied, comparing post-passages to the pre-passaged condition (i.e. Day 5, 7, and 10 in A and confluent cells in B). Significance is noted as p<0.05 * and p<0.001 **.
3.3. BMMΦ proliferation over 21 days
Primary BMMΦs initially increased and then later decreased their percent proliferation over the course of 21 days, peaking at day 7 and 10 (Figure 1). This same trend in proliferation was seen previously by our lab [36]. The colorimetric senescence assay inversely correlated with the Ki-67 assay, while the fluorescent quantitative senescent assay diverged, showing a general increasing trend over 21 days. Cells passaged and re-plated after 21 days were not capable of being restimulated to divide, even with mitogenic stimulation (Figure 3).
3.4. Phagocytosis
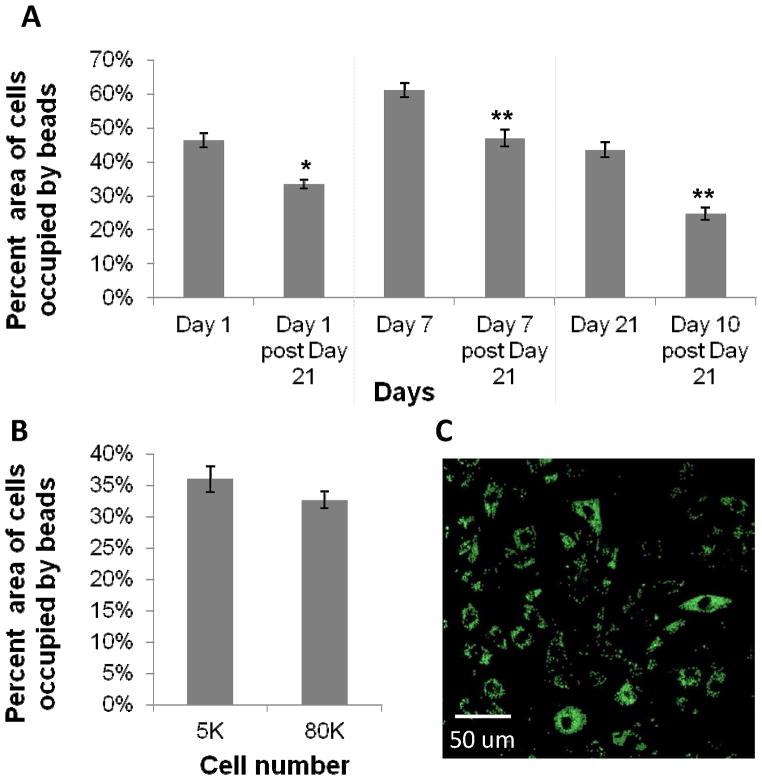
Primary BMMΦs showed reduced ability to phagocytose subsequent to passaging after 21 days (Figure 4), under the same conditions by which the majority of cells within the culture were shown to be senescent (Figure 3). Cells at high confluence (i.e., those deemed quiescent) compared to those at low confluence showed a non-significant reduction in phagocytosis (Figure 4).
Figure 4.
A) Bead phagocytic uptake in macrophages passaged after 21 days (shown in Figure 3 to be senescent) and cultured for 10 days were compared with bead uptake of their corresponding time point prior to 21 days. Data show a reduction in phagocytosis in cells at time points past 21 days. B) Bead uptake in macrophages at low and high confluency, indicating no significant change in phagocytosis at high confluence (shown in Figure 3 to be quiescent). One-way ANOVA with post-hoc Student’s t-test was applied, comparing post passages to the prepassaged condition (i.e. Day 1, 7, and 21). Significance is noted as p<.05 * and p<.001 **. C) Representative confocal image of BMMΦs with internalized beads. This image is an overlay of 10 z-sections through the cell, indicating beads are internalized by phagocytosis.
3.5. LPS stimulation
LPS stimulation in primary BMMΦs delayed SA beta-gal staining over the course of 21 days, but did not eliminate it. The colorimetric senescence assay showed least amounts of staining for SA beta-gal from day 5 to day 10, with proliferation rates decreasing after day 14 (Figure 5). Similar but delayed trends for both senescence assays were seen in the LPS-stimulated condition compared to the non-LPS stimulated condition described above.
Figure 5.
Proliferative capacity of primary BMMΦs over 21 days in culture in the presence of continual LPS stimulation. A) Color images of SA beta-gal staining in primary BMMΦs over 21 days in the presence of LPS. Graphical representation of: B) percent SA beta-gal positive cells from the colorimetric SA beta-gal assay, C) relative fluorescence from the fluorescent SA beta-gal assay, and D) cell density shown as relative absorbance from the microBCA assay. These data show a delay in the SA beta-gal response compared to non-LPS treated macrophages (Figure 1). One-way ANOVA with post-hoc Dunnett Multiple Comparisons Test was applied, comparing all days to the day 1 time point. Significance is noted as p<0.05 * and p<0.001 **.
3.6. Passaging
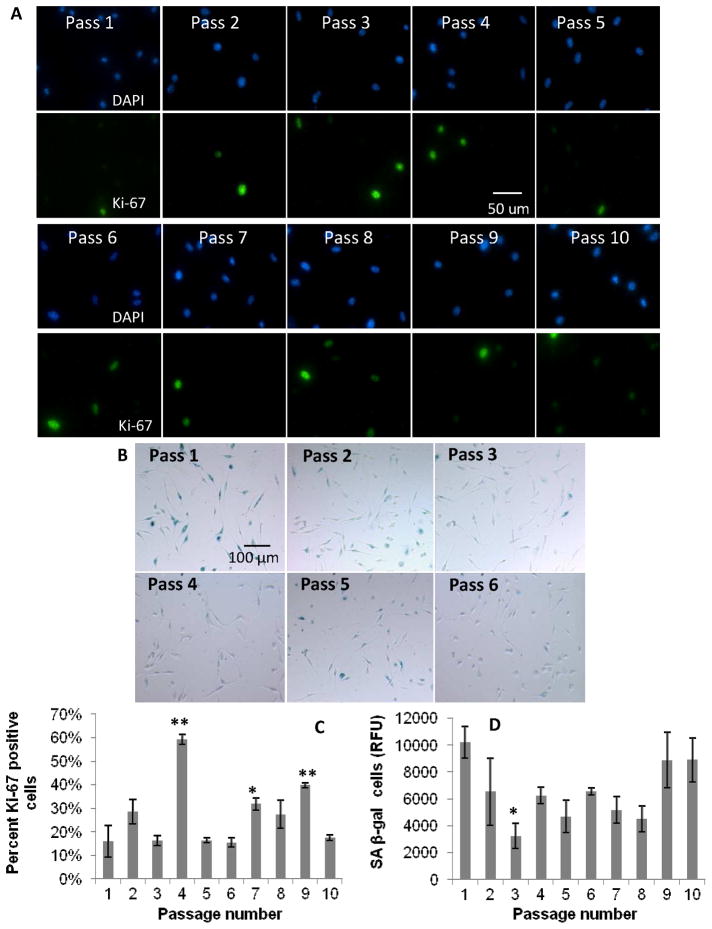
Increasing passages of BMMΦs did not correlate to a definite increasing or decreasing trend of SA beta-gal or Ki-67 expression up to 10 passages. The qualitative colorimetric percent positive cell and quantitative cell lysate senescent assays followed relatively similar trends. The Ki-67 assay was inversely related at some passage numbers, but not all (Figure 6).
Figure 6.
Effect of serial passaging on BMMΦ proliferation. Fluorescence and color images of: A) DAPI and Ki-67, and B) SA beta-gal staining, respectively, in primary BMMΦ cultures with increasing passage numbers. Graphical representation of: C) percent positive cells for Ki-67 markers, and D) SA beta-gal (from fluorescent senescence assay) with increasing passaging in BMMΦs. These data show no dramatic increasing or decreasing trends in senescence with increased passaging. One-way ANOVA with post-hoc Dunnett Multiple Comparisons Test was applied, comparing all passages to the passage 1 condition. Significance is noted as p<0.05 * and p<0.001 **.
3.7. Biochemical stimulation
After 21 days of culture, BMMΦs positive for SA beta-gal and negative for Ki-67 could not be stimulated to proliferate using cytokines, mitogens, or passaging at a lower confluency as determined by an insignificant change in either SA beta-gal or Ki-67 expression after stimulation (Figure 3, Supplementary Figure 2).
3.8. Pre-differentiated BMMΦs
SA beta-gal increased while Ki-67 decreased during the first 7 days of macrophage differentiation from bone marrow hematopoietic cell precursors (Supplementary Figure 3). Though by day 7 many now-differentiated macrophages expressed SA beta-gal, they were still able to be restimulated to divide when passaged at lower confluency. This is seen in Figure 1, representing recently differentiated macrophages after passage and plating at a lower confluency, exhibiting decreased SA beta-gal and increased Ki-67 expression after a couple days of culture.
3.9. Transformed RAW cell cultures
RAW 264.7 cells stained positive for SA beta-gal with increasing time and confluency. LPS exposure decreased SA beta-gal staining over long-term culture and with increasing confluency (Supplementary Figure 4). RAWs always responded to restimulation to proliferate regardless of culture time (data not shown) and passage number (passaged up to 30 times, Supplementary Figure 5).
4. DISCUSSION
Macrophage culture duration to 21 days was selected as a terminal time point as it is the approximate time required for the foreign body response (FBR) to mature in vivo [43]. After 21 days in culture, macrophages were found to be senescent and decrease their ability to phagocytose (Figure 4). This is consistent with the observed propensity of implants to infect [10, 11], and implies that macrophages could lose their ability to phagocytose bacteria after extended exposure to biomaterials. Importantly, Jenkins et al. recently discovered that macrophages proliferate in vivo during Th2 inflammation [9]; thus, their senescence shown in culture over time could suggest a decreased ability to properly proliferate at implant sites in the presence of infection. A previous study also found that macrophages decrease their production of inflammatory cytokines over 21 days [36]. All these findings support a phenotypic shift over time that decreases macrophage competence, contributing to their inability to respond to bacterial invasion at the surface of an implant over time. Macrophages from aged animals have also shown a decreased ability to be stimulated to divide [22] and a decreased phagocytic capacity [20]. Thus, the longer the implant resides in the body and the older the individual [44] may increase their susceptibility to implant-centered infection.
Various culture conditions are known to affect cell senescence in other cultured cell types including confluency, culture time, and passage number [17, 21, 45]. Passaging macrophages up to 10 times did not appear to have observable effects on their proliferation (Figure 6). Confluent culture disposes macrophages to quiescence but not senescence. This was substantiated by the ability of macrophages to be re-stimulated to divide upon passaging and mitogenic stimulation post-confluence (Figure 3). This result is consistent with contact inhibition-induced quiescence [21, 37] shown in other cell types to also stain positive for SA beta-gal. Decreasing SA beta-gal staining at early time points during the 21-culture is attributed to confluence-induced quiescence of pre-differentiated BMMΦ-staged cells (which were passaged and plated at lower cell confluence for the 21-day experiment, Supplementary Figure 3). Dimri et al. has also shown reduced SA beta-gal staining 2 days after highly confluent cultures of normal human fetal lung fibroblasts were passaged [37]. Confluent cultures stained positive for SA beta-gal, indicating that although this assay is specific for senescence in other cell lines [21, 37], it stains both quiescent and senescent macrophages. Interestingly, we found a slight decrease in phagocytosis in confluent versus non-confluent cultures. This decrease was not significant and was not as dramatic as in senescent cultures (Figure 4).
Two metrics were used to determine senescence: 1) senescence-associated beta-galactosidase (SA beta-gal) activity at pH 6 [37, 46], and 2) active cell proliferation (i.e., absence from the cell cycle G0 phase).[41] Many cells produce beta-galactosidase at pH 4–5 in lysosomes, but it is unique among senescent cells to exhibit beta-gal activity at pH 6 [37]. Senescent cells have been postulated to provide an environment wherein modified lysosomal beta-gal structure remains active at neutral or alkaline pH [21] and that its increased lysosomal content characteristic in senescent cells makes it detectable [25]. While macrophages frequently up-regulate lysosomal activity [47], the SA beta-gal assay used here may not selectively reflect macrophage senescence. Therefore, despite common cell culture use of the SA beta-gal assay, its validation for macrophage senescence -- to our knowledge not yet reported -- was required. Therefore, senescence used was anti-Ki-67 antibody labeling of cells to target a protein expressed only during active cell proliferation [41] in parallel with SA beta-gal staining. Significantly, anti-Ki-67 labels all cells in all active cell cycle phases (i.e., interphase and mitosis). Therefore cells were also labeled with phospho-histone H3, which only labels cells during S-phase of interphase [42]. Results showed similar trends between Ki-67 and phospho-histone H3 labeling (data not shown), validating the proliferative activity of cultured macrophages using Ki-67. Inverse correlation between cell Ki-67 expression and SA beta-gal staining suggests that SA beta-gal labels macrophages with decreasing proliferation tendency and not non-specifically as a general function of increased macrophage lysosomal activity.
As senescent cells are incapable of being re-stimulated to divide [48], passaging, mitogens, and both passaging and subsequent mitogenic stimulation were all employed in culture to determine if macrophages could be re-stimulated to divide. Macrophages were allowed to first grow for at least 7 days in the presence and absence of mitogenic stimulation after passaging to determine if cells were quiescent (capable of being re-stimulated to divide) or senescent (incapable of being re-stimulated to divide) at the time when greatest cell proliferation is seen (Figure 1).
The cell lysate-based fluorescent SA beta-gal assay did not correlate with the fixed cell colorimetric assay during extended culture time, showing instead an increase in SA beta-gal over the 21 day time-course in both LPS-stimulated and non-LPS-stimulated BMMΦ culture conditions (Figures 1 and 5, respectively). This discrepancy is attributed to the observation that over 21 days of culture, cells becoming senescent produced increasing SA beta-gal amounts within positive cells (Figure 1) so that total SA beta-gal amounts in culture wells after cell lysis increased over 21 days. This explanation is also supported by observations from increased cell confluence-dependent culture experiments where initially different plated cell densities were cultured for identical times. These results showed that both SA beta-gal senescence assays consistently correlated with each other and also inversely with Ki-67 expression. Percent of cells positive for SA beta-gal detected in fixed cells using the colorimetric SA beta-gal assay correlated inversely with percent cells positive for Ki-67, suggesting that over increased culture time, determining senescence on a percent positive cell basis is more representative of changes in proliferation than cell lysis methods.
As a positive control for Ki-67 and a negative control for senescence, secondary RAW 264.7 macrophages that also have intrinsically high lysosomal activity [49] were labeled with these markers. These immortalized cells were always shown capable of being re-stimulated to divide and subsequently expressed high levels of Ki-67 regardless of culture time or passage number, displaying 100% Ki-67 staining even at passage 30 (Supplementary Figure 5). Interestingly, these cells began to express SA beta-gal and decrease Ki-67 with increasing confluence, seen previously in immortalized fibroblast cultures at high confluence [21, 50]. This is significant as the ability of immortalized and essentially transformed cancer-like cells to quiesce is not commonly known. Primary bone-derived murine macrophage cultures on plastic have already been shown to exhibit several characteristic features distinct from the transformed macrophage-like murine RAW 264.7 cell line commonly employed as a macrophage surrogate in cultures [51–53]. These differences include variances in morphology, cytokine secretion, receptor expression, proliferation, response to LPS, and metabolic output [31, 36]. These studies now confirm integral changes in macrophage proliferative capacity.
Endotoxin, a lipopolysaccharide (LPS) found on the membrane surface of gram negative bacteria [54], is commonly used to stimulate macrophages [36, 55]. To determine if macrophages might avoid senescence during perpetual stimulation that may occur at an infected implant site, both primary and immortalized transformed macrophages were treated with LPS in serum-based culture [31]. LPS delayed the onset of SA beta-gal expression in primary BMMΦs, but did not eliminate it (Figure 5), and also ameliorated SA beta-gal expression in RAW cells (Supplementary Figure 4). We previously reported reduced inflammatory cytokine secretion from primary macrophage cultures in continued presence of LPS in 21-day cultures [36]. We attributed this phenomenon to macrophage endotoxin tolerance. However, increased cell senescence in primary macrophages over time may also contribute to their attenuated cytokine response. This idea is supported by increased senescence seen in BMMΦs over 21-day cultures, even in the persistent presence of LPS stimulation (Figure 5).
5. CONCLUSIONS
This study identified important phenotypic changes that macrophages undergo in extended culture that decrease their competence over time, including a decreased ability to proliferate and phagocytose, both integral responses for proper implant surveillance and antimicrobial activity. This finding may explain macrophages’ reduced ability to combat infection around biomaterials. Important future work should determine if macrophages become senescent and decrease their ability to phagocytose around implants in vivo. Understanding the mechanisms that predispose implant sites to infection years after device deployment will aid in better addressing this important clinical issue, perhaps also considering that senescent-state macrophages may increase throughout the duration of implantation. This study demonstrated that the commonly employed senescence-associated beta-galactosidase assay labels both quiescence and senescence cells in macrophage cultures, and revealed conditions inducing quiescence but not senescence in cultured immortalized macrophage cell lines. This is yet another difference between primary and immortalized macrophage cultures that produces inequities in their direct comparisons and perhaps their fidelity to macrophage in vivo behavior around implants. Future studies employing this SA beta-gal assay for macrophages should validate it against additional markers of senescence and proliferation. This study also found that, unlike senescent cells that display significantly decreased phagocytosis, quiescent cells have only slightly reduced but insignificantly different ability to phagocytose particles. This important functional measure distinguishes macrophage competence between normal and quiescent and senescent cells in extended culture.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Health grant R01EB000894. We acknowledge PA Tresco, A Welm, R Hitchcock, and C Terry (all University of Utah, USA) for scientific critique and expert insight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JM. Chapter 4 Mechanisms of inflammation and infection with implanted devices. Cardiovasc Pathol. 1993;2:33S–41S. [Google Scholar]
- 2.Anderson JM. Multinucleated giant cells. Curr Opin Hematol. 2000;7:40–7. doi: 10.1097/00062752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Chambers TJ, Spector WG. Inflammatory giant cells. Immunobiology. 1982;161:283–9. doi: 10.1016/S0171-2985(82)80084-3. [DOI] [PubMed] [Google Scholar]
- 4.Bernatchez SF, Parks PJ, Gibbons DF. Interaction of macrophages with fibrous materials in vitro. Biomaterials. 1996;17:2077–86. doi: 10.1016/0142-9612(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 5.Rosengren A, Bjursten LM. Pore size in implanted polypropylene filters is critical for tissue organization. J Biomed Mater Res A. 2003;67:918–26. doi: 10.1002/jbm.a.10509. [DOI] [PubMed] [Google Scholar]
- 6.Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- 7.Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. J Cell Sci. 1992;101(Pt 4):907–13. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JM, Defife K, McNally A, Collier T, Jenney C. Monocyte, macrophage and foreign body giant cell interactions with molecularly engineered surfaces. J Mater Sci Mater Med. 1999;10:579–88. doi: 10.1023/a:1008976531592. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darouiche RO. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis. 2001;33:1567–72. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 11.Murray DW, Rushton N. Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg Br. 1990;72:988–92. doi: 10.1302/0301-620X.72B6.2246303. [DOI] [PubMed] [Google Scholar]
- 12.Williams C, Aston S, Rees TD. The effect of hematoma on the thickness of pseudosheaths around silicone implants. Plast Reconstr Surg. 1975;56:194–8. doi: 10.1097/00006534-197508000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009;175:161–70. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litovchick L, Florens LA, Swanson SK, Washburn MP, DeCaprio JA. DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev. 2011;25:801–13. doi: 10.1101/gad.2034211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging. 2010;2:344–52. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gary RK, Kindell SM. Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal Biochem. 2005;343:329–34. doi: 10.1016/j.ab.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Cristofalo VJ, Volker C, Francis MK, Tresini M. Age-dependent modifications of gene expression in human fibroblasts. Crit Rev Eukaryot Gene Expr. 1998;8:43–80. doi: 10.1615/critreveukargeneexpr.v8.i1.30. [DOI] [PubMed] [Google Scholar]
- 19.Linskens MH, Feng J, Andrews WH, Enlow BE, Saati SM, Tonkin LA, et al. Cataloging altered gene expression in young and senescent cells using enhanced differential display. Nucleic Acids Res. 1995;23:3244–51. doi: 10.1093/nar/23.16.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guayerbas N, Catalan M, Victor VM, Miquel J, De la Fuente M. Relation of behaviour and macrophage function to life span in a murine model of premature immunosenescence. Behav Brain Res. 2002;134:41–8. doi: 10.1016/s0166-4328(01)00449-1. [DOI] [PubMed] [Google Scholar]
- 21.Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–71. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 22.Sebastian C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol. 2009;183:2356–64. doi: 10.4049/jimmunol.0901131. [DOI] [PubMed] [Google Scholar]
- 23.Papadimitriou JM, Robertson TA, Walters MN. An analysis of the Phagocytic potential of multinucleate foreign body giant cells. Am J Pathol. 1975;78:343–58. [PMC free article] [PubMed] [Google Scholar]
- 24.Williams GT, Williams WJ. Granulomatous inflammation--a review. J Clin Pathol. 1983;36:723–33. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113 ( Pt 20):3613–22. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 26.Holt DJ, Grainger DW. Multinucleated giant cells from fibroblast cultures. Biomaterials. 2011;32:3977–87. doi: 10.1016/j.biomaterials.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence T. Macrophages and NF-kappaB in cancer. Curr Top Microbiol Immunol. 2011;349:171–84. doi: 10.1007/82_2010_100. [DOI] [PubMed] [Google Scholar]
- 28.Deane S, Selmi C, Teuber SS, Gershwin ME. Macrophage activation syndrome in autoimmune disease. Int Arch Allergy Immunol. 2010;153:109–20. doi: 10.1159/000312628. [DOI] [PubMed] [Google Scholar]
- 29.Lynn AD, Kyriakides TR, Bryant SJ. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32595. [DOI] [PubMed] [Google Scholar]
- 30.McInnes A, Rennick DM. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988;167:598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamberlain LM, Godek ML, Gonzalez-Juarrero M, Grainger DW. Phenotypic non-equivalence of murine (monocyte-) macrophage cells in biomaterial and inflammatory models. J Biomed Mater Res A. 2009;88:858–71. doi: 10.1002/jbm.a.31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jay SM, Skokos E, Laiwalla F, Krady MM, Kyriakides TR. Foreign body giant cell formation is preceded by lamellipodia formation and can be attenuated by inhibition of Rac1 activation. Am J Pathol. 2007;171:632–40. doi: 10.2353/ajpath.2007.061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godek ML, Sampson JA, Duchsherer NL, McElwee Q, Grainger DW. Rho GTPase protein expression and activation in murine monocytes/macrophages is not modulated by model biomaterial surfaces in serum-containing in vitro cultures. J Biomater Sci Polym Ed. 2006;17:1141–58. doi: 10.1163/156856206778530731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhoades ER, Orme IM. Similar responses by macrophages from young and old mice infected with Mycobacterium tuberculosis. Mech Ageing Dev. 1998;106:145–53. doi: 10.1016/s0047-6374(98)00113-4. [DOI] [PubMed] [Google Scholar]
- 35.Kim JB, Han AR, Park EY, Kim JY, Cho W, Lee J, et al. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264. 7 macrophage cells. Biol Pharm Bull. 2007;30:2345–51. doi: 10.1248/bpb.30.2345. [DOI] [PubMed] [Google Scholar]
- 36.Holt DJ, Chamberlain LM, Grainger DW. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 2010;31:9382–94. doi: 10.1016/j.biomaterials.2010.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 39.Duong LT, Rodan GA. PYK2 is an adhesion kinase in macrophages, localized in podosomes and activated by beta(2)-integrin ligation. Cell Motil Cytoskeleton. 2000;47:174–88. doi: 10.1002/1097-0169(200011)47:3<174::AID-CM2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 41.Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–73. [PMC free article] [PubMed] [Google Scholar]
- 42.Gown AM, Jiang JJ, Matles H, Skelly M, Goodpaster T, Cass L, et al. Validation of the S-phase specificity of histone (H3) in situ hybridization in normal and malignant cells. J Histochem Cytochem. 1996;44:221–6. doi: 10.1177/44.3.8648081. [DOI] [PubMed] [Google Scholar]
- 43.Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17:669–87. doi: 10.1163/156856206777346340. [DOI] [PubMed] [Google Scholar]
- 44.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 45.Lloberas J, Celada A. Effect of aging on macrophage function. Exp Gerontol. 2002;37:1325–31. doi: 10.1016/s0531-5565(02)00125-0. [DOI] [PubMed] [Google Scholar]
- 46.Bassaneze V, Miyakawa AA, Krieger JE. A quantitative chemiluminescent method for studying replicative and stress-induced premature senescence in cell cultures. Anal Biochem. 2008;372:198–203. doi: 10.1016/j.ab.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Sutton JS, Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966;28:303–32. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherwood SW, Rush D, Ellsworth JL, Schimke RT. Defining cellular senescence in IMR-90 cells: a flow cytometric analysis. Proc Natl Acad Sci U S A. 1988;85:9086–90. doi: 10.1073/pnas.85.23.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010;184:7040–6. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yegorov YE, Akimov SS, Hass R, Zelenin AV, Prudovsky IA. Endogenous beta-galactosidase activity in continuously nonproliferating cells. Exp Cell Res. 1998;243:207–11. doi: 10.1006/excr.1998.4169. [DOI] [PubMed] [Google Scholar]
- 51.Sandeep Varma R, Ashok G, Vidyashankar S, Patki P, Nandakumar KS. Anti-inflammatory properties of Septilin in lipopolysaccharide activated monocytes and macrophage. Immunopharmacol Immunotoxicol. 2011;33:55–63. doi: 10.3109/08923971003739236. [DOI] [PubMed] [Google Scholar]
- 52.Lin NJ, Hu H, Sung L, Lin-Gibson S. Quantification of cell response to polymeric composites using a two-dimensional gradient platform. Comb Chem High Throughput Screen. 2009;12:619–25. doi: 10.2174/138620709788681943. [DOI] [PubMed] [Google Scholar]
- 53.Yoon WJ, Ham YM, Yoo BS, Moon JY, Koh J, Hyun CG. Oenothera laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264. 7 macrophages. J Biosci Bioeng. 2009;107:429–38. doi: 10.1016/j.jbiosc.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 55.Ung DY, Woodhouse KA, Sefton MV. Tumor necrosis factor (TNFalpha) production by rat peritoneal macrophages is not polyacrylate surface-chemistry dependent. J Biomed Mater Res. 1999;46:324–30. doi: 10.1002/(sici)1097-4636(19990905)46:3<324::aid-jbm3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.