Abstract
Objective
Nitric Oxide (NO) regulation during shock and sepsis is complex. NO production by endothelial NO synthase (eNOS) maintains microvascular perfusion and prevents shock-induced organ injury. However, overproduction of NO by inducible NO synthase (iNOS) contributes to liver dysfunction after shock, and is associated with increased tissue damage and mortality. Estrogen improves organ function and outcome after shock and sepsis but the mechanism is unknown. We hypothesized that 17β-Estradiol would improve organ function by regulating the production of hepatocyte nitric oxide.
Methods
Isolated rat hepatocytes were stimulated in-vitro with pro-inflammatory cytokines to induce NO synthesis with or without estrogen. Nitrite was detected after 24hours. INOS protein was determined using western blot.
Results
Cytokine stimulation increased nitrite and iNOS protein in a dose-dependent manner. The cytokine-induced nitrite increase was significantly decreased by estrogen. Inducible nitric oxide synthase expression was also diminished in cultures with the higher doses of estrogen.
Conclusion
17β-Estradiol decreases cytokine-stimulated iNOS expression and NO production. Downregulation of iNOS expression may account for the beneficial role of estrogens in models of sepsis and shock.
BACKGROUND
The number of new cases of severe sepsis has been on the rise over the last two decades1,2. As a result, mortality remains high, at about 30% for the most severe cases despite advances in critical care management2. Death from complications of sepsis is still the leading cause of mortality in the surgical ICU3. Gender has been reported to influence mortality after sepsis. Weinstein and his colleagues were able to demonstrate a statistically significant difference in mortality between male and female patients hospitalized with sepsis4. Several studies also indicate that women may be immunologically better positioned to tolerate septic challenges compared to men5, 6. This has been largely attributed to the differential cell-mediated and humoral immune reactivity to infection between both sexes 7, 8. Clinical studies demonstrate a more favorable disposition in women of reproductive age after severe sepsis and shock. There is a decreased incidence of severe sepsis of 36% in women compared to 64% in men9, women also have a 14% survival advantage after severe sepsis, and are better compensated hemodynamically after shock 10, 11. These age groups of women have a reduced length of ICU and hospital stay 12, 13, and are 40% less likely to progress onto multiple organ dysfunction syndrome (MODS) after sepsis and shock7, 13, 14. Trials also show that men are 30% more likely to develop sepsis compared to women2. Additionally, after trauma, women with a higher injury severity score have been demonstrated to fare better than their male counterparts14. Also worthy of note is that women have been shown to have better outcomes in other disease entities such as cirrhosis, hepatocellular cancers and after cerebrovascular events.
Laboratory findings have shown that female rats tolerate sepsis and trauma better than male rats but the specific mechanisms responsible for these findings are still being investigated. A persistent observation has been that these advantages seen in female rats are more pronounced during the estrogenic phase of their reproductive cycles when estrogen in circulation peaks15, 16. Some notable findings include that, female rats have a survival advantage after caecal ligation and puncture17, an immune advantage has also been reported in female mice in hemorrhage - resuscitation models18. There is however, loss of any survival advantage after ovariectomy in these female rats15, 19. Additionally, in trauma-hemorrhage models, estrogen administration to rats improved cardiac and hepatocellular function and protected against pulmonary injury16, 19 - 22.
Excessive nitric oxide (NO) production during sepsis plays a significant role in the pathophysiologic sequelae of sepsis and hemorrhagic shock23. The production of NO by inducible nitric oxide synthase (iNOS) has deleterious effects on tissues and organs in sepsis, shock and other inflammatory conditions24, 25. Estrogen administration improves shock induced hepatocellular injury and has been suggested to alter hepatic dysfunction through modulation of NO production26 but whether estrogen regulates iNOS production by hepatocytes has not been proven. We hypothesized that 17β-Estradiol will attenuate pro-inflammatory cytokine-induced nitric oxide production in rat hepatocyte.
METHODS
Animals and Hepatocyte Isolation
Adult male Sprague Dawley rats weighing 200 to 300 g (Harlan Sprague-Dawley Madison, WI) were used in all experiments. All experiments were performed in adherence to National Institutes of Guide for the Care and Use of Laboratory Animals and approved by University of Louisville Institutional Animal Care and Use Committee. Hepatocytes were harvested using a modification of the Seglen in-situ perfusion technique, as previously described27,28. Briefly, the liver was perfused with a 0.05% collagenase solution (Type IV, Sigma, St. Louis, MO) for 20 minutes. The liver capsule was dissected out after perfusion and the parenchyma homogenized to produce a cell suspension. Hepatocytes were then separated from non-parenchymal cells by repeated centrifugation, filtering and resuspension. Cells were enumerated and assessed by tryptan blue exclusion and ensured to be consistently more than 90% viable.
Hepatocyte Culture and Treatment
Hepatocyte were plated at a concentration of 1 × 106 cells/mL in 6-well plates (Corning) in Williams medium E (0.24mmol/L L-arginine) supplemented with 15 mmol/L HEPES, 2 mmol/L L-glutamine, 105 U/L penicillin, 100mg/L streptomycin (Life Technologies, Grand Island NY), and 10% heat-inactivated, low endotoxin calf serum (Hyclone Laboratories, Logan, UT) . The cells were incubated at 37°C in a 5% CO2 incubator, medium was replaced 3 hours after plating and the cells allowed to recover overnight. After an 18-hour incubation, the medium was aspirated and the cells were treated with a cytokine mix (cm) consisting of recombinant rat interferon gamma (rrIFNg) at 100U/mL and recombinant human interleukin 1 beta (rrIL-1b) at 200U/mL (Life Technologies, Grand Island NY) in serum-free antibiotic-free Williams medium E. Where indicated, different concentrations of 17β-estradiol (Sigma, St. Louis, MO) were added to the culture medium at the same time. Dimethyl sulfoxide (DMSO) or ethanol (Sigma, St. Louis, MO) was used as the vehicle control.
In further experiments, cells were separately treated with the estrogen alpha receptor (ER-α) agonist, propyl pyrazole triol (PPT, 10μM) and ER-α antagonist methyl-piperidino-pyrazole (MMP, 10μM) purchased from Tocris Bioscience, Ellisville, MO.
Nitrite (NO −2) Determination
Twenty-four hours after exposure of hepatocytes to recombinant cytokines and estrogen, production of NO −2, which is a stable and measurable metabolite of NO was assessed. The concentration of NO2− in culture supernatant was measured using an automated colorimetric procedure based on the Griess reaction.
Western blot analysis
Western blots were performed to measure hepatocyte iNOS protein levels. Samples were separated using the BioRad Mini-PROTEAN Tetra electrophoresis system on 8% polyacrylamide gels prepared in the lab with reagents also from BioRad (Hercules, CA). Proteins were transferred onto nitrocellulose membranes. The membranes were incubated with iNOS antibody (Santa Cruz Biotechnology, Inc., Santa Cruz CA) overnight at 4°C followed by horseradish peroxidase-conjugated secondary anti-rabbit antibody for 1 hour at room temperature. Membranes were stripped and then reblotted with β-actin using mouse monoclonal antibody (Millipore, Billerica, MA) to confirm equal protein loading in each lane. Signal densities were evaluated by NIH ImageJ medical imaging software (ImageJ, U.S. National Institutes of Health, Bethesda, MD, http://imagej.nih.gov/ij/). Protein levels were quantified by densitometric evaluation and were corrected to the corresponding β-actin densities.
INOS mRNA Expression
INOS mRNA was determined by real time PCR using Applied Biosystems StepOnePlusTM Real-Time PCR Systems (Applied Biosystems, Foster City CA). Total RNA was isolated from hepatocytes using TRIzol Reagent (Life Technologies) according to the manufacturer’s protocol. cDNA was generated from the total RNA samples using TaqMan Reverse Transcription Reagents (Applied Biosystems). TaqMan Gene Expression Assays (Applied Biosystems) for iNOS genes was purchased as probe and primer sets. Samples were tested in triplicate for each different set of experiment, and the average values were used for quantification. 18S rRNA was used as endogenous control. The comparative CT method (ΔΔCT, cycle threshold) was used for quantification of gene expression. Analysis was performed using the StepOne software (Applied Biosystems) according to the manufacturer’s instructions.
Statistical Analysis
Each set of experiments was performed separately and independently using livers from at least three different rats, with each rat constituting a separate experiment. All conditions in each of these separate rats were tested in triplicates. Data are presented as mean + SEM. Statistical difference between treatment groups was determined using two-tailed student’s t-test. Level of statistical significant difference was set at p<0.05.
This work was supported in part by NIH Grant R01DK055664.
RESULTS
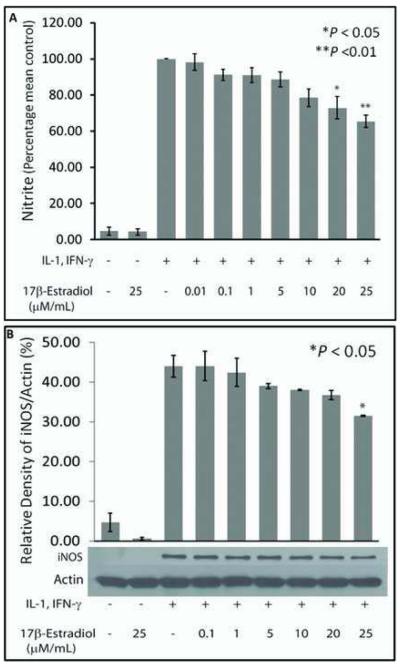
To evaluate the effect of estrogen on hepatocyte NO production, cultured hepatocytes were stimulated to produce NO in the presence of increasing concentrations of 17β-Estradiol (E2). Simultaneous incubation with E2 inhibited cytokine-stimulated hepatocyte NO production in a dose dependent manner (Fig. 1A). The MTT assay was performed to measure cell viability. This demonstrated that there were no cytotoxic effects of E2 on the hepatocytes, with no significant difference between groups treated with E2 and controls (data not shown). Western blot analysis of iNOS protein demonstrated that E2 dose-dependently suppressed cytokine-induced iNOS protein expression (Fig. 1B). Significant attenuation was observed at the 25μM dose of E2.
Figure 1.
Nitrite (NO −2) production of hepatocytes simultaneously treated with rhIL-1β, rrIFN-□, and different concentrations of E2 in serum-containing medium. Results are the mean + SEM of 3 independent rat experiments performed separately in which NO −2 was measured from supernatant of hepatocytes cultured in triplicates for each rat experiment. (A) NO −2 measured from cells stimulated with the cytokines alone minus E2 (3rd column from Y-axis) was used as positive control and all other treatments expressed as a percentage of the control. Asterisk represents a significant (p < 0.05) difference from control cells. (B) Western blot analysis of iNOS protein. A dose-dependent suppression of cytokine-stimulated iNOS protein expression by E2 is demonstrated.
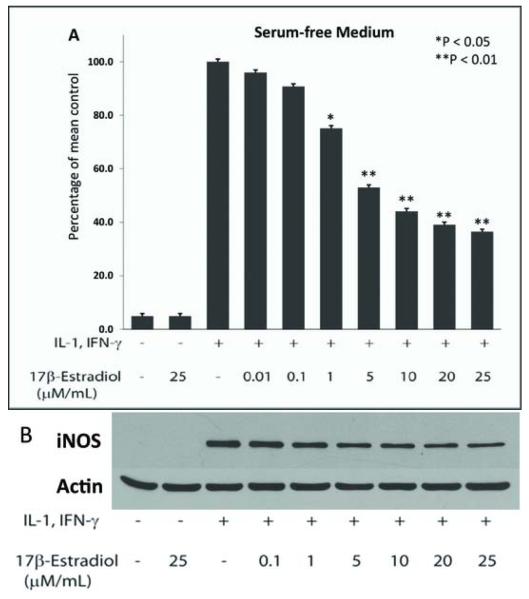
Hepatocytes are normally cultured in media containing 5-10% low endotoxin calf serum but calf serum may contain endogenous hormones. Therefore, we compared hepatocytes cultured in serum-containing and serum-free media for their ability to produce NO in the presence of E2 and found that smaller doses of E2 were required to produce a comparable lowering of NO production when hepatocytes were cultured in serum-free media (Fig. 2). Phenols have also been shown to have estrogenic effects on breast cancer cells29 and Williams media E contains phenol red as a dye. We therefore, tested the effect of E2 on NO production by cytokine-stimulated hepatocytes cultured in phenol red-free vs phenol red-containing media. We observed that there was reduced suppressive effect of E2 on NO production when using phenol red-free media, however, this was not significantly different from cells cultured in phenol red-containing media (data not shown). Thus, for further experiments we used serum-free Williams medium E containing phenol red and selected E2 concentrations of 10μM and 25μM.
Figure 2.
NO −2 production in cytokine-stimulated hepatocytes cultured in serum-free antibiotic-free medium: (A) Statistically significant suppression of NO −2 by estradiol begins to occur at the 1uM (p < 0.05) in cells cultured in serum-free, antibiotic-free medium. B. Western blot demonstrating iNOS expression in hepatocytes cultured in serum-free medium. Significant decrease in iNOS protein was noted at 10-25 uM dose of E2 (p<0.05).
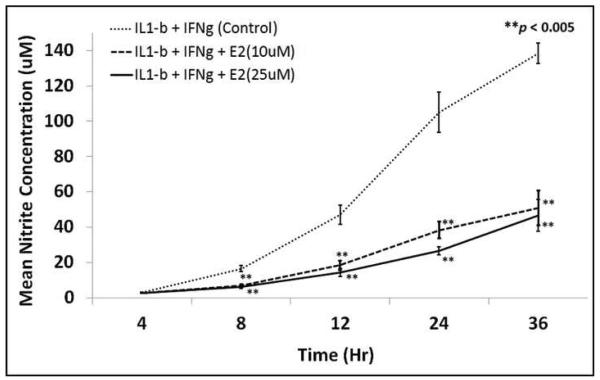
In a time course experiment, exposure of hepatocytes to E2 significantly diminished cytokine-stimulated NO production, starting at 8 hours (Fig. 3). This suppression of NO production by E2 persisted up to 36 hours of culture.
Figure 3.
Time-course relationship of cytokine-induced NO −2 production in hepatocyte exposed to two different concentrations of E2 (the two bottom line graphs) versus hepatocytes cultured without E2. Results are the mean + SEM of 3 different rats in which NO −2 was measured from supernatant of hepatocytes cultured in triplicates. The NO −2 measured are absolute values in uM.
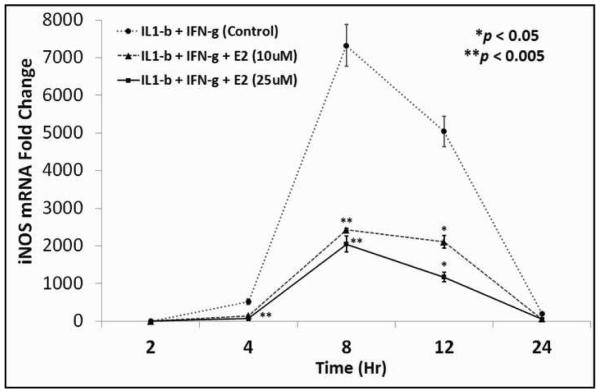
To evaluate the mechanism by which E2 regulates cytokine-stimulated hepatocyte NO production, we examined iNOS mRNA as an index of iNOS gene expression. Cytokines increased iNOS mRNA levels starting at about 2hrs, peaking at 8hrs and declining at later time points, corresponding with previous studies 30, 31. E2 decreased iNOS mRNA expression at each time point studied (Fig. 4).
Figure 4.
Line graph demonstrating iNOS mRNA expression in cytokine-stimulated hepatocytes exposed to two different doses of E2 (the two bottom line graphs) versus hepatocytes not exposed to E2 (upper line graph).
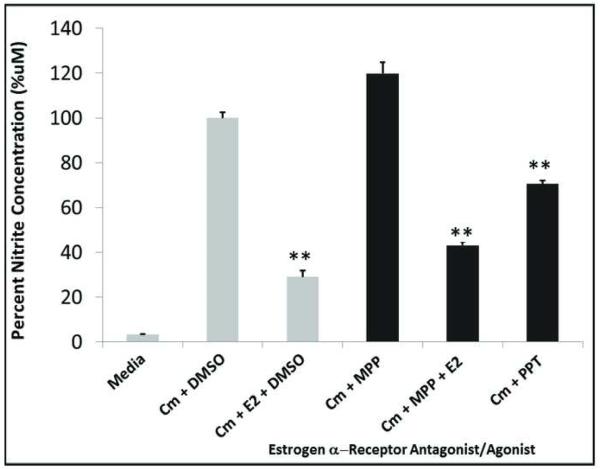
Hepatocytes contain both estrogen receptors alpha and beta (ER-α and ER-β), which are the primary estrogen receptors mediating the physiological effects of estradiol32. ER-α has been shown to regulate iNOS expression in the liver26. We therefore tested the role of ER-α in mediating the effect of E2 on hepatocyte iNOS activation. MPP, a specific ER-α antagonist had no effect on cytokine-induced NO production. PPT, a selective ER-α agonist, suppressed cytokine-induced hepatocyte NO production but with lower efficacy than estradiol (Fig. 5), suggesting that the effects of E2 may be partially but not completely mediated through ER-α
Figure 5.
Suppression of NO production involves ER-α. Hepatocytes were treated as described in Fig. 1. Concentrations of ER-α ligands were 10uM E2, 10uM PTT, a selective ER-α agonist and 10uM MMP, a specific ER-α antagonist. CM= Cytokine mix of rhIL-1b plus rrIFN-g. DMSO = Vehicle used as negative control.
DISCUSSION
Excessive NO production has damaging effects during inflammatory processes and significantly contributes to acute liver dysfunction in severe sepsis and shock23. The present study demonstrates that E2 attenuates pro-inflammatory cytokine-induced NO production in hepatocytes. This effect was most apparent at the higher doses of E2 used. This finding may represent one mechanism through which estrogens influence gender based differences in liver damage and other organ injury after sepsis22. Hepatocytes are capable of sustained iNOS expression, producing excessive amounts of NO in the process24. Our study demonstrates that E2 regulates hepatocyte NO production by decreasing iNOS mRNA levels and iNOS protein expression in cytokine stimulated cells. Hepatocyte NO production is primarily regulated at the level of iNOS gene expression33-35 and we demonstrated decreased iNOS mRNA and protein levels in E2-cultured hepatocytes. Whether E2 regulates iNOS mRNA production, mRNA stability, post-transcriptional modification or post-translational protein stability or degradation in hepatocytes will require further investigation.
A recent study showed that following hepatic injury after trauma-hemorrhage, ER-α activation was able to mediate a reduction in iNOS expression26. However, our results demonstrate that in this in-vitro model, ER-α did not account for the full extent of estradiol’s action. The reasons for the differences in these studies are not clear. Several tissue and cell types may be affected by E2 administration in an in-vivo model of inflammation that would not play a role in our in-vitro hepatocyte culture model. These factors and the mechanisms involved will need to be elucidated in further studies to give a complete picture of estradiol’s effect on iNOS regulation.
As with any experimental finding, this study has limitations. The doses of E2 used in this study were higher than doses of E2 used in other studies of E2 effects on cellular function36. However, these doses approximate the doses used in several in-vivo studies when E2 is used as a pharmacologic agent15, 37. Additionally, it is known that estrogen undergoes rapid metabolism in the liver by the cytochrome P-450 system38. The proportion of the E2 administered that might be rapidly metabolized and therefore inactivated by the hepatocytes in these experiments is unknown. Using NO production as an index of ongoing cellular injury may not best represent the several alterations in cellular function that occur after noxious insults. Other markers of cell injury may need to be assessed, validated and shown to reflectt the global picture of overall cell health. Finally, results from in-vitro cell culture studies may be difficult to translate into clinical effects, without the necessary transition to in-vivo models. Clearly, evidence supports a role of sex steroids in influencing progression of organ dysfunction, but a link between the mechanisms of these effects and outcome is obscure10, 39. The process by which sex hormones and their receptors modulate organ function remains the focus of considerable research studies, as this is yet to be fully elucidated. Further experimental studies will need to be carried out using older rats and/or female rats to determine how this will impact the results.
In conclusion, these data demonstrate that E2 dose-dependently decreases cytokine-stimulated NO production in hepatocytes and does so, at least in part, by decreasing iNOS mRNA and protein levels. The specific mechanism for the E2-mediated effect on hepatocyte NO production will require further studies to identify. E2 mediated effects on hepatocyte iNOS may represent one mechanism for the beneficial effects of estrogens in in-vivo models of shock and sepsis.
ACKNOWLEDGEMENT
This work was supported by NIH Grant R01DK055664.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;346:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein MP, Murphy JR, Reller LB, Lichenstein KA. The Clinical Significance of Positive Blood Cultures: A Comprehensive Analysis of 500 Episodes of Bacteremia and Fungemia in Adults. II. Clinical Observations, with Special Reference to Factors Influencing Prognosis. Clin Infect Dis. 1983;5:54–70. doi: 10.1093/clinids/5.1.54. [DOI] [PubMed] [Google Scholar]
- 5.Wichmann MW, Müller C, Meyer G, Adam M, Angele MK, Eisenmenger SJ, Schildberg FW. Different immune responses to abdominal surgery in men and women. Langenbecks Arch Surg. 2003;387:397–401. doi: 10.1007/s00423-002-0346-2. [DOI] [PubMed] [Google Scholar]
- 6.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27:151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 8.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 9.Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 10.Deitch EA, Livingston DH, Lavery RF, Monaghan SF, Bongu A, Machiedo GW. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg. 2007 Sep;246:447–253. doi: 10.1097/SLA.0b013e318148566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider AH, Crompton JG, Chang DC, Efron DT, Haut ER, Handly N, Cornwell EE., 3rd Evidence of hormonal basis for improved survival among females with trauma-associated shock: an analysis of the National Trauma Data Bank. J Trauma. 2010;69:537–540. doi: 10.1097/TA.0b013e3181efc67b. [DOI] [PubMed] [Google Scholar]
- 12.Reinikainen M, Niskanen M, Uusaro A, Ruokonen E. Impact of gender on treatment and outcome of ICU patients. Acta Anaesthesiol Scand. 2005;49:984–990. doi: 10.1111/j.1399-6576.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 13.Mostafa G, Huynh T, Sing RF, Miles WS, Norton HJ, Thomason MH. Gender-related outcomes in trauma. J Trauma. 2002;53:430–434. doi: 10.1097/00005373-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Knorferl MW, Jarrar D, Angele MK, Ayala A, Schwacha MG, Bland KI, Chaudry IH. 17β -Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. Am J Physiol Cell Physiol. 2002;282:C1087–C1092. doi: 10.1152/ajpcell.2001.281.4.C1131. [DOI] [PubMed] [Google Scholar]
- 16.Caruso JM, Xu DZ, Lu Q, Dayal SD, Deitch EA. The female gender protects against pulmonary injury after trauma hemorrhagic shock. Surg Infect (Larchmt) 2001;2:231–240. doi: 10.1089/109629601317202713. [DOI] [PubMed] [Google Scholar]
- 17.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Knöferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarrar D, Wang P, Knoferl MW, Kuebler JF, et al. Insight into the mechanism by which estradiol improves organ function after trauma-hemorrahge. Surgery. 2000;128:246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- 20.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000;279:H1015–H1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular function in male animals. Ann Surg. 2000;232:673–679. doi: 10.1097/00000658-200011000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananthakrishnan P, Cohen DB, Xu DZ, Lu Q, Feketeova E, Deitch EA. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. doi: 10.1016/j.surg.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 23.Chen T, Zamora R, Zuckerbraun B, Billiar TR. Role of Nitric Oxide in Liver Injury. Curr Mol Med. 2003;3:519–526. doi: 10.2174/1566524033479582. [DOI] [PubMed] [Google Scholar]
- 24.Billiar TR, Curran RD, Harbrecht BG, et al. Modulation of nitrogen oxide synthesis in vivo: NG-monomehtyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990;48:565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- 25.Kröncke K-D, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu T, Yu HP, Suzuki T, Szalay L, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. The role of estrogen receptor subtypes in ameliorating hepatic injury following trauma-hemorrhage. J Hepatol. 2007;46:1047–1054. doi: 10.1016/j.jhep.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seglen PO. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 28.Harbrecht BG, Wirant EM, Kim YM, Billiar TR. Glucagon inhibits hepatocyte nitric oxide synthesis. Arch Surg. 1996;131:1266–1272. doi: 10.1001/archsurg.1996.01430240020002. [DOI] [PubMed] [Google Scholar]
- 29.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC, Simmons RL, Billiar TR. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993;90:522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salkowski CA, Detore G, McNally R, van Rooijen N, Vogel SN. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo: the roles of macrophages, endogenous IFN-gamma, and TNF receptor-1-mediated signaling. J Immunol. 1997;158:905–912. [PubMed] [Google Scholar]
- 32.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 33.Lowenstein C, Alley E, Raval P. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vera ME, Shapiro RA, Nussler AK. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc atl Acad Sci USA. 1996;93:1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor BS, Kim Y, Wang Q, Shapiro RA, Billiar TR, Geller DA. Nitric Oxide Down-regulates Hepatocyte–Inducible Nitric Oxide Synthase Gene Expression. Arch Surg. 1997;132:1177–1183. doi: 10.1001/archsurg.1997.01430350027005. [DOI] [PubMed] [Google Scholar]
- 36.Kauser K, Sonnenberg D, Tse J, Rubanyi GM. 17 β-Estradiol attenuates endotoxin-induced excessive nitric oxide production in ovariectomized rats in vivo. Am J Physiol. 1997;273(1 Pt 2):H506–9. doi: 10.1152/ajpheart.1997.273.1.H506. [DOI] [PubMed] [Google Scholar]
- 37.Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of estrogen-mediated intestinal protection following trauma-hemorrhage: p38 MAPK-dependent upregulation of HO-1. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1825–31. doi: 10.1152/ajpregu.00112.2008. [DOI] [PubMed] [Google Scholar]
- 38.Schneider J, Sassa S, Kappas A. Metabolism of estradiol in liver cell culture. Differential responses of C-2 and C-16 oxidations to drugs and other chemicals that induce selective species of cytochrome P-450. J Clin Invest. 1983;72:1420–1426. doi: 10.1172/JCI111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George RL, McGwin G, Jr., Windham ST, et al. Age-Related Gender Differential in Outcome after Blunt or Penetrating Trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]