Abstract
Mammals contain a diverse set of secreted phospholipases A2 (sPLA2s) that liberate arachidonic acid from phospholipids for the production of eicosanoids and exert a variety of physiological and pathological effects. We report the cloning, recombinant expression, and kinetic properties of a novel human sPLA2 that defines a new structural class of sPLA2s called group XII. The human group XII (hGXII) cDNA contains a putative signal peptide of 22 residues followed by a mature protein of 167 amino acids that displays homology to all known sPLA2s only over a short stretch of amino acids in the active site region. Northern blot and reverse transcription-polymerase chain reaction analyses show that the tissue distribution of hGXII is distinct from the other human sPLA2s with strong expression in heart, skeletal muscle, kidney, and pancreas and weaker expression in brain, liver, small intestine, lung, placenta, ovaries, testis, and prostate. Catalytically active hGXII was produced in Escherichia coli and shown to be Ca2+-dependent despite the fact that it is predicted to have an unusual Ca2+-binding loop. Similar to the previously characterized mouse group IIE sPLA2s, the specific activity of hGXII is low in comparison to that of other mammalian sPLA2, suggesting that hGXII could have novel functions that are independent of its phospholipase A2 activity.
Secreted phospholipases A2 (sPLA2)1 are Ca2+-dependent disulfide-rich 14–18-kDa enzymes that catalyze the hydrolysis of phospholipids at the sn 2-position to release fatty acids and lysophospholipids (1–3). In mammalian cells stimulated with proinflammatory agonists, a subset of sPLA2s is involved in the release of arachidonic acid for eicosanoid production (4, 5). The first mammalian sPLA2 to be identified was the pancreatic sPLA2. This sPLA2 is found at high levels in pancreatic juice where it has a well known function in the digestion of dietary phospholipids (6). However, sPLA2 is also found at lower levels in lung, liver, spleen, kidney, and ovary where it has been proposed to play a role in cell proliferation, acute lung injury, cell migration, and endotoxic shock (7–9). The first nonpancreatic mammalian sPLA2 to be identified was the group IIA enzyme, which is expressed at high levels during inflammation (10) and is the principal bactericidal agent against Gram-positive bacteria in human tears (11).
In addition to the above evidence, it is becoming clear that sPLA2s are involved in a diverse set of physiological functions (7, 12–14). In the last few years, six mouse and five human sPLA2s structurally related to GIB and GIIA sPLA2s (mGIIC, hGIID, mGIID, hGIIE, mGIIE, mGIIF, hGIIF, hGV, mGV, hGX, and mGX)2 have been identified (15–20).3 All of these group I/II/V/X sPLA2s have similar primary structures, including identical catalytic site residues and partially overlapping sets of disulfides (21). However, they are not closely related isoforms because the level of amino acid identity is typically 20–50% among these sPLA2s. More recently, a novel human group III sPLA2 was identified (22), which is structurally distinct from the group I/II/V/X sPLA2s but related to the group III sPLA2s found in bee and lizard venoms. This diversity of sPLA2 structures and the fact that the tissue distribution of the different sPLA2s are distinct argue for a diversity of physiological functions for these lipolytic enzymes.
It is also clear that mammals contain a collection of proteins that tightly bind sPLA2s. Two types of sPLA2 receptors (M- and N-type) as well as other soluble sPLA2-binding proteins have been identified (7, 13, 21, 23–25) and are likely to play a role in the physiological functions of mammalian sPLA2s and in the toxicity of a wide variety of myotoxic and neurotoxic sPLA2s found in reptile and invertebrate venoms. Very recently, the cell surface proteoglycan glypican was also identified as a sPLA2-binding protein able to facilitate arachidonic acid release by GIIA and GV sPLA2s in fibroblastic cells (26).
Because of the presence of a large collection of sPLA2s in both mammals and many reptile and invertebrate venoms, we have been searching nucleic acid databases for the presence of novel mammalian sPLA2s with homology to all known types of these enzymes, including structurally distinct ones like the group IX sPLA2 (Conodipine-M) from the venom of the cone snail Conus magus (27). In this work, we report the cloning, recombinant expression, tissue distribution, and enzymatic properties of a novel human sPLA2. Because this sPLA2 clearly belongs to a new structural class, we propose to name it human group XII sPLA2 (hGXII) to follow the recently identified group XI plant sPLA2s (21, 28, 29).
EXPERIMENTAL PROCEDURES
Molecular Cloning of hGXII sPLA2
Searching for mammalian and venom sPLA2 homologs in genomic databases stored at the National Center for Biotechnology using the tBLASTn sequence alignment program (30) resulted in the identification of different human ESTs (Gen-Bank™/EBI BE271092, AW468813, AI189300) and a human genomic BAC clone (GenBank™/EBI AC004067) that display low homology with various mammalian and venom sPLA2s (27). None of the ESTs were found to contain the full-length cDNA coding for the new sPLA2 candidate, but a putative complete open reading frame could be constructed from the alignment of the different ESTs and the appropriately spliced genomic sequence. A forward primer (5′-TTTGCGGCCGCATATGGAGCTGGCTGCTGCCAAGT) and a reverse primer (5′-TTTAAGCTTCTAGAATCTGTCACTAGCTGTCGGCATC) flanking the above open reading frame and containing appropriate restriction sites were used to amplify by RT-PCR the cDNA fragment coding for hGXII sPLA2. The expected 717-nucleotide hGXII cDNA fragment could be amplified from human fetal lung, pancreas, and testis cDNAs (CLONTECH) using a Taq Pwo polymerase mixture (Hybaid, United Kingdom). The PCR fragments were digested with NotI and XbaI, ligated into the mammalian expression vector pRc/CMVneo (Invitrogen), and sequenced in its entirety. Several clones were found to be identical to the consensus sequence described above.
Recombinant Expression of hGXII sPLA2
The pRc/CMVneo-hGXII construct was used as template in a PCR reaction with a forward primer (5′-TTTGGATCCATCGAAGGTCGTCAGGAGCAGGCCCAGACCGAC), which contains a BamHI site and a factor Xa protease site (Ile-Glu-Gly-Arg) adjacent to the predicted N-terminal Gln residue of mature hGXII sPLA2 (see Fig. 1) and the reverse primer given above. The purified PCR product was digested with BamHI and HindIII and was subcloned in frame with the truncated glutathione S-transferase (~10 kDa) encoded by the modified pGEX-2T vector (pAB3), which had been previously used to express several sPLA2s in Escherichia coli (17). Protein production in E. coli BL21, purification of inclusion bodies, and refolding and cleavage of the fusion protein with factor Xa were carried out as described previously (17). Cleaved hGXII was purified by HPLC on a Spherogel TSK SP-5PW column (10 μm, 0.75 × 7.5 cm, Altex) using a gradient of 1% acetic acid to 1 M ammonium acetate over 50 min (elution at 28 min) and was further purified on a reverse phase column (Waters RP8 Symmetry Shield, 5 μm, 100 Å, 0.46 × 25 cm) using a gradient of 10–60% acetonitrile in water with 0.1% trifluoroacetic acid over 50 min (elution at 36 min). The hGXII preparation appeared 100% pure when analyzed by SDS-polyacrylamide gel electrophoresis. MALDI-TOF (Applied Biosystems DE-Pro) was carried out in the linear mode using sinapinic acid.
FIG. 1. Alignment of the amino acid sequences of sPLA2s.
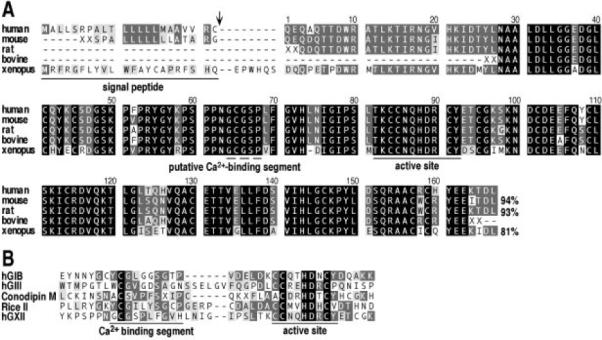
A, the full-length sequence of hGXII is aligned with the amino acid sequences of mouse, rat, bovine, and Xenopus GXII sPLA2s. (Sequences were deduced from the alignment of different ESTs and from the BAC clone). For some sPLA2s, the XX residues indicate that the sequence is partial. The arrowhead indicates the predicted signal peptide cleavage site (32). The active site region containing catalytic site residues that are found in all sPLA2s, and the putative Ca2+-binding segment GCGSP are indicated. The level of identity between the mature protein sequence of hGXII and other GXII sPLA2s is shown. B, alignment of the Ca2+-binding and active site regions of hGXII with a representative member of the four other structural classes of sPLA2s (hGIB for GI/II/V/X sPLA2s, hGIII for GIII sPLA2s,Conodipine-M for GIX sPLA2, and Rice II for GXI sPLA2s).
Analysis of the Tissue Distribution of hGXII sPLA2
The presence of mRNA for hGXII sPLA2 in different human tissues was explored by Northern blot and RT-PCR analyses. A human Northern blot (CLONTECH catalog no. 7780-1) was probed as described previously (18) with a 32P-labeled riboprobe corresponding to the hGXII coding sequence. For RT-PCR, reactions were performed with an internal forward primer (5′-GCCTTTCCCACGTTATGGTT) and the reverse primer described above (200 ng each), Taq polymerase, and 1 μl of human cDNA as template (Human Multiple Tissue cDNA Panels I and II, CLONTECH catalog nos. K1420-1 and K1421-1). PCR was carried out at 94 °C for 2 min followed by 45 cycles of 94 °C/30 s, 60 °C/30 s, and 72 °C/1 min, which was followed by 72 °C for 5 min. PCR reactions were analyzed by Southern blotting using a 32P-labeled hGXII oligonucleotide probe (5′-GGATGTGGCTCTCCACTGTT).
Kinetic Studies
Large unilamellar vesicles (0.1 μm) of POPC, POPG, and POPS (31) were used to measure the initial rates of hydrolysis by hGXII in Hank's balanced salt solution with 1.2 mM CaCl2 and 0.9 mM MgCl2 using the fatty acid-binding protein assay (17). The pH-rate profile and Ca2+ dependence for the action of hGXII on POPG and POPC vesicles, respectively, were obtained as described previously (17).
RESULTS AND DISCUSSION
Molecular Cloning of a Structurally Novel Human sPLA2
Screening of nucleic acid databases with all known types of mammalian and venom sPLA2s (groups I, II, III, V, IX, and X) led us to identify various human ESTs and a large human BAC clone of 161,326 nucleotides coding for a putative novel sPLA2 (hGXII) that displays homology with other sPLA2s only in the active site region. A cDNA sequence containing a possible complete open reading frame was deduced from the alignment of the various ESTs and the genomic sequence and was then used to design primers for RT-PCR experiments with cDNA from various human tissues. The expected 717-nucleotide cDNA fragment containing an open reading frame of 567 nucleotides was amplified at a high level from human fetal lung cDNA and at lower levels from pancreas and testis cDNAs (not shown). The open reading frame was found to display some of the expected features for a sPLA2 (Fig. 1A). The initiator methio-nine is followed by a 22-amino acid sequence presenting the features of a signal peptide (32) and a mature protein sequence of 167 residues. The calculated molecular mass and pI values for the mature protein are 18,702.1 Da and 6.26, respectively, and no consensus site for N-glycosylation was found. Like several other sPLA2s, the mature hGXII sequence contains 14 cysteines and a central catalytic domain with a HD catalytic diad (Fig. 1B). Comparison of the 717-nucleotide cDNA sequence with the genomic sequence indicates that the hGXII sPLA2 gene is composed of at least 4 exons and 3 introns spanning about 15 kilobases in length. The human BAC clone containing the hGXII gene was also found to contain different sequence-tagged sites positioned at the 4q25 locus, thus assigning the hGXII gene to this location on chromosome 4. Further screening of the EST databases with the hGXII cDNA sequence led to the identification of several other ESTs partially coding for mouse (GenBank™/EBI AA020156 and AA204520), rat (GenBank™/EBI AW918074), and bovine (GenBank™ /EBI AW353546) GXII sPLA2s (Fig. 1A). A full-length amino acid sequence coding for Xenopus laevis GXII sPLA2 was deduced from the alignment of two ESTs (GenBank™/EBI AW641606 and AW639634). Interestingly, the level of identity of this novel GXII sPLA2 among species is very high (Fig. 1A) as compared with those of other sPLA2s (18, 21).
A BLASTp search with the amino acid sequence of hGXII sPLA2 against the protein databases stored at the National Center for Biotechnology reveals matches to a variety of sPLA2s from mammals, Caenorhabditis elegans, plants, and animal venoms, suggesting that this protein belongs to the sPLA2 family. The homology however appears to be weak (<35% identity with BLAST scores lower than 35) and restricted to a short stretch of less than 60 amino acid residues containing the active site domain and the HD catalytic diad. This indicates that the hGXII sPLA2 is unique among all known sPLA2s (Fig. 1B). The histidine of HD is thought to function as a general base to deprotonate a water molecule as it attacks the substrate ester carbonyl carbon, and the β-carboxyl group of the adjacent aspartate coordinates directly to the catalytic Ca2+ cofactor (6, 33). Except for 3 cysteines in the active site consensus sequence CCXXHDXC, which match those of other groups of sPLA2s, the location of the other 11 cysteines residues in hGXII is distinct from that of other sPLA2s (Fig. 1B). Because the structural arrangement of disulfides has been the main basis for designating the different sPLA2 group numbers, the naming of the new sPLA2 as hGXII seems appropriate.
The homology between hGXII and all known sPLA2s is so low that it is difficult to find the Ca2+-binding loop, which is usually highly conserved and provides three of the four amino acid ligands for the catalytic Ca2+ (34). All mammalian group I, II, V, and X sPLA2s contain 19 amino acid residues between the most N-terminal residue that serves as a ligand to the active site Ca2+ (i.e. His-27 of hGIIA) and the catalytic histidine (i.e. His-47 of hGIIA). In contrast, the corresponding distances for hGIII and plant GXI sPLA2s are 25 and 23 residues, respectively. hGXII contains a potential Ca2+-binding segment GCGSP with 23 residues between the N-terminal glycine and the putative catalytic histidine as shown in Fig. 1. This segment is perfectly conserved among all of the GXII proteins found in genomic databases. The x-ray structures of groups I, II, and III sPLA2s reveal that the Ca2+ loop contains the consensus segment X-Cys-Gly-X-Gly with the amino acids designated as X1CG1X2G2, respectively. The backbone carbonyl oxygens of residues X1, G1, and G2 coordinate to Ca2+, and the backbone NH of G1 is proposed to donate a hydrogen bond to the carbonyl oxygen of the enzyme-susceptible substrate ester (33, 35). The fact that this residue is glycine in catalytically active sPLA2s and that mutating this residue to serine lowers catalytic activity by about 10- to 20-fold (35) argues that steric bulk is poorly tolerated at this position. The putative Ca2+-coordinating segment of hGXII shown in Fig. 1B fits the consensus sequence of other sPLA2s with the exception that G2 is a proline in hGXII. The prediction based on examination of the x-ray structures of sPLA2s is that the hGXII Ca2+-binding segment should be functional. It contains G1, and the backbone carbonyl of the C-terminal proline can coordinate to Ca2+ because its three extra methylenes, compared with glycine, are sterically allowed because of the location of this residue on the enzyme surface away from the substrate-binding cavity. Interestingly, sPLA2 isozymes with relatively low sPLA2 activity from the venom of the banded krait also contain proline in place of G2 (36).
Tissue Distribution of hGXII sPLA2
The tissue distribution of hGXII was first analyzed by hybridization at high stringency to a human Northern blot (Fig. 2). hGXII is expressed as several transcripts including a major one of ~1.4 kilobase, which is abundant in heart, skeletal muscle, and kidney. hGXII transcripts are also present at lower levels in brain, liver, small intestine, lung, and placenta, and expressed poorly, if at all, in colon, thymus, spleen, and peripheral blood leukocytes. Furthermore, analysis by RT-PCR with commercial human tissue cDNA panels indicates a pattern of hGXII expression that is consistent with the Northern blot data and additionally shows that this sPLA2 is strongly expressed in pancreas and weakly in ovaries, testis, and prostate (not shown). The pattern of expression of hGXII thus appears distinct from that of other known human sPLA2s (16, 19, 22),3 suggesting specific function(s) for this novel sPLA2.
FIG. 2. Northern blot analysis of the tissue distribution of hGXII.

A commercial Northern blot containing 2 μg of poly (A)+ RNA from different human adult tissues was hybridized at high stringency with a 32P-labeled hGXII RNA probe as described under “Experimental Procedures.” ske. muscle, skeletal muscle; small intest., small intestine; PBL, peripheral blood leukocytes; kb, kilobase.
Recombinant Expression of hGXII and Enzymatic Properties
A mammalian expression vector containing the full-length hGXII cDNA was first used to transiently transfect HEK293 cells. The amount of sPLA2 activity (as measured with an assay using radiolabeled E. coli membranes, Ref. 16) secreted into the culture medium 1–5 days after transfection was barely above that measured in medium from cells transfected with vector lacking the hGXII insert, suggesting that hGXII may have a low specific activity. To further analyze if hGXII is a catalytically active sPLA2, we expressed hGXII as a fusion protein in E. coli, and the inclusion body fraction was submitted to a refolding strategy previously used to produce catalytically active mGIID sPLA2 (17). After digesting the fusion protein with factor Xa protease, hGXII was purified to homogeneity by HPLC and was found to migrate as a pure protein of about 18 kDa on a Laemmli SDS gel (not shown). Mass spectrometry analysis gave an experimental mass of 18,702.6 ± 0.5 Da, which agrees well with the mass of 18,702.1 Da calculated from the sequence of mature hGXII shown in Fig. 1A. This result indicates that all 14 cysteines are engaged in disulfide bonds, and thus it is assumed that recombinant hGXII is properly folded.
Recombinant hGXII was found to be a catalytically active sPLA2 when assayed with the radiolabeled E. coli membrane assay (16) and with POPG, POPS, and POPC vesicles using the fatty acid-binding protein assay (17). As shown in Fig. 3A, sPLA2 activity toward POPC vesicles was strictly Ca2+-dependent (Kcalcium = 30 ± 10 μM). hGXII activity is maximal near pH 8.0 and decreases at higher and lower pH values (Fig. 3B). The decrease as the pH is lowered presumably reflects, in part, the protonation of the active site histidine. As for all mammalian sPLA2s examined so far, the enzymatic activity of hGXII on phosphatidylglycerol vesicles is highest (Fig. 3C), which probably reflects the tighter binding of hGXII to anionic vesicles (37). Although hGXII hydrolyzes POPC at only ~7% of the rate of POPG, this difference is small compared with the >105-fold preference of hGIIA for POPG versus POPC (18). POPS is also a good substrate for hGXII (Fig. 3C).
FIG. 3. Enzymatic properties of hGXII.

A, initial velocity for the hydrolysis of POPC vesicles containing a trace of 1-palmitoyl-2-[8,9-3H]palmitoyl-sn-glycero-3-phosphocholine (100,000 dpm of substrate per assay of 60-Ci/mmol-labeled lipid) as a function of the Ca2+ concentration. B, initial velocity for the hydrolysis of POPG vesicles containing a small amount of 1-palmitoyl-2-[8,9-3H]palmitoyl-sn-glycero-3-phosphoglycerol (100,000 dpm of substrate per assay of 60-Ci/mmol-labeled lipid) as a function of pH. C, initial velocity for the hydrolysis of large unilamellar vesicles (0.1 μm) of the indicated phospholipid. Additional assay details have been reported elsewhere (17).
Concluding Remarks
In summary, we cloned a novel catalytically active human sPLA2, hGXII, which belongs to a new structural class with homologs in other mammalian species and in Xenopus laevis. Because hGXII is expressed in a limited number of human tissues and has an expression pattern distinct from those of other human sPLA2s, it is not expected to carry out “housekeeping” functions in cells, but rather to have physiological function(s) distinct from those of other human sPLA2s. A sPLA2 gene cluster for the structurally similar hGIIA, hGIIC, hGIID, hGIIE, hGIIF, and hGV sPLA2s is present on human chromosome 1,3 whereas structurally more distant hGIB, hGX, and hGIII sPLA2s lie on different chromosomes (chromosomes 12, 16, and 22, respectively), as is also shown in this study for hGXII sPLA2 (chromosome 4). Recombinant expression of hGXII shows that it is a catalytically active, Ca2+-dependent sPLA2. However, the specific enzymatic activity of hGXII appears very low compared with those of other mammalian sPLA2s (for example hGIB, hGIIA, hGV, and hGX) and is comparable with the low specific activity reported for mGIIE sPLA2 (18). This may be the reason why hGXII sPLA2 was not detected in earlier biochemical studies despite the fact that this protein may be expressed at significant levels and the transcripts coding for this later are present in fairly high amounts in several human tissues (Fig. 2). It is also interesting to note that the putative GXII sPLA2 from zebrafish (Danio rerio) is represented in genomic databases by several ESTs that all contain a leucine in place of histidine in the catalytic HD segment. This type of mutation suggests that the zebrafish GXII sPLA2 has very little or no catalytic activity, probably lower than that of hGXII sPLA2. This in turn suggests that the catalytic activity of group XII sPLA2s may not be critical for their physiological function. Rather, they may act by serving as ligands for sPLA2-binding proteins instead of acting as lipolytic enzymes (13).
Acknowledgments
We thank Pierre Escoubas and Sabine Thaon for MALDI-TOF analysis and Catherine Le Calvez for technical assistance.
Footnotes
This work was supported in part by National Institutes of Health Grant HL36236, the CNRS, the Association pour la Recherche sur le Cancer, and the Fonds de Recherche Hoechst Marion Roussel. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) AF306567.
The abbreviations used are: sPLA2, secreted phospholipase A2; POPC/G/S, 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-choline/glycerol/serine; RT-PCR, reverse transcription-polymerase chain reaction; h, human; m, mouse; EST, expressed sequence tag; CMV, cytomegalovirus; HPLC, high pressure liquid chromatography; MALDI-TOF, matrix-assisted laser desorption ionization-time of flight mass spectrometry; HD, His-Asp catalytic diad.
A comprehensive abbreviation system for the various mammalian sPLA2s is used. Each sPLA2 is abbreviated with a lowercase letter indicating the sPLA2 species (m and h for mouse and human, respectively), followed by uppercase letters identifying the sPLA2 group (GIB, GIIA, GIIC, GIID, GIIE, GIIF, GIII, GV, GIX, GX, GXI, and GXII).
E. Valentin, et al., submitted for publication.
REFERENCES
- 1.Yuan C, Tsai M-D. Biochim. Biophys. Acta. 1999;1441:215–222. doi: 10.1016/s1388-1981(99)00156-0. [DOI] [PubMed] [Google Scholar]
- 2.Gelb MH, Jain MK, Hanel AM, Berg O. Annu. Rev. Biochem. 1995;64:653–688. doi: 10.1146/annurev.bi.64.070195.003253. [DOI] [PubMed] [Google Scholar]
- 3.Balsinde J, Balboa MA, Insel PA, Dennis EA. Annu. Rev. Pharmacol. Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. J. Biol. Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 5.Balboa MA, Balsinde J, Winstead MV, Tischfield JA, Dennis EA. J. Biol. Chem. 1996;271:32381–32384. doi: 10.1074/jbc.271.50.32381. [DOI] [PubMed] [Google Scholar]
- 6.Verheij HM, Slotboom AJ, De Haas GH. Rev. Physiol. Biochem. Pharmacol. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- 7.Hanasaki K, Arita H. Arch. Biochem. Biophys. 1999;372:215–223. doi: 10.1006/abbi.1999.1511. [DOI] [PubMed] [Google Scholar]
- 8.Rae D, Beechey-Newman N, Burditt L, Sumar N, Hermon-Taylor J. Scand. J. Gastroenterol. 1996;219(suppl.):24–27. doi: 10.3109/00365529609104995. [DOI] [PubMed] [Google Scholar]
- 9.Kundu GC, Mukherjee AB. J. Biol. Chem. 1997;272:2346–2353. [PubMed] [Google Scholar]
- 10.Pruzanski W, Vadas P, Browning J. J. Lipid Mediators. 1993;8:161–167. [PubMed] [Google Scholar]
- 11.Qu XD, Lehrer RI. Infect. Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Crit. Rev. Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 13.Lambeau G, Lazdunski M. Trends Pharmacol. Sci. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 14.Fenard D, Lambeau G, Valentin E, Lefebvre J, Lazdunski M, Doglio A. J. Clin. Invest. 1999;104:611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tischfield JA. J. Biol. Chem. 1997;272:17247–17250. doi: 10.1074/jbc.272.28.17247. [DOI] [PubMed] [Google Scholar]
- 16.Cupillard L, Koumanov K, Mattéi MG, Lazdunski M, Lambeau G. J. Biol. Chem. 1997;272:15745–15752. doi: 10.1074/jbc.272.25.15745. [DOI] [PubMed] [Google Scholar]
- 17.Valentin E, Koduri RS, Scimeca J-C, Carle G, Gelb MH, Lazdunski M, Lambeau G. J. Biol. Chem. 1999;274:19152–19160. doi: 10.1074/jbc.274.27.19152. [DOI] [PubMed] [Google Scholar]
- 18.Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G. J. Biol. Chem. 1999;274:31195–31202. doi: 10.1074/jbc.274.44.31195. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki N, Ishizaki J, Yokota Y, Higashino K, Ono T, Ikeda M, Fujii N, Kawamoto K, Hanasaki K. J. Biol. Chem. 2000;275:5785–5793. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- 20.Ishizaki J, Suzuki N, Higashino K-C, Yokota Y, Ono T, Kawamoto K, Fujii N, Arita H, Hanasaki K. J. Biol. Chem. 1999;274:24973–24979. doi: 10.1074/jbc.274.35.24973. [DOI] [PubMed] [Google Scholar]
- 21.Valentin E, Lambeau G. Biochim. Biophys. Acta. 2000 doi: 10.1016/s1388-1981(00)00110-4. in press. [DOI] [PubMed] [Google Scholar]
- 22.Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G. J. Biol. Chem. 2000;275:7492–7496. doi: 10.1074/jbc.275.11.7492. [DOI] [PubMed] [Google Scholar]
- 23.Arita H, Hanasaki K. J. Lipid Mediators. 1993;6:217–222. [PubMed] [Google Scholar]
- 24.Sartipy P, Bondjers G, Hurt-Camejo E. Arterioscler. Thromb. Vasc. Biol. 1998;18:1934–1941. doi: 10.1161/01.atv.18.12.1934. [DOI] [PubMed] [Google Scholar]
- 25.Mounier CM, Luchetta P, Lecut C, Koduri RS, Faure G, Lambeau G, Valentin E, Singer A, Ghomashichi F, Beguin S, Gelb MH, Bon C. Eur. J. Biochem. 2000;267:4960–4969. doi: 10.1046/j.1432-1327.2000.01523.x. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M, Kambe T, Shimbara S, Yamamoto S, Kuwata H, Kudo I. J. Biol. Chem. 1999;274:29927–29936. doi: 10.1074/jbc.274.42.29927. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh JM, Ghomashchi F, Gelb MH, Dooley DJ, Stoehr SJ, Giordani AB, Naisbitt SR, Olivera BM. J. Biol. Chem. 1995;270:3518–3526. doi: 10.1074/jbc.270.8.3518. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Chung YS, Ok SH, Lee SG, Chung WI, Kim IY, Shin JS. Biochim. Biophys. Acta. 1999;1489:389–392. doi: 10.1016/s0167-4781(99)00193-1. [DOI] [PubMed] [Google Scholar]
- 29.Stahl U, Ek B, Stymne S. Plant Physiol. 1998;117:197–205. doi: 10.1104/pp.117.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 31.Bayburt T, Gelb MH. Biochemistry. 1997;36:3216–3231. doi: 10.1021/bi961659d. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Scott DL, White SP, Otwinowski Z, Yuan W, Gelb MH, Sigler PB. Science. 1990;250:1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott DL, Sigler PB. Adv. Protein Chem. 1994;45:53–88. doi: 10.1016/s0065-3233(08)60638-5. [DOI] [PubMed] [Google Scholar]
- 35.Bekkers AC, Franken PA, Toxopeus E, Verheij HM, de Haas GH. Biochim. Biophys. Acta. 1991;1076:374–378. doi: 10.1016/0167-4838(91)90479-j. [DOI] [PubMed] [Google Scholar]
- 36.Liu C-S, Chen J-M, Chang C-H, Chen S-W, Tsai I-H, Lu H-S, Lo T-B. Toxicon. 1990;28:1457–1468. doi: 10.1016/0041-0101(90)90159-5. [DOI] [PubMed] [Google Scholar]
- 37.Jain MK, Ranadive G, Yu B-Z, Verheij HM. Biochemistry. 1991;30:7330–7340. doi: 10.1021/bi00243a038. [DOI] [PubMed] [Google Scholar]


