Abstract
Background & Aims
The molecular mechanism underlying epithelial metaplasia in Barrett's esophagus remains unknown. Recognizing that Hedgehog signaling is required for early esophageal development, we sought to determine if the Hedgehog pathway is reactivated in Barrett's esophagus, and if genes downstream of the pathway could promote columnar differentiation of esophageal epithelium.
Methods
Immunohistochemistry, immunofluorescence, and quantitative real-time PCR were used to analyze clinical specimens, human esophageal cell lines, and mouse esophagi. Human esophageal squamous epithelial (HET-1A) and adenocarcinoma (OE33) cells were subjected to acid treatment and used in transfection experiments. Swiss Webster mice were used in a surgical model of bile reflux injury. An in vivo transplant culture system was created using esophageal epithelium from Sonic hedgehog transgenic mice.
Results
Marked upregulation of Hedgehog ligand expression, which can be induced by acid or bile exposure, occurs frequently in Barrett's epithelium and is associated with stromal expression of the Hedgehog target genes PTCH1 and BMP4. BMP4 signaling induces expression of SOX9, an intestinal crypt transcription factor, which is highly expressed in Barrett's epithelium. We further show that expression of DMBT1, the human homologue of the columnar cell factor Hensin, occurs in Barrett's epithelium and is induced by SOX9. Finally, transgenic expression of Sonic hedgehog in mouse esophageal epithelium induces expression of stromal Bmp4, epithelial Sox9 and columnar cytokeratins.
Conclusions
Epithelial Hedgehog ligand expression may contribute to the initiation of Barrett's esophagus through induction of stromal BMP4, which triggers reprogramming of esophageal epithelium in favor of a columnar phenotype.
Keywords: Hedgehog signaling, Barrett's esophagus, BMP4, SOX9
Introduction
Despite a decrease in overall cancer incidence in the United States, the incidence of esophageal cancer continues to rise1. The disease ranks as the sixth leading cause of cancer death worldwide and the seventh leading cause of cancer death in American men2. Adenocarcinoma, the predominant form in the Western world, typically arises from Barrett's esophagus (BE), a metaplastic change of the distal esophageal epithelium from stratified squamous to specialized columnar1. BE is associated with a 30-125 fold increased risk of developing adenocarcinoma because progression with increasing degrees of accompanying dysplasia can occur3. Management of BE patients with dysplasia ranges from intense surveillance to localized endoscopic ablation of Barrett's epithelium to esophagectomy, which has relatively high operative mortality and complication rates4.
BE is thought to represent a protective response to chronic acid and bile injury from gastroesophageal reflux disease (GERD)5. Animal studies suggest that BE originates from native esophageal cells, rather than from rostral migration of gastric columnar epithelium6. Multiple genetic and epigenetic changes occur in BE, BE with dysplasia, and adenocarcinoma including silencing, loss or mutation of P16, P53 and APC and overexpression of cyclin D15. Other reported genetic changes include aneuploidy; C-ERB2, EGFR, and P21 overexpression; SRC and telomerase activation; K-RAS mutations; and P27 and E-cadherin underexpression2. Despite these findings, the basic mechanism underlying the conversion of squamous to columnar epithelium remains unknown.
Recognizing that the esophagus develops from a primitive gut tube that resembles intestine7, we took a developmental approach in an attempt to elucidate the molecular mechanism underlying Barrett's metaplasia. The Hedgehog (Hh) signaling pathway, critical for normal gut development, represents a leading candidate as a molecular mediator of BE. In mammals, Hh signaling is initiated by the binding of Hh ligands, Sonic (Shh), Indian (Ihh), or Desert (Dhh), to the transmembrane receptor Patched1 (Ptch1)8. This leads to release of Ptch1's constitutive repression of Smoothened (Smo), another transmembrane protein. Unrepressed Smo activates a cytoplasmic protein complex containing Gli transcription factors, leading to nuclear translocation of the Gli proteins and activation of pathway targets8. In the developing gut these include Ptch1 and Bone morphogenetic protein 4 (Bmp4)9, 10.
Hh signaling characterizes developing intestinal columnar epithelium, including the early esophagus11, 12. As esophageal development progresses, squamous epithelium appears as Hh signaling is downregulated12. We hypothesized that aberrant reactivation of esophageal Hh signaling in adulthood could lead to BE. Others' experimental observations support this hypothesis. First, Shh expression in the adult gut is limited to the stomach and small intestine13. Second, intestinal metaplasia of pancreatic ducts occurs when Shh is overexpressed in a gastrointestinal organ that normally does not have Hh signaling14. Third, activation of Hh signaling has been demonstrated in several injury repair/tissue regeneration models15-17. Finally, the Hh pathway target BMP4 has been shown to be expressed in BE18.
Materials and Methods
Clinical specimens
Tissue microarrays representing 96 esophagectomy cases and containing esophageal squamous epithelium, stomach, BE, BE with low/high-grade dysplasia, adenocarcinoma, lymph node metastases, and connective tissue were obtained from the Johns Hopkins Tissue Microarray Core. Sixteen additional esophagectomy cases were obtained from a Department of Oncology frozen tissue bank and included diagnoses of achalasia, squamous epithelium, BE, and adenocarcinoma. Frozen tissue was cut on a cryostat and upper and lower sections were stained with H&E to confirm the labeled diagnosis and that the majority of each section was epithelium. Exemptions and/or approval were obtained from the IRB for use of de-identified patient materials.
Immunohistochemistry/immunocytochemistry, immunofluorescence
Following epitope retrieval with citrate and blocking of endogenous peroxidase, paraffin-embedded sections were submitted to immunohistochemistry/immunocytochemistry using the Vectastain ABC system (Vector Labs) or the EnVision FLEX system (Dako). Frozen sections were air-dried and permeabilized with methanol. Species-specific secondary fluorescent antibodies and DAPI were used to visualize proteins and cell nuclei. Antibodies are listed in Supplemental Table 1.
Quantitative real-time PCR
RNA was isolated using Trizol (Invitrogen) and quantitated with a Nanodrop spectrophotometer. 1μg RNA was reverse transcribed using Superscript II (Invitrogen) or the Quantitect RT kit (Qiagen). Quantitative real-time PCR was performed using Biorad's SYBR-Green Supermix on an I-cycler. Relative amounts of cDNA were calculated using the ΔΔCt method and normalized to β-actin. For clinical samples, expression was compared to normal human whole esophageal total RNA (BioChain). Human and mouse primers are listed in Supplemental Table 2.
Cell culture, acid/BMP4 treatment, transfections
HET-1A (ATCC) and OE33 (Sigma) cells were cultured in BEGM and advanced RPMI with 1% FBS, respectively. Acid treatment experiments based on a previous report19 were repeated twice with three biological replicates. Recombinant human BMP4 (R&D Systems 314-BP) was used to treat cells at 100ng/ml for three hours. Transfections were performed using Lipofectamine LTX and Plus reagent (Invitrogen) and repeated a minimum of three times. Because of poor transfection efficiency (5% by FACS analysis), HET-1A cells were co-transfected with a plasmid containing enhanced green fluorescent protein and a neomycin resistance gene. HET-1A cells were then selected in G418 for one week and harvested. Experimental plasmids utilized included pcDNA3.1, pFLAG-Gli1, pcDNA3.1-SOX9, and constitutively active BMPRIA.
RNA interference
Sixty percent confluent OE33 cells were transfected with 25nM non-targeting or SOX9-specific siRNAs (Dharmacon) using Lipofectamine LTX and Plus reagent. Cells were transfected again 24 hours later and split 1:3 at 30 hours for collection of total protein and RNA at 48 hours. For Western blot, cells were lysed using 50mM Tris and 2% SDS containing protease inhibitors. Following centrifugation, equal amounts of protein (quantitated with Pierce BCA) were loaded on a 4-12% NuPage Bis-Tris gel (Invitrogen). Proteins were then transferred to a PVDF membrane and blocked with 5% milk in TBST. Following overnight incubation with primary antibodies and one hour incubation with species-specific HRP-conjugated secondary antibodies, proteins were visualized using chemiluminescence (Pierce).
Mouse esophagojejunostomies
Ten Swiss Webster mice and ten Ptch1-LacZ/Swiss Webster mice underwent esophagojejunostomies as previously described according to an IACUC approved protocol20, 21. Half of each esophagojejunal anastomosis was taken for formalin fixation and H&E staining; the other half was placed in Trizol for subsequent RNA isolation or stained with X-gal. For RNA isolation, the distal esophagus was carefully dissected from the anastomosis to prevent intestinal contamination. To determine β-galactosidase activity, tissue was dissected in ice-cold PBS and fixed with fresh 4% paraformaldehyde for 30-60 minutes. Following multiple washes with PBS, the tissue was incubated with X-gal at 37°C overnight. Tissue was then fixed in 4% paraformaldehyde for an additional 24 hours.
In vivo transplant culture system
3-D tissue reconstitution assays were performed as previously described22. Briefly, epithelial cells were isolated from esophagi taken from conditional Shh-transgenic mice or wild-type control littermates. Shh-transgenic mice harbor a lox-EGFP-STOP-lox-Shh allele under the control of a β-actin promoter. Digestion of the esophagi in 6mg/ml Dispase II (Roche) was followed by removal of the mucosal layer and disaggregation in 0.05% trypsin, 0.02% EDTA. Esophageal epithelial cells were grown in CNT-07 media and transduced with adenoviral-cre recombinase. Devitalised and denuded rat tracheas were injected with 5×105 epithelial cells and 1×105 cultured primary C57Bl/6 mouse esophageal fibroblasts. The tracheas were sealed with ligature clips and surgically implanted under the dorsal skin of SCID mice. Epithelia were allowed to reconstitute over 4 weeks, then the tracheas were excised and fixed in formalin.
Results
Hh pathway expression in BE and associated adenocarcinoma
To determine the prevalence of Hh ligand expression in BE, we analyzed esophageal tissue microarrays by immunohistochemistry using a SHH antibody (staining controls shown in Supplemental Figure 1). While squamous epithelium did not express SHH, Barrett's epithelium from 55/56 (98%) patients and adenocarcinomas from 83/89 (93%) patients were SHH positive (Figure 1A-D). This supports earlier findings of active Hh signaling in human esophageal cancer cell lines23. Quantitative real-time PCR (qRT-PCR) analysis of 15 frozen esophagectomy cases, with paired normal squamous and Barrett's epithelium obtained from the same patient, was performed for expression of SHH, IHH, PTCH1, and BMP4. We included IHH because of its expression in the distal gastrointestinal tract8. Marked increases in both SHH and IHH expression (Figure 1E-F) were accompanied by modest increases in the expression of the Hh pathway targets PTCH1 and BMP4 in BE (Figure 2A-B). Immunofluorescence staining in the same frozen samples shows that PTCH1 and BMP4 expression is limited to the stromal compartment surrounding BE (Figure 2C, E), whereas no PTCH1 or BMP4 was observed in stroma surrounding squamous epithelium obtained from a patient with achalasia (Figure 2D, F). These data are consistent with epithelial-mesenchymal Hh signaling in BE, which would closely resemble that seen in the developing intestine9, 24.
Figure 1. Epithelial Hh activation in BE and esophageal adenocarcinoma.
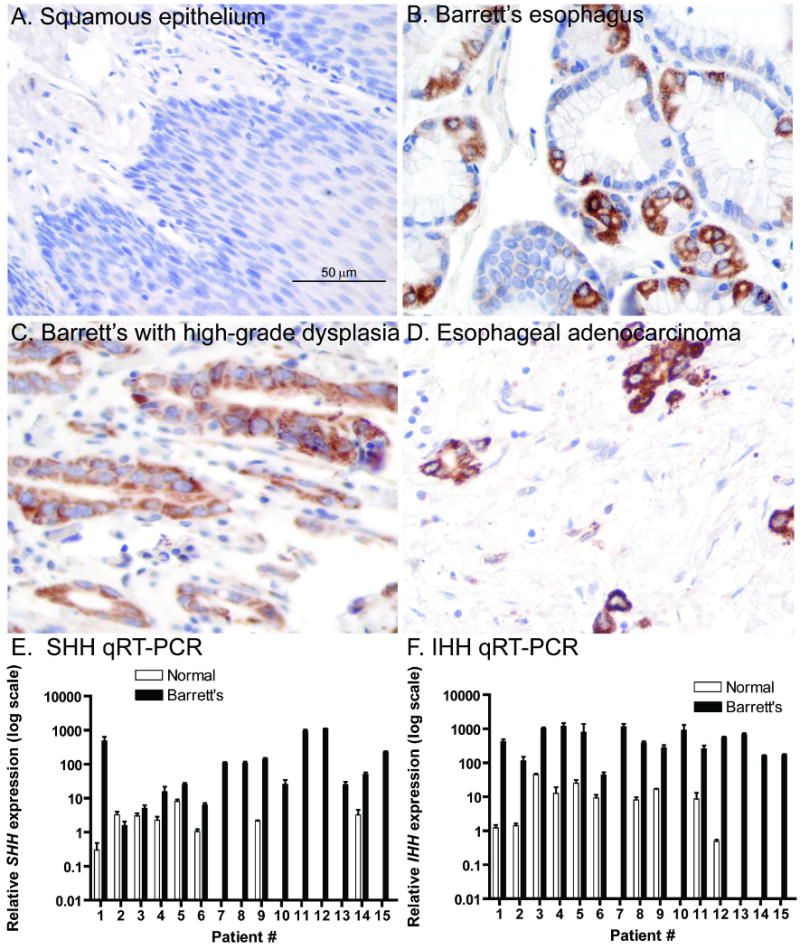
A-D) SHH immunohistochemistry. (40×) A) Esophageal squamous epithelium. B) BE. C) BE with high-grade dysplasia. D) Esophageal adenocarcinoma. E-F) SHH and IHH quantitative real-time PCR (qRT-PCR) data from 15 frozen esophagectomy cases. Note log scale of Y-axis. Absence of bar indicates undetectable expression levels.
Figure 2. Stromal Hh pathway targets are upregulated in BE.
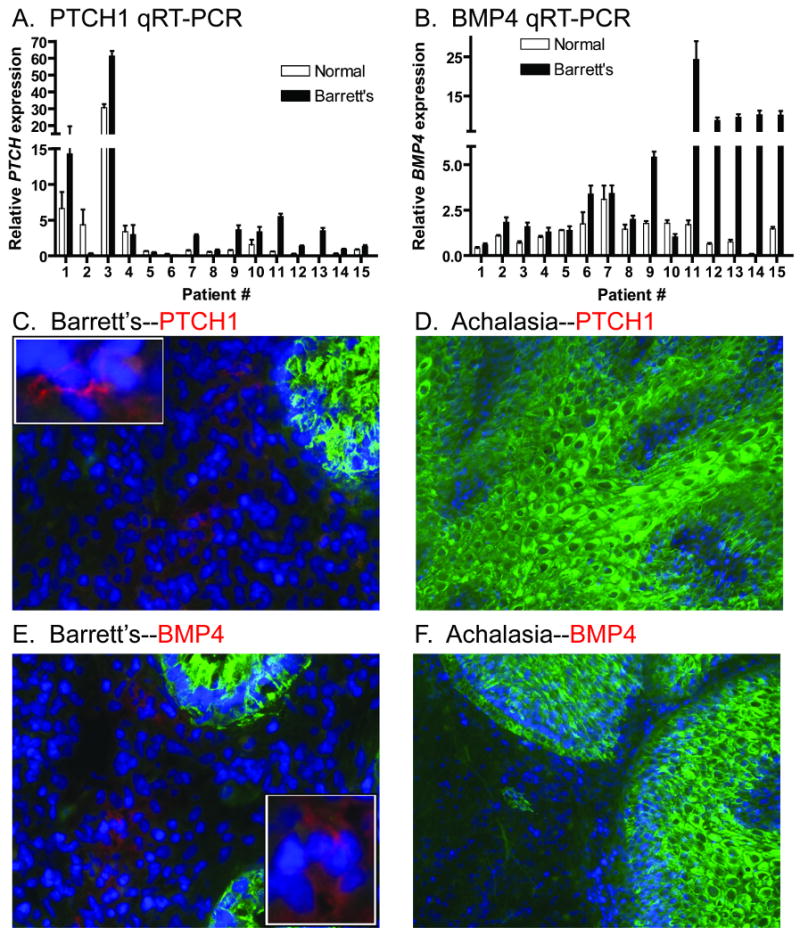
A) PTCH1 and B) BMP4 qRT-PCR data from 15 frozen esophagectomy cases. (C-F) Immunofluorescence performed on frozen esophagectomy cases. Nuclei stained with DAPI in blue. Anti-cytokeratin in green. C) BE and D) Achalasia stained with anti-PTCH1 in red. E) BE and F) Achalasia stained with anti-BMP4 in red. Insets are higher magnification of positive staining cells.
Acid and bile reflux can activate Hh signaling
Since epithelial injury can activate the Hh pathway15, 16, we sought to link Hh pathway activity to the acid and bile injury that characterize BE. HET-1A cells, an SV-40 immortalized human esophageal squamous epithelial cell line, were cultured in normal serum-free media (pH 7.6) and media acidified with HCl for 48 hours19 (Supplemental Figure 2). We found that SHH and IHH expression was markedly upregulated in response to acid (Figure 3A), confirming a previous report25. Interestingly, upregulation of Hh ligand expression occurred at pH≤4, the cutoff used to diagnose patients with GERD on ambulatory esophageal pH measurements26. Acid studies were also performed in OE33 esophageal adenocarcinoma cells with similar results (data not shown).
Figure 3. Physiologic causes of BE activate the Hh pathway.
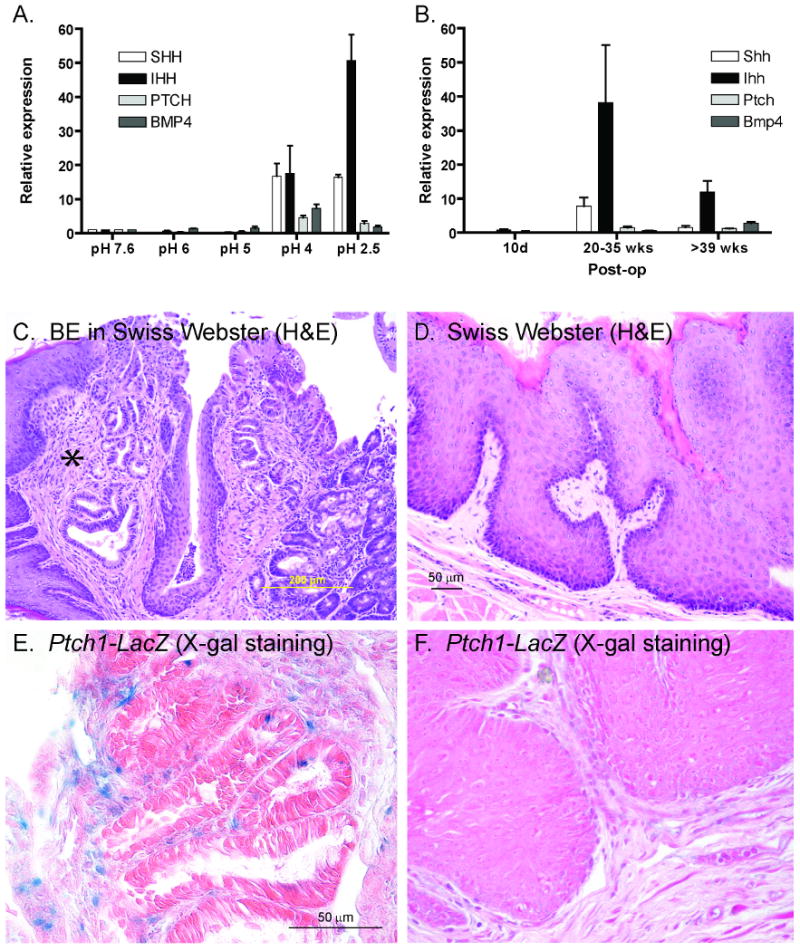
A) qRT-PCR data of HET-1A cells treated with non-acidified (pH 7.6) or acidified media for 48 hours. B) qRT-PCR data of mouse esophagi following esophagojejunostomy as compared to Swiss Webster whole esophagus. C) BE in Swiss Webster mouse 40 weeks after esophagojejunostomy. * BE between two regions of squamous epithelium. D) Hyperproliferative squamous epithelium in Swiss Webster mouse 40 weeks post-op. (20×) E-F) β-galactosidase activity in Ptch1-LacZ mouse 30 weeks post-op. (40×) E) Intestinal-like epithelium with positive blue staining in underlying stroma. F) Hyperproliferative squamous epithelium with absent staining.
To isolate the effect of bile reflux on esophageal epithelium in vivo, we utilized a mouse gastric bypass and esophagojejunal anastomosis model21. Mice sacrificed after 39 weeks following esophagojejunostomy demonstrated histologic changes consistent with BE (Figure 3C), which correlated with increases in Hh pathway gene expression (Figure 3B). Mice sacrificed at 20-35 weeks post-op demonstrated upregulation of Hh signaling in the distal esophagus prior to the appearance of BE (Figure 3B), suggesting that Hh pathway activation precedes the development of BE. To further characterize Hh pathway activation in response to bile reflux, we used mice heterozygous for an allele in which the β-galactosidase gene replaces the first two exons of endogenous Ptch1 (Ptch1-LacZ)20. Since Ptch1 is a direct Hh pathway target, β-galactosidase activity can be used to report Hh pathway activation in vivo. Following esophagojejunostomy, stromal Ptch1 expression was induced near epithelium resembling intestine (Figure 3E) but not adjacent to squamous epithelium (Figure 3D, F).
SOX9 expression is associated with aberrant Hh pathway activation in BE
We next considered what downstream targets of the Hh pathway might mediate the switch from squamous to columnar epithelium in BE. One candidate is Sox9, a transcription factor that localizes to Paneth cells and potential stem cells in intestinal crypts27-29. Sox9 is a well-defined Hh target in chondrocytes and a putative target in epithelial stem cells of the hair follicle30, 31. To pursue this potential connection, we performed SOX9 immunohistochemistry in the adult human gut. While SOX9 is not expressed in the esophagus, strong epithelial expression was seen in the gastric pit-gland transition zone and small intestinal crypts, both areas of known Hh signaling (Figure 4A-C)8, 32. To determine the expression pattern of Sox9 during esophageal development, embryonic C57Bl/6 mice were isolated from timed-pregnant females. From embryonic day 12.5 until birth, the basal esophageal epithelial cells express Sox9 (Supplemental Figure 3A-C). In contrast, keratinized adult esophageal epithelium no longer expresses Sox9 (Supplemental Figure 3D). This embryonic esophageal expression pattern follows that of Shh12 and Bmp433, and is consistent with the hypothesis that Sox9 is downstream of the Hh pathway during the columnar epithelial phase of esophageal development.
Figure 4. SOX9 expression in normal human gastrointestinal tract, BE and esophageal adenocarcinoma.
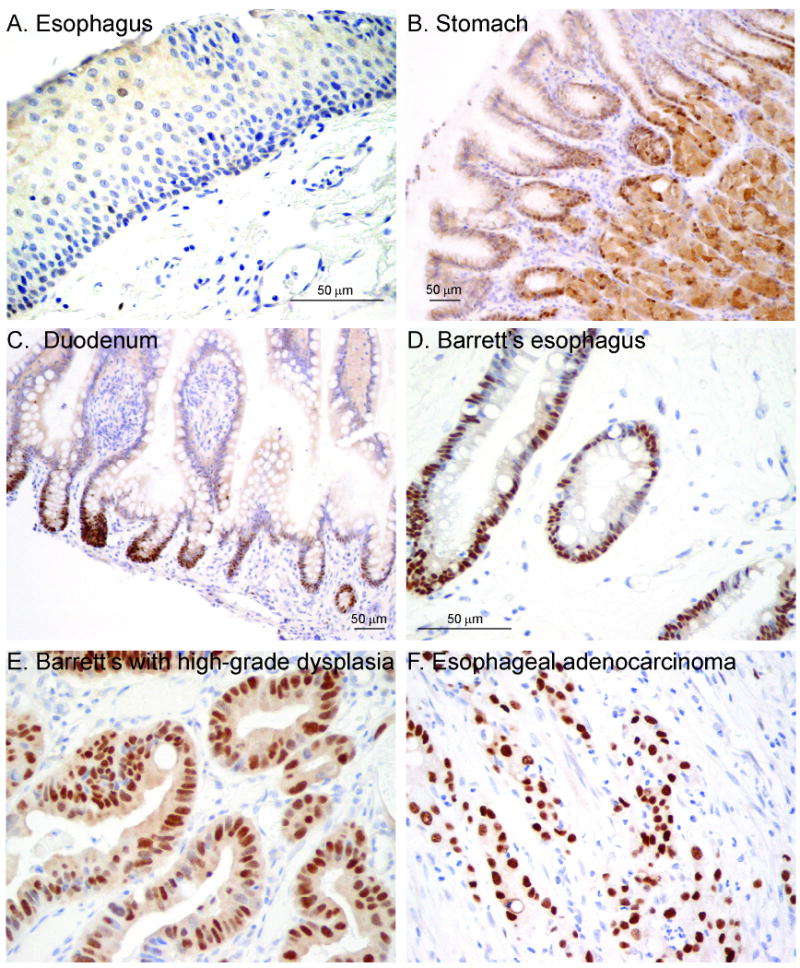
SOX9 immunohistochemistry. A) Esophagus. (40×) B) Stomach with staining in pit-gland transition zone. (20×) C) Duodenum with staining in intestinal crypts. (20×) D) BE. (40×) E) BE with high-grade dysplasia. (40×) F) Esophageal adenocarcinoma. (40×)
To determine if SOX9 is re-expressed in BE, we stained the same tissue microarrays used to examine SHH expression in patients with BE and/or adenocarcinoma with a SOX9 antibody. The epithelium of BE, BE with dysplasia, and adenocarcinoma, all stained strongly for SOX9 (Figure 4D-F) while squamous epithelium was negative. 56/56 patients (100%) with BE and 71/84 (85%) patients with adenocarcinoma were SOX9 positive. We then examined the fifteen frozen esophagectomy cases of BE by qRT-PCR and found 13/15 patients had increased SOX9 expression in their BE tissue as compared to their squamous tissue (Figure 5A).
Figure 5. BMP4 signaling activates SOX9.
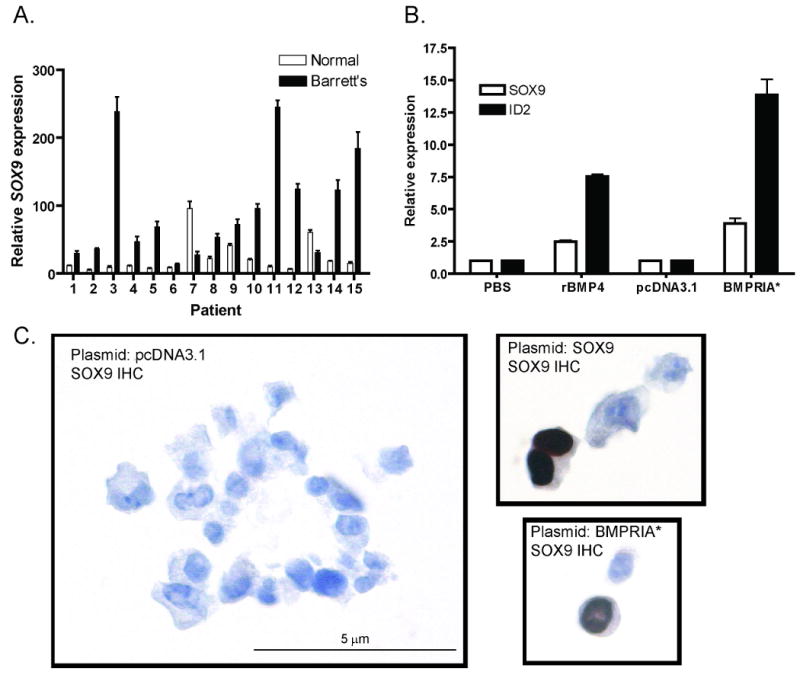
A) SOX9 qRT-PCR data from 15 frozen esophagectomy cases. B) HET-1A cells treated with PBS (vehicle) or 100ng/ml BMP4 for three hours or transfected with pcDNA3.1 (empty vector) or BMPRIA* (constitutively active BMPRIA). qRT-PCR data for expression of SOX9 and the BMP target gene ID2. C) SOX9 immunocytochemistry in transfected HET-1A cells. (60×)
BMP4 signaling induces SOX9 expression in esophageal epithelium
To determine how SOX9 and Hh signaling interact in esophageal epithelium, we treated HET-1A cells with conditioned media containing Shh, transfected them with a Gli1 expression vector, and infected them with adenovirus containing a constitutively active mutant form of Smo34. These treatments did not increase SOX9 transcript levels in HET-1A cells, though they did activate the Hh pathway in a Gli-dependent reporter cell line (data not shown)35. Noting that stromal Hh target genes signal back to epithelium during gut development24, we wondered if BMP4 might signal to epithelium to activate SOX9 in BE. BMPs, members of the TGF-β superfamily, signal through type I and type II receptors. Type II receptors bind ligand and activate type I receptors which subsequently phosphorylate cytoplasmic SMAD proteins, transcription factors which then undergo nuclear translocation36. Since a previous study established that esophageal epithelium expresses the BMP receptors BMPRIA and BMPRII18, we treated HET-1A cells with recombinant BMP4 or transfected them with a constitutively active form of the human BMPRIA type I receptor (BMPRIA*). By qRT-PCR, BMP4 induced a 2.5 fold upregulation of SOX9 expression compared to vehicle while BMPRIA* induced a 3.9 fold upregulation compared to empty vector (Figure 5B). Both also caused upregulation of the BMP4 target gene ID2. Since BMPRIA* gave a more robust response, we chose to use it in subsequent experiments. Immunocytochemical staining of HET-1A cells with a SOX9-specific antibody demonstrated nuclear SOX9 expression in response to BMPRIA* transfection (Figure 5C). Taken together, these data demonstrate that BMP4 signaling can induce expression of SOX9 in esophageal epithelial cells. Since BMP4 is a direct target of Hh signaling in gut stromal cells and is expressed in stromal cells surrounding Hh secreting Barrett's epithelium, these findings identify a potential link between Hh pathway activation and esophageal columnar metaplasia.
SOX9 upregulates expression of DMBT1, the human homologue of Hensin
Although SOX9 is expressed in intestinal crypts, its functional relevance to the induction of esophageal columnar metaplasia is unknown. We therefore looked at columnar cell factors and asked if they could be regulated by SOX9. One candidate is Hensin, an extracellular matrix protein that is sufficient to induce a columnar-like phenotype in embryonic stem cells37. Though Hensin was first discovered in association with kidney intercalated cells38, expression surveys demonstrated that it is most highly expressed in the intestine39, 40. Further studies revealed that Hensin causes intercalated cells to express Villin and Cytokeratin 19, form microvilli, and assume a columnar shape40.
The human Hensin homologue, DMBT1 (DELETED IN MALIGNANT BRAIN TUMORS 1), and two transcript variants of mouse Hensin, CRP-ductin α and β, which are expressed in intestinal crypt cells, have been identified41, 42. To test whether DMBT1 expression is upregulated in BE, we examined the same 15 frozen esophagectomy cases of BE used in previous experiments using qRT-PCR. Here, DMBT1 expression was markedly elevated in BE when compared to normal squamous epithelium from the same patient (Figure 6A). Using the Sox consensus binding sequence of 5′ - A/TA/TCAAA/TG - 3′ or its complement43, we found six binding sites in the DMBT1 promoter up to 5kb upstream from the transcriptional start site with the most proximal sites at -703 and -678. We then sought to determine if DMBT1 expression could by induced by SOX9 or BMPRIA* in esophageal epithelial cells. By qRT-PCR, SOX9 overexpression in HET-1A cells increased DMBT1 expression 3.1 fold while transfection of BMPRIA* induced a 4.3 fold increase compared to empty vector (Figure 6B). SOX9 and BMPRIA* also induced Cytokeratin 8/18 expression in HET-1A cells (Supplemental Figure 4), suggesting that they can activate other columnar genes. To demonstrate that DMBT1 is a target of SOX9 regulation, we transfected SHH and SOX9 expressing OE33 cells (Figure 6C) with a non-targeting siRNA or three SOX9-specific siRNAs. One SOX9 siRNA was able to decrease SOX9 protein expression and DMBT1 gene expression (Figure 6D-E).
Figure 6. SOX9 activates DMBT1 expression.
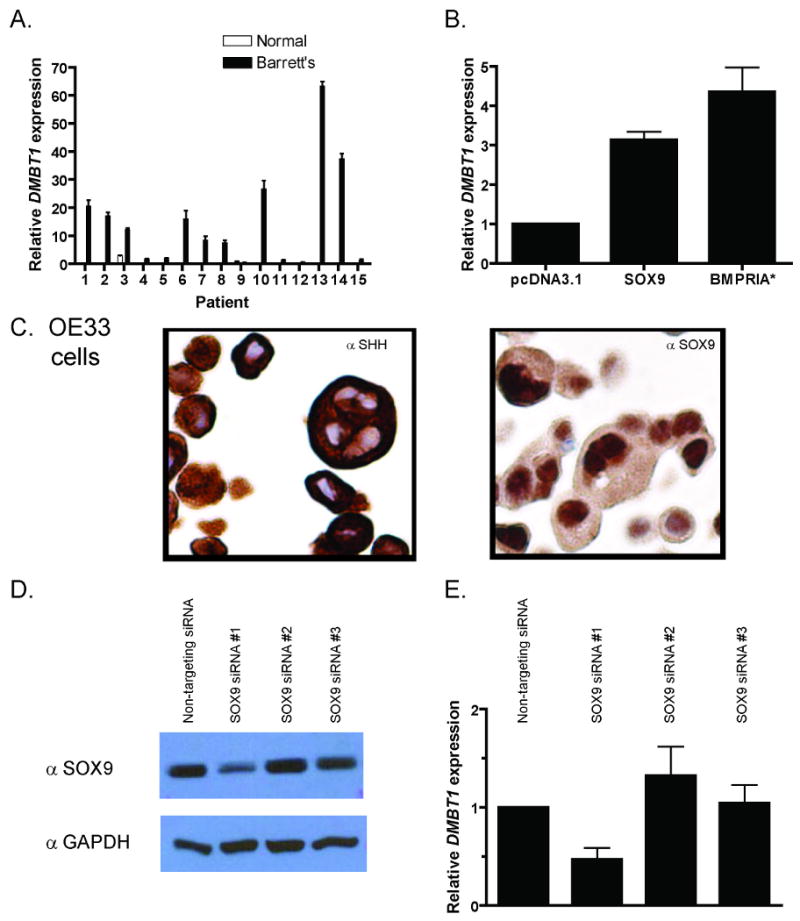
DMBT1 qRT-PCR data from A) 15 frozen esophagectomy cases and B) transfected HET-1A cells. C) SHH and SOX9 immunocytochemistry in OE33 cells. D) SOX9 Western blot with GAPDH loading control and E) DMBT1 qRT-PCR data of OE33 cells transfected with SOX9 siRNAs.
Transgenic Shh overexpression in mouse esophageal epithelia leads to columnar gene expression
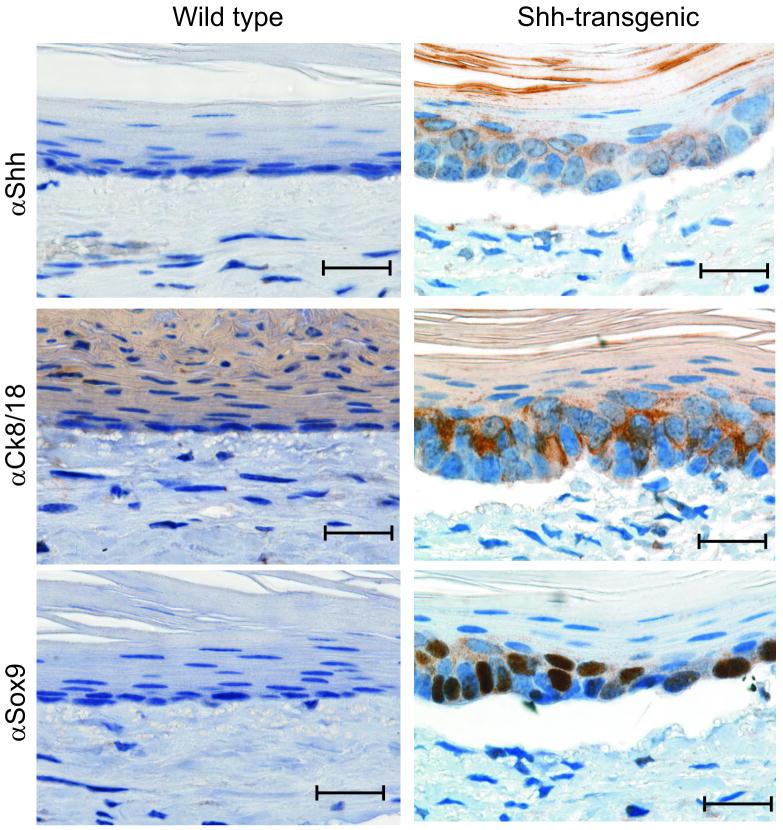
We next attempted to demonstrate that esophageal epithelial Hh ligand expression is sufficient to induce epithelial columnar gene expression. In collaboration with the NCI Mouse Cancer Genetics Program, we developed a novel conditional Shh transgenic mouse (Supplemental Figure 5). Unable to find a mouse strain with an esophageal epithelial specific promoter driving cre expression, we chose instead to utilize an in vivo transplant culture system22. In this model, esophageal epithelial cells and fibroblasts are cultured within a denuded rat trachea which is placed subcutaneously into an immunodeficient mouse. The trachea becomes vascularized allowing the cells to recapitulate the 3-D structure of esophageal mucosa with stratified epithelium overlying a stromal fibroblast layer. We transduced esophageal epithelial cells from Shh transgenic (ShhTg) or wild-type control littermates with adenoviral-cre recombinase before placing them into culture with C57Bl/6 esophageal fibroblasts. After four weeks, we found that ShhTg epithelium expressed Shh, Cytokeratin 8/18, and Sox9 (Figure 7) in the presence of Bmp4 expressing stromal cells (Supplemental Figure 6). Morphologically, the Shh expressing cells had features suggestive of a columnar phenotype, with nuclei oriented in a perpendicular direction. Cells from ShhTg mice that did not express Shh maintained a squamous morphology and did not express Cytokeratin 8/18 or Sox9 (Supplemental Figure 7) or induce stromal expression of Bmp4 (data not shown) in our culture system.
Figure 7. Shh signaling induces columnar differentiation in a 3-D tissue reconstitution model.
Shh, Ck8/18, and Sox9 immunohistochemistry in 3-dimensional culture reconstituted from primary esophageal squamous epithelial cells isolated from conditional Shh-transgenic or wild-type littermate mice. Bars represent 20μm.
Discussion
Our results demonstrate the novel findings of widespread epithelial-mesenchymal Hh pathway activation as well as SOX9 and DMBT1 expression in BE. We have shown that SOX9 expression can be induced by BMP4, a known target of Hh signaling in gut mesenchyme, which is expressed in stromal cells surrounding BE. Furthermore, SOX9 can upregulate the expression of DMBT1, a gene linked to the induction of columnar epithelial differentiation. Finally, in an in vivo 3-D reconstitution model, we demonstrated that esophageal epithelial expression of Shh can lead to epithelial expression of columnar cytokeratins in the presence of stromal fibroblasts. Our data present a compelling argument for a connection between aberrant Hh signaling and the initiation of columnar metaplasia in BE.
Epithelial-mesenchymal Hh signaling is a hallmark of gut development, regional specification, radial patterning, and differentiation in vertebrates8. In chick embryos, Shh is expressed within the endoderm of the developing gut and induces expression of Bmp4 and Hox genes in adjacent mesoderm of the hindgut9. These mesoderm genes specify gut regionalization as virally-mediated misexpression of Bmp4 in the foregut leads to formation of a stomach with hindgut features24. In epithelium-mesenchyme recombination experiments, epithelial Shh regulates radial patterning of the gut tube through activation of mesenchymal Ptch1 and Bmp410. Finally, mesenchymal Bmp4 specifies pyloric sphincter epithelium by activating Sox9 and Nkx2.544, 45. During mammalian embryogenesis, Shh is essential for foregut development and is active in the early stages of esophageal development before the conversion of the epithelium to stratified squamous. Shh, Gli2, and compound Gli2/Gli3 knockout mouse embryos all develop tracheo-esophageal defects12, 46.
Our finding 70 cases of BE with Hh pathway activation expands on the recent study which describes BMP4 signaling in BE18. Activation of Hh signaling and subsequent BMP4 signaling is attractive as an underlying mechanism of BE since both pathways are active in the developing esophagus, inactive in adult esophagus, and active in adult stomach and small intestine. Hh pathway activation by the proposed biologic causes of BE provides a basis for BMP4 activation in BE.
Our results are the first to describe Sox9's expression pattern in the developing esophagus as well as in BE and esophageal adenocarcinoma. Sox9 was first described in the gut as a Wnt pathway target in intestinal crypts and a repressor of CDX2 and MUC227. In adult small intestinal crypts, Sox9 expression overlapped that of Ki-67 in the proliferative compartment. However, Ki-67 negative cells, which were felt to be post-mitotic Paneth cells, also expressed Sox9. Two recent reports further delineate Sox9's role in the intestine through the use of Villin-Cre X Sox9flox/flox mice. In the first, loss of Paneth cells as well as increased crypt size in the mutant mice were seen29. The second study found a 40% loss of goblet cells and almost complete loss of Paneth cells in the mutant mice with extension of the proliferative compartment into the area where Paneth cells reside28. Interestingly, the phenotype of Villin-Cre X Bmpr1aflox/flox mice is similar47. These mice had increased crypt proliferation and lack of maturity of both Paneth and goblet cells. Although these phenotypes demonstrate Sox9's role in early Paneth cell development, Paneth cells are not commonly associated with BE. More likely, SOX9 is expressed in BE by a columnar cell that has the ability to differentiate into either Paneth or goblet cells depending on the expression of other transcription factors. This could potentially explain SOX9's repression of CDX2 and MUC2 in intestinal crypts, something which may not occur in BE48. SOX9 expression in the absence of nuclear β-catenin localization (Supplemental Figure 8), suggests that SOX9 induction may not be driven by Wnt signaling in BE.
Finally, we describe DMBT1 as a target of SOX9 induced transcriptional activation in esophageal squamous epithelium. Though we do not offer direct evidence that DMBT1 shares the same properties as Hensin in directing cells towards a columnar phenotype, it is worth noting that 14/15 BE patients have upregulated DMBT1 in their Barrett's tissue compared to squamous epithelium. To our knowledge, this is the first time that elevated DMBT1 expression levels have been described in BE and would be consistent with DMBT1's proposed role in epithelial differentiation. The observation that DMBT1 expression is typically absent in foregut-derived squamous cell carcinomas but present in foregut adenocarcinomas49, 50 provides indirect evidence that expression of this gene is linked to a columnar cell phenotype in gut neoplasia.
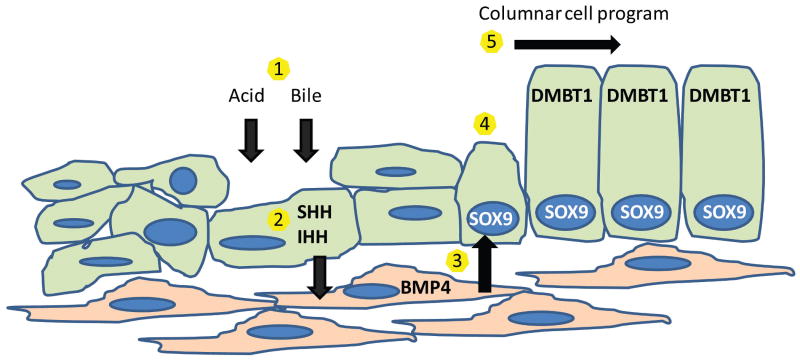
Based on the developmental pattern of crosstalk between epithelium and mesenchyme, we propose the following model of Barrett's metaplasia (Figure 8). The Hh pathway is reactivated in adult esophageal epithelium by acid and bile injury. Hh ligands then signal to surrounding mesenchyme activating the pathway target BMP4. Mesenchymal BMP4 signals to the epithelium to activate SOX9 which initiates a columnar transcriptional program including DMBT1.
Figure 8. Proposed molecular model of metaplasia.
1) Acid and bile injure esophageal squamous epithelium. 2) Injured epithelial cells secrete SHH and IHH which causes 3) mesenchymal secretion of BMP4. 4) BMP4 signals to epithelium activating SOX9. 5) SOX9 activates a columnar cell transcriptional program including DMBT1.
In summary, we believe that our proposed molecular cascade links acid and bile injury to columnar metaplasia. Re-establishment of Hh signaling, BMP4 signaling, and SOX9 expression in BE may simply reset esophageal epithelium to a time when columnar cells lined a primordial esophagus. We feel that future studies should focus on several areas. First, can Hh pathway activation and SOX9 expression in GERD patients predict which will go on to develop BE? This would allow more judicious use of endoscopic surveillance in these patients. Second, confirming that DMBT1 has the same properties as Hensin and understanding its role in esophageal epithelium should be undertaken. Third, other SOX9 targets that could play a role in the metaplastic process should be identified. In silico gene expression studies or animal studies in which Sox9 is overexpressed in esophageal epithelium could provide insight into new molecular targets in BE. Finally, our model opens the possibility for use of Hh and BMP4 antagonists as therapeutic agents in BE.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Wrana and Dr. Mark de Caestecker for the BMPRIA* plasmid, Dr. Malcolm Brock for samples from the esophagectomy tumor bank, Dr. Ross Hannan for the adenoviral-cre recombinase, and Ms. Debbie Swing for assistance in generating the ShhTg mouse. The Jerry D'Amato Charity Foundation and the Roy L. Jeannotte Foundation supported construction of the esophageal tissue microarrays.
Funding: This work was supported by an ASCO Young Investigator Award (D.H.W.), Ruth L. Kirschstein NRSA F32CA-123945 (D.H.W.), a Victorian Cancer Agency Early Career Seed Grant (N.J.C.), Australian Research Council Discovery Project Grant DP0878303 (N.J.C.), NIH 1K23DK068149 (J.S.W.), the Sidney Kimmel Cancer Research Foundation (D.N.W.), and the NHMRC Australia, Fellowship 546107/Project Grant 546098 (D.N.W.).
Footnotes
Financial Disclosures: None.
Author contributions: Study concept and design (DHW, NJC, TM, JWH, WAP, DNW), acquisition of data (DHW, NJC, TM, WZ, AS, IMC-S, DLW, JWH, WAP, DNW), analysis and interpretation of data (DHW, NJC, TM, EAM, JSW, JWH, WAP, DNW), drafting of the manuscript (DHW, DNW), critical revision of the manuscript for important intellectual content (DHW, NJC, WZ, EAM, JSW, JWH, DNW), obtained funding (DHW, NJC, JWH, WAP, DNW), technical or material support (DHW, NJC, TM, AJD, WZ, AS, IMC-S, DLW, EAM, JSW, NAJ, NAC, JWH, WAP, DNW), and study supervision (DHW, DNW).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeMeester SR. Adenocarcinoma of the esophagus and cardia: a review of the disease and its treatment. Ann Surg Oncol. 2006;13:12–30. doi: 10.1245/ASO.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Paulson TG, Reid BJ. Focus on Barrett's esophagus and esophageal adenocarcinoma. Cancer Cell. 2004;6:11–6. doi: 10.1016/j.ccr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Wijnhoven BP, Tilanus HW, Dinjens WN. Molecular biology of Barrett's adenocarcinoma. Ann Surg. 2001;233:322–37. doi: 10.1097/00000658-200103000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spechler SJ. Clinical practice. Barrett's Esophagus. N Engl J Med. 2002;346:836–42. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 5.Jankowski JA, Harrison RF, Perry I, et al. Barrett's metaplasia. Lancet. 2000;356:2079–85. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 6.Gillen P, Keeling P, Byrne PJ, et al. Experimental columnar metaplasia in the canine oesophagus. Br J Surg. 1988;75:113–5. doi: 10.1002/bjs.1800750208. [DOI] [PubMed] [Google Scholar]
- 7.Yu WY, Slack JM, Tosh D. Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev Biol. 2005;284:157–70. doi: 10.1016/j.ydbio.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Lees C, Howie S, Sartor RB, et al. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology. 2005;129:1696–710. doi: 10.1053/j.gastro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DJ, Johnson RL, Burke AC, et al. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–74. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- 10.Sukegawa A, Narita T, Kameda T, et al. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–80. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- 11.Madison BB, Braunstein K, Kuizon E, et al. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 12.Litingtung Y, Lei L, Westphal H, et al. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 13.van den Brink GR, Hardwick JC, Nielsen C, et al. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628–33. doi: 10.1136/gut.51.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins DN, Berman DM, Burkholder SG, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–7. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 16.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 17.Beachy PA, Karhadkar SS, Berman DM. Mending and malignancy. Nature. 2004;431:402. doi: 10.1038/431402a. [DOI] [PubMed] [Google Scholar]
- 18.Milano F, van Baal JW, Buttar NS, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007;132:2412–21. doi: 10.1053/j.gastro.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald RC, Omary MB, Triadafilopoulos G. Acid modulation of HT29 cell growth and differentiation. An in vitro model for Barrett's esophagus. J Cell Sci. 1997;110(Pt 5):663–71. doi: 10.1242/jcs.110.5.663. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran RB, Scott MP. A mouse model for medulloblastoma and basal cell nevus syndrome. J Neurooncol. 2001;53:307–18. doi: 10.1023/a:1012260318979. [DOI] [PubMed] [Google Scholar]
- 21.Sui G, Bonde P, Dhara S, et al. Epidermal growth factor receptor and hedgehog signaling pathways are active in esophageal cancer cells from rat reflux model. J Surg Res. 2006;134:1–9. doi: 10.1016/j.jss.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Croagh D, Cheng S, Tikoo A, et al. Reconstitution of stratified murine and human oesophageal epithelia in an in vivo transplant culture system. Scand J Gastroenterol. 2008;43:1158–68. doi: 10.1080/00365520802102489. [DOI] [PubMed] [Google Scholar]
- 23.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 24.Roberts DJ, Smith DM, Goff DJ, et al. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- 25.Dimmler A, Brabletz T, Hlubek F, et al. Transcription of sonic hedgehog, a potential factor for gastric morphogenesis and gastric mucosa maintenance, is up-regulated in acidic conditions. Lab Invest. 2003;83:1829–37. doi: 10.1097/01.lab.0000101729.25140.0c. [DOI] [PubMed] [Google Scholar]
- 26.Rokkas T, Sladen GE. Ambulatory esophageal pH recording in gastroesophageal reflux: relevance to the development of esophagitis. Am J Gastroenterol. 1988;83:629–32. [PubMed] [Google Scholar]
- 27.Blache P, van de Wetering M, Duluc I, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastide P, Darido C, Pannequin J, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–48. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori-Akiyama Y, van den Born M, van Es JH, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–46. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Tavella S, Biticchi R, Schito A, et al. Targeted expression of SHH affects chondrocyte differentiation, growth plate organization, and Sox9 expression. J Bone Miner Res. 2004;19:1678–88. doi: 10.1359/JBMR.040706. [DOI] [PubMed] [Google Scholar]
- 31.Vidal VP, Chaboissier MC, Lutzkendorf S, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–51. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 32.van den Brink GR, Hardwick JC, Tytgat GN, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 33.Que J, Choi M, Ziel JW, et al. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–37. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 34.Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 35.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 36.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 37.Takito J, Al-Awqati Q. Conversion of ES cells to columnar epithelia by hensin and to squamous epithelia by laminin. J Cell Biol. 2004;166:1093–102. doi: 10.1083/jcb.200405159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Adelsberg J, Edwards JC, Takito J, et al. An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell. 1994;76:1053–61. doi: 10.1016/0092-8674(94)90382-4. [DOI] [PubMed] [Google Scholar]
- 39.Takito J, Hikita C, Al-Awqati Q. Hensin, a new collecting duct protein involved in the in vitro plasticity of intercalated cell polarity. J Clin Invest. 1996;98:2324–31. doi: 10.1172/JCI119044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vijayakumar S, Takito J, Hikita C, et al. Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: processes that resemble terminal differentiation. J Cell Biol. 1999;144:1057–67. doi: 10.1083/jcb.144.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takito J, Yan L, Ma J, et al. Hensin, the polarity reversal protein, is encoded by DMBT1, a gene frequently deleted in malignant gliomas. Am J Physiol. 1999;277:F277–89. doi: 10.1152/ajprenal.1999.277.2.F277. [DOI] [PubMed] [Google Scholar]
- 42.Cheng H, Bjerknes M, Chen H. CRP-ductin: a gene expressed in intestinal crypts and in pancreatic and hepatic ducts. Anat Rec. 1996;244:327–43. doi: 10.1002/(SICI)1097-0185(199603)244:3<327::AID-AR5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 43.Genzer MA, Bridgewater LC. A Col9a1 enhancer element activated by two interdependent SOX9 dimers. Nucleic Acids Res. 2007;35:1178–86. doi: 10.1093/nar/gkm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theodosiou NA, Tabin CJ. Sox9 and Nkx2.5 determine the pyloric sphincter epithelium under the control of BMP signaling. Dev Biol. 2005;279:481–90. doi: 10.1016/j.ydbio.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Moniot B, Biau S, Faure S, et al. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. Development. 2004;131:3795–804. doi: 10.1242/dev.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motoyama J, Liu J, Mo R, et al. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–7. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 47.Auclair BA, Benoit YD, Rivard N, et al. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007;133:887–96. doi: 10.1053/j.gastro.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 48.Steininger H, Pfofe DA, Muller H, et al. Expression of CDX2 and MUC2 in Barrett's mucosa. Pathol Res Pract. 2005;201:573–7. doi: 10.1016/j.prp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Mori M, Shiraishi T, Tanaka S, et al. Lack of DMBT1 expression in oesophageal, gastric and colon cancers. Br J Cancer. 1999;79:211–3. doi: 10.1038/sj.bjc.6690035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollenhauer J, Herbertz S, Helmke B, et al. Deleted in Malignant Brain Tumors 1 is a versatile mucin-like molecule likely to play a differential role in digestive tract cancer. Cancer Res. 2001;61:8880–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.