Abstract
Oral leukoplakia and other potentially malignant disorders (PMD) may progress to oral squamous cell carcinoma (OSCC). The gold standard for assessing the potential for malignant transformation remains histologic examination with the aim of grading the dysplastic changes. However, not all lesions with dysplasia will progress to OSCC. DNA ploidy has been suggested as a method to predict the clinical behaviour of PMD. This study reports on the use of high-resolution flow cytometry to determine the ploidy status of formalin-fixed, paraffin-embedded material from PMD compared to their dysplasia grade on histology. Aneuploidy was found in 13 % of mild, 31 % of moderate, and 54 % of severe dysplasia cases. This difference was statistically significant (p = 0.011). The differences in ploidy status were more significant when grouping the dysplasia into low-risk and high-risk categories (p = 0.008). These findings indicate that the ploidy status of PMD as determined by high-resolution flow cytometry may be of value in predicting biological behaviour in PMD such as leukoplakia.
Keywords: Leukoplakia, DNA ploidy, Dysplasia, Potentially malignant disorders, Oral mucosa, Flow cytometry
Introduction
Many oral squamous cell carcinomas (OSCC) are associated with precursor lesions in the form of potentially malignant disorders (PMD), the majority of which present as leukoplakia lesions. The current best practice of care is to remove these lesions for histologic analysis. The presence of epithelial dysplasia, albeit with well-documented limitations, is still the gold standard in evaluating the malignant potential of suspicious lesions of the oral mucosa [1–4]. It is also well-documented that a number of PMDs, regardless of the presence of dysplasia, do not progress to carcinoma [5].
Contradictory reports and lack of information in the literature to reliably predict the behaviour of these lesions are the main reasons why the management of patients with PMD is still controversial. Several recent studies have reported the potential use of DNA ploidy analysis to predict the behaviour of oral PMD [6–12]. Aneuploidy in oral dysplastic lesions, as detected by DNA image cytometry, was found to be indicative of a high risk of OSCC development [10]. The ploidy status of OSCC pertaining to different clinical settings also has been evaluated with high-resolution flow cytometry. DNA aneuploidy in OSCC is an independent predictive factor of local recurrence [13] and a useful tool to predict regional lymph node metastases [14]. It has also been demonstrated as an independent prognostic factor in OSCC [15].
High-resolution flow cytometers are equipped with a high-pressure mercury lamp or UV laser for fluorochrome excitation allowing DAPI (4′6-diamidino-2-phenylindole-dihydrochloride) to be used for DNA staining. This allows for a lower coefficient of variance (CV) to be obtained to enable the detection of peri-diploid aneuploidy cell populations that would have been reported as diploid with less sensitive techniques [16]. The aim of this study was to evaluate the use of high-resolution flow cytometry on formalin-fixed, paraffin-embedded tissue of leukoplakia from the tongue and floor of mouth and to correlate the findings with the histologic grading.
Materials and Methods
Tissue blocks from all cases from the floor of mouth and lateral and ventral surfaces of the tongue diagnosed with epithelial dysplasia during the period 2000–2008 were retrieved from the archives of the Department of Oral Pathology and Oral Biology, University of Pretoria. The haematoxylin and eosin-stained sections of all the cases were examined by two experienced oral pathologists for agreement on the diagnosis and grade of dysplasia. Grading of epithelial dysplasia was done according to the 2005 WHO classification of epithelial dysplasia [17] and was then further defined as low-risk or high-risk according to the workshop coordinated by the WHO Collaborating Centre for Oral Cancer and Precancer in 2005 [1]. Cases where there was disagreement on the histologic grading of the dysplasia, ulceration with extensive inflammation, or an inadequate amount of tissue for flow cytometric evaluation were excluded from the study. Thirty-nine cases of mild dysplasia, 35 cases of moderate dysplasia, and 28 cases of severe dysplasia were selected. Eight cases showing basal cell hyperplasia only, were also included.
Sections were prepared according to the modified Hedley method [18]. Four to six 40 μm sections were cut from a paraffin block, wrapped in 50 μm nylon mesh, placed in a histology cassette, manually de-waxed and hydrated with distilled water. After being washed in distilled water overnight, the enwrapped sections were digested in subtilisin Carlsberg solution at 37 °C in a centrifuge tube for 120 min and stirred for 20 min thereafter. The Carlsberg solution was then filtered through a 50 μm nylon mesh and DAPI (Research Organics, Cleveland, OH, USA) staining solution was added. Flow cytometry was carried out using a PAS II flow cytometer equipped with a high-pressure 100 W mercury lamp (Partec, Münster, Germany). DNA histograms of at least 10 000 cells were plotted. The diploid cell populations were used as an internal reference standard for the identification of aneuploid clones. The DNA index (DI) was defined as the ratio of the mean channel number of the G0/G1 aneuploid peak to the mean channel number of the G0/G1 diploid peak. The CV of the measurements was obtained by a Gaussian curve fitting of the G0/G1 peaks (FloMax Software, 2.7, Partec, Münster, Germany).
Data collection and statistical analysis were done using Microsoft Office Excel (Microsoft Corporation, USA) and the SPSS 19.0 software package (Apache Software Foundation, Chicago, IL, USA). The association between ploidy status and the histologic grade of dysplasia was evaluated with the Chi-square test. The differences in the mean values of the DNA index in the different dysplasia groups were evaluated with the Student’s t test. A p value of less than or equal to 0.05 was considered statistically significant.
Ethics approval to conduct this study was obtained from the Research Ethics Committee of the Faculty of Health Sciences of the University of Pretoria.
Results
Aneuploidy was found in 13 % of mild, 31 % of moderate, and 54 % of severe epithelial dysplasia cases (Figs. 1, 2). These differences were statistically significant (p = 0.011). The differences in ploidy status were even more prominent when grouping the cases into low-risk and high-risk categories (p = 0.008) (Table 1). All the lesions showing only basal cell hyperplasia were diploid.
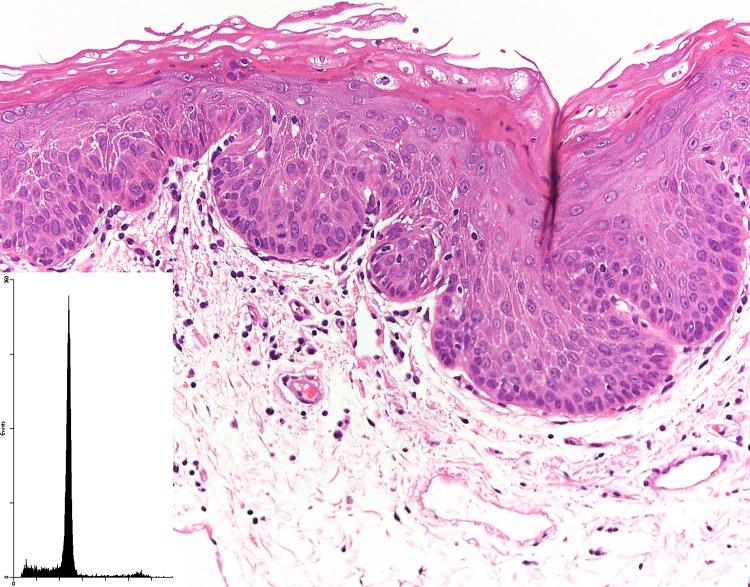
Fig. 1.
A case of moderate epithelial dysplasia that had a diploid DNA measurement (inset)
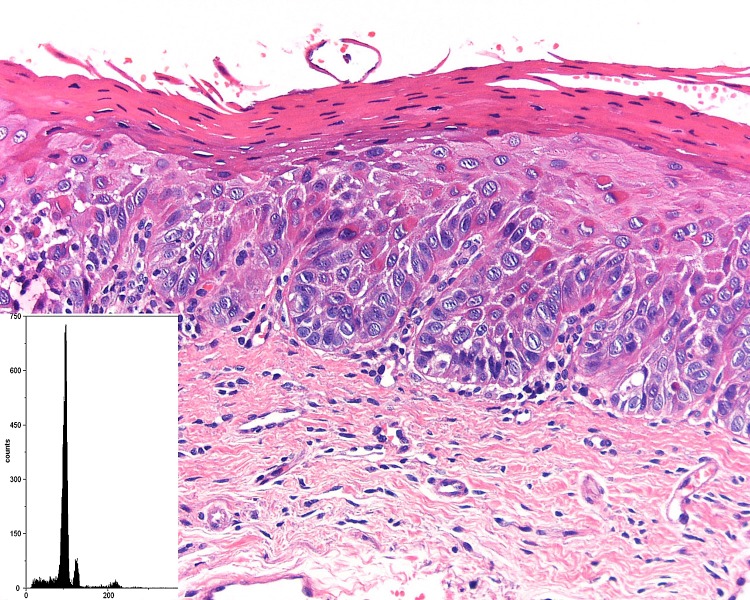
Fig. 2.
A case of severe epithelial dysplasia that demonstrated an aneuploid DNA histogram (inset)
Table 1.
Ploidy status of the different degrees of dysplasia
| Diploid | Aneuploid | Mean DNA index (DI) | |
|---|---|---|---|
| Basal cell hyperplasia (n = 8) | 8 (100 %) | 0 | – |
| Mild dysplasia (n = 39) | 34 (87 %) | 5 (13 %) | 1.13 (±0.05) |
| Moderate dysplasia (n = 35) | 24 (69 %) | 11 (31 %) | 1.38 (±0.33) |
| Severe dysplasia (n = 28) | 13 (47 %) | 15 (54 %) | 1.54 (±0.33) |
| Low-risk dysplasia (n = 47)a | 42 (89 %) | 5 (11 %) | 1.13 (±0.05) |
| High-risk dysplasia (n = 63)b | 37 (59 %) | 26(41 %) | 1.47 (±0.33) |
aCombination of basal cell hyperplasia and mild dysplasia
bCombination of moderate and severe dysplasia
The aneuploid peaks of the mild dysplasia cases were predominantly in a peri-diploid position while the mean DI values gradually increased with the degree of dysplasia (Figs. 3, 4). The differences in the mean DI between mild and moderate dysplasia and between mild and severe dysplasia were statistically significant (p = 0.033 and p = 0.017, respectively). Two of the aneuploid cases with moderate dysplasia and three cases associated with severe dysplasia were tetraploid. All the aneuploid cases consisted of a single clone alone. The mean CV of the analyses was 3.7 ± 0.9.
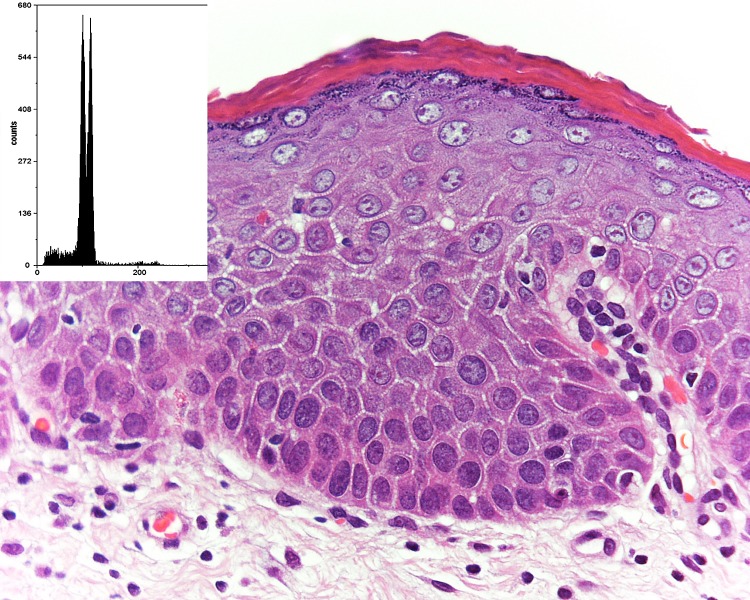
Fig. 3.
A case of mild epithelial dysplasia that had an aneuploid DNA histogram (inset). The DI was 1.16
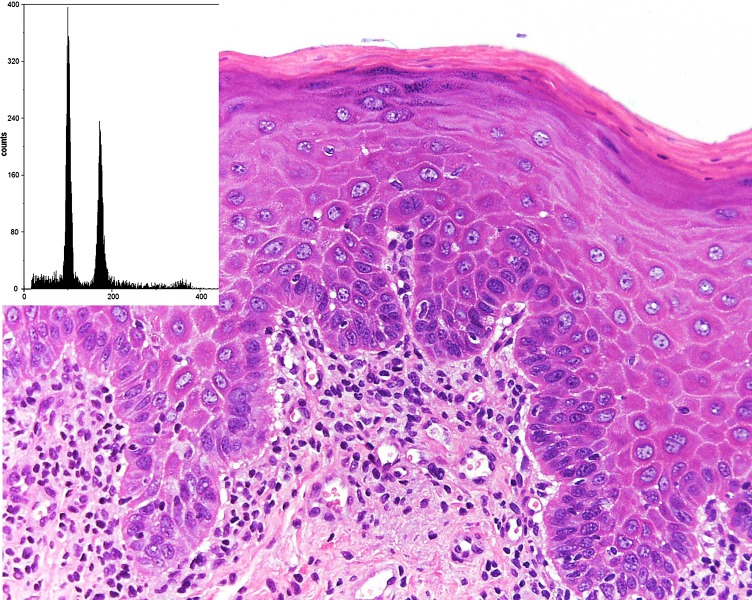
Fig. 4.
The aneuploid DNA histogram (inset) of a case of moderate epithelial dysplasia had a DI of 1.75
Discussion
DNA aneuploidy can be defined as any imbalance of chromosomal material. Any amplification or deletion of DNA influences the expression of a multitude of genes, regardless of whether such changes occur in chromosomal structure or number. High-resolution DNA flow cytometry has been performed on OSCC in a variety of clinical settings [13–15, 19, 20]. About 80 % of OSCC were found to be aneuploid and 20 % diploid with high-resolution flow cytometric analysis. It can be assumed that diploid OSCC develops from diploid oral mucosa. An aneuploid OSCC would in all likelihood develop from an aneuploid precursor area, although flow cytometrically detectable changes might occur in a diploid PMD leading to an aneuploid OSCC. It should be emphasised that even high-resolution flow cytometry cannot detect all the molecular changes associated with carcinogenesis.
A large number of the published reports evaluating the DNA ploidy status of PMD have used image cytometry. This study confirmed that high-resolution flow cytometry on archival material might add potentially valuable information for the clinician treating patients with PMD. A clear correlation between histologic grade of the leukoplakias and DNA ploidy status was found in the present study. This is in contrast to the results from Torres-Rendon et al. [10] and Bremmer et al. [12] who found no correlation between histologic grade and DNA aneuploidy. The aneuploid dysplastic lesions in the study of Torres-Rendon et al. [10] had a significantly increased risk to progress to OSCC compared to diploid dysplastic lesions. Donadini and co-workers, on the other hand, found aneuploid clones in 23 % of PMDs with no histologic evidence of dysplasia compared to 46 % of PMDs with dysplasia using high-resolution flow cytometry [8]. No aneuploidy was detected in our eight basal cell hyperplasia cases while aneuploidy was present in 11 % of the low-risk dysplasia group and in 41 % of the high-risk dysplasia group. This binary grading system was proposed to improve the well-documented inter-observer disagreement regarding grading of epithelial dysplasia [21].
The aneuploid lesions associated with mild dysplasia in this study were characterised by peri-diploid aneuploid clones, suggesting early changes. Similar observations were reported by Donadini et al. [8]. This might suggest that peri-diploid aneuploid changes represent an early step in the carcinogenesis of oral cancer. It is possible that the presence of peri-diploid aneuploid clones might be interpreted as a diploid histogram if the CV is not low enough to distinguish between the two peaks. Although the use of formalin-fixed, paraffin-embedded tissue is not ideal, the sensitivity of high-resolution flow cytometry was still able to detect aneuploid clones with a small DI.
All the aneuploid cases consisted of a single aneuploid clone. This is in line with data indicating that only about 10 % of OSCC cases are bi-clonal or tri-clonal [22]. The multiclonal clones in OSCC are not related to tumour size and, therefore, argues against the continuous development of additional aneuploid clones during tumour development [22]. The absence of multiple aneuploid clones in the high-risk dysplasia cases also supports this argument.
It is our management protocol to remove all leukoplakia lesions where possible, irrespective of histologic grading. These patients are then followed on a regular basis (every 6 months) for clinical examination assisted by visual diagnostic aids. Any new suspicious lesion is biopsied. It is thus not possible to comment on disease progression of any leukoplakic lesion. OSCC was subsequently diagnosed in 11 patients included in this study. Five were diagnosed as severe dysplasia with an aneuploid DNA content, three as severe dysplasia with a diploid DNA content, two as moderate dysplasia with aneuploid DNA content, and one as mild dysplasia with diploid DNA content. It was obviously not possible to describe these as malignant transformation of the leukoplakia lesions as in most cases the OSCC did not develop from the same site.
The association between the grade of epithelial dysplasia and OSCC development is supported by several other studies [1, 3, 4, 23]. It is important to note that patients with mild dysplasia may develop OSCC [9]. The presence of DNA aneuploidy in a lesion showing mild epithelial dysplasia suggests that it may be relevant for the clinician in determining a management strategy for these patients. A direct correlation between aneuploidy, as determined with high-resolution flow cytometry, and disease progression was not possible due to our management protocol described above, but the demonstrated association between DNA ploidy status and the presence of epithelial dysplasia suggested that regular follow-up for the early detection of any possible mucosal alteration should be the minimum standard of care.
It must be emphasised that PMD should only be regarded as indicator lesions reflecting the status of the oral mucosa. It is furthermore wrong to assume that OSCC will always develop from a pre-existing PMD. This concept is in all likelihood one of the reasons why removal of PMD has not significantly influenced the development of OSCC over the last few decades. Although there are uncertainties regarding the most appropriate treatment of patients with PMD, DNA ploidy determination by high-resolution flow cytometry might be a marker to predict biological behaviour in PMD and could be an additional tool in determining the prognosis of oral dysplastic lesions.
Acknowledgments
The authors would like to thank Prof Sonja Boy who participated with the histological grading and the National Research Foundation of South Africa for financial support.
References
- 1.Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37(3):127–133. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 2.Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37(1):1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 3.Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long-term treatment outcome of oral premalignant lesions. Oral Oncol. 2006;42(5):461–474. doi: 10.1016/j.oraloncology.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Silverman S, Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53(3):563–568. doi: 10.1002/1097-0142(19840201)53:3<563::AID-CNCR2820530332>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Eversole LR. Dysplasia of the upper aerodigestive tract squamous epithelium. Head Neck Pathol. 2009;3(1):63–68. doi: 10.1007/s12105-009-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna R, Agarwal A, Khanna S, Basu S, Khanna AK. S-phase fraction and DNA ploidy in oral leukoplakia. ANZ J Surg. 2010;80(7–8):548–551. doi: 10.1111/j.1445-2197.2009.05196.x. [DOI] [PubMed] [Google Scholar]
- 7.Islam MN, Kornberg L, Veenker E, Cohen DM, Bhattacharyya I. Anatomic site based ploidy analysis of oral premalignant lesions. Head Neck Pathol. 2010;4(1):10–14. doi: 10.1007/s12105-009-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donadini A, Maffei M, Cavallero A, Pentenero M, Malacarne D, Nallo E, et al. Oral cancer genesis and progression: DNA near-diploid aneuploidization and endoreduplication by high resolution flow cytometry. Cell Oncol. 2010;32(5–6):373–383. doi: 10.3233/CLO-2010-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley G, Odell EW, Raphael S, Ho J, Le LW, Benchimol S, et al. Abnormal DNA content in oral epithelial dysplasia is associated with increased risk of progression to carcinoma. Br J Cancer. 2010;103(9):1432–1442. doi: 10.1038/sj.bjc.6605905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Rendon A, Stewart R, Craig GT, Wells M, Speight PM. DNA ploidy analysis by image cytometry helps to identify oral epithelial dysplasias with a high risk of malignant progression. Oral Oncol. 2009;45(6):468–473. doi: 10.1016/j.oraloncology.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Pentenero M, Giaretti W, Navone R, Demurtas A, Rostan I, Bertolusso G, et al. DNA aneuploidy and dysplasia in oral potentially malignant disorders: association with cigarette smoking and site. Oral Oncol. 2009;45(10):887–890. doi: 10.1016/j.oraloncology.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Bremmer JF, Brakenhoff RH, Broeckaert MA, Belien JA, Leemans CR, Bloemena E, et al. Prognostic value of DNA ploidy status in patients with oral leukoplakia. Oral Oncol. 2011;47(10):956–960. doi: 10.1016/j.oraloncology.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Hemmer J, Heerden W, Raubenheimer E, Kreidler J, Schon E. Flow cytometric DNA ploidy and recurrence development in squamous cell carcinoma of the oral cavity. Int J Oncol. 1996;8(1):113–116. doi: 10.3892/ijo.8.1.113. [DOI] [PubMed] [Google Scholar]
- 14.Hemmer J, Thein T, Heerden WF. The value of DNA flow cytometry in predicting the development of lymph node metastasis and survival in patients with locally recurrent oral squamous cell carcinoma. Cancer. 1997;79(12):2309–2313. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2309::AID-CNCR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Hemmer J, Nagel E, Kraft K. DNA aneuploidy by flow cytometry is an independent prognostic factor in squamous cell carcinoma of the oral cavity. Anticancer Res. 1999;19(2B):1419–1422. [PubMed] [Google Scholar]
- 16.Heerden WF, Raubenheimer EJ, Dreyer L. The role of DNA ploidy and Ki-67 in the grading of mucoepidermoid carcinomas. Anticancer Res. 2005;25(3c):2589–2592. [PubMed] [Google Scholar]
- 17.Pathology & genetics. Head and neck tumours. Lyon: International Agency for Research on Cancer (IARC) IARC Press; 2005. [Google Scholar]
- 18.Heiden T, Wang N, Tribukait B. An improved Hedley method for preparation of paraffin-embedded tissues for flow cytometric analysis of ploidy and S-phase. Cytometry. 1991;12(7):614–621. doi: 10.1002/cyto.990120705. [DOI] [PubMed] [Google Scholar]
- 19.Hemmer J, Kraft K. High-resolution DNA flow cytometry in oral verrucous carcinoma. Oncol Rep. 2000;7(2):433–435. [PubMed] [Google Scholar]
- 20.Hemmer J, Kreidler J. Flow cytometric DNA ploidy analysis of squamous cell carcinoma of the oral cavity. Comparison with clinical staging and histologic grading. Cancer. 1990;66(2):317–320. doi: 10.1002/1097-0142(19900715)66:2<317::AID-CNCR2820660220>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(10):987–993. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Hemmer J, Schon E. Cytogenetic progression and prognosis in oral-carcinoma—a DNA flow cytometric study on 317 cases. Int J Oncol. 1993;3(4):635–640. doi: 10.3892/ijo.3.4.635. [DOI] [PubMed] [Google Scholar]
- 23.Speight PM. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007;1(1):61–66. doi: 10.1007/s12105-007-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]