Abstract
Middle ear adenomas (MEAs) are benign neoplasms along a spectrum with neuroendocrine neoplasms (carcinoid tumors). Immunohistochemical (IHC) staining for myoepithelial markers has not been reported in these tumors. The archives of the Cleveland Clinic, University of Virginia and Armed Forces Institute of Pathology were retrospectively searched for tumors arising within the middle ear with material available for IHC staining. Twelve cases of MEAs, four cases of jugulotympanic paragangliomas (JPGs), 10 cases of ceruminous adenomas (CAs) and four cases of ceruminous adenocarcinomas (CACs) were obtained. IHC staining was performed for smooth muscle actin (SMA), p63, S-100 protein, cytokeratin 5/6 (CK5/6), and cytokeratin 7 (CK7). The MEAs were positive for: CK7 (92 %, luminal), CK5/6 (92 %, abluminal), p63 (83 %, abluminal), and negative for SMA and S-100 protein. The JPGs were negative for CK7, CK5/6, p63 and SMA; S-100 protein highlighted sustentacular cells. The CAs were positive for: CK7 (100 %, luminal), CK5/6 (100 %, abluminal), S-100 protein (80 %, abluminal), p63 (100 %, abluminal), and SMA (90 %, abluminal). CACs demonstrated two patterns, (1) adenoid cystic carcinoma-type: positive for CK7 (100 %, luminal), CK5/6, S-100 protein, p63, and SMA (all 100 %, abluminal); and (2) conventional-type: CK7 (50 % luminal), and no CK5/6, SMA, S-100 protein, or p63 expression. The IHC profile of MEAs suggests that these tumors harbor at least two cell populations, including luminal and basal cells. However, unlike ceruminous adenomas, MEAs lack true myoepithelial differentiation given the absence of S-100 protein and SMA staining in all cases.
Keywords: Ear, Middle ear adenoma, Neuroendocrine adenoma of the middle ear, Myoepithelial, Basal cell, Immunohistochemistry, Ceruminous adenoma, Ceruminous adenocarcinoma, Paraganglioma, Differential diagnosis
Introduction
Middle ear adenomas (MEAs) are rare neoplasms that are generally considered to be benign. They were first described in 1976 [1–3], and were given a variety of names, including “ceruminoma, ceruminous adenoma, monomorphic adenoma, and carcinoid tumor” [3]. However, many of these terms were incorrectly applied (e.g., no true ceruminous differentiation), and these lesions are now unified under the name “middle ear adenoma” or neuroendocrine adenoma of the middle ear (NAME).
The clinical differential diagnosis of MEAs is extensive including schwannoma, schneiderian-type papilloma, squamous cell carcinoma, rhabdomyosarcoma, papillary adenocarcinoma, meningioma, and paraganglioma [3, 4]. While most of these lesions can be distinguished based on histology alone, some distinctions may require immunohistochemistry (e.g., crushed paraganglioma vs. MEA).
In addition, the true nature of MEAs is debatable as to whether they represent an epithelial-derived adenoma or a carcinoid tumor [5, 6]. The histologic variability seen includes different degrees of glandular and neuroendocrine differentiation [7]. Since MEAs are often crushed and distorted by the resection technique, accurate diagnosis can be difficult, particularly when the immunohistochemical staining pattern can vary [8].
Previous studies have suggested a biphasic appearance to MEAs with a luminal/abluminal pattern [7, 8]. In this study, we evaluated MEAs for the presence of an abluminal cell population, using immunohistochemistry for basal cell and myoepithelial markers.
Materials and Methods
The archives of Cleveland Clinic and the University of Virginia were retrospectively searched from 1990 to 2010 for tumors arising within the middle ear that had histologic material available for review and paraffin blocks for additional immunohistochemical staining. Four cases of jugulotympanic paraganglioma (JPG), six cases of middle ear adenoma (MEA), and two cases of ceruminous adenoma (CA) were obtained from the files of the Cleveland Clinic. Six cases of MEA were obtained from the files of the University of Virginia. Four cases of ceruminous adenocarcinoma (CAC) and 10 cases of CA were obtained from the files of the Armed Forces Institute of Pathology (AFIP). Information regarding clinical presentation, demographic features, and imaging studies were available for all but two patients. This clinical investigation was conducted in accordance and compliance with an Institutional Review Board authorization from the Cleveland Clinic.
The H&E-stained glass slides were reviewed to confirm the diagnosis. Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue using the Ventana Medical Systems Benchmark XT and Benchmark Ultra automated immunostaining platforms for the following markers: smooth muscle actin (1A4, 1:50 dilution, Dako), p63 (4A4, Ventana), S-100 protein (polyclonal, 1:200 dilution, Dako), cytokeratin 5/6 (D5/16B4, 1:150 dilution, Millipore), and cytokeratin 7 (OV-TL 12/30, 1:40 dilution, Dako). Nuclear staining was considered positive for p63 and S-100 protein, and cytoplasmic/membranous staining was considered positive for all other stains. The pattern of immunohistochemical staining within each tumor was recorded as negative (<1 % of cells), focal (<25 % of cells) or diffuse (>25 % of cells), and sub-classified as luminal or abluminal where appropriate. All immunohistochemical slides were reviewed independently by two pathologists, and discrepant results were resolved by dual review at a multi-headed microscope.
Results
Clinical Data
A total of 30 cases of MEAs, CAs, CACs, and JPGs were examined. Clinical data was unavailable in one patient with MEA, and one patient with CA. The patients with clinical information available included 15 men and 13 women aged 15–75 years (mean 47 years) (Table 1). The patients presented clinically with ear fullness, tinnitus, vertigo, and pain. By radiographic imaging, the MEAs were centered in the middle ear and did not show evidence of bony invasion or erosion. The JPGs were also located in the middle ear but consistently showed bone erosion of either the mastoid air cells or jugular tubercle. The CAs were centered in the external ear canal, and showed no evidence of bone erosion or invasion. The imaging studies for CACs were unavailable.
Table 1.
Demographic information
| MEA (n = 12)a | CA (n = 10)a | CAC (n = 4) | JPG (n = 4) | |
|---|---|---|---|---|
| Age, years (mean) | 36.2 | 54.3 | 58.5 | 52.5 |
| Gender | M = 5; F = 6 | M = 6; F = 3 | M = 4; F = 0 | M = 0; F = 4 |
| Presenting symptoms | Pain, vertigo, tinnitus | Progressive hearing loss | Mass, hearing loss, pain | Fullness, pressure, tinnitus, vertigo, popping |
| Site | Middle ear L (n = 5); R (n = 6) |
External ear canal L (n = 5); R (n = 4) |
External ear canal L (n = 2); R (n = 2) |
R ear (n = 2); R middle ear (n = 2) |
| Bone erosion | None | None | Not available | Present |
M male, F female, L left, R right, MEA middle ear adenoma, CA ceruminous adenoma, CAC ceruminous adenocarcinoma, JPG jugulotympanic paraganglioma
aClinical data unavailable in one case
Morphology
Histologically, the MEAs showed a range of morphologic patterns including cribriform, glandular, nested, lobular and trabecular, which we divided broadly into solid (Fig. 1, left) and glandular (Fig. 2, left) patterns. There was variation in pattern within each tumor, and the lesional cells were present in a fibroblastic stroma. In most cases, the presence of nested tumor cells in a fibrous stroma gave the impression of an infiltrative growth pattern, although true invasion into surrounding structures was not demonstrated. The cells had variable amounts of eosinophilic cytoplasm. The nuclei had inconspicuous nucleoli and neuroendocrine-like “salt-and-pepper” chromatin. There was no endocrine-type pleomorphism seen. Crush artifact was present in all specimens.
Fig. 1.
Middle ear adenoma, solid pattern: H&E ×200 (left), CK7 ×200 (right)
Fig. 2.
Middle ear adenoma, glandular pattern: H&E ×200 (left), CK7 ×200 (right)
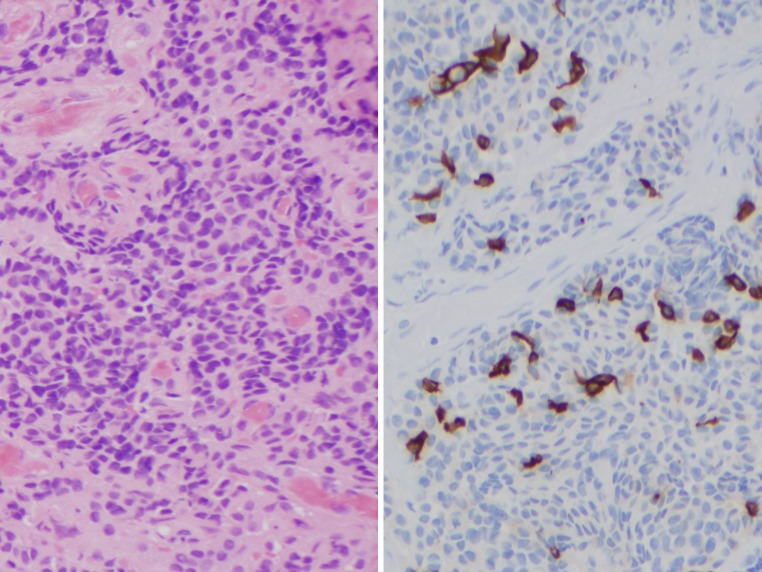
Jugulotympanic paragangliomas (JPGs) displayed lobular nests of cells within a vascular network (Fig. 3, left). The cytoplasm was eosinophilic and vacuolated. The nuclei were round with speckled chromatin and inconspicuous nucleoli, and showed isolated pleomorphism.
Fig. 3.
Jugulotympanic paraganglioma: H&E ×200 (left), CK7 ×200 (right upper), and S-100 protein ×200 (right lower)
Ceruminous adenomas (CAs) showed true gland formation with eosinophilic luminal secretions. The cells displayed focal nuclear pleomorphism with eosinophilic cytoplasm and apical (apocrine) snouts (Fig. 4, left). The nuclei were round to oval with vesicular to condensed chromatin. Cytoplasmic yellow-brown cerumen pigment was present in a few cells in several cases. A distinct myoepithelial cell layer was identifiable by H&E.
Fig. 4.
Ceruminous adenoma: H&E ×200 (left), CK7 ×200 (right upper), p63 ×200 (right lower)
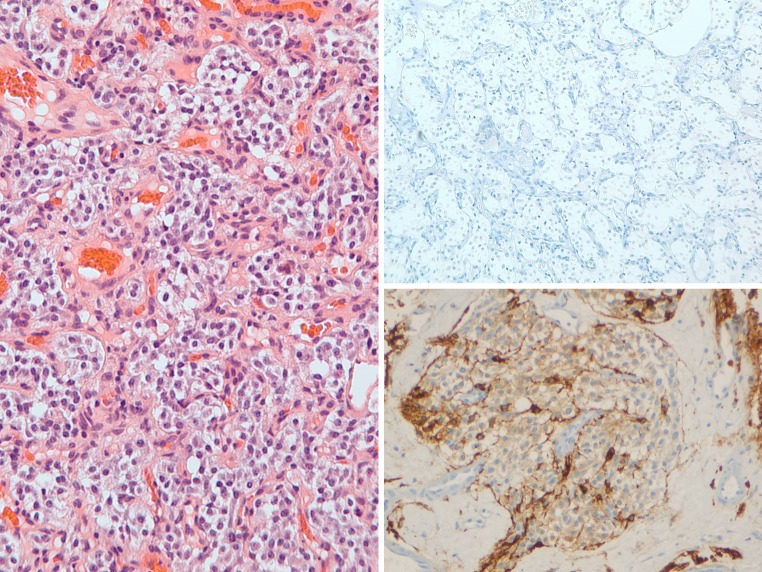
Ceruminous adenocarcinomas (CACs) demonstrated two patterns. The first pattern (conventional type) showed an infiltrating solid to cystic carcinoma composed of glandular to cribriform epithelial cells (Fig. 5, left). The second pattern (adenoid cystic carcinoma-type) demonstrated an appearance very similar to adenoid cystic carcinoma of the salivary gland, with infiltrative nests containing two distinct cell layers (Fig. 6, left). In both patterns, the cells displayed infiltration into nearby structures, along with nuclear pleomorphism and increased mitotic figures.
Fig. 5.
Ceruminous adenocarcinoma, conventional-type: H&E ×100 (left), CK7 ×200 (right upper), p63 ×200 (right lower)
Fig. 6.
Ceruminous adenocarcinoma, adenoid cystic carcinoma-type: H&E ×200 (left), CK7 ×200 (right upper), p63 ×200 (right lower)
Immunohistochemistry
The immunohistochemical results for all cases are reported in Table 2.
Table 2.
Immunohistochemical staining
| CK7 | CK5/6 | p63 | S-100 protein | SMA | |
|---|---|---|---|---|---|
| MEA (n = 12) | Neg (8 %) Focal (59 %)a Diffuse (33 %)a |
Neg (8 %) Focal (42 %)b Diffuse (50 %)b |
Neg (17 %) Focal (58 %)b Diffuse (25 %)b |
Neg (100 %) | Neg (100 %) |
| CA (n = 10) | Diffuse (100 %)a | Diffuse (100 %)b | Diffuse (100 %)b | Neg (20 %) Focal (30 %) Diffuse (50 %) |
Neg (10 %) Diffuse (90 %)b |
| CAC ACC-type (n = 2) | Diffuse (100 %)a | Diffuse (100 %)b | Diffuse (100 %)b | Diffuse (100 %)b | Diffuse (100 %)b |
| CAC conventional-type (n = 2) | Neg (50 %) Diffuse (50 %) |
Neg (100 %) | Neg (100 %) | Neg (100 %) | Neg (100 %) |
| JPG (n = 4) | Neg (100 %) | Neg (100 %) | Neg (100 %) | Focal (100 %)c | Neg (100 %) |
MEA middle ear adenoma, CA ceruminous adenoma, CAC ceruminous adenocarcinoma, ACC adenoid-cystic carcinoma type, JPG jugulotympanic paraganglioma, Neg negative
aPredominantly luminal pattern
bPredominantly abluminal pattern
cStaining of sustentacular cells
All but one of the MEAs (92 %) was at least focally positive for CK7, predominately in a luminal pattern best seen in tumors with a glandular growth pattern (Fig. 1, right, Fig. 2, right). The abluminal cells stained at least focally for CK5/6 (92 % of cases) and p63 (83 % of cases) (Figs. 7, 8, right upper and left upper, respectively). The luminal and abluminal cells showed nonspecific cytoplasmic S-100 protein staining, which was interpreted as negative; all cases were negative for SMA (Figs. 7, 8, right lower and left lower, respectively).
Fig. 7.
Middle ear adenoma (solid pattern) myoepithelial/basal cell immunohistochemistry: CK5/6 ×200 (left upper), p63 ×200 (right upper), S-100 protein ×200 (left lower), SMA ×200 (right lower)
Fig. 8.
Middle ear adenoma (glandular pattern) myoepithelial/basal cell immunohistochemistry: CK5/6 ×200 (left upper), p63 ×200 (right upper), S-100 protein ×200 (left lower), SMA ×200 (right lower)
The JPGs were uniformly negative for CK7 (Fig. 3, right upper), CK5/6, and p63. The S-100 protein highlighted sustentacular cells, but also showed nonspecific cytoplasmic staining in the remaining cells in all cases (Fig. 3, right lower). SMA was negative in the lesional cells.
The CAs stained positive for CK7 (Fig. 4, right upper), CK5/6, S-100 protein, p63 (Fig. 4, right lower), and SMA. CK7 stained the luminal cells strongly in 100 % of cases. Abluminal cells were strongly positive for CK5/6 (100 % of cases), p63 (100 % of cases), and SMA (90 % of cases). S-100 protein was positive in both luminal and abluminal cells in 80 % of cases.
The CACs displayed two distinct patterns. In the conventional-type, one case was negative for all markers and the other case showed CK7 staining (Fig. 5, right upper), and no staining for CK5/6, SMA, S-100 protein, and p63 (Fig. 5, right lower). In the adenoid cystic carcinoma-type, both cases stained positive for CK7 in the luminal cells (Fig. 6, right upper), and positive for CK5/6, S-100 protein, p63 (Fig. 6, right lower), and SMA in abluminal cells.
Discussion
MEAs are rare, benign tumors that have been called by many different names since they were first described [1, 2]. Most recently, the term NAME has been suggested to demonstrate the nature of the tumor and the site of occurrence [7]. The true nature of these neoplasms has been the topic of several papers [3, 4, 6–12]. Previous immunohistochemical studies have demonstrated that MEAs can have both epithelial and neuroendocrine differentiation [3]. However, in contrast to neuroendocrine tumors at other body sites, MEAs have not been shown to metastasize or be associated with a paraneoplastic syndrome [7].
Histologically, MEAs can have many different patterns, including solid, glandular or trabecular. The associated stromal fibrosis can give the impression of an infiltrative growth pattern, but this should not be considered true invasion unless normal structures are involved. The tumor cells are usually uniform with eosinophilic cytoplasm, and have a round to oval nucleus with delicate, “salt-and-pepper” chromatin. Mucin production can occasionally be demonstrated by PAS stains [2, 4, 8]. The cases in this series showed a similar variety of histologic patterns including glandular, trabecular, and solid, along with bland cytology. Frequently, there was more than one pattern displayed by the tumors, most commonly mixed solid and glandular patterns. In many cases, there was a significant amount of crush artifact making it difficult to classify the histology.
To date, the reported immunohistochemical staining pattern of MEAs includes epithelial markers, including cytokeratin cocktails, CAM5.2 and CK7, although the staining may be patchy and focal [7, 8]. Vimentin is consistently positive [7]. MEAs stain with neuroendocrine markers including chromogranin, synaptophysin, CD56, CD57, and neuron-specific enolase [7]. Selected peptide markers, such as human pancreatic peptide [7], are also reactive. S-100 protein may be positive, with variable staining intensity and distribution [7]. CK7 has been reported to be positive in the luminal cell population previously [7].
Considerable confusion is present in the literature regarding the distinction between myoepithelial and basal cells, as both are present in an abluminal location and there is overlap in the immunohistochemical phenotype of these cells. Immunohistochemical studies of the salivary gland have demonstrated that intercalated ducts contain an abluminal population of cells which is designated myoepithelial, due to the co-expression of epithelial and smooth muscle proteins [13]. Myoepithelial cells show immunohistochemical staining with antibodies for high molecular weight keratins (including CK5/6 and CK14), smooth muscle markers (including smooth muscle actin, smooth muscle myosin, calponin, and caldesmon), and S-100 and p63. In contrast, the striated and excretory ducts of the salivary gland contain an abluminal cell population that is designated basal, as it lacks expression of smooth muscle proteins. Basal cells show immunohistochemical staining with antibodies for high molecular weight keratins (including CK5/6 and CK14) and p63, but are negative for smooth muscle actin, smooth muscle myosin, calponin, caldesmon, and S-100. Therefore, CK5/6 and p63 immunohistochemical staining are seen in both myoepithelial and basal cells.
Myoepithelial cells are found in a variety of other organ systems, including breast, lacrimal gland, and skin adnexal structures. It is unclear whether these sites show a transition from myoepithelial to basal cells in the larger ducts, such as seen in salivary glands. In contrast, basal cells that lack myoepithelial differentiation are uncommon in other tissue sites, with the exception of the prostate. Prostatic basal cells show strong immunohistochemical staining for p63 and high molecular weight keratins, but lack features of smooth muscle differentiation by electron microscopy [14] and are negative for S-100 by immunohistochemistry [15].
In the current study, we find a similar pattern to prior reports, with CK7 staining a luminal population, best seen in cases with a glandular pattern. In addition, we demonstrate a basal cell population staining with CK5/6 and p63, but not with other myoepithelial markers, including S-100 protein and SMA. This is in contrast to prior reports of S-100 staining in MEAs; this may be related to the type of S-100 immunohistochemical stain used (polyclonal vs. monoclonal) or the interpretation of cytoplasmic staining as negative in this study. This immunohistochemical profile would support a degree of both ductal luminal and basal cell differentiation. This contention is further supported by electron microscopy, which highlights two cell types (type A and type B) in MEAs [9]. Type A cells were apical with exocrine activity with mucus granules, while type B cells were basilar in location and contained neurosecretory granules [7].
In our study, the presence of basal but not myoepithelial cells suggests that MEAs are likely not derived from minor salivary gland tissues, which are not normally present in this site. MEAs are postulated to arise from the epithelial lining of the middle ear, but a corresponding neuroendocrine cell precursor has not been definitively identified. It is thought that MEAs could arise from an undifferentiated, pluripotent endodermal stem cell of putative neural crest origin [7, 8, 12, 16, 17].
Most middle ear tumors are easily distinguished from MEA. JPGs demonstrate S-100 protein positive sustentacular cells, and lack an epithelial phenotype, including CK7 and CK5/6 staining [18]. This pattern was confirmed in our study.
For comparison, we included CAs and CACs in this study. Although these tumors do not arise in the middle ear, they may involve the middle ear through direct extension. Furthermore, the corollary can be true: MEAs may involve the external auditory canal by direct extension. CAs are known to contain a well-developed myoepithelial layer which stains immunohistochemically with CK5/6, S-100 protein, and p63 [19]. In this study, the CAs consistently showed luminal staining with CK7, and abluminal staining with CK5/6, p63, S-100 protein and SMA. CACs could be separated both histologically and immunohistochemically into two groups. Two cases of CAC were histologically adenoid cystic carcinoma-type CACs. In both of these tumors, the invasive tumor retained the myoepithelial cell layer, similar to adenoid cystic carcinomas of salivary gland origin. However, the remaining two cases of conventional-type CAC showed loss of a myoepithelial layer in the invasive component [20].
In summary, MEAs are rare benign tumors that can show a range of histologic patterns. This is the first study to evaluate the expression of myoepithelial markers in MEAs. The presence of a dual cell population with luminal epithelial and abluminal basal cells correlates with what has been observed in other studies. However, we also demonstrated that this abluminal population shows ductal-type basal cell differentiation but lacks true myoepithelial differentiation, as seen in CAs.
Contributor Information
Abberly A. Lott Limbach, Phone: +1-216-4440291, FAX: +1-216-4453707, Email: lottlia@ccf.org
Aaron P. Hoschar, Phone: +1-216-445877, FAX: +1-216-4453707, Email: hoschaa@ccf.org
Lester D. R. Thompson, Phone: +1-818-7192613, FAX: +1-818-7192309, Email: Lester.D.Thompson@kp.org
Edward B. Stelow, Phone: +1-434-9824185, FAX: +1-434-9826130, Email: edstelow@yahoo.com
Deborah J. Chute, Phone: +1-216-4440291, FAX: +1-216-4453707, Email: dchute1@gmail.com, Email: chuted@ccf.org
References
- 1.Hyams VJ, Michaels L. Benign adenomatous neoplasm (adenoma) of the middle ear. Clin Otolaryngol Allied Sci. 1976;1:17–26. doi: 10.1111/j.1365-2273.1976.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 2.Derlacki EL, Barney PL. Adenomatous tumors of the middle ear and mastoid. Laryngoscope. 1976;86:1123–1135. doi: 10.1288/00005537-197608000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Berns S, Pearl G. Middle ear adenoma. Arch Pathol Lab Med. 2006;130:1067–1069. doi: 10.5858/2006-130-1067-MEA. [DOI] [PubMed] [Google Scholar]
- 4.Mills SE, Fechner RE. Middle ear adenoma. A cytologically uniform neoplasm displaying a variety of architectural patterns. Am J Surg Pathol. 1984;8:677–685. doi: 10.1097/00000478-198409000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Sahan M, Yildirim N, Arslanoglu A, Karslioglu Y, Kazikdass KC. Carcinoid tumor of the middle ear: report of a case. Am J Otolaryngol. 2008;29:352–356. doi: 10.1016/j.amjoto.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey MJ, Nadol JB, Jr, Pilch BZ, McKenna MJ. Carcinoid tumor of the middle ear: clinical features, recurrences, and metastases. Laryngoscope. 2005;115:1660–1666. doi: 10.1097/01.mlg.0000175069.13685.37. [DOI] [PubMed] [Google Scholar]
- 7.Torske KR, Thompson LD. Adenoma versus carcinoid tumor of the middle ear: a study of 48 cases and review of the literature. Mod Pathol. 2002;15:543–555. doi: 10.1038/modpathol.3880561. [DOI] [PubMed] [Google Scholar]
- 8.Leong K, Haber MM, Divi V, Sataloff RT. Neuroendocrine adenoma of the middle ear (NAME) Ear Nose Throat J. 2009;88:874–879. [PubMed] [Google Scholar]
- 9.Bailey QR, Weiner JM. Middle ear adenoma: a case report with ultrastructural findings. J Laryngol Otol. 1986;100:467–470. doi: 10.1017/S0022215100099497. [DOI] [PubMed] [Google Scholar]
- 10.Devaney KO, Ferlito A, Rinaldo A. Epithelial tumors of the middle ear—are middle ear carcinoids really distinct from middle ear adenomas? Acta Otolaryngol. 2003;123:678–682. doi: 10.1080/00016480310001862. [DOI] [PubMed] [Google Scholar]
- 11.Thompson LD. Neuroendocrine adenoma of the middle ear. Ear Nose Throat J. 2005;84:560–561. [PubMed] [Google Scholar]
- 12.Wassef M, Kanavaros P, Polivka M, Nemeth J, Monteil JP, Frachet B, Tran Ba Huy P. Middle ear adenoma. A tumor displaying mucinous and neuroendocrine differentiation. Am J Surg Pathol. 1989;13:838–847. doi: 10.1097/00000478-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Cheuk W, Chan JK. Advances in salivary gland pathology. Histopathology. 2007;51:1–20. doi: 10.1111/j.1365-2559.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 14.El-Alfy M, Pelletier G, Hermo LS, Labrie F. Unique features of the basal cells of human prostate epithelium. Microsc Res Tech. 2000;51:436–446. doi: 10.1002/1097-0029(20001201)51:5<436::AID-JEMT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Constantinides C, Manousakas TH, Pavlaki K, Zizi D, Kyriakou G, Alamanis CH, Dimopoulos C. The distribution of S-100 protein in hyperplastic and neoplastic prostatic epithelium. Int Urol Nephrol. 2000;32:259–261. doi: 10.1023/A:1007118210759. [DOI] [PubMed] [Google Scholar]
- 16.McNutt MA, Bolen JW. Adenomatous tumor of the middle ear. An ultrastructural and immunocytochemical study. Am J Clin Pathol. 1985;84:541–547. doi: 10.1093/ajcp/84.4.541. [DOI] [PubMed] [Google Scholar]
- 17.Amble FR, Harner SG, Weiland LH, McDonald TJ, Facer GW. Middle ear adenoma and adenocarcinoma. Otolaryngol Head Neck Surg. 1993;109:871–876. doi: 10.1177/019459989310900516. [DOI] [PubMed] [Google Scholar]
- 18.Wieneke JA, Smith A. Paraganglioma: carotid body tumor. Head Neck Pathol. 2009;3:303–306. doi: 10.1007/s12105-009-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson LD, Nelson BL, Barnes EL. Ceruminous adenomas: a clinicopathologic study of 41 cases with a review of the literature. Am J Surg Pathol. 2004;28:308–318. doi: 10.1097/00000478-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Crain N, Nelson BL, Barnes EL, Thompson LD. Ceruminous gland carcinomas: a clinicopathologic and immunophenotypic study of 17 cases. Head Neck Pathol. 2009;3:1–17. doi: 10.1007/s12105-008-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]