Abstract
Hyalinizing clear cell carcinoma (HCCC) is a rare, low-grade salivary gland tumor with clear cells and hyalinized stroma. Prognosis of HCCC is excellent with few cases metastasizing to the lymph nodes and lung. We present a case of a 61-year-old male with recurrent HCCC on the base of tongue. Histologic examination revealed sheets of clear and eosinophilic cells with a background of a myxoid-like matrix. In addition, large, bizarre malignant cells, focal necrosis, and atypical mitotic figures were identified. By immunohistochemistry, the clear cells were positive for CK18, EMA and vimentin, focally positive for CK7 and CD10, but negative for p63, HMWK, SMA and calponin. A metastatic renal cell carcinoma was considered a possibility but the tumor was called “poorly-differentiated carcinoma, NOS”. The patient underwent primary radiotherapy. A recurrence was identified at 10 months follow-up. A biopsy of the recurrent tumor showed clear cell differentiation and a predominant cribriform pattern with focal cords of eosinophilic cells invading the stroma. In contrast to the original tumor, no mitotic figures, atypia or necrosis were identified. The combination of lower grade and different architectural patterns appeared markedly different than the previous biopsy and the immunohistochemical pattern was also different. The recurrent tumor showed diffuse positivity for p63 and HMWK. It was negative for CD10, vimentin, SMA and calponin. Fluorescence in situ hybridization (FISH) analysis was positive for rearrangement of the EWSR1 gene in both samples, confirming that this represented a recurrence of the same tumor. It also confirmed that the initial tumor was a HCCC with high-grade transformation.
Keywords: Hyalinizing clear cell carcinoma, Dedifferentiation, High-grade transformation, EWSR1, Salivary
Introduction
Hyalinizing clear cell carcinoma (HCCC) was first described by Milchgrub et al. [1]. It is a rare, low-grade salivary gland tumor with clear cell morphology typically showing a distinctive hyalinized stroma [1]. The hyalinized stroma often mimics a cribriform pattern within the tumor and surrounds the clear cell nests and cords. HCCC most frequently arises in minor salivary and seromucous gland locations in middle-aged females [1]. The most common sites of occurrence are palate, buccal mucosa, tongue, and the floor of the mouth. Rare cases have also arisen in the sinonasal tract/nasopharynx and larynx [1, 2]. Prognosis of HCCC is excellent with a minority showing recurrence and only a few cases metastasizing to lymph nodes [1]. Metastases and mortality are distinctly unusual [1–7].
Tumor cells are characteristically positive for cytokeratins and epithelial membrane antigen (EMA), but negative for S100, smooth muscle actin (SMA), carcinoembryonic antigen (CEA) and vimentin [1, 8, 9]. The combination of positivity for squamous markers (p63, HMWK) and presence of tonofilaments and desmosomes ultrastructurally has led to the conclusion that HCCC shows squamous differentiation [2, 10]. It has also been noted to show occasional gland formation and mucinous differentiation [2]. Although the combination of histology and immunohistochemical staining can distinguish HCCC from the majority of other clear cell tumors of the salivary gland, such as epithelial-myoepithelial carcinoma (EMC), mucoepidermoid carcinoma (MEC) and myoepithelial carcinoma, establishing the correct diagnosis can be challenging in difficult cases or small biopsies. Our previous study indicated that HCCC has a consistent EWSR1 rearrangement by FISH as a result of a EWSR1-ATF1 fusion oncogene [2]. This consistent finding in HCCC can be used to separate it from its potential mimics, as none of the most common clear cell tumors showed either EWSR1 or ATF1 rearrangement by FISH [2].
In this report, we describe a rare case of recurrent HCCC of the base of tongue that was not initially recognized on initial presentation owing to its high-grade morphology. Interestingly, the more typical features of HCCC were seen in the recurrence, which was positive for rearrangement of the EWSR1 gene by FISH. Retrospective FISH on the original case showed a similar EWSR1 rearrangement, thus confirming that this second lesion represented a recurrence of the same tumor. In the original tumor, the presence of high-grade features (anaplasia, mitotic figures and necrosis) coupled with loss of the typical immunohistochemical profile in the high-grade areas, warrants the designation “HCCC with high-grade transformation (HGT) (dedifferentiation)”.
Materials and Methods
The surgical specimens were fixed in 10% neutral buffered formalin and processed routinely. Hematoxylin and eosin stains were performed on 3–4 μ thick sections of formalin-fixed paraffin-embedded tissue. Immunohistochemical stains were performed in sections from paraffin blocks, using the ultrastreptavidin-HRP detection system (ID Labs Biotechnology, London, Ontario, Canada). Color development was performed using the NovaRed substrate kit (Vector Labs, Burlingame, California, USA). All the immunohistochemical studies were performed in an automated Ventana BenchMark® instrument (Ventana Systems, Tucson AZ). Immunohistochemistry was performed for the following antibodies: CK7 (Dako, 1/2000), CK18 (Novacastra, 1/20), CK14 (Vector, 1/100), HMWK (34BE12) (Ventana, prediluted), p63 (Vector, 1/50), EMA (Ventana, prediluted), S100 (Ventana, prediluted), CD10 (Ventana, prediluted), SMA (Ventana, prediluted), calponin (Dako, 1/100), and vimentin (American Research Products, 1/100).
Interphase Fluorescence in situ hybridization (FISH) analysis was performed at the University Health Network, from paraffin blocks. FISH for the rearrangement of the EWSR1 gene was performed using dual color break apart probes (Vysis®, Downer’s Grove, IL) for the 5′ region of the EWSR1 gene (spectrum green telomeric to gene) and the corresponding 3′ region of the gene (spectrum orange centromeric to gene). A total of 200 lesional cells were examined and a positive result was scored when ≥10% of cells showed a break-apart signal.
Results
Clinical Findings
A 61-year-old male had a mass on the left side of the base of tongue. Physical examination and staging imaging confirmed a left tongue base primary measuring 3.0 cm in diameter. An MRI scan confirmed a large exophytic mass predominantly involving the left tongue base with extension to the right tongue base and extending onto the lateral pharyngeal wall and pre-epiglottic space. The patient had a palpable ipsilateral level II neck mass, which on MRI scan imaging measured 2.0 cm in size, as well as small lymph nodes in the contralateral neck. A chest CT scan at the time of presentation was negative for metastases. He was staged as a T4N2c poorly-differentiated carcinoma of the tongue base and was treated with primary radiation. During the early course of treatment, the pathology review suggested a poorly-differentiated carcinoma with clear cell differentiation, thereby raising the possibility of a malignancy of salivary gland etiology. At this point, the patient had received a dosage of 48 Gy of radiation treatment. Discussions were held with the patient regarding primary surgical resection, i.e. a near-total glossectomy and total laryngectomy. However, based on the morbidity of treatment, the patient deferred surgical treatment and completed a full course of radiation treatment to 64 Gy in 40 fractions. Approximately 10 months after completion of radiation therapy, a mass recurred at the primary site and the patient underwent a biopsy confirming recurrence. On re-staging work-up, the patient was confirmed to have local disease and chest CT imaging confirmed the development of pulmonary metastases. The patient is currently being managed symptomatically.
Histological and Immunohistochemical Findings
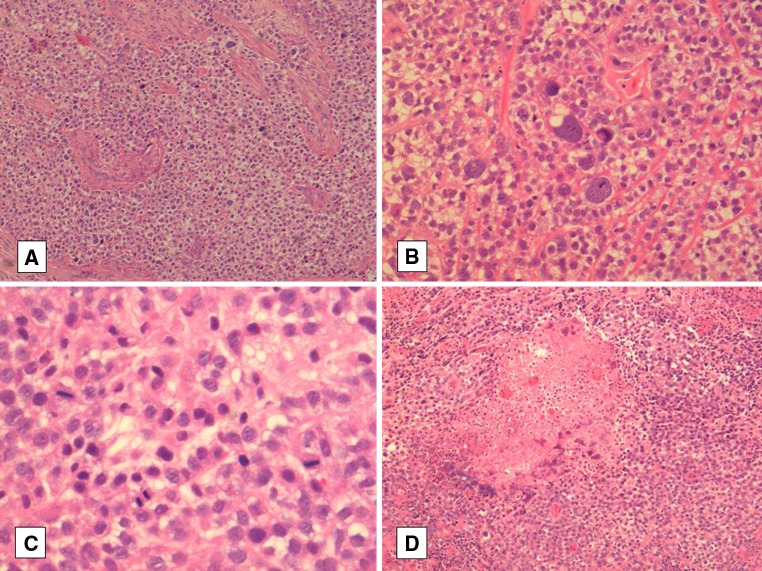
Microscopically, the biopsy of the original tumor showed sheets of clear and eosinophilic cells with a background of a myxoid-like matrix and focal neutrophilic infiltrates. The nuclei had a stippled chromatin pattern and were largely uniform but there were also bizarre pleomorphic cells with irregularly-shaped nuclei throughout the tumor. In addition, focal necrosis was present and mitotic figures were easily identified, including atypical forms (Fig. 1). There was no overlying dysplasia but the tumor appeared to focally connect to the surface. Immunohistochemical staining demonstrated that the tumor cells were positive for CK18, EMA and vimentin and focally positive for CK7 and CD10. They were completely negative for p63, HMWK (34BE12), SMA and calponin (Table 1). The overlying mucosal epithelium was strongly positive for p63 and HMWK, as expected. The tumor was considered a poorly-differentiated carcinoma with clear cell differentiation. The differential diagnoses considered included a clear cell carcinoma, poorly-differentiated squamous cell carcinoma (despite lack of squamous differentiation) and a metastatic renal cell carcinoma. No clinical evidence of a renal mass was identified, however.
Fig. 1.
a The high-grade component showing sheets of neoplastic cells with prominent clear cytoplasm and notable nuclear pleomorphism. b High-power view of large bizarre tumor cells. c High-power view of mitotic figures in the high-grade component. d An area of clear cell carcinoma with focal necrosis
Table 1.
Comparison of immunostaining between the original and recurrent tumour biopsy
| Staining | Original | Recurrrent |
|---|---|---|
| CK7 | + | ND |
| CK14 | ND | − |
| CK18 | ++ | + |
| HMWK | − | ++ |
| P63 | − | ++ |
| EMA | ++ | ND |
| Vimentin | ++ | − |
| CD10 | + | − |
| SMA | − | − |
| Calponin | − | − |
−, Negative; +, focally or weakly positive; ++, strongly or diffusely positive; ND not done
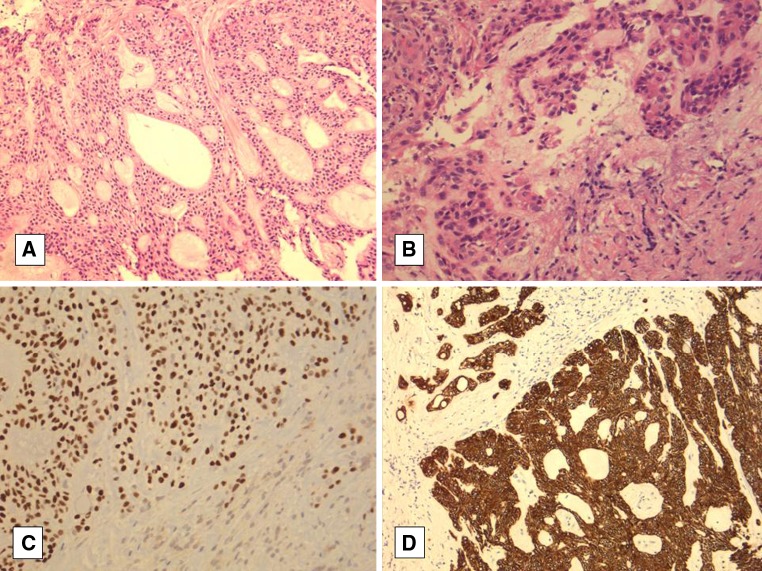
The biopsy of the recurrent tumor showed an epithelial proliferation with a predominant cribriform pattern. There was no overlying dysplasia or connection to the surface epithelium. There were also infiltrative tumor cells arranged in small nests and cords in the fibrous stroma surrounding these cribriform nests. Cells with eosinophilic cytoplasm were also identified. Focal squamous-like differentiation was noted. However, there was no evidence of nuclear atypia, mitotic figures or necrosis identified (Fig. 2). Isolated mucinous cells were seen within the tumor on careful inspection. The recurrent tumor sample stained strongly and diffusely positive for p63 and HMWK. It was focally or weakly positive for CK18, but was negative for SMA, calponin, CK14, vimentin, and CD10 (Table 1).
Fig. 2.
a Recurrent hyalinizing clear cell carcinoma with prominent cribriform pattern. b Recurrent hyalinizing clear cell carcinoma with eosinophilic cords of cells. c, d Diffuse positivity was seen with p63 (nuclear) and HMWK (cytoplasmic), respectively
Fluorescence in Situ Hybridization Findings (FISH)
Both the primary tumor (retrospectively tested) and the recurrence were examined by FISH for the EWSR1 rearrangement and were positive (Fig. 3), confirming the diagnosis of HCCC including the high-grade areas.
Fig. 3.
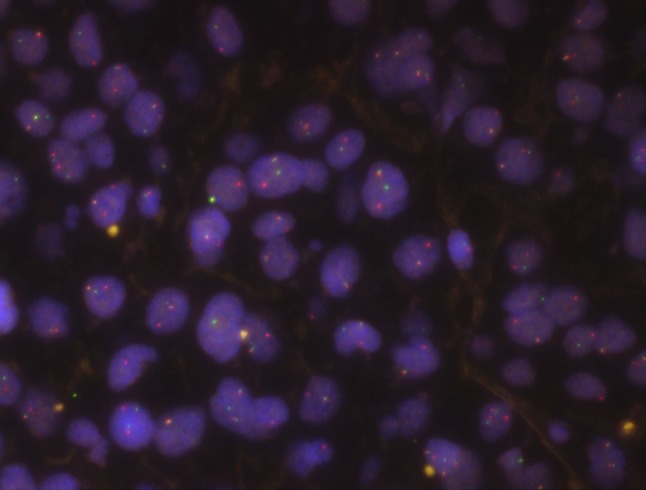
Fluorescence in situ hybridization analysis in both tumors revealed a break-apart signal (the primary tumor shown here). Each cell shows one fused normal signal (yellow) and one break apart showing separate spectrum green and spectrum orange signals, indicating rearrangement of the EWSR1 gene
Discussion
Hyalinizing clear cell carcinoma is a rare neoplasm with a predilection for intraoral sites in middle-aged females. It was first described by Milchgrub et al. [1] and is characterised by bland infiltrating clear cells forming nests and cords within a hyalinizing or myxoid matrix. The tumors most often involve the tongue and palate, but other sites, such as the buccal mucosa, the floor of mouth, parotid gland, nasal cavity and larynx have also been reported [1, 2, 4–6]. Clinically, local recurrence or distant metastases can occur despite its indolent character, albeit rarely [3–6]. Histologically, HCCC is a low-grade carcinoma, but an aggressive case with focal necrosis, atypical large cells, and mitotic figures was reported by O’Regan [7].
In this case report, the original biopsy showed a high-grade clear cell carcinoma with focal necrosis, large bizarre tumor cells, and mitotic figures. The high-grade histopathologic appearance of the tumor was verified by the clinical course of the patient with local recurrence and distant metastases. Although the later biopsy showed much blander characteristics with only mild nuclear atypia, absent necrosis and no mitotic figures, the clear cell differentiation and location linked these two tumors. Upon retrospective review, the original tumor also had a low-grade component similar to the later biopsy but this was not appreciated given the overall high-grade appearance of the tumor. In addition to varying architecture and grade, the immunostaining of the two biopsies was completely different with HMWK and p63 positivity only in the recurrence. The initial biopsy, which was negative for p63 and HMWK, showed strong positivity of the mucosal epithelium as an internal control, demonstrating that this immunonegativity was not due to fixation or technical issues. The histologic features and immunoprofile of the recurrence raised the possibility of a HCCC and subsequent FISH was positive for rearrangement of the EWSR1 gene, thus confirming the diagnosis. EWSR1 rearrangement is not a feature of other clear cell tumors of salivary gland, such as EMC, clear cell myoepithelial carcinoma, MEC and clear cell squamous carcinoma [2]. In our recent experience since our initial study, we have also found that polymorphous low-grade adenocarcinoma (PLGA) is negative for EWSR1 rearrangement (unpublished observations).
The persistent EWSR1 rearrangement in both biopsies clearly linked these two tumors together despite their somewhat disparate features. There are several possibilities that could explain these differences. First, it could be that the tumor was always made up of a combination of the typical low-grade HCCC as well as the high-grade areas and that this was not recognized due to tumor sampling issues. Similarly, it could represent residual low-grade HCCC left behind after the successful radiation response of only the most high-grade proliferative areas of the neoplasm. Finally, it could have represented a hybrid salivary gland carcinoma, which consisted of two histologically distinct types of carcinoma within the same area. However, this latter possibility is discounted owing to the shared genetics of the two tumor biopsies.
The possibility of a carcinoma arising in a mixed tumor was considered a possible explanation for the dual morphologies and grade; however, the presence of EWSR1 rearrangement in both elements appears to exclude that possibility. A metastasis from renal clear cell carcinoma was also initially considered, particularly in light of the CD10, vimentin and CK18 staining without CK7 or squamous marker positivity. However, the clear cut squamous marker expression (p63 and HWMK) in the recurrence, the FISH findings and the lack of a kidney mass seem to exclude this explanation as well.
The features all appear to point to a tumor with HGT, also known as “dedifferentiation”. This conclusion does not rest on the presence of high-grade areas within the tumor alone, but more importantly, the apparent loss of the usual morphology and typical immunohistochemical features of HCCC in the higher grade regions, making the entity initially unrecognizable in these areas. The term HGT is preferred in this case since the tumor was recognizably of epithelial and, specifically, salivary gland origin, rather than showing true dedifferentiation, which arguably requires loss of a clear cut line of differentiation. HGT in a salivary carcinoma has previously been described mainly in adenoid cystic carcinoma [11], but has also been seen in EMC [12], MEC [13], acinic cell carcinoma [14] and PLGA [15]. Like most salivary carcinomas with HGT, this tumor appeared to have more aggressive behavior with early recurrence and radiologic evidence of metastatic disease. It is not clear at this time what the long-term prognosis will be for this variant of HCCC.
In summary, we report a rare case of HCCC from the base of tongue, which showed HGT/dedifferentiation. The presence of only the high-grade areas in the initial biopsy led to a less specific diagnosis, as the true nature of the tumor was not recognized. The tumor recurrence, which showed low-grade classic HCCC features, and the subsequent molecular findings allowed for a refining of the diagnosis. HCCC with HGT should, therefore, be considered in the differential diagnosis of poorly-differentiated carcinomas of the oral cavity showing clear cell differentiation.
References
- 1.Milchgrub S, Gnepp DR, Vuitch F, Delgado R, Albores-Saavedra J. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994;18(1):74–82. doi: 10.1097/00000478-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, Perez-Ordoñez B, Have C, Asa SL, Leong IT, Bradley G, Klieb H, Weinreb I. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 3.Tang SK, Wan SK, Chan JK. Hyalinizing clear cell carcinoma of salivary gland: report of a case with multiple recurrences over 12 years. Am J Surg Pathol. 1995;19(2):240–241. doi: 10.1097/00000478-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Solar AA, Schmidt BL, Jordan RC. Hyalinizing clear cell carcinoma: case series and comprehensive review of the literature. Cancer. 2009;115(1):75–83. doi: 10.1002/cncr.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Sullivan-Mejia ED, Massey HD, Faquin WC, Powers CN. Hyalinizing clear cell carcinoma: report of eight cases and a review of literature. Head Neck Pathol. 2009;3(3):179–185. doi: 10.1007/s12105-009-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang B, Brandwein M, Gordon R, Robinson R, Urken M, Zarbo RJ. Primary salivary clear cell tumors-a diagnostic approach: a clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial-myoepithelial carcinoma. Arch Pathol Lab Med. 2002;126(6):676–685. doi: 10.5858/2002-126-0676-PSCCTA. [DOI] [PubMed] [Google Scholar]
- 7.O’Regan E, Shandilya M, Gnepp DR, Timon C, Toner M. Hyalinizing clear cell carcinoma of salivary gland: an aggressive variant. Oral Oncol. 2004;40(3):348–352. doi: 10.1016/j.oraloncology.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Angiero F, Stefani M. Hyalinizing clear cell carcinoma arising on the anterior palatoglossal arch. Anticancer Res. 2007;27(6):4271–4277. [PubMed] [Google Scholar]
- 9.Lai G, Nemolato S, Lecca S, Parodo G, Medda C, Faa G. The role of immunohistochemistry in the diagnosis of hyalinizing clear cell carcinoma of the minor salivary gland: a case report. Eur J Histochem. 2008;52(4):251–254. doi: 10.4081/1224. [DOI] [PubMed] [Google Scholar]
- 10.Dardick I, Leong I. Clear cell carcinoma: review of its histomorphogenesis and classification as a squamous cell lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):399–405. doi: 10.1016/j.tripleo.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Seethala RR, Hunt JL, Baloch ZW, Livolsi VA, Leon Barnes E. Adenoid cystic carcinoma with high-grade transformation: a report of 11 cases and a review of the literature. Am J Surg Pathol. 2007;31(11):1683–1694. doi: 10.1097/PAS.0b013e3180dc928c. [DOI] [PubMed] [Google Scholar]
- 12.Roy P, Bullock MJ, Perez-Ordoñez B, Dardick I, Weinreb I. Epithelial-myoepithelial carcinoma with high grade transformation. Am J Surg Pathol. 2010;34(9):1258–1265. doi: 10.1097/PAS.0b013e3181e366d2. [DOI] [PubMed] [Google Scholar]
- 13.Nagao T, Gaffey TA, Kay PA, Unni KK, Nascimento AG, Sebo TJ, Serizawa H, Minato H, Lewis JE. Dedifferentiation in low-grade mucoepidermoid carcinoma of the parotid gland. Hum Pathol. 2003;34(10):1068–1072. doi: 10.1053/S0046-8177(03)00418-0. [DOI] [PubMed] [Google Scholar]
- 14.Skálová A, Sima R, Vanecek T, Muller S, Korabecna M, Nemcova J, Elmberger G, Leivo I, Passador-Santos F, Walter J, Rousarova M, Jedlickova K, Curik R, Geierova M, Michal M. Acinic cell carcinoma with high-grade transformation: a report of 9 cases with immunohistochemical study and analysis of TP53 and HER-2/neu genes. Am J Surg Pathol. 2009;33(8):1137–1145. doi: 10.1097/PAS.0b013e3181a38e1c. [DOI] [PubMed] [Google Scholar]
- 15.Simpson RH, Pereira EM, Ribeiro AC, Abdulkadir A, Reis-Filho JS. Polymorphous low-grade adenocarcinoma of the salivary glands with transformation to high-grade carcinoma. Histopathology. 2002;41(3):250–259. doi: 10.1046/j.1365-2559.2002.01439.x. [DOI] [PubMed] [Google Scholar]