Abstract
A 16-year-old previously asymptomatic boy presented with complaints of fatigue, weight loss, and back pain for several months. Imaging studies revealed a large superior mediastinal mass, numerous bilateral pulmonary nodules, and multiple lytic bone lesions. A needle biopsy from a sternal lesion showed a poorly differentiated carcinoma, immunoreactive for cytokeratins and EMA and immunonegative for various organ/tissue-specific markers. His past medical history was significant for excision of a parotid gland tumor 5 years earlier. Histologic review of the salivary gland tumor revealed a pleomorphic adenoma containing a microscopic focus of invasive carcinoma (carcinoma ex pleomorphic adenoma). By immunohistochemistry, both the salivary gland tumor and the disseminated carcinoma expressed PLAG1 with a strong nuclear pattern. Fluorescence in situ hybridization (FISH), using dual-color, break-apart probes for PLAG1, showed rearrangement of the gene in both the salivary gland and the disseminated tumors. FISH demonstrated additional cytogenetic aberrations in the carcinoma, including polysomy for chromosome 8 (in both the primary salivary gland and the metastatic tumors) and PLAG1 amplification (in the metastatic tumor). We conclude that in the proper clinicopathologic setting, application of PLAG1 immunohistochemistry and FISH for PLAG1 gene rearrangement may be valuable in establishing the diagnosis of carcinoma ex pleomorphic adenoma as the source of a cancer of unknown primary site.
Keywords: Carcinoma ex pleomorphic adenoma, Carcinoma of unknown primary, PLAG1, Salivary gland
Introduction
Pleomorphic adenoma, or “mixed tumor”, accounts for nearly two-thirds of all salivary gland neoplasms. Although considered to be a benign tumor, pleomorphic adenoma has a high potential for local recurrence and a nearly 6% risk of malignant transformation [1, 2]. Carcinoma ex pleomorphic adenoma (CA ex PA) is the term applied to describe an epithelial cancer arising in a pleomorphic adenoma. Although much less common, a malignancy can also develop in the mesenchymal, or both the epithelial and mesenchymal, components of pleomorphic adenoma (sarcoma or carcinosarcoma ex PA, respectively) [1]. CA ex PA accounts for less than 4% of all salivary gland neoplasms and less than 12% of malignant salivary gland tumors [2]. It is often a high-grade and clinically aggressive tumor, commonly resulting in regional lymph node and/or distant metastasis [3].
Despite extensive investigation, the primary site of approximately 4–5% of disseminated malignant tumors remains unknown [4]. How often CA ex PA may be the source of carcinoma of unknown origin is unclear. While we found only a rare report of this clinical scenario in the literature [5], it is not inconceivable that a small deep-seated CA ex PA may rapidly disseminate before manifesting at the primary site. We report the case of an adolescent presenting with widespread carcinoma, a case in which the finding of PLAG1 overexpression helped to establish the diagnosis of CA ex PA in the setting of metastatic disease of unknown origin, highlighting the potential value of this marker in similar settings.
Case Report
Clinical History
A 16 year-old African-American boy presented to St. Jude Children’s Research Hospital (SJCRH) with complaints of fatigue, weight loss, low back pain, bilateral knee swelling and an enlarging sternal mass over 6 months. A CT scan revealed multiple lesions, including a 4-cm destructive mass involving the second and third sternal segments, a bulky mass in the left upper anterior thoracic cavity (8.8 cm maximum diameter), and numerous bilateral pulmonary nodules, of which some appeared to be partly calcified (Fig. 1c–e).
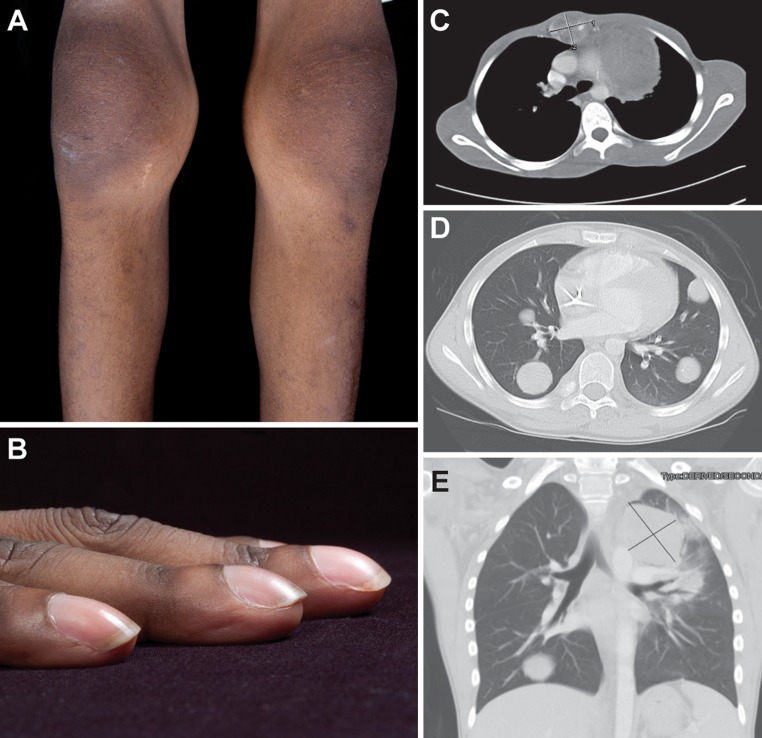
Fig. 1.
a Bilateral knee joint swelling without inflammation, clinically consistent with hypertrophic osteoarthropathy; b severe digital clubbing; c–e computed tomography scan of the chest showing: c a destructive mass, 4.1 cm × 3.2 cm, involving the second and third sternal segments; d numerous pulmonary nodules involving all lobes of both lungs, ranging from 0.2 to 3 cm; e a large mass in the left upper mediastinum (8.8 cm × 6.4 cm), abutting the aortic arch, the left lateral aspect of the main pulmonary outflow tract, and the left pulmonary artery
The patient had been healthy until 6 months prior to this presentation. His past medical history was remarkable for a right neck mass excised 5 years earlier, with no evidence of local recurrence. The pathologic diagnosis at that time was pleomorphic adenoma with negative margins. On physical examination, he was a cachectic, frail young man whose activity was limited by severe pain, and his gait was unsteady. The most notable findings were a 4-cm firm tender mass anterior to his sternum, a 5-cm surgical scar at the site of his previous neck surgery, marked clubbing of all digits, and bilateral swelling without signs of inflammation in his wrists, ankles, and knees (Fig. 1a, b). No cervical mass and no lymphadenopathy were apparent. Further diagnostic imaging studies with technetium-99 bone scan and FDG-PET/CT revealed multiple bony lesions in his vertebrae, ribs and long bones, consistent with metastatic disease. Laboratory studies revealed anemia of chronic illness, with a low serum iron, and an elevated ferritin level. The patient was admitted and subsequently underwent a CT-guided biopsy of his sternal mass.
Materials and Methods
Immunohistochemistry and Cytogenetic Studies
Sections of formalin-fixed paraffin-embedded (FFPE) tissue (4 μm) were processed for immunohistochemistry using heat- and/or enzyme-induced epitope retrieval with the Leica Refine Polymer™, Ventana iVIEW™ or Ventana ultraView™ detection systems. Antibodies to the following antigens were used: cytokeratins (clone AE1/AE3, DAKO), cytokeratins (clone Cam5.2, BD), CK7 (clone OV-TL/12/30, DAKO), CK20 (clone Ks20.8, DAKO), CK5/6 (clone D5&16B4, Ventana), p63 (clone 4A4, Ventana), EMA (clone Mc5, Ventana), CD5 (clone 4C7, Leica Microsystems), CEA (11-7, DAKO), PLAP (clone 8A9, DAKO), OCT3/4 (clone C-10, Leica Microsystems), TTF1 (clone SPT24, Leica Microsystems), HepPar1 (OCHIE5, RTU, Ventana), inhibin (R1, DAKO), chromogranin A (LK2H10(3), Leica Microsystems), synaptophysin (27G12, Leica Microsystems), CD99 (013, Signet), muscle specific actin (HHF35, ENZO, RTU), desmin (clone D33, DAKO), S100 (DAKO), monoclonal anti-PLAG1 (clone 3B7, Novus, 1:50, 2-h incubation) and anti-IGF-II (polyclonal, Sigma, 1:100, 15-min incubation). Immunohistochemistry for NUT expression was performed at Brigham and Women’s Hospital.
A dual-color, break-apart FISH assay for PLAG1 (pleomorphic adenoma gene 1) was developed using bacterial artificial chromosome (BAC) probes to include the RP11-22E14 clone, flanking the 3′ end, and the RP11-1130K23 clone, flanking the 5′ end of the PLAG1 gene. BAC DNA was isolated using a modified Qiagen plasmid extraction protocol. The RP11-22E14 sequence was labeled with AlexaFluor 488 (green fluorochrome), and the RP11-1130K23 sequence with Rhodamine (red fluorochrome). Dual-color FISH was performed on FFPE tissue sections (4 μm), as previously described [6]. A normal control produced two overlapping green and red (yellow) signals on chromosome 8 in each nucleus. A centromeric probe for chromosome 8 (Vysis CEP8, orange fluorochrome) was included to establish chromosome 8 copy number. In addition, a FISH probe for CTNNB1, a common partner gene with PLAG1, was developed using the CTD2544D3 clone.
RNA was extracted from fresh tissue from the sternal mass biopsy for RT-PCR assays for the EWS-FLI1, EWS-ERG, PAX3-FKHR, PAX7-FKHR, and EWS-WT1 fusion transcripts, as previously described [7].
Results
Pathologic Findings
The needle biopsy of the sternal mass contained a malignant neoplasm, with medium to large cells and a high mitotic count, arranged in nests or solid sheets among necrotic areas (Fig. 3a, b). The neoplastic cells had round, slightly pleomorphic nuclei, with a moderate amount of pale cytoplasm (Fig. 3c), showing no obvious tubular, glandular, or squamous differentiation. The tumor cells were immunopositive for cytokeratins AE1/AE3, Cam5.2, CK7 (Fig. 3d), and EMA and were negative for CK20, CK5/6, p63, CD5, PLAP, OCT3/4, TTF1, HepPar1, CEA, inhibin, chromogranin A, synaptophysin, CD99, actin, desmin, S100, and NUT. Tumor cells showed intense immunoreactivity for PLAG1 (Fig. 3e) and IGF-II. By RT-PCR, the tumor was negative for common fusion transcripts in Ewing sarcoma, desmoplastic small round cell tumor, and alveolar rhabdomyosarcoma, respectively.
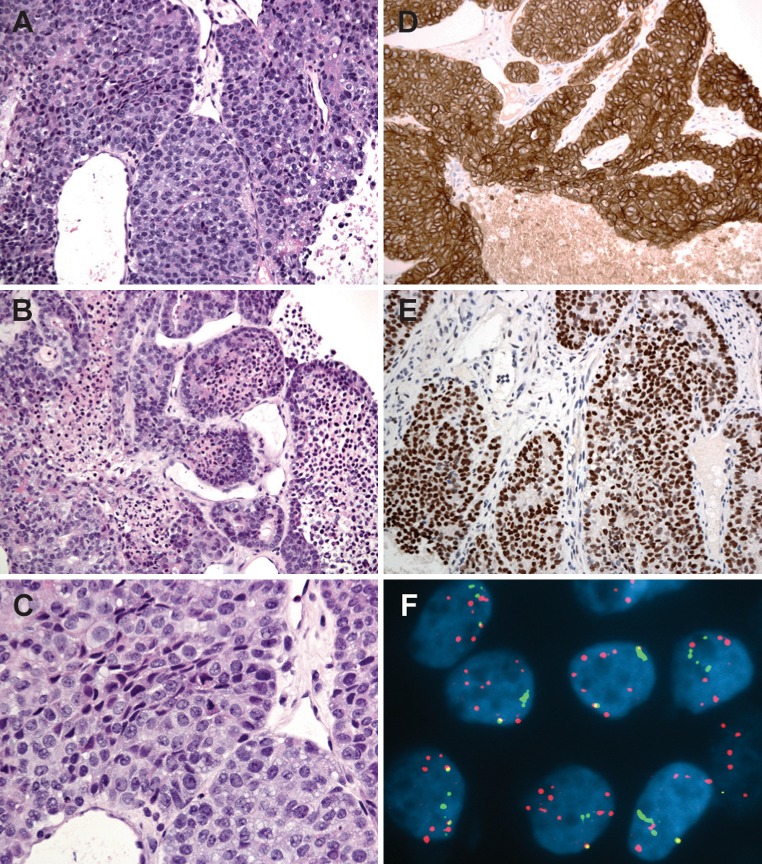
Fig. 3.
a–c Photomicrographs of a needle biopsy from the sternal lesion showing a poorly differentiated carcinoma composed of cohesive malignant epithelioid cells arranged in solid sheets with necrotic areas; d Tumor is diffusely positive for cytokeratin 7; e Strong nuclear staining for PLAG1 in the poorly-differentiated carcinoma; f FISH for PLAG1 shows multiple copies of split green and red signals and a homogenously staining region, indicating PLAG1 rearrangement and amplification in carcinoma cells
The pathology of the previously excised parotid gland tumor was reviewed at SJCRH. According to the report, this was a 4.5-cm well circumscribed and encapsulated mass arising in the parotid gland. Microscopically, the majority of the tumor had features of a typical pleomorphic adenoma with a heterogeneous growth pattern and variable cellularity, ranging from hypocellular areas with a predominance of myxoid and chondromyxoid matrix (Fig. 2a) to highly cellular zones composed of solid sheets of epithelial and plasmacytoid myoepithelial cells with little intervening stroma. The tumor appeared to be confined within a fibrous capsule except in one focus where clusters of irregular epithelial cords and tubules with enlarged hyperchromatic nuclei extended outside the tumor capsule within a desmoplastic stroma (Fig. 2d). The tumor extended 4 mm beyond the capsule, a finding diagnostic for a microscopic focus of invasive moderately differentiated adenocarcinoma, arising in a pleomorphic adenoma (CA ex PA). The surgical margins were negative for tumor. By immunohistochemistry, neoplastic cells in the extracapsular component were positive for cytokeratins (AE1/AE3, Cam5.2, and CK7) and EMA. In the benign component, only few cells labeled for MIB-1, whereas in the small extracapsular malignant component, the MIB-1 proliferative index was up to 20%. PLAG1 immunoreactivity was evident in both intracapsular pleomorphic adenoma cells (Fig. 2b) and extracapsular malignant cells (Fig. 2e), but not in the surrounding salivary gland. Both pleomorphic adenoma and CA ex PA expressed IGF-II with a cytoplasmic pattern (with weak and strong intensity, respectively).
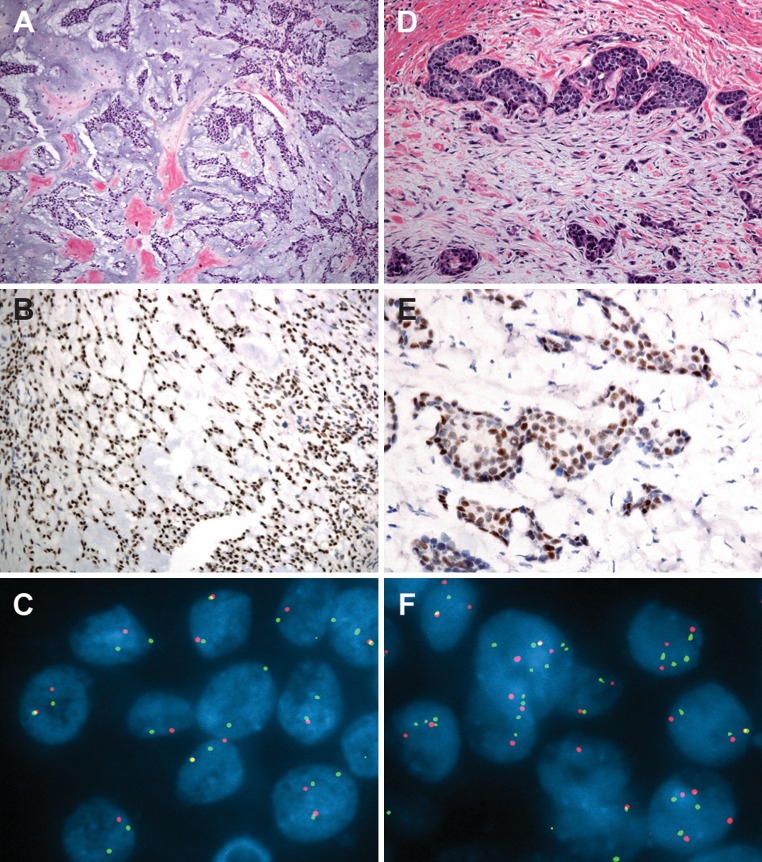
Fig. 2.
a Photomicrographs of the right parotid gland tumor showing a typical pleomorphic adenoma composed of cords and nests of epithelial and myoepithelial cells within an abundant chondromyxoid to mucoid stroma; b Immunohistochemistry for PLAG1 showing strong nuclear immunoreactivity in pleomorphic adenoma cells; c FISH for PLAG1 with a break-apart probe set shows one pair of split signals (distinct red and green signals) indicating rearrangement and one pair of overlapping signals (yellow signal) indicating an intact gene locus in most tumor cells ; d Microscopic focus of carcinoma ex pleomorphic adenoma (CA ex PA) composed of poorly formed tubular structures and clusters of hyperchromatic cells in a desmoplastic stroma; e PLAG1 immunostaining is strongly positive in CA ex PA cells. f FISH for PLAG1 in the carcinomatous component shows multiple copies of split red and green signals, consistent with polysomy with gene rearrangement
FlSH demonstrated rearrangement of PLAG1 in 62% of cells in the pleomorphic adenoma (Fig. 2c). In the focus of carcinoma, in addition to gene alteration, 78% of the cells showed a profile of polysomy, with 3–4 copies of split red and green signals (rearrangement in the setting of polysomy; Fig. 2f). Analysis of the sternal mass showed PLAG1 rearrangement with additional chromosomal complexity. The majority of neoplastic cells (89%) had 6–8 copies of split green and red signals. Nearly 50% of cells showed a broad green signal, consistent with a homogenously staining region and PLAG1 amplification (Fig. 3f). Using a probe targeting the centromeric region of chromosome 8, polysomy with 3–5 signals was evident in 80% of cells. FISH targeting the CTNNB1 locus was negative for gene rearrangement.
Discussion
The case presented in this report is unique for several reasons. Patients with CA ex PA are typically elderly adults (mean age of 61 years) [3]. While 16% of parotid masses in children are malignant, most are mucoepidermoid or acinic cell carcinoma [8, 9]. To our knowledge, CA ex PA in a child has never been documented. Risk factors associated with malignant change in pleomorphic adenoma, such as prolonged history, large tumor size, and extensive hyalinization were not features of the tumor in our patient [9]. CA ex PA, when arising in a pleomorphic adenoma, is typically the dominant component of the mass. By contrast, in our patient, there was only a microscopic focus of invasive carcinoma [3]. Another unusual aspect of the case is the development of widespread distant metastasis in the absence of a history of local recurrence—an event seen in 23% of CA ex PA after complete removal [3]. In addition, malignancy-associated hypertrophic osteoarthropathy (a paraneoplastic syndrome characterized by digital clubbing, polyarthritis, and periosteal new bone formation) developed in our patient and has only rarely been described in children [10].
The most significant prognostic indicator in CA ex PA is the degree of invasion [3, 9, 11, 12]. According to most series, when penetration beyond the capsule is <5 mm, a favorable outcome should be anticipated (as long as the lesion is excised with clear margins) [3, 11]. In contrast, a few reports of metastasis or death in patients with minimally invasive CA ex PA (<1.5 mm invasion outside the capsule) [2], or non-invasive CA ex PA (also known as carcinoma in situ or intracapsular CA ex PA) support the notion that even minimal invasion may carry the potential for aggressive clinical behavior [13, 14], as was observed in our patient.
The diagnosis of CA ex PA in our case was suggested by matching histologic and immunohistochemical features between the small focus of carcinoma in the original salivary gland tumor and the later disseminated carcinoma, and subsequently proven by genetic analysis. In addition, we were able to confirm the pathologic progression of the disease in our case by assaying the status of PLAG1 in both tumors.
The pathogenic role of PLAG1 has been well established in pleomorphic adenoma. Conventional cytogenetic analysis first demonstrated translocations in approximately 70% of pleomorphic adenomas [15–18]. Subsequent research discovered two target genes involved in these translocations, PLAG1 [19, 20] at 8q12 in approximately 39%, and HMGA2 [21, 22] at 12q14-15 in 8%. The remaining (23%) karyotypically abnormal pleomorphic adenomas showed various sporadic clonal aberrations [23]. Translocations involving PLAG1 lead to gene overexpression through the mechanism of promoter swapping with a variety of partner genes, the most common of which is the gene for beta-catenin (CTNNB1). The pathogenic role of PLAG1, however, is not limited to tumors with cytogenetic evidence of PLAG1 alteration. Immunohistochemical and Northern blot studies have shown overexpression of PLAG1 in nearly all pleomorphic adenomas, supporting a major pathogenic role for this gene in tumor development [23–25]. It seems plausible that PLAG1 rearrangement persists in the associated carcinomatous element, and this is supported by cytogenetic evaluation of a few examples of CA ex PA [26].
PLAG1 encodes a nuclear oncoprotein (PLAG1) that functions as a DNA-binding transcription factor [27, 28]. Several genes upregulated by PLAG1 are growth factors, including human IGF-II. Activation of the IGF-II signaling pathway is one of the main molecular mechanisms mediating PLAG1-induced oncogenesis [29, 30]. We were able to demonstrate IGF-II expression in this patient’s tumor by immunohistochemistry. This finding also suggests that blocking the IGF-II pathway may offer potential therapeutic benefit in these patients.
Overexpression of PLAG1 is not exclusive to pleomorphic adenoma; it has also been described in lipoblastoma, with a mechanism analogous to pleomorphic adenoma but with different partner genes [31], and in subsets of acute myeloid leukemia [32] and hepatoblastoma, presumably through other mechanisms such as gene amplification without rearrangement [33]. However, none of these possibilities was of diagnostic consideration in our case, given the morphologic and immunohistochemical characteristics of the tumor.
Our report highlights the potential for disseminated disease to occur in the setting of CA ex PA with only a microscopic focus of invasion. The corollary of this has implications for pathologists and clinicians. There should be extensive sampling and careful microscopic examination of pleomorphic adenomas to avoid missing a malignant component. If detected, clinical staging and post-diagnostic surveillance should be initiated. In addition, we demonstrate the utility of evaluating PLAG1 status by immunohistochemistry and FISH in the clinicopathologic setting of disseminated carcinoma of unknown primary site, albeit with a history of parotid gland tumor. Once the extent of PLAG1 immunohistochemistry has been explored in normal and neoplastic tissues, consideration should be given to including it as a marker for CA ex PA in the situation of neoplastic disease of unknown origin.
Acknowledgments
The authors would like to thank Dr. Christopher French for performing NUT immunohistochemistry. This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
The study was performed with the approval of the Institutional Review Board of St. Jude Children’s Research Hospital. IRB number: NR11-046.
References
- 1.Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993;28(Pt 1):279–328. [PubMed] [Google Scholar]
- 2.Gnepp DR, Brandwein M, El-Naggar AK, et al. Pleomorphic adenoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World health organization classification of tumours. Pathology and genetics of head and neck tumours. IARC Press: France; 2005. pp. 242–244.
- 3.Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001;23:705–712. doi: 10.1002/hed.1100. [DOI] [PubMed] [Google Scholar]
- 4.Greco FA, Hainsworth JD. Introduction: unknown primary cancer. Semin Oncol. 2009;36:6–7. doi: 10.1053/j.seminoncol.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Bourke JL, Langer SW, Jensen HL. Metastasizing malignant pleomorphic adenoma in a young man. APMIS. 2007;115:866–868. doi: 10.1111/j.1600-0463.2007.apm_534.x. [DOI] [PubMed] [Google Scholar]
- 6.Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Athale UH, Shurtleff SA, Jenkins JJ, et al. Use of reverse transcriptase polymerase chain reaction for diagnosis and staging of alveolar rhabdomyosarcoma, Ewing sarcoma family of tumors, and desmoplastic small round cell tumor. J Pediatr Hematol Oncol. 2001;23:99–104. doi: 10.1097/00043426-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Auclair PL, Ellis GL. Atypical features in salivary gland mixed tumors: their relationship to malignant transformation. Mod Pathol. 1996;9:652–657. [PubMed] [Google Scholar]
- 9.Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596–604. doi: 10.1053/hupa.2001.25000. [DOI] [PubMed] [Google Scholar]
- 10.Miller RE, Illing RO, Whelan JS. Lung carcinoma with hypertrophic osteoarthropathy in a teenager. Rare Tumors. 2011;3:e8. doi: 10.4081/rt.2011.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tortoledo ME, Luna MA, Batsakis JG. Carcinomas ex pleomorphic adenoma and malignant mixed tumors. Histomorphologic indexes. Arch Otolaryngol. 1984;110:172–176. doi: 10.1001/archotol.1984.00800290036008. [DOI] [PubMed] [Google Scholar]
- 12.Brandwein M, Huvos AG, Dardick I, et al. Noninvasive and minimally invasive carcinoma ex mixed tumor: a clinicopathologic and ploidy study of 12 patients with major salivary tumors of low (or no?) malignant potential. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:655–664. doi: 10.1016/S1079-2104(96)80071-0. [DOI] [PubMed] [Google Scholar]
- 13.Felix A, Rosa-Santos J, Mendonca ME, et al. Intracapsular carcinoma ex pleomorphic adenoma. Report of a case with unusual metastatic behaviour. Oral Oncol. 2002;38:107–110. doi: 10.1016/S1368-8375(01)00023-9. [DOI] [PubMed] [Google Scholar]
- 14.Katabi N, Gomez D, Klimstra DS, et al. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: a clinicopathologic study of 43 cases. Hum Pathol. 2010;41:927–934. doi: 10.1016/j.humpath.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Bullerdiek J, Wobst G, Meyer-Bolte K, et al. Cytogenetic subtyping of 220 salivary gland pleomorphic adenomas: correlation to occurrence, histological subtype, and in vitro cellular behavior. Cancer Genet Cytogenet. 1993;65:27–31. doi: 10.1016/0165-4608(93)90054-P. [DOI] [PubMed] [Google Scholar]
- 16.Mark J, Dahlenfors R. Cytogenetical observations in 100 human benign pleomorphic adenomas: specificity of the chromosomal aberrations and their relationship to sites of localized oncogenes. Anticancer Res. 1986;6:299–308. [PubMed] [Google Scholar]
- 17.Mark J, Sandros J, Wedell B, et al. Significance of the choice of tissue culture technique on the chromosomal patterns in human mixed salivary gland tumors. Cancer Genet Cytogenet. 1988;33:229–244. doi: 10.1016/0165-4608(88)90033-7. [DOI] [PubMed] [Google Scholar]
- 18.Sandros J, Stenman G, Mark J. Cytogenetic and molecular observations in human and experimental salivary gland tumors. Cancer Genet Cytogenet. 1990;44:153–167. doi: 10.1016/0165-4608(90)90042-9. [DOI] [PubMed] [Google Scholar]
- 19.Kas K, Voz ML, Roijer E, et al. Promoter swapping between the genes for a novel zinc finger protein and beta-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat Genet. 1997;15:170–174. doi: 10.1038/ng0297-170. [DOI] [PubMed] [Google Scholar]
- 20.Kas K, Roijer E, Voz M, et al. A 2-Mb YAC contig and physical map covering the chromosome 8q12 breakpoint cluster region in pleomorphic adenomas of the salivary glands. Genomics. 1997;43:349–358. doi: 10.1006/geno.1997.4819. [DOI] [PubMed] [Google Scholar]
- 21.Geurts JM, Schoenmakers EF, Roijer E, et al. Expression of reciprocal hybrid transcripts of HMGIC and FHIT in a pleomorphic adenoma of the parotid gland. Cancer Res. 1997;57:13–17. [PubMed] [Google Scholar]
- 22.Geurts JM, Schoenmakers EF, Roijer E, et al. Identification of NFIB as recurrent translocation partner gene of HMGIC in pleomorphic adenomas. Oncogene. 1998;16:865–872. doi: 10.1038/sj.onc.1201609. [DOI] [PubMed] [Google Scholar]
- 23.Stenman G. Fusion oncogenes and tumor type specificity–insights from salivary gland tumors. Semin Cancer Biol. 2005;15:224–235. doi: 10.1016/j.semcancer.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Astrom AK, Voz ML, Kas K, et al. Conserved mechanism of PLAG1 activation in salivary gland tumors with and without chromosome 8q12 abnormalities: identification of SII as a new fusion partner gene. Cancer Res. 1999;59:918–923. [PubMed] [Google Scholar]
- 25.Matsuyama A, Hisaoka M, Nagao Y, et al. Aberrant PLAG1 expression in pleomorphic adenomas of the salivary gland: a molecular genetic and immunohistochemical study. Virchows Arch. 2011. [DOI] [PubMed]
- 26.Martins C, Fonseca I, Roque L, et al. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18:1048–1055. doi: 10.1038/modpathol.3800386. [DOI] [PubMed] [Google Scholar]
- 27.Voz ML, Van de Ven WJ, Kas K. First insights into the molecular basis of pleomorphic adenomas of the salivary glands. Adv Dent Res. 2000;14:81–83. doi: 10.1177/08959374000140011301. [DOI] [PubMed] [Google Scholar]
- 28.Kas K, Voz ML, Hensen K, et al. Transcriptional activation capacity of the novel PLAG family of zinc finger proteins. J Biol Chem. 1998;273:23026–23032. doi: 10.1074/jbc.273.36.23026. [DOI] [PubMed] [Google Scholar]
- 29.Voz ML, Agten NS, Van de Ven WJ, et al. PLAG1, the main translocation target in pleomorphic adenoma of the salivary glands, is a positive regulator of IGF-II. Cancer Res. 2000;60:106–113. [PubMed] [Google Scholar]
- 30.Voz ML, Mathys J, Hensen K, et al. Microarray screening for target genes of the proto-oncogene PLAG1. Oncogene. 2004;23:179–191. doi: 10.1038/sj.onc.1207013. [DOI] [PubMed] [Google Scholar]
- 31.Hibbard MK, Kozakewich HP, Dal CP, et al. PLAG1 fusion oncogenes in lipoblastoma. Cancer Res. 2000;60:4869–4872. [PubMed] [Google Scholar]
- 32.Landrette SF, Kuo YH, Hensen K, et al. Plag1 and Plagl2 are oncogenes that induce acute myeloid leukemia in cooperation with Cbfb-MYH11. Blood. 2005;105:2900–2907. doi: 10.1182/blood-2004-09-3630. [DOI] [PubMed] [Google Scholar]
- 33.Zatkova A, Rouillard JM, Hartmann W, et al. Amplification and overexpression of the IGF2 regulator PLAG1 in hepatoblastoma. Genes chromosomes. Cancer. 2004;39:126–137. doi: 10.1002/gcc.10307. [DOI] [PubMed] [Google Scholar]