Abstract
Rationale
Drug users often report using drugs to enhance social situations, and empirical studies support the idea that drugs increase both social behavior and the value of social interactions. One way drugs may affect social behavior is by altering social processing, for example by decreasing perceptions of negative emotion in others.
Objectives
We examined effects of d-amphetamine on processing of emotional facial expressions, and on the social behavior of talking. We predicted amphetamine would enhance attention, identification and responsivity to positive expressions, and that this in turn would predict increased talkativeness.
Methods
Over three sessions, 36 healthy normal adults received placebo, 10mg, and 20mg d-amphetamine under counterbalanced double-blind conditions. At each session we measured processing of happy, fearful, sad and angry expressions using an attentional visual probe task, a dynamic emotion identification task, and measures of facial muscle activity. We also measured talking.
Results
Amphetamine decreased the threshold for identifying all emotions, increased negative facial responses to sad expressions, and increased talkativeness. Contrary to our hypotheses, amphetamine did not alter attention to, identification of or facial responses to positive emotions specifically. Interestingly, the drug decreased the threshold to identify all emotions, and this effect was uniquely related to increased talkativeness, even after controlling for overall sensitivity to amphetamine.
Conclusions
The results suggest that amphetamine may encourage sociability by increasing sensitivity to subtle emotional expressions. These findings suggest novel social mechanisms that may contribute to the rewarding effects of amphetamine.
Keywords: amphetamine, emotional faces, attentional bias, social interaction, psychophysiology
Introduction
Drugs are commonly used in social settings, especially in early stages of use. Indeed, drug users report they often use drugs in order to enhance social situations (Boys et al. 2001). Laboratory studies also suggest drugs increase social behavior and the value of social interaction. For example, amphetamine and alcohol increase preference for social rewards over monetary rewards, and increase the social behavior of talking (Griffiths et al. 1975; Higgins et al. 1989). Changes in the processing of social cues may partially underlie drug-produced increases in social behavior and the value of social interaction. For example, alcohol facilitates perception of positive emotional expressions in others (Kano et al. 2003), and blunts perception of negative emotions (Attwood et al. 2009). Either of these effects might increase social behaviors like talking, enhance rewarding aspects of social interaction, and thus increase desire to use the drug again in a social situation.
Facial expressions of emotion are particularly potent and relevant social cues, and drugs appear able to alter several aspects of emotional expression processing. As we describe below, drugs may change attention to emotional expressions, they may change the ability to identify emotional expressions, or they may change the way an individual responds to emotional stimuli, each of which might in turn alter social behavior and the value of social interactions.
Both abused and non-abused drugs change attention to emotional expressions in visual probe tasks. The visual probe task is an established reaction time task used to quantify biases in selective attention (i.e. the differential allocation of attentional resources across competing stimuli), and has been extensively used to examine biases in orienting to emotional relative to neutral facial expressions in healthy and clinical populations (see Bar-Haim et al. 2007 for review). Combining the visual probe task with concurrent eye-tracking provides additional measures of attention including initial orienting (e.g. direction of first eye-movement) and maintenance of attention (e.g. durations of fixations/dwell time). The antidepressant citalopram and the anxiolytic diazepam both decrease attentional bias to negative expressions in this task, consistent with their therapeutic effects (Murphy et al. 2008; Murphy et al. 2009). Further, in social phobics, alcohol reduces attention to anxious faces in the visual probe task (although not in non-phobic controls; Stevens et al. 2009).
Several drugs also alter the ability to identify emotional expressions in others. For example, alcohol impairs identification of sadness and improves identification of happiness (Attwood et al. 2009; Kano et al. 2003). The ‘designer’ stimulant ±3,4-methylenedioxy-methamphetamine (MDMA, ecstasy) which produces feelings of empathy and closeness with others, impairs identification of fear (Bedi et al. 2010). In a recent study, MDMA also improved “mind reading” of positive emotions based on pictures of the eye region of the face (Hysek et al. in press).
Finally, drugs may alter affective responses to emotional expressions, as assessed by subtle facial muscle activity indicative of emotional state. Typically, viewing negative emotional stimuli (including negative faces) increases electromyographic (EMG) measures of activity in the corrugator (frown) muscle, whereas viewing positive stimuli (including positive faces) decreases corrugator activity and increases zygomatic (smile) muscle activity (Dimberg and Karlsson 1997; Dimberg et al. 2000; Larsen et al. 2003; Moody et al. 2007). In the same sample of participants used here, we found that d-amphetamine enhances positive facial EMG responses to emotionally positive pictures (Wardle and de Wit 2012). We will now examine a measure of reactivity to facial emotions, to determine whether amphetamine similarly alters EMG responses to emotional facial expressions. Facial muscle activity in response to viewing facial expressions in others is involved in empathy, strategic interaction and emotional contagion (Barger and Grandey 2006; McIntosh et al. 2006; Oberman et al. 2007; Sonnby–Borgström 2002). Thus, findings that drugs can produce alterations in facial responsivity to emotional expressions would have important implications for social experience and behavior. In summary, abused drugs may alter attention to, identification of, and facial responses to emotional expressions in ways that could impact social behavior and the value of social interactions.
Stimulants such as cocaine and amphetamine increase the value of social rewards in humans and nonhumans (Higgins et al. 1989; Thiel et al. 2008), and increase social behaviors (e.g., talkativeness) in humans (Griffiths and et al. 1977; Marrone et al. 2010). However, it is unknown whether the “pro-social” effects of stimulants include changes in social processing like those described above. Thus, the purpose of the current study was to examine the effect of a prototypical stimulant, d-amphetamine, on processing of facial emotion expressions, and to determine whether the effects of d-amphetamine on social processing related to the increase in talkativeness typically produced by d-amphetamine. We had three primary predictions about facial expression processing: First, we predicted d-amphetamine would increase attentional bias to positive faces. Induction of positive mood by non-pharmacological means biases attention towards positive stimuli (Tamir and Robinson 2007), suggesting euphoric drugs like amphetamine might also produce positive biases. Second, we predicted d-amphetamine would improve participants’ ability to identify dynamically developing positive emotional expressions. Third, we predicted d-amphetamine would enhance EMG activity in response to dynamically presented positive emotional expressions, just as it enhanced responses to generally positive pictures in this same sample. Finally, we examined whether the effects of amphetamine on emotional face processing were related to its effect on talkativeness. We hypothesized that d-amphetamine would alter emotional expression processing, and that this would be related to its effects on talkativeness, independent of the drug’s effects on positive mood, arousal and blood pressure.
Methods
Study design
The within-subject design consisted of three sessions, separated by at least 72h, at which participants received capsules containing placebo, 10mg or 20mg d-amphetamine (Mallinckrodt, St. Louis, MO) in counterbalanced order under double-blind conditions. At each session they completed visual probe, dynamic emotional identification and talking tasks. The participants also completed measures of affective responses to generally emotional pictures, but these results are reported elsewhere (Wardle and de Wit 2012).
Participants
Healthy participants (20 female, 16 male) ages 18–35 were recruited through flyers and online advertisements. Screening included a physical examination, electrocardiogram, modified Structured Clinical Interview for DSM-IV (SCID; First et al. 1996), and self-reported health and drug use. Inclusion criteria were: Body Mass Index of 18 to 35, no medical conditions/contraindications, not pregnant, nursing, or trying to become pregnant, no past year DSM-IV Axis I Disorders or lifetime history of drug dependence, mania or psychosis, some previous recreational drug use, no previous adverse amphetamine reactions, smoking < 10 cigarettes per week, and high school education level. Participants were primarily Caucasian (n = 26, 72%), in their twenties (M = 24.3 years, SD = 4.5) with some college education (M = 15.4 years, SD = 1.5) and light to moderate recreational drug use (see Table 1).
Table 1.
Demographic and Substance Use Characteristics.
| n(%) or M(SD) | |
|---|---|
| Demographic Variables | 16 (44%) male |
| Sex | |
| Ethnicity | 35 (97%) Non-Hispanic |
| Race | 26 (72%) Caucasian |
| 5 (13%) African-American | |
| 2 (6%) Asian | |
| 3 (8%) Other/Mixed Race | |
| Age | 24.3 (4.5) |
| Education in years | 15.4 (1.5) |
| Current Substance Use | |
| Typical alcoholic drinks/week | 7.5 (6.0) |
| Smoking at all in past month | 3 (8.3%) |
| Lifetime Occasions Recreational Use | |
| Cannabis | 4 (11%) never |
| 18 (50%) 1–10x | |
| 12 (33%) 11–50x | |
| 0 (0%) 50–100x | |
| 2 (6%) > 100x | |
| Tranquilizers | 35 (97%) never |
| 1 (3%) 1–10x | |
| Stimulants | 28 (78%) never |
| 7 (19%) 1–10x | |
| 1 (3%) 11–50x | |
| Opiates | 34 (94%) never |
| 2 (6%) 1–10x | |
| Hallucinogens | 29 (81%) never |
| 7 (19%) 1–10x | |
| Entactogens | 31 (86%) never |
| 5 (14%) 1–10x | |
| Other Drugs | 34 (94%) never |
| 2 (6%) 1–10x | |
Participants were instructed to refrain from recreational and over-the-counter drugs for 24 hours before the sessions, with compliance verified using breath alcohol (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests (ToxCup, Branan Medical Corporation, Irvine, CA). Participants were asked to maintain normal caffeine and nicotine intake for 24 hours before the sessions, and fast for 9 hours prior to sessions. Female participants provided urine samples for pregnancy tests before each session. Women not on hormonal birth control were scheduled only during the follicular phase (White et al. 2002). Participants were informed that they might receive a stimulant, tranquilizer, marijuana-like drug, or placebo. All participants provided informed consent. All procedures were approved by The University of Chicago Institutional Review Board.
Procedure
Participants attended an orientation during which they were familiarized with tasks and psychophysiological equipment, and completed baseline anxiety and depression measures. They then completed three 4-hour individual study sessions separated by at least 72 hours. For each study session participants arrived at 9:00am. They provided breath and urine samples for recent drug use and pregnancy (women), consumed a standard snack and completed baseline measures of typical subjective and cardiovascular drug effects. At 9:30am they took two opaque size 00 gelatin capsules containing 10mg or 20mg of d-amphetamine with dextrose filler, or placebo (dextrose only). From 9:30am to 11:00am participants relaxed (watching a movie from a selection available or reading). Measures of subjective and cardiovascular effects were obtained at 10:00 and 11:00am. Then psychophysiological sensors were attached, and participants completed the visual probe task, dynamic emotional identification task, and an emotional responsivity task using the International Affective Picture Set (IAPS; Lang et al. 1999), in counterbalanced order. The results of the IAPS task are reported elsewhere, as this task did not directly address the topic of this report, i.e. processing of emotional expressions (Wardle and de Wit 2012). After the tasks (approximately 12:30pm), sensors were removed, and subjective and cardiovascular drug effects re-assessed. Participants then completed the talking task, and returned to relaxing. Subjective and cardiovascular effects were assessed for the last time at 1pm. At 1:30pm participants completed an end of session questionnaire and left the laboratory.
Measures
Visual Probe Task (VPT)
VPT stimuli were color pictures from the Karolinska Directed Emotional Faces Set (Goeleven et al. 2008). Anger, fear, happiness and sadness were each posed by 8 female and 8 male actors. Each actor posed only one emotion. Each trial consisted of a 1,000ms fixation cross, then a neutral and an emotional face posed by the same actor, one to the right and one to the left of the screen, for 2,000ms. Images measured 520mm × 760mm with the inner edges 173mm apart, and were viewed at a distance of approximately 65cm. Both faces then disappeared, and were replaced by grey rectangles of the same size, one of which contained an 80mm × 80mm white visual probe that the participant classified as quickly as possible (as circle or square) by pressing a key on the keyboard. After a response, or 10s with no response, an intertrial interval of 750 to 1,250ms began. Within each pair-type, probe shape, probe location and emotional face location were counterbalanced. The 64 trials were presented in random order.
The primary VPT outcome was attentional bias to emotional faces, quantified as: 1. Initial attentional bias - direction of the first gaze when the faces appeared, and 2. Total attentional bias - total gaze time at the emotional picture minus total gaze time at the neutral picture. Reaction time is not a valid indicator of attentional bias at this length of stimulus presentation, as multiple eye-movements could occur prior to the onset of the visual probe. Therefore RT data was not analyzed. Gaze was quantified using electrooculography (EOG), with a 4mm Ag/AgCl electrode filled with electrolyte gel attached 1.5 cm from the outer canthus of each eye, and an 8mm gel-filled Ag/AgCl ground sensor on the forehead. Sites were cleaned with alcohol and exfoliant, and any with impedance above 20k (Model 1089 MK III Checktrode; UFI, Morro Bay, CA, USA) were reapplied. EOG was amplified, digitized and sampled at 1000 Hz using an EOG100C amplifier, Biopac MP150 system and AcqKnowledge software (Biopac, Goleta, CA USA). Trained raters discarded trials in which: 1. Gaze was not centrally fixated prior to the trial 2. Initial fixation was < 100ms after picture onset (reflecting anticipatory eye-movements) 3. Noise obscured eye movements. Three percent of trials were noise contaminated or not centrally fixated, while 10% showed no valid gazes.
Dynamic Emotional Identification Task (DEIT)
The DEIT was generated from a database of actors performing facial expressions in front of a Dalsa DS-25-02M30 color camera (Benton et al. 2007). These images have previously been used in static format to examine effects of alcohol on emotion identification (Attwood et al. 2009). Five female and 5 male actors performed angry, fearful, sad and happy expressions, for a total of 40 sequences. Each sequence consists of 2s of video from neutral to full emotional expression captured at 25 Hz, creating 50 “frames” from 0–100% emotional intensity at 2% steps that follow the actual expression time course, rather than assuming all elements appear at the same gradual and simultaneous rate (as do morphs between 0 and 100% images). Sequences were presented in random order, and each frame was presented for an average of 250ms, within a random range of 100–400ms, giving the impression of a color video of an emotional expression developing. Participants were instructed to “press the space bar as soon as you know what expression is being displayed.” This ended the sequence, and presented multiple-choices of “Angry,” “Fearful,” “Sad,” and “Happy.” Dynamically developing emotional faces are more ecologically valid, and more strongly activate brain areas associated with emotion perception than static pictures (LaBar et al. 2003; Platt et al. 2010; Walter et al. 2011). Thus dynamic presentations may allow detection of more subtle drug effects.
Emotion identification was quantified as the intensity (0–100%) of the face when the participant pressed the space bar (for correctly identified sequences). Accuracy was very high (94%), and not sufficiently variable for analysis. Facial responses were quantified as mean electromyographic activity (EMG) in the corrugator and zygomatic for the final 1s of face presentation (during correctly identified sequences), minus mean EMG for a 1s pre-picture baseline. EMG was measured over left brow and cheek with 4mm Ag/AgCl electrodes and the same site preparation and ground as EOG. EMG signals were amplified, 10Hz – 500Hz band pass filtered, digitized at 1000 Hz, 60 Hz band stop filtered, rectified, and integrated over 20ms using EMG100C amplifiers, an MP150 Data Acquisition System and Acqknowledge software from Biopac Systems Inc. (Goleta, CA USA). Trained raters excluded trials with excessive baseline activity or artifactual activations. The top and bottom 1% of responses were trimmed to remove outliers, and EMG was square root transformed, to correct typical positive skew.
Talking Task
The talking task was a 5 min. modification (Wardle et al. 2011) of the Interpersonal Perception Task previously used by Janowsky (2003) to study psychoactive drugs. At orientation, participants nominated three “important people in your life.” At each session, one person was selected (in counterbalanced order), and the participant talked with the research assistant about this individual for 5min. Research assistants were trained in reflective listening. Speech samples were transcribed, then scored using the Linguistic Inquiry and Word Count software, a standardized and validated dictionary (Pennebaker et al. 2007), to produce total word count.
Covariates
Depression and anxiety
Because clinical anxiety and depression can affect attentional bias, we collected the Trait scale of the State-Trait Anxiety Inventory (STAI; Spielberger et al. 1983) and the Beck Depression Inventory (BDI-II; Beck et al. 1988) for use as covariates in attentional analyses. These are widely used and well-validated measures.
Subjective and cardiovascular drug effects
We also measured typical mood and cardiovascular effects of amphetamine, to help rule out the possibility that drug effects on social processing and talking were related simply due to differences in overall sensitivity to the drug. The Profile of Mood States (POMS; Johanson and Uhlenhuth 1980) measured typical effects on subjective mood. It is a 72-adjective list rated on 5-point Likert scales from 0 (“not at all”) to 4 (“extremely”), with 8 subscales. For this study we used the Elation and Arousal subscales, which are sensitive to amphetamine (Gabbay 2003). Elation measures positive mood, whereas Arousal measures general emotional activation. We also measured blood pressure using portable monitors (Life Source, A&D Company, Tokyo, Japan), with Mean Arterial Pressure (MAP; [Systolic BP + 2*Diastolic BP]/3) as our measure of cardiovascular effects. Results reported elsewhere (Wardle and de Wit 2012) indicate typical, dose-dependent effects of increased elation, arousal and blood pressure were present before and after the tasks analyzed in this paper. To summarize the effects of amphetamine on subjective mood and MAP for each session, we calculated relative area under the curve (AUC) scores for Elation, Arousal and MAP by subtracting baseline scores from all subsequent scores in the same session and calculating the area under those difference scores.
Statistical Analyses
We conducted one Linear Mixed-Effect (LME) model using lme4 (v 0.999375-42; Bates et al. 2011) in the R statistical computing environment (v. 2.14.0; R Development Core Team 2011) for each face-processing variable, as follows: 1. Direction of initial gaze on each dot-probe trial, modeled using a logit link function 2. Total emotional gaze bias on each dot-probe trial. 3. Intensity of the face at the time of correct identifications in the DEIT. 4. Corrugator responsivity to DEIT emotional expressions and 5. Zygomatic responsivity to DEIT emotional expressions. Linear mixed-effects models offer significant advantages relative to traditional repeated-measures ANOVA in power and handling of missing data (participants with missing trials do not need to be excluded), easy generalization to binary outcomes, and relaxation of assumptions of normality and homogeneity of variance (Raudenbush and Bryk 2002).
All face-processing models used Dose, Emotion Displayed and Stimulus Sex as independent (fixed) factors. (The addition of Participant Sex to these models did not alter any results, and is omitted from reporting.) Dose effects were examined using polynomial contrasts, with significant linear effects followed up by treatment contrasts comparing each dose to placebo. Emotion effects were examined using Helmert contrasts: 1. Happy vs. all negative emotions, 2. Fear vs. other negative emotions (anger and sadness), and 3. Anger vs. sadness. The first captures our primary hypothesis, while subsequent contrasts distinguish specific negative emotions. Stimulus and participant sex were included because drug effects on face-processing may depend on these factors (Attwood et al. 2009). Attentional analyses additionally included STAI and BDI totals, but these did not alter results and are omitted. EMG analyses additionally included grand-mean-centered intensity at display termination as a predictor, because display face intensity might influence response intensity, and as the participant chose when to terminate the sequence, display face intensity might be systematically affected by drug or other factors. All models included random effects for Participant and Dose Within Participant to allow individual differences in overall level of the dependent variable and responses to the drug. DEIT analyses also included a random effect for Actor (Baayen et al. 2008), to allow for the possibility that some actors were better at posing (potentially important given the smaller number of actors in this task). Effect sizes are reported as unstandardized coefficients (B) with standard errors (SE). 1
We examined the effect of amphetamine on total word count in a separate LME model using Dose as an independent (fixed) effect, and random effects for Participant and Dose Within Participant. (The addition of Participant Sex did not alter any results, and is omitted from reporting.) We then examined the relationship between talkativeness and face processing as follows: For each significant amphetamine effect on face processing, we conducted individual linear regressions on each participant’s data using Dose, Emotion Displayed, and Stimulus Sex as independent variables, and the face-processing variable as the dependent variable. The unstandardized estimate of the linear dose effect from each regression represents a per-participant estimate of the strength of the amphetamine effect on that face-processing variable. We then produced per-participant estimates of the strength of the amphetamine effect on word count, POMS Elation, POMS Arousal and MAP using the same procedure. We first entered per-participant effects of amphetamine on face-processing, POMS Elation, POMS Arousal and MAP separately in linear regressions to see whether any of these predicted amphetamine’s effect on talkativeness. Then we entered the per-participant effects on face processing, mood and blood pressure simultaneously into a linear regression predicting per-subject effects on word count, to examine if any uniquely predicted amphetamine’s effect on talkativeness, after shared variance due to general amphetamine sensitivity was removed.
One participant was missing gaze data for all sessions, and 8 others for a single session or part of a single session due to equipment failure. Thus n = 35 participants with complete or partially complete data were included in attentional analyses. One participant was excluded from all DEIT analyses for unusually poor accuracy (> 3 S.D. from the mean), and a second for waiting until the end of all videos (100% intensity) to identify the faces. Zygomatic EMG data for one participant for one session was missing due to improperly attached sensors. Thus n = 34 participants with complete or partially complete data were included in identification of emotion and response to emotion analyses.
Results
Attentional Bias to Emotional Expressions (VPT)
Amphetamine did not affect either initial attentional bias (direction of the first gaze) or total attentional bias (total gaze times) and did not interact with emotion displayed, stimulus sex or participant sex. Independent of drug condition, participants showed greater bias in total gaze time for fearful faces compared to other negative faces; B = 43.37, SE = 20.19, t(34) = 2.15, p = .03.
Identification of Emotional Expressions (DEIT)
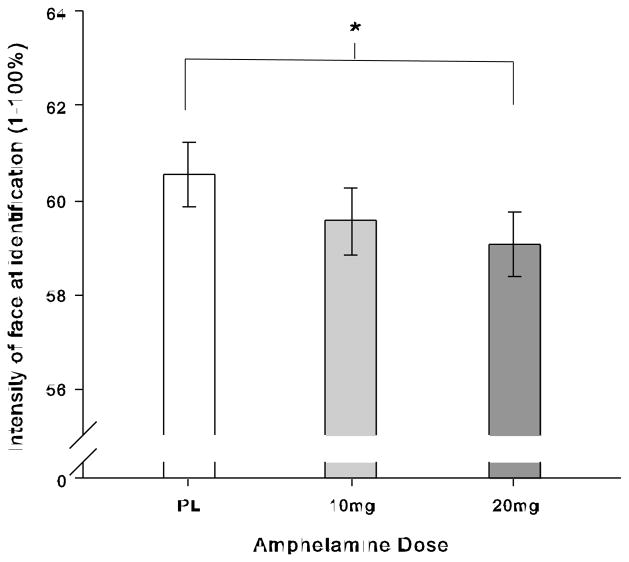
Amphetamine (20mg) slightly but significantly lowered the intensity required to identify emotional faces (emotions were identified approximately one frame sooner, see Table 2 Drug contrast and Fig. 1). Although emotions differed in the intensity needed for identification, the drug did not affect identification of any one emotion more than the others. Negative emotions were identified at lower intensity than happiness, while fear was identified at higher intensity than anger and sadness, and anger was identified at lower intensity than sadness (see Table 2 Emotion contrasts). Further, most emotions were identified at slightly lower intensity in female than male faces, except anger, which was identified at slightly lower intensity in male than female faces (see Table 2 Emotion x Stimulus Sex contrasts).
Table 2.
Effect size (B and SE), and significance of fixed effects from LME model of identification of emotional expressions (percent intensity at time of identification).
| Fixed Effects | B (% intensity) | SE | t(33) | p |
|---|---|---|---|---|
| Drug (linear) | −1.46 | 0.69 | 2.13 | 0.04 |
| Emotion | ||||
| Happy vs. negative | 2.32 | 0.40 | 5.85 | <.001 |
| Fear vs. other negative | 4.04 | 0.43 | 9.47 | <.001 |
| Sad vs. angry | 2.40 | 0.50 | 4.80 | <.001 |
| Stimulus sex | 2.91 | 3.38 | 0.86 | 0.40 |
| Drug x Emotion | ||||
| Drug x Happy vs. negative | 0.72 | 0.97 | 0.74 | 0.46 |
| Drug x Fear vs. other negative | −0.13 | 1.04 | 0.12 | 0.91 |
| Drug x Sad vs. angry | 0.41 | 1.23 | 0.34 | 0.74 |
| Drug x Stimulus sex | 0.70 | 0.85 | 0.82 | 0.42 |
| Emotion x Stimulus sex | ||||
| Happy vs. negative x Stimulus sex | 4.40 | 0.79 | 5.57 | <.001 |
| Fear vs. other negative x Stimulus sex | 2.00 | 0.85 | 2.34 | 0.03 |
| Sad vs. angry x Stimulus sex | 16.15 | 1.00 | 16.13 | <.001 |
| Drug x Emotion x Stimulus sex | ||||
| Drug x Happy vs. negative x Stimulus sex | −0.36 | 1.94 | 0.18 | 0.86 |
| Drug x Fear vs. other negative x Stimulus sex | 1.56 | 2.09 | 0.75 | 0.46 |
| Drug x Sad vs. angry x Stimulus sex | −1.19 | 2.45 | 0.48 | 0.63 |
Fig. 1.
Drug effects on intensity of expression needed for identification of emotion, shown as estimated marginal means from LME model, ±SE of linear drug effect (n = 34). Amphetamine (20mg) decreased the intensity of expression needed to identify emotions.
Note: * p < .05 follow up treatment effect between 20mg and placebo.
Responses to Emotional Expressions (DEIT)
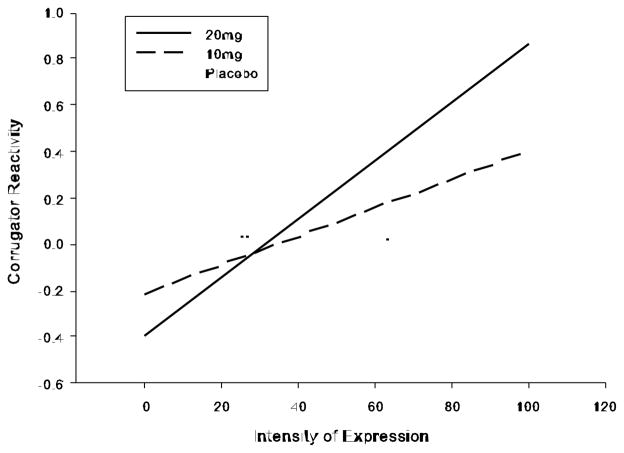
Viewing emotional faces affected activity in the corrugator muscle, as expected. Happy faces reduced corrugator activity while negative faces increased it, and Fear faces produced less corrugator activity than other negative emotions (see Table 3 Emotion contrasts). Further, intensity of display face related to corrugator activity, supporting inclusion of intensity as a predictor. Specifically, corrugator activity decreased as the intensity of happy faces increased, but increased as the intensity of negative faces increased (see Table 3 Emotion x Intensity contrasts). In contrast to our predictions, amphetamine did not reduce corrugator responses to happy faces (which would indicate more positive reactions). Rather, amphetamine increased the amount of corrugator activity produced in relation to the intensity of specific negative emotion expressions (see Table 3 Drug x Emotion x Intensity contrasts). Post-hoc testing suggested amphetamine specifically increased responsivity of the corrugator muscle to the degree of sadness displayed in the stimuli – in other words, under amphetamine conditions the corrugator muscle responded much more as the sadness of faces increased, while under placebo corrugator responses were similar regardless of degree of sadness shown in the display face; B = 0.007, SE = 0.002, t(33) = 3.00, p = .002, see Fig. 2.
Table 3.
Effect size (B and SE), and significance of fixed effects from LME model of corrugator activity during emotional expressions (square root of microvolts)
| Fixed Effects | B (sqrt uV) | SE | t(33) | p |
|---|---|---|---|---|
| Drug (linear) | 0.0002 | 0.003 | 0.04 | 0.97 |
| Emotion | ||||
| Happy vs. negative | −0.02 | 0.002 | 13.59 | <.001 |
| Fear vs. other negative | −0.01 | 0.002 | 6.33 | <.001 |
| Sad vs. angry | 0.003 | 0.002 | 1.41 | 0.17 |
| Stimulus sex | 0.0004 | 0.002 | 0.19 | 0.85 |
| Intensity | 0.0003 | 0.0001 | 3.71 | <.001 |
| Drug x Emotion | ||||
| Drug x Happy vs. negative | −0.004 | 0.004 | 1.11 | 0.28 |
| Drug x Fear vs. other negative | 0.002 | 0.004 | 0.45 | 0.66 |
| Drug x Sad vs. angry | 0.009 | 0.005 | 1.82 | 0.08 |
| Drug x Stimulus sex | −0.002 | 0.003 | 0.67 | 0.51 |
| Drug x Intensity | 0.0002 | 0.0002 | 1.21 | 0.23 |
| Emotion x Stimulus sex | ||||
| Happy vs. negative x Stimulus sex | 0.002 | 0.003 | 0.61 | 0.55 |
| Fear vs. other negative x Stimulus sex | −0.004 | 0.003 | 1.07 | 0.29 |
| Sad vs. angry x Stimulus sex | −0.005 | 0.004 | 1.17 | 0.25 |
| Emotion x Intensity | ||||
| Happy vs. negative x Intensity | −0.0007 | 0.0002 | 3.76 | <.001 |
| Fear vs. other negative x Intensity | −0.0002 | 0.0001 | 1.76 | 0.09 |
| Sad vs. angry x Intensity | 0.0002 | 0.0001 | 1.41 | 0.17 |
| Stimulus Sex x Intensity | 0.00005 | 0.0001 | 0.38 | 0.71 |
| Drug x Emotion x Stimulus sex | ||||
| Drug x Happy vs. negative x Stimulus sex | −0.004 | 0.008 | 0.55 | 0.59 |
| Drug x Fear vs. other negative x Stimulus sex | 0.002 | 0.008 | 0.21 | 0.83 |
| Drug x Sad vs. angry x Stimulus sex | 0.008 | 0.01 | 0.85 | 0.40 |
| Drug x Emotion x Intensity | ||||
| Drug x Happy vs. negative x Intensity | 0.0007 | 0.0005 | 1.40 | 0.17 |
| Drug x Fear vs. other negative x Intensity | 0.0004 | 0.0003 | 1.20 | 0.24 |
| Drug x Sad vs. angry x Intensity | 0.0009 | 0.0003 | 3.01 | 0.004 |
| Emotion x Stimulus Sex x Intensity | ||||
| Happy vs. negative x Stimulus sex x Intensity | −0.0001 | 0.0004 | 0.28 | 0.78 |
| Fear vs. other negative x Stimulus sex x Intensity | 0.0002 | 0.0003 | 0.63 | 0.53 |
| Sad vs. angry x Stimulus sex x Intensity | 0.0005 | 0.0003 | 1.80 | 0.08 |
| Drug x Emotion x Stimulus sex x Intensity | ||||
| Drug x Happy vs. negative x Stimulus sex x Intensity | 0.002 | 0.0009 | 1.90 | 0.07 |
| Drug x Fear vs. other negative x Stimulus sex x Intensity | 0.001 | 0.0006 | 1.65 | 0.11 |
| Drug x Sad vs. angry x Stimulus sex x Intensity | 0.0002 | 0.0006 | 0.32 | 0.75 |
Fig. 2.
Drug effects on corrugator activation to sad facial expressions, shown as partial effects from LME model (n = 34). Amphetamine (10 and 20mg) increased activity in the corrugator muscle relative to the intensity of the facial expression displayed.
Viewing emotional faces also affected activity in the zygomatic muscle, with greater activity during happy as compared to negative faces (see Table 4 Emotion contrasts). Intensity of the display face related to zygomatic activity, such that zygomatic activity increased as intensity of happy faces increased, but not as intensity of negative faces increased (see Table 4 Emotion x Intensity contrasts). However, in contrast to our predictions, there were no significant effects of amphetamine on zygomatic activity in response to the faces.
Table 4.
Effect size (B and SE), and significance of fixed effects from LME model of zygomatic activity during emotional expressions (square root of microvolts)
| Fixed Effects | B (sqrt uV) | SE | t(33) | p |
|---|---|---|---|---|
| Drug (linear) | 0.002 | 0.001 | 1.27 | 0.21 |
| Emotion | ||||
| Happy vs. negative | 0.006 | 0.001 | 5.73 | <.001 |
| Fear vs. other negative | −0.001 | 0.001 | 1.42 | 0.16 |
| Sad vs. angry | −0.002 | 0.001 | 1.64 | 0.11 |
| Stimulus sex | 0.0008 | 0.001 | 0.72 | 0.48 |
| Intensity | 0.0001 | 0.00004 | 3.40 | 0.001 |
| Drug x Emotion | ||||
| Drug x Happy vs. negative | 0.001 | 0.002 | 0.59 | 0.56 |
| Drug x Fear vs. other negative | −0.001 | 0.002 | 0.51 | 0.61 |
| Drug x Sad vs. angry | 0.002 | 0.003 | 0.72 | 0.48 |
| Drug x Stimulus sex | 0.004 | 0.002 | 1.75 | 0.09 |
| Drug x Intensity | 0.0002 | 0.0001 | 1.91 | 0.06 |
| Emotion x Stimulus sex | ||||
| Happy vs. negative x Stimulus sex | −0.003 | 0.002 | 1.53 | 0.14 |
| Fear vs. other negative x Stimulus sex | 0.002 | 0.002 | 1.09 | 0.28 |
| Sad vs. angry x Stimulus sex | −0.008 | 0.002 | 3.09 | 0.004 |
| Emotion x Intensity | ||||
| Happy vs. negative x Intensity | 0.0004 | 0.0001 | 3.65 | <.001 |
| Fear vs. other negative x Intensity | −0.0002 | 0.0001 | 2.47 | 0.02 |
| Sad vs. angry x Intensity | 0.0001 | 0.0001 | 0.89 | 0.38 |
| Stimulus Sex x Intensity | 0.00001 | 0.0001 | 0.23 | 0.82 |
| Drug x Emotion x Stimulus sex | ||||
| Drug x Happy vs. negative x Stimulus sex | −0.002 | 0.005 | 0.46 | 0.65 |
| Drug x Fear vs. other negative x Stimulus sex | −0.001 | 0.005 | 0.29 | 0.77 |
| Drug x Sad vs. angry x Stimulus sex | −0.01 | 0.006 | 1.69 | 0.10 |
| Drug x Emotion x Intensity | ||||
| Drug x Happy vs. negative x Intensity | 0.0002 | 0.0002 | 0.72 | 0.48 |
| Drug x Fear vs. other negative x Intensity | 0.0001 | 0.0001 | 0.29 | 0.77 |
| Drug x Sad vs. angry x Intensity | 0.0003 | 0.0002 | 1.58 | 0.12 |
| Emotion x Stimulus Sex x Intensity | ||||
| Happy vs. negative x Stimulus sex x Intensity | −0.0002 | 0.0002 | 0.74 | 0.46 |
| Fear vs. other negative x Stimulus sex x Intensity | 0.00005 | 0.0001 | 0.33 | 0.74 |
| Sad vs. angry x Stimulus sex x Intensity | 0.00003 | 0.0002 | 0.22 | 0.83 |
| Drug x Emotion x Stimulus sex x Intensity | ||||
| Drug x Happy vs. negative x Stimulus sex x Intensity | 0.0009 | 0.0006 | 1.54 | 0.13 |
| Drug x Fear vs. other negative x Stimulus sex x Intensity | 0.0002 | 0.0004 | 0.58 | 0.57 |
| Drug x Sad vs. angry x Stimulus sex x Intensity | 0.0005 | 0.0004 | 1.22 | 0.23 |
Talking Task
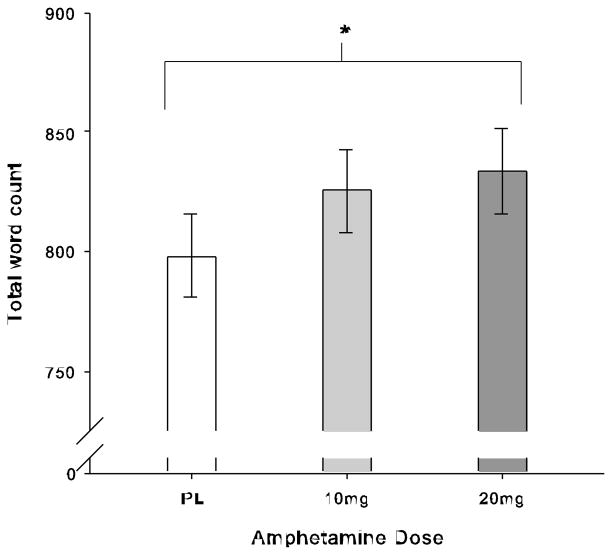
Amphetamine slightly but significantly increased talkativeness; B = 35.47, SE = 17.50, t(35) = 2.03, p = .05, see Fig. 3. To determine if effects of amphetamine on face processing (lowered intensity needed to identify all emotions, and increased corrugator responsiveness to sad faces) were related to the effect of the drug on talkativeness, we used per-participant estimates of the effect of amphetamine on face processing, mood and blood pressure as independent variables in regressions predicting the per-participant effects of amphetamine on talkativeness (per-participants estimates were derived as described in the analysis section). Independent variables were entered both individually and simultaneously, to examine first whether any predicted amphetamine’s effect on talkativeness, and second whether any uniquely predicted amphetamine’s effects on talkativeness after controlling for general amphetamine sensitivity. As shown in Table 5, identifying emotions at a lower intensity and greater Elation and Arousal on amphetamine all predicted greater talkativeness on amphetamine when entered individually. Further, identifying emotions at a lower intensity uniquely predicted greater talkativeness on amphetamine, even when other amphetamine effects were entered simultaneously. In contrast, Elation and Arousal no longer predicted talking, suggesting these measures did not have unique explanatory power for talkativeness when the other variables in the regression were controlled for.
Fig. 3.
Drug effects on total word count in talking task, shown as estimated marginal means from LME model, ±SE of linear drug effect (n = 36). Amphetamine (20mg) increased talkativeness.
Note: * p < .05 follow-up treatment effect between 20mg and placebo
Table 5.
Linear regressions using effects of amphetamine on face-processing, mood and cardiovascular variables both individually and simultaneously to predict effects of amphetamine on talkativeness.
| Effect of Amphetamine | Standardized beta and p entered individually | Standardized beta and p entered simultaneously |
|---|---|---|
| Face Processing Effects | ||
| Intensity of faces at ID | b = −0.35, p = .04* | b = −0.38, p = .02* |
| Corrugator response to sad faces | b = −0.06, p = .74 | b = 0.003, p = .99 |
| Mood Effects | ||
| POMS Elation | b = 0.39, p = .02* | b = 0.27, p = .26 |
| POMS Arousal | b = 0.36, p = .04* | b = 0.27, p = .27 |
| Cardiovascular Effects | ||
| Mean Arterial Pressure | b = 0.02, p = .92 | b = −0.23, p = .19 |
Discussion
Amphetamine altered certain aspects of emotional processing in ways that were related to the social behavior of talking. Contrary to our predictions, amphetamine did not alter attention, identification or reactivity specifically to happy faces. Indeed, it increased corrugator responses to sad faces, indicating more responsiveness to negative emotions, rather than positive emotions. Nevertheless, the drug lowered the intensity needed to identify emotional expressions in general. The discrepancy between the emotion-specific effects on responsivity and the non-emotion specific effects on identification may indicate that these behavioral measures are mediated by different processes. That is, identifying an emotion in a target stimulus may be distinct from the process of generating an emotional response to the depicted emotion. Importantly, the amphetamine-produced improvement in detection of all emotional expressions was related to amphetamine-induced increases in talkativeness. That is, the extent to which amphetamine decreased the threshold of detection for all emotions predicted its ability to increase talking. This effect was apparent above and beyond the relations between talkativeness and other typical amphetamine effects like increased positive mood and arousal. In contrast, amphetamine’s effects on facial responsiveness to sadness did not seem to contribute to its effects on talkativeness.
These findings suggest stimulants may increase sensitivity to subtle cues of emotion in others, and that this may contribute to drug-induced increases in social behavior, in this case, the social behavior of talking. Although we found that amphetamine increased sensitivity to emotional cues regardless of their valence, it could be argued that most of the cues present during recreational drug use are positive. In this way, increased detection of subtle emotional cues may encourage social interaction by enhancing responses to cues in the environment. In our study, research assistants were instructed to smile and be pleasant to participants during the talking task, creating presumably positive social cues. In pleasant, social drug-taking environments, increased detection of positive social cues may increase talkativeness, increase the value of the social interactions, and, indirectly increase desire to take the drug again. The converse prediction is that if amphetamine is used in a setting where negative emotions are present, this process may contribute to amphetamine-induced paranoia (Dawe et al. 2009). Even though amphetamine also appeared to increase responsivity to sad faces, this effect did not appear to contribute to talkativeness, perhaps because the talking measure was obtained in the context of primarily positive social stimuli. This explanation fits with the value that many drug-users put on “setting the scene” for a good drug experience, which includes using the drug only around individuals they like and trust (Quintero 2009). Interestingly, the stimulant and euphoric effects of amphetamine do not seem to be highly dependent on either context or presence of others (de Wit et al. 1997; Zacny et al. 1992). Further, in this study there was not a close correlation between stimulant/euphoric and social processing effects of the drug, suggesting some dissociation. However, future research systematically varying the positivity of the environment during use will be required to establish the importance of the environment to the social (vs. euphoric) effects of the drug.
The extent to which variations in social processing contribute to desire to take the drug again is also unknown. Although drug users report that they use drugs in part to enhance social situations, it remains to be determined to what extent drug effects on social perception and contribute to use or abuse of the drug. Our findings that amphetamine altered emotional perception and that this predicted talkativeness support the idea that drug-induced alterations in social perception may influence social behavior. Thus, the ability of amphetamine to enhance social situations may independently contribute to its rewarding effects and make the drug more attractive to users. It has been reported that individuals with substance abuse disorders have deficits in emotion processing (Kornreich et al. 2003). Although these deficits are typically attributed to the chronic exposures to drugs, it is also possible that drug users had pre-existing deficits in emotion processing, that they attempted to ameliorate with the use of drugs. If this is the case, emotion-processing deficits may represent a vulnerability factor for drug dependence.
Amphetamine also altered face processing in ways that differ from other drugs of abuse, suggesting drug-specific mechanisms. In the current study amphetamine improved the detection of emotions regardless of the type of emotion. In contrast, as noted in the introduction, alcohol both impairs identification of sadness (Attwood et al. 2009), and improves identification of happiness (Kano et al. 2003; Walter et al. 2011) whereas MDMA, which is pharmacologically quite similar to amphetamine, impairs identification of fear, and improves identification of positive emotions (Bedi et al. 2010; Hysek et al. in press). Taken together these suggest that the effects of drugs on processing of social cues are fairly drug-unique, such that even two stimulant drugs such as MDMA and amphetamine have different effects. One future direction is to examine whether these unique social drug effects contribute to the selection of one drug vs. another as a “drug of choice.”
In this same sample we also tested the effects of amphetamine on emotional reactivity to positive and negative images that were not necessarily social in nature (IAPS pictures), and found that the drug enhanced responses to these positive stimuli (Wardle and de Wit 2012). On the surface, the present finding that amphetamine did not specifically enhance responses to positive facial expressions seems inconsistent with the enhanced reactivity to generally positive pictures. There are several possible explanations for these differences. First, the IAPS task did not measure responses only to faces, but rather positive pictures in general, which included positive nonsocial scenes containing no people at all (e.g. pictures of food and cute animals). There is evidence that neural processing of facial expressions is distinct from processing of other stimuli (Bruce and Humphreys 1994), thus one possibility is that amphetamine may affect these various processes differently. A second possibility has to do with differences in the display and measurement in the two tasks. In the DEIT, the faces were presented dynamically, and the presentations ended when the participant identified the emotion. In contrast, in the IAPS task, participants had 6s to look at and process static pictures. It is possible that more time is needed for amphetamine to “amplify” responses to external positive stimuli and thus the IAPS measure may have been more sensitive to that effect. Third, differences in the attentional demand of the two tasks may have influenced the results. The DEIT is attentionally demanding, whereas in the IAPS task, participants were simply instructed to “look at the picture and think about how it makes you feel.” It is possible that the high attentional demands of the DEIT highlighted the cognitive effects of the drug more than its emotional effects.
There are several limitations of the current study, including a relatively small participant sample with light drug use. The findings might not apply in heavier users either because of pre-existing characteristics or neural adaptations taking place over the course of dependence. Further, as noted above, we did not systematically vary the drug-taking environment, and thus the contribution of a positive environment to these effects is speculative.
The findings of this study emphasize the importance of studying social effects when assessing the rewarding properties of a drug. We found that amphetamine enhanced sensitivity to emotional expressions, regardless of the valence of the emotional expression, and that this effect contributed to increased talkativeness. This mechanism could increase the reward value of the drug when the drug-taking context is rich in positive emotional cues (e.g., friends, other drugs, laughter, sex). Conversely, this mechanism could also contribute to unpleasant drug effects in negative social environments. Thus, the interaction between the pharmacological effect and the social environment may drive use and abuse in ways that would be missed in a neutral laboratory setting. As drugs are typically used in social settings, it is important to study the potentially synergistic effects between drugs and positive or negative social environments.
Acknowledgments
The authors thank Dr. John Cacioppo and his staff at the University of Chicago Center for Cognitive and Social Neuroscience for their technical assistance, Adam D. I. Kramer for statistical consulting and Cassandra Esposito, Celina Joos and Megan Leino for their work on this study. The National Institute on Drug Abuse supported this work through grant R01 DA02812 to Dr. Harriet de Wit. Dr. Wardle is supported by a National Institute on Drug Abuse Training grant, T32 DA007255.
Footnotes
P-values were calculated using the t-distribution with degrees of freedom (d.f.) = n -1, except the binomial eye-gaze data, which uses a z-distribution. D.f. in mixed models are somewhat controversial (Baayen et al. 2008), with a proposed upper bound of the number of trials (over 3,000 in our datasets) minus number of fixed effects. Our selected d.f. was considerably more conservative; however, p-values must be considered estimates.
The authors declare no potential financial or other conflicts of interest.
References
- Attwood AS, Ohlson C, Benton C, Penton-Voak IS, Munafò MR. Effects of acute alcohol consumption on processing of perceptual cues of emotional expression. J Psychopharmacol (Oxf) 2009;23:23–30. doi: 10.1177/0269881108089604. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language. 2008;59:390–412. doi: 10.1016/j.jml.2007.12.005. [DOI] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barger PB, Grandey AA. Service with a smile and encounter satisfaction: Emotional contagion and appraisal mechanisms. Acad Manage J. 2006;49:1229–1238. doi: 10.5465/AMJ.2006.23478695. [DOI] [Google Scholar]
- Bates DM, Meachler M, Bolker B. lme4: Linear mixed-effects model using S4 classes 2011 [Google Scholar]
- Beck AT, Steer R, Carbin M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68:1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CP, Etchells PJ, Porter G, Clark AP, Penton-Voak IS, Nikolov SG. Turning the other cheek: The viewpoint dependence of facial expression after-effects. Proceedings of the Royal Society B: Biological Sciences. 2007;274:2131–2137. doi: 10.1098/rspb.2007.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boys A, Marsden J, Strang J. Understanding reasons for drug use amongst young people: A functional perspective. Health Educ Res. 2001;16:457. doi: 10.1093/her/16.4.457. [DOI] [PubMed] [Google Scholar]
- Bruce V, Humphreys GW. Recognizing objects and faces. Visual Cognition. 1994;1:141–180. [Google Scholar]
- Dawe S, Davis P, Lapworth K, McKetin R. Mechanisms underlying aggressive and hostile behavior in amphetamine users. Current Opinion in Psychiatry. 2009;22:269–273. doi: 10.1097/YCO.0b013e32832a1dd4. [DOI] [PubMed] [Google Scholar]
- de Wit H, Clark M, Brauer LH. Effects of d-Amphetamine in Grouped Versus Isolated Humans. Pharmacology Biochemistry and Behavior. 1997;57:333–340. doi: 10.1016/s0091-3057(96)00316-4. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Karlsson B. Facial reactions to different emotionally relevant stimuli. Scand J Psychol. 1997;38:297–303. doi: 10.1111/1467-9450.00039. [DOI] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Strutured clinical interview for DSM-IV axis I disorders. Biometrics Research Department; New York: 1996. [Google Scholar]
- Gabbay FH. Variations in affect following amphetamine and placebo: Markers of stimulant drug preference. Experimental and Clinical Psychopharmacology. 2003;11:91–101. doi: 10.1037/1064-1297.11.1.91. [DOI] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska Directed Emotional Faces: A validation study. Cognition and Emotion. 2008;22:1094–1118. doi: 10.1080/02699930701626582. [DOI] [Google Scholar]
- Griffiths RR, Bigelow G, Liebson I. Effect of ethanol self-administration on choice behavior: Money vs. socializing. Pharmacology Biochemistry and Behavior. 1975;3:443–446. doi: 10.1016/0091-3057(75)90054-4. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, et al. Drug-produced changes in human social behavior: Facilitation by d-amphetamine. Pharmacol Biochem Behav. 1977;7:365–372. doi: 10.1016/0091-3057(77)90233-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Hughes JR, Bickel WK. Effects of d-amphetamine on choice of social versus monetary reinforcement: A discrete-trial test. Pharmacol Biochem Behav. 1989;34:297–301. doi: 10.1016/0091-3057(89)90315-8. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology (Berl) doi: 10.1007/s00213-012-2645-9. in press. [DOI] [PubMed] [Google Scholar]
- Janowsky DS. Depression and dysphoria effects on the interpersonal perception of negative and positive moods and caring relationships: Effects of antidepressants, amphetamine, and methylphenidate. Current Psychiatry Reports. 2003;5:451–459. doi: 10.1007/s11920-003-0084-3. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology (Berl) 1980;71:275–279. doi: 10.1007/bf00433062. [DOI] [PubMed] [Google Scholar]
- Kano M, Gyoba J, Kamachi M, Mochizuki H, Hongo M, Yanai K. Low doses of alcohol have a selective effect on the recognition of happy facial expressions. Human Psychopharmacology: Clinical and Experimental. 2003;18:131–139. doi: 10.1002/hup.440. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Foisy M-L, Philippot P, Dan B, Tecco J, Noël X, Hess U, Pelc I, Verbanck P. Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatry Res. 2003;119:251–260. doi: 10.1016/s0165-1781(03)00130-6. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cereb Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the study of emotion and attention, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- Larsen JT, Norris CJ, Cacioppo JT. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40:776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Marrone GF, Pardo JS, Krauss RM, Hart CL. Amphetamine analogs methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) differentially affect speech. Psychopharmacology (Berl) 2010;208:169–177. doi: 10.1007/s00213-009-1715-0. [DOI] [PubMed] [Google Scholar]
- McIntosh DN, Reichmann-Decker A, Winkielman P, Wilbarger JL. When the social mirror breaks: Deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Developmental Science. 2006;9:295–302. doi: 10.1111/j.1467-7687.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- Moody EJ, McIntosh DN, Mann LJ, Weisser KR. More than mere mimicry? The influence of emotion on rapid facial reactions to faces. Emotion. 2007;7:447–457. doi: 10.1037/1528-3542.7.2.447. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Downham C, Cowen PJ, Harmer CJ. Direct effects of diazepam on emotional processing in healthy volunteers. Psychopharmacology (Berl) 2008;199:503–513. doi: 10.1007/s00213-008-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ. Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. The International Journal of Neuropsychopharmacology. 2009;12:169–179. doi: 10.1017/S1461145708009164. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Winkielman P, Ramachandran VS. Face to face: Blocking facial mimicry can selectively impair recognition of emotional expressions. Social Neuroscience. 2007;2:167–178. doi: 10.1080/17470910701391943. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Booth RJ, Francis ME. Linguistic Inquiry and Word Count: LIWC 2007. LIWC; Austin, TX: 2007. [Google Scholar]
- Platt B, Kamboj S, Morgan CJA, Curran HV. Processing dynamic facial affect in frequent cannabis-users: Evidence of deficits in the speed of identifying emotional expressions. Drug Alcohol Depend. 2010;112:27–32. doi: 10.1016/j.drugalcdep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Quintero G. Rx for a party: A qualitative analysis of recreational pharmaceutical use in a collegiate setting. J Am Coll Health. 2009;58:64–72. doi: 10.3200/jach.58.1.64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Sage Publications, Inc; 2002. [Google Scholar]
- Sonnby–Borgström M. Automatic mimicry reactions as related to differences in emotional empathy. Scand J Psychol. 2002;43:433–443. doi: 10.1111/1467-9450.00312. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacob GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, California: 1983. [Google Scholar]
- Stevens S, Rist F, Gerlach AL. Influence of alcohol on the processing of emotional facial expressions in individuals with social phobia. Br J Clin Psychol. 2009;48:125–140. doi: 10.1348/014466508x368856. [DOI] [PubMed] [Google Scholar]
- Tamir M, Robinson MD. The happy spotlight: Positive mood and selective attention to rewarding information. Personality and Social Psychology Bulletin. 2007;33:1124–1136. doi: 10.1177/0146167207301030. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter NT, Mutic S, Markett S, Montag C, Klein AM, Reuter M. The influence of alcohol intake and alcohol expectations on the recognition of emotions. Alcohol Alcohol. 2011 doi: 10.1093/alcalc/agr082. [DOI] [PubMed] [Google Scholar]
- Wardle M, Cederbaum K, de Wit H. Quantifying talk: Developing reliable measures of verbal productivity. Behavior Research Methods. 2011;43:168–178. doi: 10.3758/s13428-010-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M, de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology (Berl) 2012;220:143–153. doi: 10.1007/s00213-011-2498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Bodker BK, de Wit H. Effects of setting on the subjective and behavioral effects of d-amphetamine in humans. Addict Behav. 1992;17:27–33. doi: 10.1016/0306-4603(92)90050-6. [DOI] [PubMed] [Google Scholar]