Abstract
Background
Alström Syndrome (ALMS) is an extremely rare multiorgan disease caused by mutations in ALMS1. Dilated Cardiomyopathy (DCM) is a common finding but only one series has been investigated by Cardiac Magnetic Resonance (CMR).
Methods
Eight genetically proven ALMS patients (ages 11–41) underwent CMR performed by standard cine steady state, T1, T2 and Late Gadolinium Enhancement (LGE) sequences. Ejection fraction (EF), Diastolic Volume (EDV) and Systolic Volume normalized for body surface area (ESV), and Mass indices were determined, as well as EDV/Mass ratio, an index expressing the adequacy of cardiac mass to heart volume. Regional fibrosis was assessed by LGE; diffuse fibrosis was measured by a TI scout sequence acquired at 5, 10 and 15 min after gadolinium by comparing inversion time values (TI) at null time in ALMS and control group.
Results
In one patient severe DCM was present with diffuse LGE. There were seven cases without clinical DCM. In these patients, EF was at lower normal limits or slightly reduced and ESV index increased; six patients had decreased Mass index and EDV/Mass ratio. Mild regional non ischemic fibrosis was detected by LGE in three cases; diffuse fibrosis was observed in all cases, as demonstrated by shorter TI values in ALMS in comparison with controls (5 min:152±12 vs 186±16, p 0,0002; 10 min: 175±8 vs 204±18, p 0,0012; 15 min: 193± 9 vs 224±16, p 0,0002).
Conclusions
Cardiac involvement in ALMS is characterized by progressive DCM, associated with systolic dysfunction, myocardial fibrosis and reduced myocardial mass.
Keywords: Alström Syndrome, ALMS1, Dilated cardiomyopathy, Cardiac Magnetic Resonance, fibrosis
Introduction
Alström Syndrome [ALMS, Online Mendelian Inheritance in Man (OMIM #203800)] is an extremely rare autosomal recessive genetic disease caused by mutations in ALMS1, a novel gene of unknown function and expressed in all major tissues (1,2).
ALMS is multisystemic and characterized by progressive pigmentary retinopathy leading to blindness, sensorineural hearing impairment, childhood obesity, short adult stature, dyslipidemia and severe insulin resistance followed by type 2 diabetes mellitus (T2DM). Very high incidences of additional disease phenotypes that may severely affect prognosis and survival include endocrine abnormalities, restrictive lung disease, urinary bladder instability, progressive hepatic and renal failure.
Congestive heart failure (CHF) secondary to dilated cardiomyopathy (DCM) occurs at some time in life in the majority of patients. In 40% there is acute onset in infancy, often followed by atypical clinical normalization of cardiac function. After variable intervals, these patients remain at risk for future recurrence. Another subset of patients, with no previous history of CHF in infancy, develop later-onset progressive DCM or restrictive cardiomyopathy.
Pathologically the most prominent finding is interstitial fibrosis, which has been observed in the kidney, bladder, pancreas, lungs, liver, and ovaries (3,4). Severe to moderate interstitial fibrosis was found in the hearts of seven ALMS cases with medical history of DCM (3,5,6), while mild interstitial myocardial fibrosis was present in one patient without a prior history of DCM (3).
Cardiac Magnetic Resonance (CMR) is a well known powerful tool in the evaluation of cardiomyopathies, in acute and chronic settings (7–12). CMR studies in ALMS have been previously reported in only one series of 7 patients (13). We present in this paper CMR evaluation of myocardial function and the assessment of myocardial tissue in an additional group of ALMS patients.
Materials and Methods
The authors of this manuscript have certified that they comply with the Principles of ethical Publishing in the International Journal of Cardiology (14).
Our series is derived from a group of eleven ALMS patients aged 11–41 years who have been followed for medical management and therapy since 2007 in the Department of Medical and Surgical Sciences of University of Padua. In all cases diagnosis of ALMS was initially based on revised major and minor clinical criteria (15) and subsequently proven by genetic evaluation of ALMS1 (16). Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinky and was approved by the the Ethical Committee of Padua Hospital.
For mutation analysis gDNA was isolated from peripheral blood samples of ALMS patients, was amplified using a standard PCR protocol and amplicons corresponding to exons 8, 10, and 16 of ALMS1 gene were sequenced using ABI 3100 Sequencing Analyzer (Applied Biosystems, Forster City, CA, USA). Sequences were compared with the unaffected control and the mRNA reference sequence (NM_015120.4). The mutations identified were reported in Table 1. All patients carry 2 mutations in the homozygous or in heterozygous (heterozygous compound) form except for patient 6 in which the full sequence of the entire open reading frame (exons 1–23) reveals the presence of only 1 mutation in the heterozygous form.
Table 1. Description of ALMS Patients.
Major, minor criteria and supportive evidence for diagnosis of ALMS in our series are listed, as derived by history, clinical examination and laboratory findings. In children aged 3–14 two major plus one minor criteria are requested for diagnosis; at 15 through adulthood, two major plus two minor or one major plus four minor criteria prove the diagnosis. The mutation nomenclature was established according to den Dunnen et al. (47).
| PATIENT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| GENDER | F | M | M | F | M | F | M | F |
|
| ||||||||
| AGE | 41 years | 40 years | 31 years | 26 years | 20 years | 25 years | 18 years | 11 years |
|
| ||||||||
| Section 1: MAJOR CRITERIA | ||||||||
|
| ||||||||
| MUTATION | ||||||||
| Description | c.[8938C>T] | c.[3425C>G] | c.[2164A>T] c.[11313_11316 delTAGA] |
c.[7304_7305 delAG] c.[10975C>T] |
c.[8782C>T] c.[9901_9902 insC] |
c.[11313_11316 delTAGA] | c.[1769T>A] c.[3425C>G] |
c.[1769T>A] c.[3425C>G] |
|
|
||||||||
| Exon position | 10 | 8 | 8 and 16 | 8 and 16 | 10 and 12 | 16 | 8 | 8 |
|
|
||||||||
| Genotype | homozygous | homozygous | compoud heterozygous | compoud heterozygous | compoud heterozygous | heterozigous | compoud heterozygous | compoud heterozygous |
|
| ||||||||
| RETINOPATHY | ● | ● | ● | ● | ● | ● | ● | ● |
|
| ||||||||
| FAMILY HISTORY OF ALMS | ● | ● | ● | ● | ||||
|
| ||||||||
| Section 2: MINOR CRITERIA | ||||||||
|
| ||||||||
| SENSORINEURAL HEARING IMPAIRMENT | ● | ● | ● | ● | ● | ● | ● | ● |
|
| ||||||||
| CHILDHOOD OBESITY | ● | ● | ● | ● | ● | ● | ● | ● |
|
| ||||||||
| HYPERINSULINEMIA/TYPE 2 DIABETES MELLITUS | ● | ● | ● | ● | ● | ● | ● | ● |
|
| ||||||||
| HYPOGONADISM (Male) HYPERANDROGENISM (Female) | ● | ● | ● | ● | ● | ● | ● | ● |
|
| ||||||||
| HEPATIC DISORDERS | ● | ● | ● | ● | ● | ● | ● | ● |
|
| ||||||||
| SHORT STATURE | ● | ● | ● | ● | ● | ● | ||
|
| ||||||||
| INFANTILE CARDIOMYOPATHY | ● | ● | ● | ● | ||||
|
| ||||||||
| NEPHROPATHY | ● | ● | ● | ● | ||||
|
| ||||||||
| Section 3: SUPPORTIVE EVIDENCES | ||||||||
|
| ||||||||
| RESPIRATORY DISORDERS | ● | ● | ● | ● | ||||
|
| ||||||||
| DYSLIPIDEMIA | ● | ● | ● | ● | ● | ● | ● | |
|
| ||||||||
| GH DEFICIENCY | ● | ● | ● | ● | ● | |||
|
| ||||||||
| HYPERTENSION | ● | ● | ● | ● | ||||
|
| ||||||||
| HYPOTHIROIDISM | ● | ● | ||||||
|
| ||||||||
| SCOLIOSIS/FLAT WIDE FEETS | ● | ● | ● | ● | ● | ● | ● | ● |
|
| ||||||||
| ALOPECIA | ● | ● | ● | ● | ● | ● | ||
|
| ||||||||
| UTI/URINARY DYSFUNCTION | ● | |||||||
UTI: urinary tract infection.
Three out of eleven patients were excluded from the study due to claustrophobia (1 case) or inadequate compliance to CMR examination (2 cases). Therefore our series is comprised of 8 patients (age range 11–41; 4 males) in whom a good quality CMR study could be performed successfully.
All features contributing to diagnosis in this group of patients, as derived by history and thorough clinical and laboratory investigation, are listed in Table 1. In all patients at least 2 major criteria required for diagnosis were present (ALMS1 mutation; family history of ALMS; history of nystagmus, legal blindness, cone and rod dystrophy) (Table 1, section 1). Minor criteria and supporting features are detailed for all patients in Table 1, (sections 2 and 3). Routine non-invasive laboratory and cardiac evaluation prior to CMR was performed in all patients. In addition, all patients underwent CMR using a 1T unit (Siemens Harmony, Maestro class) equipped with cardiologic software, 4 channel phased array body coil and gradient system with maximum strength of 50 T/m/sec. In each study, function was evaluated in breathold by ECG retrogated balanced free precession sequences (SSFP, TR 5 ms, TE 2,5 ms, pixel size 1,6×1,3 mm, temporal resolution 53 ms) by acquiring three long axis (2,3 and 4 chambers views, thickness 6 mm) and a stack of short axis cine images (thickness 8 mm; gap 2 mm) from base to apex according to commonly used protocols.
Morphologic evaluation was achieved by ECG gated T1 and T2 black blood TSE sequences in three long axis and three short axis views (basal, middle and apical, voxel size of 1,8×1,3×7 mm.) A rest perfusion study was then performed by injection of a bolus of gadobutrol (Gadovist, Bayer-Schering) 0,05 mmol/kg followed by saline using a saturation recovery turboFlash sequence (voxel size 2,7×1,4×8 mm, temporal resolution after preparation pulse 161 ms) and acquiring one short axis slice /heartbeat at basal, middle and apical level; further 0,15 mmol/kg of contrast medium were then given immediately after first pass (total amount 0,2 mmol/kg) to detect regional fibrosis by late gadolinium enhancement (LGE) sequences. The LGE study was started 10 min after contrast injection by acquiring the same short and long axis views as in cine study. A conventional segmented turbo flash inversion recovery sequence (voxel size 1,8×1,4×8 mm − TR= 2R−R) was used, adjusting time inversion (TI) for optimal nulling. In addition to the standard protocol to detect diffuse fibrosis an inversion recovery SSFP with incrementally increasing inversion time sequence (TI scout) was acquired in short axis at midventricular level 5,10 and 15 min after contrast injection; in order to maximize temporal resolution and minimize detectable differences in TI segmentation during acquisition was set at 5 and resolution at 128 × 50, resulting in a temporal resolution and increasing TI steps of 16,7 ms, voxel size 5,5 × 2,7 × 8 mm and average breathold time of 18–22 sec, depending on heart rate.
To compare ALMS patients with controls, the same TI scout protocol was performed in a selected group of 10 patients aged 16–52 years that underwent CMR study for minor indications and were considered as normal because of the absence of significant cardiovascular history or risk factors and negative CMR findings.
Image analysis
All images were analyzed in post processing by a radiologist ( FC, with ten year experience in CMR) and a cardiologist ( FT, with seven year experience in CMR) in consensus. Cardiac left ventricular (LV) function was evaluated both visually and by end-systolic (ES)/end-diastolic (ED) thickness ratio (17) with reference to a 16 segment model (18) and segments were graded as a) normal; b) hypokinetic; c) akinetic ; d) dyskinetic. Right ventricular (RV) kinetic was evaluated visually. Images were then transferred to a dedicated workstation (Syngo, Siemens Healthcare, version 2007 c) and epicardial (LV) and endocardial (both ventricles) borders in short axis end systolic and end diastolic cine images were manually traced, thus deriving the following standard functional parameters a) Ejection Fraction (EF); b) End Diastolic Volume (EDV); c) End Systolic Volume (ESV) and d) Myocardial Mass by custom software (Argus). EDV, ESV and Mass values were normalized for body surface area and recorded as volume and mass indices; the mass of the right ventricle was not calculated. For evaluation of our data we referred to normal values (mean, range) provided by our software, which correspond quite closely to values reported by Bellenger (19).
However, because somewhat different reference values have been reported by other authors (20), potentially leading to different interpretation of the same data, the LV mass/EDV ratio (gr/ml, normal values 1–1,2) was calculated, an index that evaluates the relationship between cardiac remodelling and function (21), and expresses the adequacy of cardiac mass to heart volume (22), thus providing an additional criterion for evaluation of cardiac function unrelated to the aforementioned routine parameters.
T1, T2 and first pass images were evaluated visually; on postcontrast late images areas of LGE were first identified visually and then accepted after thresholding with the public domain NIH image program (developed at the U.S. National Institute of Health Bethesda Maryland) when their signal intensity (SI) was above 2 SD of the mean SI of nonenhancing myocardium (7,23).
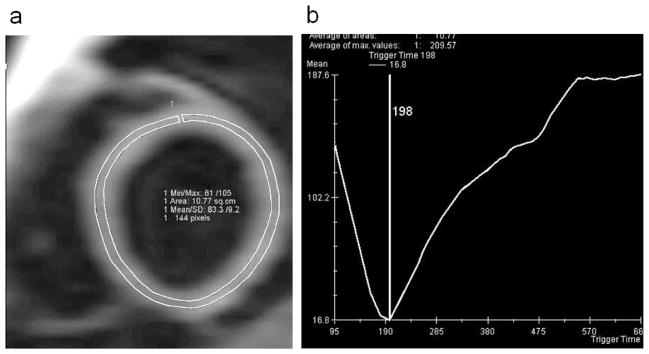
For TI scout images evaluation, a single ROI encompassing all the left ventricle was manually traced at midventricular level, excluding the inner and outer thirds of myocardial wall to avoid artifacts from flowing blood and epicardial fat (Figure 1a). In all series, data acquired at various TI were then fitted to exponential curve using custom software (Mean Curve) and, in each case, a definite value of null time was obtained (Figure 1b). For statistical purposes values of TI obtained at 5, 10, 15 min after contrast medium, as well ROI size and mean age of ALMS patients were compared with control group by Student T test using Windows XP Statistical analysis.
Figure 1. TI scout images evaluation.
a) ROI drawn on time inversion (TI) scout image obtained at midventricular level 10 min after contrast administration in a 16 year normal male. b) data acquired at progressively increasing TI are fitted to exponential curve and a definite myocardial null point is identified (198 msec in the example), reflecting myocardial T1.
Results
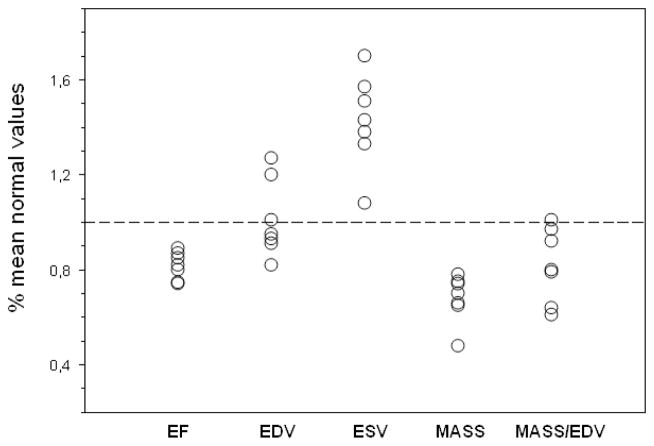
The ages of ALMS patients (27± 10) and the control group (32±15) were not significantly different (p 0,42). Data from routine patient evaluation performed before CMR are listed in Table 2. On kinetic evaluation (Table 3) three patients (case 1,6, and 7) had normal LV systolic thickening of all segments. Mild segmental hypokinesia (ES/ED thickness ratio <1,4) involving septum and/or inferior wall, was observed in 4 cases (case 2,3,4 and 8) and was limited to one or two segments. No obvious segmental dysfunction was observed in the RV in these cases. In the last patient (case 5), septal akinesia and thinning were present, with marked hypokinesia of all other LV segments and of the RV. Results of functional analysis are listed in Table 3. As shown, severe DCM was present in case 5. In the remaining 7 patients, cases without LV segmental dysfunction had preserved EF values, although approximating at the lower limits of normal range; patients with segmental dysfunction had slight reduction of EF. Excluding case 5 because of his obvious and severe DCM, all other patients had preserved LV EDV index but ESV index exceeding normal limits. Moreover, all these patients, except case 2, had a reduction of LV mass index, and mass/EDV ratios were at various degrees below normal limits. A comprehensive overview illustrating LV function in these 7 patients is displayed in the diagram of Figure 2.
Table 2. Clinical, laboratory and cardiological features of ALMS Patients.
Main clinical, laboratory and cardiologic features are listed, as assessed by routine evaluation before cardiac magnetic resonance study. Abnormal values in block letters.
| PATIENT EVALUATION | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Clinical: | ||||||||
| Blood Pressure (mmHg) | 120/70 | 100/70 | 140/100 | 130/80 | 120/75 | 125/70 | 100/70 | 110/75 |
| Pheripheral oedema | no | no | no | no | no | no | no | no |
| Pulmonary crackles | no | no | no | no | no | no | no | no |
| Laboratoristic | ||||||||
| NT-BNP (ng/L) | 16 | 48 | 17 | 35 | 1980 | 75 | 25 | 28 |
| HbA1c% | 8,3 | 5,5 | 9 | 4,8 | 4,8 | 5,9 | 5,2 | 5,3 |
| P-Creatinine (umol/L) | 84 | 108 | 109 | 59 | 97 | 88 | 63 | 51 |
| Tryglicerides (mmol/L) | 1,7 | 3,32 | 6,08 | 3,43 | 0,81 | 1,16 | 1,21 | 1,49 |
| Chol:LDL (mmol/L) | 3,15 | 3,73 | 1,89 | 5,49 | 3,46 | 2,99 | 2,57 | 2,22 |
| Cardiologic | ||||||||
| NYHA Class | I–II | I–II | I–II | I–II | III | I–II | I–II | I–II |
| Echo LV EF% | 58 | 62 | 50 | 49 | 38 | 55 | 54 | 65 |
| ECG | negative | negative | negative | negative | LBBB, parossistic AF | negative | negative | negative |
LBBB: Left bundle branch block; AF: atrial fibrillation ; NT-BNP: N-terminal brain natriuretic peptide; LV EF: left ventricular ejection fraction
Table 3. Cardiac Magnetic Resonance of ALMS Patients.
Left ventricular (LV) and right ventricular (RV) cardiac magnetic resonance functional data in our 8 patients series. Abnormal values in block letters.
| PATIENT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Wall motion abnormalities | |||||||||
|
| |||||||||
| - hypokinetic segments | 2 | 1 | 1 | 11 | 2 | ||||
|
| |||||||||
| - akinetic segments | 5 | ||||||||
|
| |||||||||
| LV function | normal range | ||||||||
|
| |||||||||
| EF | 56–78 | 60,2 | 50,1 | 54 | 55,2 | 23,7 | 58,5 | 57,2 | 53,8 |
|
| |||||||||
| EDV | male 47–92 | 57 | 69,8 | 64 | 74 | 99,8 | 72,9 | 71,5 | 77,6 |
| ml/m2 | female 41–81 | ||||||||
|
| |||||||||
| ESV | male 12,75–30 | 22,7 | 34,9 | 31,8 | 33,1 | 76,2 | 30,2 | 30,6 | 35,8 |
| ml/m2 | female 11,9–20,74 | ||||||||
|
| |||||||||
| LV mass | male 70–113 | 55,6 | 71,1 | 58,4 | 59,8 | 89,3 | 58,6 | 43,9 | 51,6 |
| (ED) g/m2 | female 63–95 | ||||||||
|
| |||||||||
| Mass/EDV | 1–1.2 | 0,97 | 1,01 | 0,91 | 0,8 | 0,89 | 0,8 | 0,61 | 0,66 |
| ratio (g/ml) | |||||||||
|
| |||||||||
| RV function | normal range | ||||||||
|
| |||||||||
| EF | 47–74 | 60,1 | 49,2 | 52,6 | 61,1 | 15,1 | 53,8 | 49 | 44 |
|
| |||||||||
| EDV | male 55–105 | 59,2 | 74,6 | 65,4 | 73,1 | 95,4 | 68,1 | 58,8 | 71,7 |
| ml/m2 | female 48–87 | ||||||||
|
| |||||||||
| ESV | male 15,43–42,91 | 23,6 | 37,9 | 31 | 28,4 | 80,9 | 31,5 | 30 | 40,2 |
| ml/m2 | female 11,01–27,6 | ||||||||
EF: ejection fraction; EDV: end diastolic volume index; ESV: end-systolic volume index; LV Mass: LV myocardial mass index
Figure 2. Cardiac function in seven ALMS patients without clinical dilated cardiomyopathy.
For ejection fraction (EF), end-diastolic volume index (EDV), end-systolic volume index (ESV) and Myocardial Mass index (Mass) values are presented as percent of normal mean values (dotted line). For Mass/EDV ratio a mean reference normal value = 1 was assumed
RV evaluation confirmed severe reduction of EF and increase of ESV index in the patient with severe DCM; in the other 7 cases reduced EF was present in only one patient (case 8) but increased ESV index was documented in three (cases 4, 6 and 8).
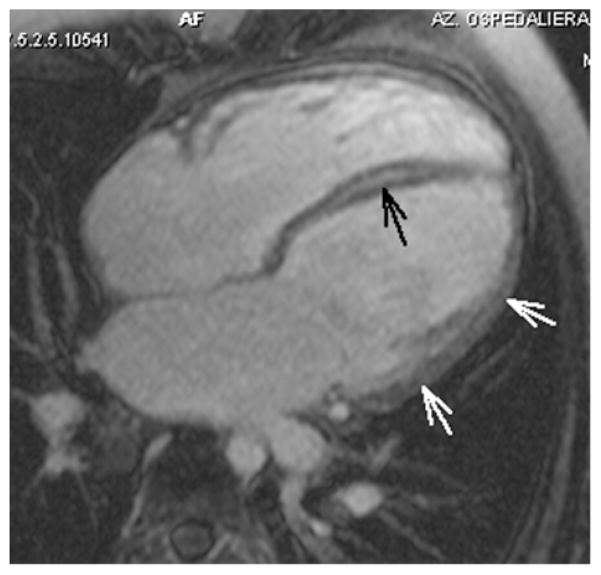
T1, T2 studies were normal in all cases, as well as rest perfusion. On post contrast late images, a diffuse quite homogeneous transmural high signal consistent for LGE involving all segments was observed in case 5, with a thick midwall stria of higher intensity in the septum (Figure 3).
Figure 3. Case 5: four chambers view.
Image acquired 10 min after gadolinium using a routine TI= 190 msec, which nulls normal myocardium at 10 min on our 1T unit. Mild diffuse homogeneous LGE in the LV lateral wall (white arrows), and thick midwall stria in the septum (black arrow). Nulling time at 10 min in this patient, as derived by TI scout sequence, was 113 msec. The data indicate slow gadolinium wash out, suggesting severe diffuse fibrosis.
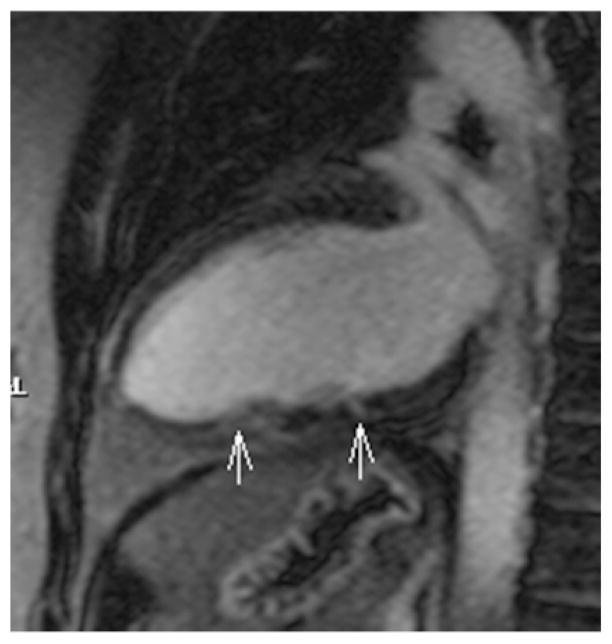
Small focal midwall areas of non ischemic LGE or minimal midwall striae were seen in three patients in the LV, in all cases with partial involvement of only one (case 4 and 6) or two segments (case 2) (Figure 4); in two cases, segments showing LGE were hypokinetic. No LGE was found in four patients in the LV and in all cases in the RV.
Figure 4. Case 2: two chambers view.
Tiny focal LGE and midwall stria in the middle and apical segments of LV inferior wall respectively (white arrows).
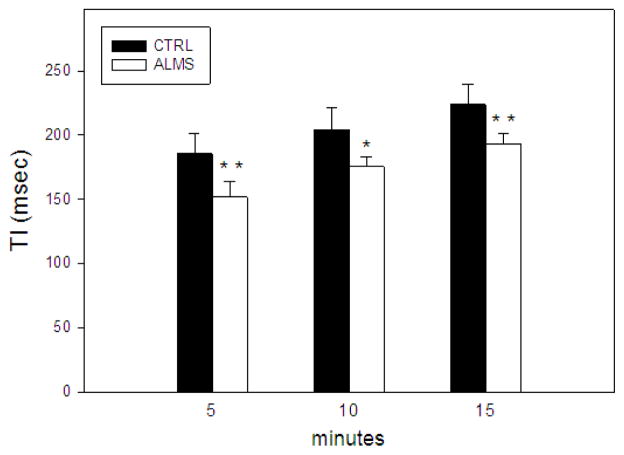
As far as TI scout image evaluation was concerned, no significant differences in number of pixels sampled in ROI drawn at 5, 10 and 15 minutes after contrast injection was found between ALMS patients and the control group (5 min:141±14 vs 148±15, p 0,38; 10 min: 138±15 vs 143±16, p 0,56; 15 min: 138±11 vs 145±13, p 0,29). In no case the ROI drawn at midventricular level encompassed areas of focal LGE. In the 7 patients without or with only mild focal LGE, TI values were significantly lower in ALMS in comparison with controls in all series (Figure 5). When patient 5 with diffuse LGE and markedly reduced TI values was included in the analysis, significant differences were confirmed, (5 min: p=0,0004 ;10min: p=0,0017; 15min p=0,0007) with a somewhat wider standard deviation.
Figure 5. Inversion time sequence analysis.
Significant differences in TI (inversion time) between control group (black bar) and ALMS patients (white bar) without late enhancement (LGE) in midventricular ROI at 5, 10 and 15 min after contrast administration are shown, consistent with interstitial fibrosis. ALMS patients vs control group: *p=0,001; **p=0,0002.
Discussion
ALMS is a monogenic recessive multiorgan disorder with an estimated prevalence < 1:1000000 and approximately 750 cases known worldwide (24). The disease is progressive and is caused by mutations in ALMS1, a novel gene in human chromosome 2 p13 encoding a protein of 4169 aminoacids. Studies have identified more than 100 disease-causing mutations in ALMS1, mostly clustered in exons 8, 10 and 16 (16), but the function of ALMS1 protein remains unclear. However, since it is ubiquitously expressed and localizes subcellularly to basal bodies and centrosomes of ciliated cells, it has been proposed that ALMS1 protein is involved in the functioning of intraflagellar trafficking (25, 26). We recently studied dermal fibroblast cultures from some of the ALMS patients described here and showed that they displayed a constitutively activated myofibroblast phenotype with the up-regulation of collagens’ expression and secretion, thus supporting a genetic basis for ALMS fibrosis (27).
ALMS DCM, which is the most common cause of morbidity and mortality in ALMS (3, 28), is usually related to myocardial fibrosis, given the multiorgan fibrotic involvement that characterizes this syndrome; however, pathologic evidence of cardiac fibrosis has been provided only in a limited number of cases (3, 5,6).
CMR is a widely accepted tool to detect myocardial fibrosis, with excellent correlation with pathology (29, 30). Visualization of fibrosis by LGE sequences relies on delayed washout of gadolinium from tissues with collagen deposition due to increased extracellular space, leading to shorter T1 relaxation time in comparison with normal myocardium and higher signal intensity on T1 weighted images (7). Although LGE sequences depict regional fibrosis accurately, spatial resolution is limited and they do not allow detection of histological diffuse fibrosis. To overcome this limit, the evaluation of T1 relaxation time has been proposed as a quantitative marker of diffuse fibrosis, and validation has been achieved by histology (23). Among methods for T1 quantification, various techniques of T1 mapping have been developed but they require dedicated or experimental software (23), research sequences (31), complicated computation (32, 33) or long examination time (34). In clinical practice an alternative easy and more widely applicable method for T1 estimation is based on values of TI derived by the commercially available TI scout sequences. Since mathematically T1= TI /ln2 (or simplifying T1= TI/≅0,693), an estimation of T1 is possible starting from values of TI at null point in TI scout sequences, and this method has been used by some authors to evaluate T1 effects of contrast agents in coronary angiography (35), myocardial infarct depiction (36) as well as in detection of diffuse fibrosis in DCM (37, 38). By applying this method, values of TI in our ALMS patients were significantly lower than in the control group, reflecting shorter T1 relaxation times in areas without LGE. This supports the presence of diffuse fibrosis seen histologically in ALMS myocardium, and gives strength, despite the limited pathologic evidence reported in literature, to the current opinion that multiorgan fibrotic involvement in ALMS includes myocardium even in the absence of overt clinical DCM. In this context, the mild signs of LGE partially involving one or two segments that we observed in three cases may represent a more extensive focal fibrotic substitution, and explain the mild dysfunction observed in the same segments in two cases.
It should be highlighted that in our functional analysis, excluding patient 5 because of his severe biventricular impairment, the following findings were observed in the other seven patients (summarized in fig 2). First, in all cases EF was slightly reduced or approximated lower normal limits, thus confirming the “lower normal function” previously reported in ALMS patients without clinical DCM (15). Secondly, we observed in all these patients increased ESV index confirming the observations of Loudon (13) and Makaryus (39). Since the increase of ESV index is a main predictor of heart failure in patients with or without history of myocardial infarction (40, 41) and may occur before EF changes (42), the finding of increased ESV index in all our patients suggests a subclinical systolic dysfunction and may represent an additional feature of ALMS cardiomyopathy. Finally, most of these patients had a reduction of LV mass, as noted also by Loudon (13), and normal EDV index, resulting in a decrease of mass/EDV ratio. In this condition an increased afterload may occur, due to a decreased LV wall thickness, and may contribute to systolic dysfunction as the result of a myocardial mass inadequate to ventricular volume. Whether myocardial mass of functional myocytes is reduced by substitutive fibrosis, or other factors play a role can be only speculated. Additionally, given the well known decreased cardiac function and mass in patients with hypopituitarism and the incremental effects of GH therapy (43, 44), it should be noted that GH hormone deficiency is common in ALMS (45) and we observed this condition in 5 of our patients.
Unlike LV, functional impairment of right ventricle was observed in only one patient (case 8); however, the number of patients with increased ESV index was not negligible (3 out of 7), and make feasible the hypothesis of a common pathway for both left and right ventricular dysfunction.
Limitations
The extreme rarity of ALMS and the small number of patients, made a more detailed statistical analysis of data impossible. In our study the evaluation of rest perfusion is of limited value. The low temporal resolution (one slice/heartbeat) of our machine due to the limits in gradient performance is inappropriate for perfusion studies by itself. Moreover, as previously reported by Loudon (13), no resting perfusion defect was observed in our patients and in no case LGE showed a subendocardial ischemic pattern. However, we did not perform any stress perfusion imaging and therefore the possibility of inducible ischemia cannot be excluded.
In no case endomyocardial biopsy confirming the diffuse fibrosis suggested by reduced TI was performed, as it was felt ethically inappropriate in young patients with diagnosis of ALMS, with generally normal or only mildly impaired cardiac function.
Conclusions
Despite that it has not been specifically mentioned in the recent classification of cardiomyopathies by AHA (46), cardiac involvement in ALMS merits consideration as a cardiomyopathy, whose main feature is progressive DCM, in association with systolic dysfunction, diffuse fibrosis and reduced myocardial mass. Causes of the ALMS cardiac involvement remain to be determined, and more than one factor might play a role; unfortunately the extreme rarity of the syndrome makes thorough investigation difficult.
CMR plays an important role as a supporting tool for the diagnosis, the functional quantification and fibrosis detection; moreover, follow up studies for these patients seem advisable, not only to assess progression of heart disease, but also because medical strategies guided by accurate evaluation of cardiac function may mitigate ventricular dilatation and may improve long term outcome.
Acknowledgments
Grant support:
Support for this work was provided by EURO-WABB agreement number 2010 12 05 (VB, GM and PM), and National Institutes of Health HD036878 (JDM and JN).
We are grateful to the patients with Alström Syndrome who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collin GB, Marshall JD, Ikeda A, et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alström syndrome. Nature Genetics. 2002;31:74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- 2.Hearn T, Renforth GL, Spalluto C, et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nature Genetics. 2002;31:79–83. doi: 10.1038/ng874. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JD, Bronson RT, Collin GB, et al. New Alström Syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165:675–683. doi: 10.1001/archinte.165.6.675. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JL, Fialkow P. The Alström Syndrome. Report of three cases with further delineation of the clinical, pathophysiological and genetic aspects of the disorder. Medicine. 1973;52:53–71. doi: 10.1097/00005792-197301000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Warren SE, Schnitt SJ, Bauman AJ, et al. Late onset dilated cardiomyopathy in a unique familial syndrome of hypogonadism and metabolic abnormalities. Am Heart J. 1987;114(6):1522–1524. doi: 10.1016/0002-8703(87)90561-8. [DOI] [PubMed] [Google Scholar]
- 6.Michaud JL, Héon E, Guilbert F, Weill J. Natural history of Alström syndrome in early childhood: onset with dilated cardiomyopathy. J Pediatr. 1996;128:225–229. doi: 10.1016/s0022-3476(96)70394-3. [DOI] [PubMed] [Google Scholar]
- 7.Kim HV, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2010;55:1–16. doi: 10.1016/j.jacc.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 8.Sarafoff N, Vochem R, Fichtner S, et al. Diagnostic value of standard and extended ECG leads for the detection of acute myocardial infarction as compared to contrast-enhanced magnetic resonance imaging. Int J Cardiol. 2011;152:103–105. doi: 10.1016/j.ijcard.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Bielecka-Dabrowa A, Mikhailidis DP, Hannam S, et al. Takotsubo cardiomyopathy. The current state of knowledge. Int J Cardiol. 2010;142:120–125. doi: 10.1016/j.ijcard.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Okayama S, Uemura S, Soeda T, et al. Clinical significance of papillary muscle late enhancement detected via cardiac magnetic resonance imaging in patients with single old myocardial infarction. Int J Cardiol. 2011;146:73–79. doi: 10.1016/j.ijcard.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 11.Kummings KV, Balla S, Javidan-Nejad C, et al. A pattern-based approach to assessment of delayed enhancement to nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29:89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu I, Iguchi N, Watanabe H, et al. Delayed enhancement cardiovascular magnetic resonance as a novel technique to predict cardiac events in dilated cardiomyopathy patients. Int J Cardiol. 2010;142:224–229. doi: 10.1016/j.ijcard.2008.12.189. [DOI] [PubMed] [Google Scholar]
- 13.Loudon MA, Bellenger NG, Carey CM, et al. Cardiac magnetic resonance imaging in Alström syndrome. Orphanet J Rare Dis. 2009;4:14–19. doi: 10.1186/1750-1172-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coats AJ, Shevan LG, Coats AJ. Statement on authorship and publishing ethics in the international journal of cardiology. Int J Cardiol. 2011;153:239–240. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JD, Beck S, Maffei P, et al. Alström Syndrome. Eur J Hum Genet. 2007;15:1193–1202. doi: 10.1038/sj.ejhg.5201933. [DOI] [PubMed] [Google Scholar]
- 16.Marshall JD, Hinman EG, Collin GB, et al. Spectrum of ALMS1 variants and evaluation of genotype-phenotype correlations in Alström Syndrome. Hum Mutat. 2007;28:1114–1123. doi: 10.1002/humu.20577. [DOI] [PubMed] [Google Scholar]
- 17.Bogaert J. Cardiac function. In: Bogaert J, Dymarkowski S, Taylor AM, editors. Clinical Cardiac MRI. Springer Verlag; Berlin Heidelberg: 2005. pp. 99–141. [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 19.Bellenger NG, Pennell DJ. Ventricular function. In: Manning WJ, Pennell DJ, editors. Cardiovascular Magnetic Resonance. Churchill Livingstone; New York: 2002. pp. 99–111. [Google Scholar]
- 20.Maceira AM, Prasad SK, Khan M, et al. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 21.De Castro S, Caselli S, Maron M, et al. Left ventricular remodelling index (LVRI) in various pathophysiological conditions: a real time three-dimensional echocardiographic study. Heart. 2007;93:205–209. doi: 10.1136/hrt.2006.093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field BJ, Baxley WA, Russell RO, et al. Left ventricular function and hypertrophy in cardiomyopathy with depressed ejection fraction. Circulation. 1973;47:1022–1031. doi: 10.1161/01.cir.47.5.1022. [DOI] [PubMed] [Google Scholar]
- 23.Iles L, Pfuger h, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1080. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JD, Maffei P, Collin GB, Naggert JK. Alstrom Syndrome: Genetics and Clinical Overview. Current Genomics. 2011;12:225–235. doi: 10.2174/138920211795677912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hearn T, Spalluto C, Phillips VJ, et al. Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insuline resistance and type 2 diabetes. Diabetes. 2005;54:1581–1587. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Vega R, Nelms K, et al. A Role for Alstrom Syndrome Protein, Alms1, in Kidney Ciliogenesis and Cellular Quiescence. PLoS Genetics. 2007;3(1):e8. doi: 10.1371/journal.pgen.0030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zulato E, Favaretto F, Veronese C, Campanaro S, Marshall JD, Romano S, Cabrelle A, Collin GB, Zavan B, Belloni AS, Rampazzo E, Naggert JK, Abatangelo G, Sicolo N, Maffei P, Milan G, Vettor R. ALMS1-deficient fibroblasts over-express extra-cellular matrix components, display cell cycle delay and are resistant to apoptosis. PLoS One. 2011;6:e19081. doi: 10.1371/journal.pone.0019081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minton JAL, Owen KR, Ricketts CJ, et al. Syndromic obesity and diabetes: changes in body composition with age and mutation analysis of ALMS1 in 12 United Kingdom kindreds with Alström syndrome. J Clin Endocrinol Metab. 2006;91:3110–3116. doi: 10.1210/jc.2005-2633. [DOI] [PubMed] [Google Scholar]
- 29.Kim RJ, Fieno DS, Parrish DB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 30.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 31.Piechnik SK, Ferreira VM, Dall’Armellina E, et al. Shortened modified Look-Locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1. 5 and 3 T within a 9 heartbeat breathold. J Cardiov Magn Reson. 2010;12:69–79. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparrow P, Messroghli DR, Reid S, et al. Myocardial TI mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR. 2006;187:W630–W635. doi: 10.2214/AJR.05.1264. [DOI] [PubMed] [Google Scholar]
- 33.Messroghli DR, Plein s, Higgins DM, et al. Human myocardium: single breat-hold MR T1 mapping with high spatial resolution-Reproducibility study. Radiology. 2006;238:1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 34.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis. Preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 35.Nassenstein K, Waltering K-U, Kelle S, et al. Magnetic resonance coronary angiography with vasovist™: In vivo T1 estimation to improve image quality of navigator and breath-hold techniques. Eur Radiol. 2008;18:103–109. doi: 10.1007/s00330-007-0720-0. [DOI] [PubMed] [Google Scholar]
- 36.Schlosser T, Hunold P, Herborn CU, et al. Myocardial infarct: depiction with contrast-enhanced MR imaging. Comparison of gadopentetate and gadobenate. Radiology. 2005;236:1041–1046. doi: 10.1148/radiol.2363040220. [DOI] [PubMed] [Google Scholar]
- 37.Han Y, Peters DC, Dokhan B, et al. Shorter difference between myocardium and blood optimal inversion time suggests diffuse fibrosis in dilated cardiomyopathy. J Magn Reson Imaging. 2009;30:967–972. doi: 10.1002/jmri.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sueyoshi E, Sakamoto I, Uetani M. Contrast enhanced myocardial inversion time at the null point for detection of left ventricular myocardial fibrosis in patients with dilated and hypertrophic cardiomyopathy: a pilot study. AJR. 2010;194:W293–W298. doi: 10.2214/AJR.09.3414. [DOI] [PubMed] [Google Scholar]
- 39.Makaryus AN, Zubrow ME, Marshall JD, et al. Cardiac manifestation in Alström sindrome: echocardiographic findings. J Am Soc Ecocardiogr. 2007;20:1359–1363. doi: 10.1016/j.echo.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Hamer AW, Takayama M, Abraham MD, et al. End-systolic volume and long-term survival after coronary artery by pass graft surgery in patients with impaired left ventricular function. Circulation. 1994;90:2899–2904. doi: 10.1161/01.cir.90.6.2899. [DOI] [PubMed] [Google Scholar]
- 41.Vasan RS, Larson MG, Benjamin EJ, et al. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 42.White HD, Norris RM, Brown MA, et al. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 43.Amato G, Carella C, Fazio S, et al. Body composition, bone metabolism and heart structure and function in growth hormone (GH) deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab. 1993;77:1671–1676. doi: 10.1210/jcem.77.6.8263158. [DOI] [PubMed] [Google Scholar]
- 44.Maison P, Chanson P. Cardiac effects of growth hormone in adults with growth hormone deficiency. A meta analysis. Circulation. 2003;108:2648–2652. doi: 10.1161/01.CIR.0000100720.01867.1D. [DOI] [PubMed] [Google Scholar]
- 45.Maffei P, Boschetti M, Marshall JD, et al. Characterization of the IGF system in 15 patients with Alstrom syndrome. Clin Endocrinol (Oxf) 2007;66:269–275. doi: 10.1111/j.1365-2265.2007.02721.x. [DOI] [PubMed] [Google Scholar]
- 46.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies, An American Heart Association Scientific Statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 47.den Dunnen JT, Antonarakis SE. Nomenclature for the description of human sequence variations. Hum Genet. 2001;109:121–124. doi: 10.1007/s004390100505. [DOI] [PubMed] [Google Scholar]