Abstract
BACKGROUND
There is a gap of knowledge in the long-term outcomes of patients who have complete recovery of kidney function after an episode of acute kidney injury (AKI). We sought to determine if complete recovery of kidney function after an episode of AKI is associated with development of incident stage 3 chronic kidney disease (CKD) and mortality in patients with normal baseline kidney function.
DESIGN
Retrospective cohort study.
SETTING & PARTICIPANTS
3,809 patients from an integrated healthcare delivery system that had a hospitalization between January 1, 1999 and December 31, 2009 with follow-up through March 31, 2010.
PREDICTOR
AKI defined by ICD-9 codes and using the Acute Kidney Injury Network (AKIN) definition with complete recovery defined by reduction in serum creatinine to less than 1.10 times the baseline value.
OUTCOMES AND MEASUREMENTS
Incident stage 3 CKD persistent for 3 months and all-cause mortality.
RESULTS
After a median follow-up of 2.5 years, incident stage 3 CKD occurred in 15% and 3% of those with and without AKI, respectively, with an unadjusted HR of 5.93 (95% CI, 4.49-7.84) and a HR of 3.82 (95% CI, 2.81-5.19) in propensity score-stratified analyses. Deaths occurred in 35% and 24% of those with and without AKI, respectively, with an unadjusted HR of 1.46 (95% CI, 1.27-1.68). In the propensity score stratified analyses, HR decreased to 1.08 (95% CI, 0.93-1.27).
LIMITATIONS
Measurements of albuminuria were not available.
CONCLUSIONS
Complete recovery of kidney function after an episode of AKI in subjects with normal baseline kidney function is associated with an increased risk of development of incident stage 3 CKD but not all-cause mortality.
Acute kidney injury (AKI) is a condition that is growing more common and that is associated with high in-hospital mortality. Approximately 17 million hospital admissions a year are complicated by AKI leading to an additional health care cost estimated at 10 billion dollars [1]. Furthermore, the incidence of AKI from all causes has increased from 60 to 500 events per 100,000 persons over the past decade [1]. Previous studies have shown that an episode of AKI, independent of the cause, leads to higher rates of in-hospital mortality [2,3]. Recent studies have also demonstrated an increased risk of long-term mortality associated with an episode of AKI [1,4-6]. However, it is important to note that only two studies to date have examined the risk of reversible AKI on all-cause mortality and both only evaluated surgical populations [4,5].
In addition to an increased risk of death, prior studies have also shown that an episode of AKI leads to an increased risk of progression to end stage kidney disease [7,8]. In both of these studies, a significant percentage of subjects had underlying chronic kidney disease (CKD) at baseline. Furthermore, Wald and colleagues only examined severe cases of AKI requiring dialysis [7]. However, less is known about patients that have complete recovery of kidney function after an episode of AKI and there is a gap of knowledge regarding the risk of incident CKD in subjects who have complete recovery of kidney function after an episode of AKI.
We conducted an analysis investigating the association between complete recovery of AKI with incident stage 3 CKD and all-cause mortality in an integrated health care delivery system. We constructed a propensity score in an attempt to estimate an unbiased estimate of the association between AKI and the outcomes of interest.
METHODS
Data Source
A retrospective cohort study was performed using the Intermountain Healthcare Enterprise Data Warehouse, which incorporates comprehensive electronic health and administrative data [9]. Intermountain Healthcare is a non-profit organization with 23 hospitals and over 150 outpatient clinics and averages 130,000 admissions annually [9]. Its facilities range from major adult tertiary-level care centers to small clinics and hospitals that are the only source of care in rural communities.
Cohort Definition
The study sample included all adult patients who had at least one hospitalization between January 1, 1999 to December 31, 2009. All participants (AKI and controls) were required to have administrative and clinical data in the Intermountain Healthcare system at least 90 days prior to the index hospital admission with at least one serum creatinine value to obtain a baseline kidney function. We defined the first AKI hospitalization in this period as the index admission with follow-up beginning at the time of discharge from the inpatient service. All participants (AKI and controls) were also required to have administrative and clinical data in the Intermountain Healthcare system at least one year after discharge. We excluded individuals who were < 18 years old; pregnant; who had an outpatient or inpatient diagnosis of end stage renal disease (ESRD) or an inpatient procedure code for acute hemodialysis; and those with a prior diagnosis of AKI or an estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73m2 by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) prediction equation [10]. Of note, all serum creatinine measurements in the Intermountain Health Care system use a modified kinetic Jaffé method [11]. Figure 1 shows the number of participants with AKI excluded at each step and the final study population. The institutional review board at Intermountain Healthcare System and University of Colorado approved the project.
Figure 1.
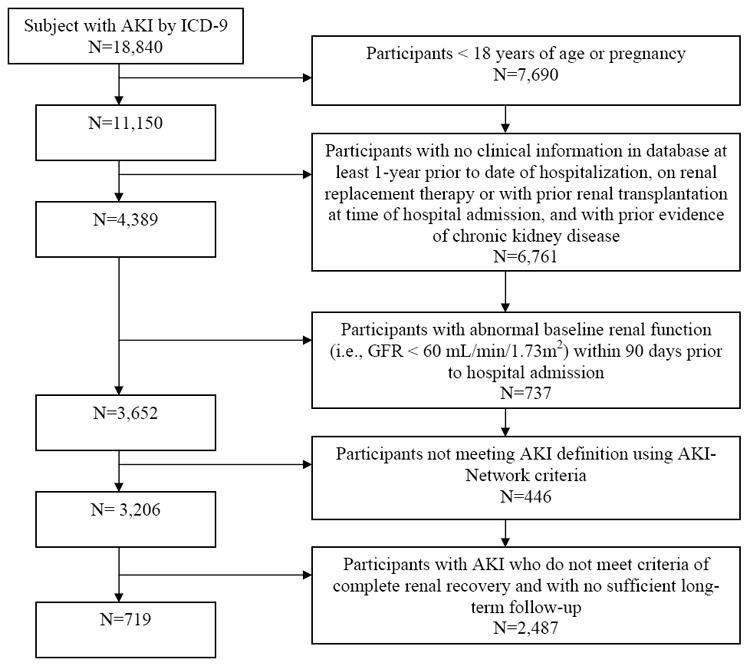
Cohort definition
Definitions
Cases of AKI were identified by having both an ICD9-CM code for AKI (584.0; 584.5; 584.9; 580.9) in an inpatient claim [12] and an AKI-Network (AKIN) definition for AKI. The AKI-Network definition classifies AKI according to changes in serum creatinine concentration and urine output and has been validated in hospitalized patients [13]. Using this definition, AKI participants were identified within the hospitalization by comparing the highest serum creatinine during the hospitalization period (between admission and discharge dates) with the lowest serum creatinine concentration available 90 days before the index admission (baseline creatinine). When the ratio of those two serum creatinine values was ≥1.5, the hospitalization was classified as an AKI participant. AKI events were further categorized by AKIN stage (I, II and III) according to this ratio (1.5 to 2.0, 2.0 to 3.0, and >3.0, respectively). When the creatinine increased by ≥ 0.5 mg/dL and reached 4.0 mg/dL, the encounter was also categorized as with AKIN stage III. The approach of identifying AKI participants using both ICD-9 codes and applying the AKIN definition allowed us to confirm both (a) the presence of AKI based on objective criteria and (b) that a physician noted the AKI such that it was documented administratively.
Patients identified as an AKI participant were required to have complete recovery of kidney function at the time of discharge. Complete recovery of kidney function occurred if the serum creatinine within 7 days of the discharge date returned to a level less than 1.10 (10%) times the baseline serum creatinine. This criterion is significantly more conservative than what has been utilized in prior published studies to define complete recovery of kidney function after an episode of AKI [4,5]. Additional analyses were performed for different definitions of recovery: a return of the serum creatinine to a level less than 1.25 (25%) and 1.50 (50%) times the baseline serum creatinine.
All potential control participants were required to have at least one serum creatinine 90 days prior to the index hospitalization and one serum creatinine during the hospitalization. Thus, the numbers of participants included in the final analysis were 719 participants with AKI and 3,090 controls.
Outcomes
Our main objective was to examine if patients with complete recovery of kidney function after an episode of AKI were at increased risk for incident CKD stage 3 when compared to controls using a propensity score analyses. Change in eGFR was dichotomized to analyze clinically relevant declines in kidney function and was defined as the occurrence of incident cases of stage 3 CKD (defined as a CKD-EPI eGFR < 60 mL/min/1.73m2) that persisted for at least 3 months prior to the end of the follow-up. This outcome was chosen based on previous studies evaluating relevant outcomes for kidney disease progression [14].
A secondary objective was to investigate whether AKI cases with complete recovery of kidney function were at higher risk for all-cause mortality than controls. In addition, the main causes of death in both groups were examined using data collected from death certificates using the State of Utah death Index records.
Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics of AKI and control participants. Baseline characteristics of AKI and control participants were compared using analyses of variance for continuous variables and X2 statistics for categorical variables. Follow-up started at the time of discharge for the index hospitalization and terminated on March 31, 2010, time of event, or, for incident CKD, at the time of death.
We used a logistic regression model to calculate a propensity score estimate of the probability of being in the control or AKI group. The covariates used for the development of propensity score included: age, sex, race, Charlson comorbid conditions (shown in Table 1), hypertension, prior inpatient visits, admission day, and baseline serum creatinine. Performance of the propensity score model was evaluated by assessing whether any important relationships between exposure group and the covariates remained after adjustment.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | AKI Group (n=719) | Control Group (n=3090) | P§ | P for Balance∞ |
|---|---|---|---|---|
| Age, years | 64 [62-66] | 59 [58-59] | <0.001 | 0.9 |
| Female | 46% | 53% | 0.001 | 0.7 |
| Race, White | 91% | 91% | 0.8 | 0.9 |
| Charlson comorbid condition | ||||
| Myocardial Infarction | 20% | 9% | <0.001 | 0.3 |
| Heart Failure | 39% | 15% | <0.001 | 0.1 |
| Peripheral Vascular Disease | 22% | 11% | <0.001 | 0.4 |
| Cerebrovascular Disease | 23% | 13% | <0.001 | 0.5 |
| Dementia | 3% | 3% | 0.9 | 0.8 |
| Pulmonary Disease | 55% | 33% | <0.001 | 0.4 |
| Connective Tissue Disorders | 8% | 6% | 0.01 | 0.9 |
| Peptic Ulcer Disease | 17% | 9% | <0.001 | 0.3 |
| Mild liver disease | 28% | 15% | <0.001 | 0.3 |
| Diabetes | 42% | 21% | <0.001 | 0.5 |
| Diabetes with Complications | 42% | 21% | <0.001 | 0.5 |
| Paralysis | 6% | 3% | 0.001 | 0.6 |
| Cancer | 25% | 23% | 0.3 | 0.9 |
| Severe Liver disease | 4% | 1% | <0.001 | 0.3 |
| Cancer with metastases | 11% | 12% | 0.7 | 0.9 |
| HIV/AIDS | <1 % | <1 % | 0.09 | 0.4 |
| Hypertension | 75% | 45% | <0.001 | 0.9 |
| No. of Hospitalizations before Index Admission | 3 [2-3] | 1 [1-1] | <0.001 | 0.4 |
| Baseline serum creatinine (mg/dL) | 0.90 [0.89-0.90] | 0.90 [0.89-0.90] | 0.4 | 0.9 |
Values for continuous variables given as median [25th-75th percentile]; values for categorical variables given as percentage.
P-value prior applying propensity score stratified analyses
P-value for balance demonstrates that the propensity score stratified analyses was able to correct for all imbalances in the baseline characteristics
AKI, acute kidney injury; HIV/AIDS= Human immunodeficiency virus/ acquired immunodeficiency syndrome
Once the propensity score was built for each participant, we used 3 different methods of analysis to examine the propensity-adjusted relationship between complete recovery of AKI and incident stage 3 CKD and all-cause mortality [15,16]:
Propensity Stratified Analyses:AKI and control participants were grouped into quintiles based on the propensity score. Analysis was based on the pooled estimate after observing homogeneity in the risk estimate across strata. Main result of our analyses represents findings obtained from the propensity-stratified analyses.
Propensity Matched Analyses: AKI participants were matched one-to-one to a control on the basis of the propensity score. For this approach a prespecified greedy algorithm with a 0.01 caliper distance was used. Cases without an adequate match were discarded, as were controls for which another control could be found.
Propensity Regression Analyses: In this approach, the final adjusted Cox proportional model included the continuous propensity score as a covariate for statistical adjustment.
Although main results presented in the manuscript are using the propensity-stratified analyses, results obtained using other propensity score techniques are also reported.
We considered a finding to be statistically significant if the two-sided P value was less than 0.05. All statistical analyses were conducted using R v2.12.1 (R Development Core Team, Vienna, Austria).
RESULTS
Baseline Characteristics
We identified 3,809 participants who met inclusion criteria during the study period. The mean age of the cohort was 58 ± 18 (SD) years and 52% of patients were women. The study population was primarily Caucasian. Of this cohort, 719 (19%) participants were identified on the basis of having complete recovery of kidney function after an episode of AKI. The AKI population included 224 with AKIN stage I, 261 with AKIN stage II, and 234 with AKIN stage III. The baseline characteristics of the sample by AKI status are depicted in Table 1. AKI participants were more likely to be older, male, and have a higher number of admissions prior to index hospitalization. As expected, AKI participants had a higher prevalence of Charlson comorbid conditions including myocardial infarction, heart failure, peripheral vascular disease, cerebrovacular disease, pulmonary disease, peptic ulcer disease, liver disease, diabetes and hypertension. Importantly, no difference in baseline kidney function, as assessed by serum creatinine, was observed. Neither the matched nor pooled stratified propensity score analysis demonstrated residual evidence of confounding at the p=0.05 level (Table 1).
By the definition of inclusion criteria of the cohort, serum creatinine values at baseline and during the index hospitalization were available in 100% of AKI and controls participants. The proportion of patients with serum creatinine available within the 7 days of the discharge date was 100% and 66% for the AKI and control group, respectively. Finally, the median number of serum creatinine measurements during the follow-up period were 11 (25th-75th percentile, 5-24)] and 3 (25th-75th percentile, 1-7) for the AKI and the control group, respectively. The median serum creatinine levels for the control group and each of AKIN stages for the baseline, encounter and discharge period are shown in Table 2.
Table 2.
SCr values During Study Period and by AKIN Stage
| Control (n=3,090) | AKIN Stage I (n=224) | p-value | AKIN Stage II (n=261) | p-value | AKIN Stage III (n=234) | p-value | |
|---|---|---|---|---|---|---|---|
| Minimum Baseline SCr (mg/dL) | 0.90 [0.74-1.00] | 0.92 [0.80-1.02] | <0.001 | 0.90 [0.80-1.00] | 0.7 | 0.80 [0.70- 1.00] | 0.05 |
| Maximum Encounter SCr (mg/dL) | 0.90 [0.70-1.00] | 1.58 [1.40-1.80] | <0.001 | 2.03 [1.80-2.50] | <0.001 | 3.50 [2.80-4.39] | <0.001 |
| Minimum Recovery SCr (mg/dL)* | 0.80 [0.68, 0.90] | 0.80 [0.70, 0.99] | 0.10 | 0.80 [0.68, 0.90] | 0.08 | 0.70 [0.60, 0.90] | 0.001 |
Values given as median [25th-75th percentile]. The p-value= statistical comparison of each AKIN stage with the control group.
recovery defined within 7 days of discharge
AKIN, Acute Kidney Injury Network; SCr, serum creatinine
Relationship between AKI and Progression to CKD
During the median follow-up period of 2.5 (25th-75th percentile, 1.1-5.3) years, 108 (15%) patients with complete recovery of kidney function after an episode of AKI developed incident stage 3 CKD. In contrast, only 97 (3%) of the control patients developed incident stage 3 CKD during the follow-up period. AKI patients were associated with a greater risk of incident stage 3 CKD in crude analysis (unadjusted Hazard Ratio [HR], 5.93; 95% confidence interval [CI], 4.49-7.84; p < 0.001, Table 3). Complete recovery of kidney function after an episode of AKI remained associated with almost a 4-fold increase in the risk of developing incident stage 3 CKD in propensity-stratified analyses (HR, 3.82; 95% CI, 2.81 to 5.19; p < 0.001, Table 3). Similar results were obtained with propensity-matched analyses (HR, 4.08; 95% CI, 2.61-6.36; p <0.001) and propensity-regression analyses (HR, 4.50; 95% CI, 3.29-6.14; p < 0.001).
Table 3.
Association of Complete Recovery of AKI with Incident Stage 3 CKD and All-Cause Mortality
| Crude | Propensity Stratified Analyses | |
|---|---|---|
| Incident Stage 3 CKD | ||
| Control (n=3,090) | 1.00 (Reference) | 1.00 (Reference) |
| AKI group (n=719) | 5.93 (4.49-7.84) | 3.82 (2.81-5.19) |
| All-Cause Mortality | ||
| Control (n=3,090) | 1.00 (Reference) | 1.00 (Reference) |
| AKI group (n=719) | 1.46 (1.27-1.68) | 1.08 (0.93-1.27) |
Values given as hazard ratio (95% confidence interval).
AKI, acute kidney injury; CKD, chronic kidney disease
Figure 2 demonstrates the risk of incident stage 3 CKD in the AKI and control groups according to specific patient characteristics. For all these clinical characteristics, the hazard ratio between the AKI and control group were only significantly different for peripheral vascular disease (p for interaction < 0.001), cerebrovascular disease (p for interaction < 0.05), pulmonary disease (p for interaction <0.01), hypertension (p for interaction <0.01) and baseline serum creatinine (p for interaction < 0.05).
Figure 2.
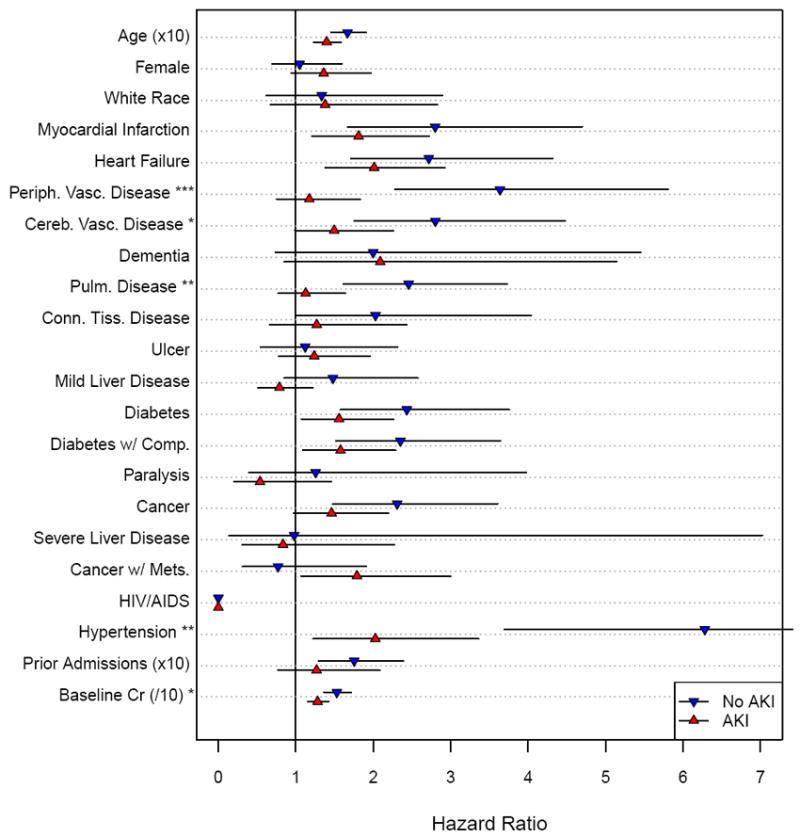
Hazard for Incident Stage 3 CKD by Baseline Participant Characteristics in the AKI and Control groups. ∇= Control (no AKI) group; Δ=AKI group; lines indicate 95% confidence intervals. Asterisks next to characteristic definition indicate p-value for the interaction between group (AKI versus control) and the characteristic (*** = p<0.001, ** = p<0.01, * = p<0.05).
Relationship Between AKI and Risk of Long-Term All-Cause Mortality
During the follow-up period, patients with complete recovery of kidney function after an episode of AKI had a greater risk of all-cause mortality in crude analysis (Table 3). Patients with reversible AKI had an unadjusted HR of 1.46 (95% CI, 1.27-1.68; p < 0.001). This increased risk of long-term mortality did not persist with the use of propensity-stratified analyses (adjusted HR, 1.08; 95% CI, 0.93-1.27; p=0.3). Similar results were obtained with the propensity-matched (adjusted HR, 1.13; 95% CI, 0.93-1.37; p=0.2) and propensity-regression analyses (adjusted HR, 1.14; 95% CI, 0.97-1.34; p=0.1). The main causes of death were malignancy, cardiovascular disease, and infection.
Sensitivity Analyses
To evaluate the strength of our definition of complete renal recovery within 10% of the baseline serum creatinine, we performed additional analyses defining renal recovery as a reduction in serum creatinine to less than 1.25 (25%) and 1.50 (50%) times the baseline serum creatinine. When using these definitions of renal recovery after an episode of AKI, we observed an increase in the hazard for incident stage 3 CKD in propensity-stratified analyses (HRs of 3.87 [95% CI, 2.87-5.21] and 4.21 [95% CI, 3.17-5.60], respectively, p < 0.001 for both cutoffs). Similar to the primary analyses, we only observed an association with death in the unadjusted analyses but not in the propensity-stratified analyses (HRs of 1.09 [95% CI, 0.95-1.27] and 1.07 [95% CI, 0.93-1.23], respectively, p > 0.20 for both cutoffs). Identical results were obtained when using propensity-matched and propensity-regression analyses.
DISCUSSION
This study demonstrates the increased long-term risk of incident stage 3 CKD in patients who have complete recovery of kidney function after an episode of AKI. Even after accounting for confounding variables that are known to contribute to kidney function decline, this higher risk of new onset stage 3 CKD persisted. In these analyses we only observed a relationship between complete recovery of kidney function and all-cause mortality in unadjusted models, which was markedly attenuated in the propensity score analyses. Thus, patients who experience AKI in the hospital should be monitored closely and have aggressive management of other risk factors that may contribute to loss of further kidney function despite complete recovery of kidney function at discharge.
A recent meta-analysis of 13 cohort studies comprising more than 3,000 patients performed by Coca et al examined the risk of incident stage 3 CKD and death after an AKI episode [17]. Outcomes for kidney disease progression (i.e., incident CKD and ESRD) was reported in only 6 out of the 13 studies and showed that AKI patients had a higher hazard for developing new onset CKD (pooled adjusted HR, 8.8; 95% CI, 3.1-25.5). Only 1 of the 6 studies included in this meta-analysis stratified AKI by recovery status [18]. In this study, the investigators examined the long-term clinical consequences of AKI in human immunodeficiency virus (HIV). Interestingly, this group of investigators found that the adjusted risk for kidney disease progression was present in participants with AKI who recovered kidney function. However, recovery was defined as a decrease in serum creatinine below the threshold level for stage 1 AKI which was defined as a crude serum creatinine increase of 0.3 mg/dl or greater, or a relative increase between 150 and 200%.
In the meta-analysis by Coca and colleagues, all-cause mortality in patients with and without AKI was also assessed. The risk for all-cause mortality was 2.0 (95% CI, 1.3-3.1) for participants with AKI when compared to non-AKI participants [17]. To note, when the investigators removed a study by James and colleagues [19] because of the high rate of death in the AKI group reported in this study, the pooled adjusted HR was attenuated to 1.6 (95% CI, 1.3–2.1). Again, none of these studies included patients with complete recovery of kidney function. In our study, we only observed a relationship with all-cause mortality in unadjusted analyses, which became statistically non-significant in the propensity score analyses. These findings raise the question of whether prior studies that found AKI to be a strong risk factor for death adequately adjusted for other important confounders.
Animal models have demonstrated the underlying histologic changes that occur after an episode of AKI with recovery. Basile et al [20] treated male rats with 60 minutes of bilateral renal ischemia followed by reperfusion. In this model of ischemic AKI, rats that had recovery of kidney function had normal tubular morphology by one month. However, the onset of proteinuria developed by week 16 and progressed to approximately 275 mg/d per 100 g by week 40 (p <0.05). There was also damage noted in the peritubular capillaries. Furthermore, tubulointerstitial fibrosis developed at week 40, a finding not present at weeks 4 and 8. Hence, it is plausible that an episode of AKI followed by complete recovery of kidney function may be associated with worsening kidney function decline.
The strengths of our study include the long duration of follow-up, the detailed participant clinical data, and the ability to determine the rate of kidney function decline over time. We were able to account for important confounders that are known to increase the rate of kidney function decline, thus strengthening our ability to establish the relationship between renal recovery after AKI and progression to incident stage 3 CKD.
Nevertheless, our study does have limitations. First, despite a careful propensity score analyses, we cannot exclude the possibility of residual confounding by hemoglobin and serum phosphorus concentration and usage of drugs such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and non-steroidal anti-inflammatory drugs. Second, sicker patients had a greater number of creatinine measurements, leading to a higher chance of being identified with CKD during the follow-up period (ascertainment bias). However, in this analysis we did not require participants to have serum creatinine during the follow-up period. Third, we used ICD-9 codes and change in serum creatinine to identify patients that developed AKI during a hospitalization that may have underestimated the true number of cases of AKI. Nonetheless, our AKI classification includes both administrative and pathophysiological evidence of AKI. Fourth, we included only participants that were receiving care in the Intermountain Healthcare System. Although the selected participants could have had serum creatinine drawn outside the Intermountain system, this factor is unlikely to invalidate our findings as our analyses are based on a large number of patients from our source population and represent standard clinical practices in a large geographical region served by a large healthcare system organization. Fifth, we defined AKI as occurring from any cause and did not separate the cases based on different etiologies. Thus, we were unable to detect if the risk of progression to CKD or mortality differs amongst different causes of AKI. Furthermore, our study was a retrospective cohort design, thus causal inferences cannot be made.
In conclusion, our study demonstrates that even with complete recovery of kidney function after an episode of AKI, there is an increased long-term risk of progression to incident stage 3 CKD. Even after accounting for traditional risk factors for kidney disease progression, the increased risk persisted in this AKI group. Thus, we propose that complete recovery of kidney function after an episode of AKI should be considered an independent risk factor for decline in kidney function over the long-term in older Caucasian patients. Further studies should be done to confirm these results in other groups of patients.
Acknowledgments
Support: The research reported in this study was supported by the American Heart Association Grant 10POST423001 and a Genzyme Nephrology Fellowship Award. Additional support came from the National Institute of Diabetes and Digestive and Kidney Disease grant R01 DK081473 and R01 DK078112.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coca S, Yusuf B, Shilpak M, Garg A, Parikh C. Long-term Risk of Mortality and Other Adverse Outcomes After Acute Kidney Injury: A Systematic Review and Meta-analysis. Am J of Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Mendonca A, Vincent J, Suter P, et al. Acute Renal Failure in the ICU: Risk Factors and Outcomes Evaluated by the SOFA Score. Intensive Care Med. 2000;26(7):915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 3.Groeneveld A, Tran D, van der Meulen, Nauta J, Thijs L. Acute Renal Failure in the Medical Intensive Care Unit: Predisposing, Complicating Factors and Outcome. Nephron. 1991;59(4):602–610. doi: 10.1159/000186651. [DOI] [PubMed] [Google Scholar]
- 4.Hobson C, Yavas S, Segal M, et al. Acute Kidney Injury is Associated with Increased Long-term Mortality After Cardiothoracic Surgery. Circulation. 2009;119(18):2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 5.Bihorac A, Yavas S, Subbiah S, et al. Long-term Risk of Mortality and Acute Kidney Injury During Hospitalization After Major Surgery. Ann Surg. 2009;249(5):851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 6.Lafrance JP, Miller DR. Acute Kidney Injury Associates with Increased Long-Term Mortality. J Am Soc Nephrol. 2010;21(2):345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R, Quinn R, Luo J, et al. Chronic Dialysis and Death Among Survivors of Acute Kidney Injury Requiring Dialysis. JAMA. 2009;302(4):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 8.Ishani A, Xue J, Himmelfarb J, et al. Acute Kidney Injury Increases Risk of ESRD Among Elderly. J Am Soc Nephrol. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intermountain Healthcare. 2009 Annual Report. [January 5th, 2012]; Available at: http://intermountainhealthcare.org/about/overview/annualreport2009/Pages/ClinicalStatistics.aspx.
- 10.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Walser M. Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis. 1998;32(1):23–31. doi: 10.1053/ajkd.1998.v32.pm9669420. [DOI] [PubMed] [Google Scholar]
- 12.American Medical Association Hospital International Classification of Diseases, 9th Revision, Clinical Modification Codes. 2009 [Google Scholar]
- 13.Mehta R, Kellum J, Shah S, et al. Acute Kidney Injury Network: Report of an Initiative to Improve Outcomes in Acute Kidney Injury. Critical Care. 2007;11(2):1–811. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29(20):2137–2148. doi: 10.1002/sim.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115(17):2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 17.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int. 2010;78(5):478–485. doi: 10.1038/ki.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James MT, Hemmelgarn BR, Wiebe N, et al. Alberta Kidney Disease Network. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 20.Basile D, Donohoe D, Roethe K, Osborn J. Renal Ischemic Injury Results in Permanent Damage to Peritubular Capillaries and Influences Long-term Function. Am J Physiol Renal Physiol. 2001;281(5):F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]


