Abstract
Working memory (WM) is considered a core deficit in Attention-Deficit/ Hyperactivity Disorder (ADHD), with numerous studies demonstrating impaired WM among children with ADHD. We tested the degree to which WM in children with ADHD was improved by performance-based incentives, an analog of behavioral intervention. In two studies, WM performance was assessed using a visuo-spatial n-back task. Study 1 compared children (ages 9-12 years) with ADHD–Combined type (n=24) to a group of typically developing (TD) children (n=32). Study 1 replicated WM deficits among children with ADHD. Incentives improved WM, particularly among children with ADHD. The provision of incentives reduced the ADHD-control group difference by approximately half but did not normalize WM. Study 2 examined the separate and combined effects of incentives and stimulant medication among 17 children with ADHD-Combined type. Both incentives and a moderate dose of long-acting methylphenidate (MPH; ~0.3 mg/kg t.i.d. equivalent) robustly improved WM relative to the no-incentive, placebo condition. The combination of incentives and medication improved WM significantly more than either incentives or MPH alone. These studies indicate that contingencies markedly improve WM among children with ADHD–Combined type, with effect sizes comparable to a moderate dose of stimulant medication. More broadly, this work calls attention to the role of motivation in studying cognitive deficits in ADHD and in testing multifactorial models of ADHD.
Keywords: Attention-Deficit/Hyperactivity Disorder, ADHD, working memory, incentives, reinforcement, methylphenidate
Introduction
Though Attention-Deficit/Hyperactivity Disorder (ADHD) is diagnosed on the basis of symptoms of inattention and/or hyperactivity-impulsivity (American Psychological Association [APA], 2000), both theory and empirical research highlight a number of additional cognitive and motivational processes implicated in the disorder (e.g., Nigg, 2003; Sonuga-Barke, 2002). In the cognitive domain, there is considerable interest in working memory, a multi-component system responsible for the storage and manipulation of on-line, short-term information that is critical for learning, decision-making, and sustaining goal-directed behavior (e.g., Baddeley, 2007). Deficits in working memory among children with ADHD are robust, with the largest impairments on tasks that require executive control in dealing with visual and spatial information (i.e., visuo-spatial working memory; e.g., meta-analytic reviews of Martinussen, Hayden Hogg-Johnson, & Tannock, 2005; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2002; Willcutt et al., 2005). Of course, no single cognitive process, including working memory, is highly specific or sensitive to ADHD (Pennington et al., 2005; Willcutt et al., 2005). However, working memory does have ecological validity in predicting observable inattentive behavior in ADHD (Kofler, Rapport, Bolden, Sarver, & Raiker, 2010), and preliminary evidence suggests that training working memory may be an effective treatment for the disorder (e.g., Klingberg et al., 2005).
In the motivational domain, there are several theories of ADHD that emphasize motivation in general and reward and reinforcement in particular. Specifically, ADHD may be associated with problems in the reward threshold (e.g., Haenlein & Caul, 1987) and may be characterized by a steeper reinforcement gradient (e.g., Sagvolden et al., 2005), or general discounting of delayed consequences (e.g., Luman, Tripp, & Scheres, 2010; Shiels et al., 2009; Sonuga-Barke, 2005) The clinical parallel is the clear efficacy of contingency management for ADHD (Fabiano et al., 2009). Although traditionally cognitive and motivational models have sometimes been pitted against one another, an emerging literature is evaluating the degree to which the two domains interact. Specifically, a growing number of studies provide evidence that motivational processes may influence, or moderate, cognitive processing in ADHD (see reviews by Luman et al., 2005, 2010).
Despite the interest in motivation-cognition, there is very little published research examining incentive effects on working memory in ADHD (in contrast to response inhibition, another putative core process in ADHD, e.g., Oosterlaan and Sergeant, 1998; Scheres et al., 2001; Slusarek et al, 2001). Shiels and colleagues (2008) used a computerized spatial span task designed to separately test online storage and maintenance of visuo-spatial information (forward span condition), as well as manipulation of stored information (backward span condition). Incentive effects were only apparent during the backward span condition, suggesting that provision of immediate reward is associated with performance improvements during visuo-spatial working memory tasks that require mental storage and manipulation of visual information. However, this prior study did not include a control group, and without comparison to typically developing peers, it remains unclear whether or not a working memory deficit was present in this sample, or if improved performance in response to incentives constituted working memory normalization. Additionally, the study examined performance on a spatial span task that included only two trials for each span presented in order of increasing load. Although the authors hypothesized that the observed incentive effects should increase with task difficulty, this could not be clearly evaluated due to the small number of trials per span, and because working memory load and time on task were confounded (i.e., backward span always followed forward span). The current study aimed to address the extent to which incentives may improve or normalize working memory in children with ADHD in comparison to a control group of typically developing (TD) children across varying levels of WM load. This was accomplished with the use of a visuo-spatial n-back paradigm.
N-back Working Memory Paradigm
The visuo-spatial n-back task is a neuro-cognitive paradigm designed to measure working memory performance across multiple task loads requiring executive control of visuo-spatial information. The task requires participants to monitor a series of presented stimuli while indicating whether or not each stimulus is the same as a stimulus presented n number of trials back in the sequence (n = 0,1, 2, etc.). Typically, n varies across blocks of trials, often 0-back, 1-back, and 2-back. Task difficulty is graded across blocks, making the n-back task an excellent candidate for investigating group differences in response to varying levels of task load. Indeed, the few published studies of the n-back among children with ADHD (e.g., Karatekin Bingham & White, 2009; Klein et al., 2006; Shallice et al., 2002) have generally demonstrated working memory deficits among children with ADHD, with greater deficits often apparent as n-back load is increased (see also the work of Ehlis et al., 2008, in adults). Kobel et al. (2008) have also demonstrated that methylphenidate normalized group differences in working memory performance among children with ADHD in comparison to TD children on an n-back task, although this effect did not vary across n-back load.
Study 1
Study 1 examined the effects of incentives on WM among children with ADHD, in comparison to TD peers across varying levels of task load. Consistent with prior research (e.g., Alderson, Rapport, Hudek, Sarver, & Kofler, 2010; Kofler et al., 2010; Martinussen, Hayden-Hogg-Johnson, & Tannock, 2005; Rapport et al., 2008; Willcutt et al., 2005), we predicted that children with ADHD would exhibit poorer visuo-spatial WM, particularly when the manipulation and updating of working memory were required (i.e., 1- and 2-back compared to the 0-back). Although we hypothesized that incentives would improve WM performance among children with ADHD and TD controls, we expected children with ADHD to show the greatest improvement with incentives and when manipulation and updating of working memory were required. Finally, we evaluated the extent to which incentives normalized working memory among children with ADHD. Paralleling studies in which medicated children with ADHD are compared to unmedicated controls, our normalization test compared the performance of children with ADHD under incentive conditions to the performance of TD children under no-incentive conditions.
Study 1 Methods
Participants
Participants were recruited to participate in a “Research Camp” examining the influence of incentives on neurocognitive processes in 9 to 12-year-old children. Most participants in the ADHD group (n = 24) were clinic-referred, whereas participants in the TD control group (n = 32) were recruited through flyers in pediatricians’ offices and schools and advertisements in local periodicals1. Parents received modest monetary remuneration; children were rewarded with prizes and gift cards.
All children in the ADHD group met Diagnostic and Statistical Manual of Mental Disorders (4th ed., or DSM–IV; American Psychiatric Association, 2000) criteria for ADHD– Combined type. Following a preliminary phone screening, parents and teachers of prospective participants completed a DSM-IV symptom checklist (Disruptive Behavior Disorder Rating Scale [DBD-RS]; Pelham, Fabiano, & Massetti, 2005; Pelham, Gnagy, Greenslade, & Milich, 1992) and Impairment Rating Scale (IRS; Fabiano et al., 2006). All children included in the ADHD group were required to have 6 or more symptoms of inattention and 6 or more symptoms of hyperactivity/impulsivity on the DBD-RS (with parent/teacher overlap on at least one symptom in each domain), and clinically significant impairment on the IRS. Parents of children meeting the ratings scale criteria were invited to complete a structured computerized clinical interview (Diagnostic Interview Schedule for Children Version IV [DISC-IV]; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000); a DISC-IV diagnosis of ADHD-Combined type was required to be eligible for the ADHD group. The psychometric properties of the DISC-IV ADHD module are favorable, including adequate test-retest reliability (.79), and internal consistency (.60) (Shaffer et al., 2000). As part of this structured interview, children were also assessed for psychiatric comorbidity, and the typical pattern of high levels of comorbid externalizing disorders were observed.
Children included in the control group exhibited less than 4 symptoms of inattention and hyperactivity/impulsivity on the DBD-RS, were free of significant impairment on the IRS (all scores < 3 on the 0-6 scale), and did not meet diagnostic criteria for ADHD or any other behavioral disorder on the DISC-IV.
Exclusionary criteria for both groups included (1) estimated IQ less than 80 (using the Vocabulary and Block Design subtests from the Wechsler Intelligence Scale for Children - Fourth Edition; Wechsler, Kaplan, Fein, Kramer, Delis, & Morris, 2004), (2) parent report of lifetime diagnosis of pervasive developmental disorder, schizophrenia or other psychotic disorders, (3) history of seizures or other medical conditions that could be predictably worsened by stimulant medication , (4) and uncorrected vision/auditory problems that would make it difficult to complete the task. Additional measures of externalizing symptomatology (Child Behavior Checklist and Teacher Report Form, Achenbach & Rescorla, 2001), internalizing symptomatology (Revised Children's Manifest Anxiety Scale [RC-MAS], Reynolds & Richmond, 2005, and Child Depression Inventory [CDI], Saylor, Finch, Sporito, and Bennett, 1984), and academic achievement (Woodcock-Johnson Test of Achievement [WJTA], Woodcock, McGrew, & Mather, 2001) were used to characterize the sample, but not in determining eligibility (see Table 1).
Table 1.
Study 1 sample characteristics for each group
| Control (n=32) | ADHD (n=24) | p-value | |
|---|---|---|---|
| Age in years, mean (SD) | 10.9 (1.1) | 10.8 (1.1) | .87 |
| Gender, % female | 19% | 12% | .50 |
| Race, % minority | 16% | 12% | .98 |
| WISC estimated full scale IQ, mean (SD) | 112 (12) | 108 (12) | .17 |
| WJ Test of achievement, mean (SD) | |||
| Letter-word identification | 110 (11) | 103 (11) | .02 |
| Calculation | 112 (10) | 104 (15) | .01 |
| Spelling | 110 (13) | 99 (13) | < .01 |
| ADHD Symptoms | |||
| Inattentive, DBD-RS mean (SD)* | |||
| Parent report | <1 (.4) | 8 (1.2) | < .001 |
| Teacher report | <1 (.4) | 6 (3.9) | < .001 |
| Hyperactive/Impulsive, DBD-RS mean (SD)* | |||
| Parent report | <1 (.2) | 6 (1.7) | < .001 |
| Teacher report | <1 (.2) | 5 (2.5) | < .001 |
| Attention Problems subscale t-score (SD) | |||
| Parent report (CBCL) | 51.3 (2.4) | 72.4 (7.5) | < .001 |
| Teacher report (TRF) | 51.2 (2.9) | 66.3 (6.9) | < .001 |
| ODD Symptoms | |||
| DBD-RS mean (SD)* | |||
| Parent report | 0 (0) | 4 (2.5) | < .001 |
| Teacher report | 0 (0.2) | 2 (3.0) | < .001 |
| ODD Problems subscale t-score (SD) | |||
| Parent Report (CBCL) | 51.0 (2.3) | 67.0 (8.1) | < .001 |
| Teacher (TRF) | 51.3 (2.9) | 61.6 (8.5) | < .001 |
| CD Symptoms | |||
| DBD-RS mean (SD)* | |||
| Parent report | 0 (0) | 1 (1) | < .001 |
| Teacher report | 0 (0) | <1 (1) | .001 |
| CD Problems subscale t-score (SD) | |||
| Parent Report (CBCL) | 51.6 (3.1) | 65.7 (9.0) | < .001 |
| Teacher Report (TRF) | 51.4 (3.0) | 61.0 (8.0) | < .001 |
| Internalizing Symptoms | |||
| Child Depression Inventory (CDI) t-score (SD) | 44 (4.6) | 49 (7.9) | .004 |
| Revised Children's Manifest Anxiety Score (RC-MAS) overall t-score (SD) | 45 (9.3) | 53 (8.6) | .002 |
Note. These values represent the total number of symptoms endorsed on the DBD-RS (i.e., rated as ‘pretty much’ or ‘very much’).
Procedures
All procedures were approved by the Child and Youth Institutional Review Board at the State University of New York at Buffalo. The n-back task described here was conducted as part of a larger battery of tasks designed to assess the influence of incentives across a wide range of cognitive processes in children with and without ADHD. These tasks were administered during laboratory sessions occurring between the hours of 9:30 am to 5:30 pm. These cognitive tasks were conducted within the context of a camp-like atmosphere in which children played games, made arts and crafts, and ate lunch and snacks with 1 - 4 other children, with task periods interspersed throughout the day. All participants who were actively taking stimulant medication (n = 12) discontinued use at least 24 hours prior to each testing day.
The n-back task was performed during two separate sessions scheduled one-week apart (n = 4 completed within 2 - 4 weeks). During the first session, participants only completed the baseline version of the n-back task in order to familiarize the participants with the task and ensure understanding of task instructions, provide baseline data in the absence of incentives, and to allow testing of group differences prior to administration of the incentive manipulation version of the task. The baseline version was administered in a fixed order increasing in difficulty (i.e., starting with the 0-back, followed by the 1-back, and ending with the 2-back condition). Although this order confounds time on task and working memory load, pilot testing suggested that an ascending difficulty sequence was necessary on first exposure to the task to ensure children in this age range could reasonably understand the task. During the second session one week later, all children performed the n-back task again to evaluate the effects of incentives. Load order was randomized and counterbalanced across participants, and incentive and no-incentive conditions alternated within load. Children were given a short practice at the start of each load condition to ensure continued understanding of task instructions from the prior week. This session was completed in a sound attenuated chamber and heart rate, neck and leg EMG were collected to provide preliminary data for a future study (details available from the authors).
N-Back Visuo-spatial Working Memory Task, Baseline Version
The baseline visuo-spatial n-back task was similar to those used in recent research with children and adults (see also Ehlis et al., 2005; Jaeggi et al., 2010; Postle, D'Espisito, & Corkin, 2005; Shallice et al., 2002). The task was programmed in E-Prime (Psychology Soft ware Tools, Inc.; programs available from the authors) and run on a desktop PC. Stimuli were presented on a 16” CRT monitor, and responses were registered with the outermost buttons of a PST serial response box. Stimuli were grey circles, ~5 cm in diameter, that were presented for 100 ms, followed by a 2900 ms response window. A fixation cross was present at all times.
The baseline n-back task included 3 conditions of varying difficulty levels: the 0-back, the 1-back, and the 2-back presented in ascending order. Participants were instructed to respond on every trial (100 trials per condition), indicating whether the stimulus was a target (30% of stimuli) or non-target (70%) using 2 buttons on the response box labeled “yes” or “no.” During the 0-back, participants were instructed to press the “yes” button if the stimulus appeared in the upper left corner (i.e., target) and to press the “no” button if the stimulus appeared in any other location (i.e., non-target). The 0-back served as a benchmark condition, during which children engaged in a task that did not require manipulation or updating of information in working memory, but was otherwise analogous to the other n-back conditions. During the 1-back, participants were instructed to press the “yes” button whenever the stimulus appeared in the same location as the stimulus that immediately preceded it, and the “no” button whenever the stimulus appeared in any other location. During the 2-back, participants were instructed to press the “yes” button whenever the stimulus appeared in same location as the stimulus that preceded it by 2 trials, and to press the “no” button if the stimulus appeared in any other location.
N-Back Visuo-spatial Working Memory Task, Incentive Version
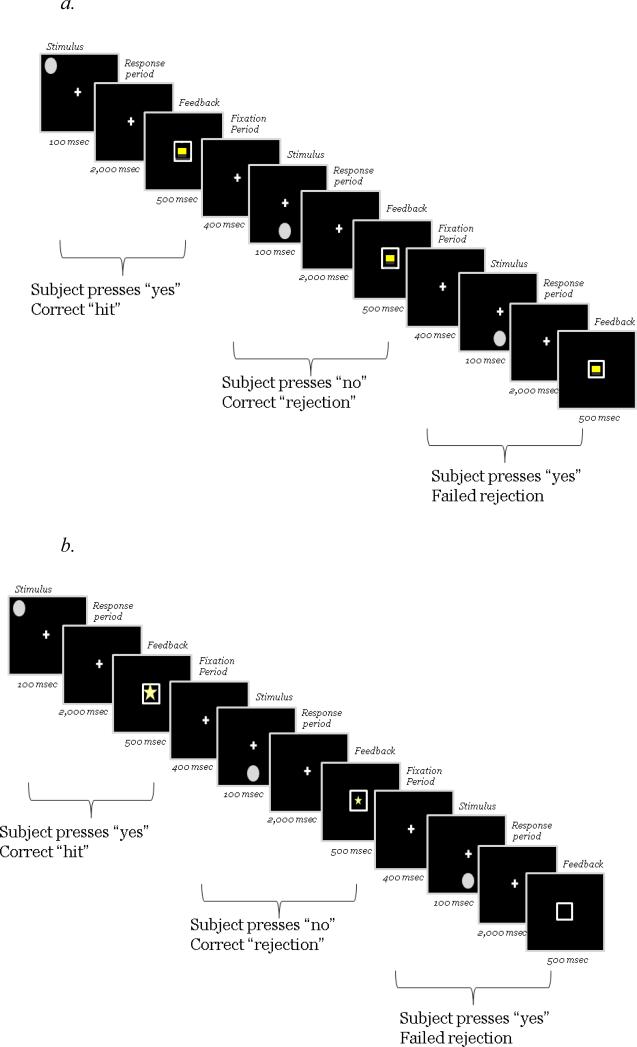
Each difficulty level (0-, 1-, 2-back; order counterbalanced) consisted of four blocks of 50 trials, alternating between blocks with incentives and blocks without incentives. Incentive order (whether a child received an incentive block 1st or a no-incentive block 1st) was counterbalanced between subjects, since incentive order may influence working memory task performance in children with ADHD (see Shiels et al., 2008). Each trial consisted of a 100-ms n-back stimulus presentation, 2000-ms response window, 500-ms feedback presentation, and 400-ms inter-stimulus interval (see Figure 1). The fixation cross was present at all times, except for during feedback. Total trial duration was identical for the baseline and incentive versions of the task, but the incentive manipulation doubled the number of trials compared to the baseline visit.
Figure 1.
Schematic of trial sequence in the 0-back condition for the no-incentive (Panel a) and incentive (Panel b) visits.
Feedback during the incentive conditions was provided inside a white box that appeared in the center of the screen (see Figure 1b). On correct trials, the box contained a large star worth five points for target hits or a small start worth two points in the case of correct rejections of non-targets. More points were provided for target hits than correct rejections in order to prevent response bias toward rejections since only 30% of stimuli were targets.2 Points (maximum of ~1800, each worth ~0.01 USD) earned during the incentive conditions were exchanged for toys, games, or gift cards available in a small laboratory “point store” at the end of the day.
Feedback following incorrect responses during incentive conditions was an empty white box (in order to evaluate the effects of reward alone prior to investigating mixed contingencies), children did not lose points in the case of an error). During the no-incentive conditions (Figure 1a), a non-informative yellow square in a white box appeared on every trial, regardless of accuracy, and no points were awarded.
Statistical Analyses
Analyses focused on percent accuracy. Non-responses and premature responses (within the 100-ms stimulus presentation) were considered inaccurate. Percent accuracy was computed for each block.
Baseline day accuracy data were analyzed with a 2 Group (ADHD vs. TD) × 3 Load (0-back, 1-back, 2-back) ANOVA. For load, single-df orthogonal contrasts examined accuracy as a function of the absence or presence of a working memory manipulation requirement (0- vs. 1- and 2-back) and of increasing demands on working memory manipulation (1- v. 2-back).3
Data from the incentive task were analyzed similarly, except that incentive condition (incentive vs. no incentive) and trial block (2 blocks of 50 trials each) were additional within-subjects factors. In order to test the extent to which incentives normalized working memory among children with ADHD, ADHD group performance during the incentive conditions was compared to control group performance during the no incentive conditions with a 2 Group (ADHD-Incentive, TD-No-Incentive) × 3 Load ANOVA.
Effect sizes are reported as Cohen's d (Cohen, 1988).
Study 1 Results
Sample Characteristics
Table 1 provides descriptive and inferential statistics on demographics, intelligence and achievement, and dimensional measures of ADHD, ODD, CD, anxiety, and depression. Importantly, children with ADHD and the TD control group were comparable on age, gender, race, and estimated IQ. As expected, the two groups differed robustly on all measures of ADHD. Children with ADHD also exhibited greater average levels of ODD and CD, as well as anxiety and depression, than did the TD control group.
Baseline visit
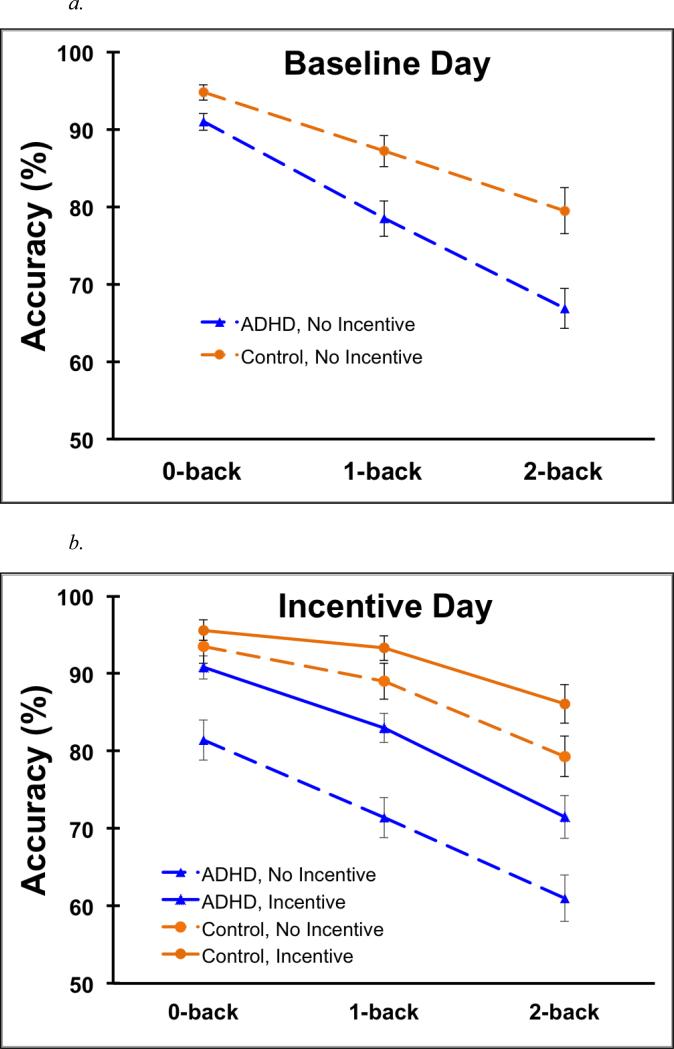
Figure 2a provides mean (SD) accuracy on the n-back for all Group × Load conditions at the baseline visit. As expected, accuracy significantly declined when working memory manipulation was required, 0-back v. 1- and 2-back, F(1,52) = 101.9, p < .001, d = 1.28; with further decline as working memory task demands increased, 1- v. 2-back, F(1,52) = 37.5, p < .001, d = .8. Also as predicted, children in the ADHD group demonstrated lower accuracy than the TD control group, F(1,52) = 12.6, p = .001, d = .85. This group difference was greater during the WM manipulation conditions (i.e., 1- and 2-back), F(1, 52) = 11.3, p < .001, d = 1.15, than during the 0-back, F(1,52) = 6.25, p = .016, d = .63, Group × 0- v. 1- and 2-back F(1,52) = 5.38, p= .024, d = .7; however, performance did not vary between WM manipulation conditions, Group × 1- v. 2-back, F(1,52) = 1.6, p= .21, d = .31.
Figure 2.
Mean n-back accuracy for all Group × Load conditions during the baseline visit (Panel a) and all Group × Incentive × Load conditions during the incentive visit (Panel b) of Study 1. Bars are standard error.
Incentive visit
Figure 2b provides mean (SD) accuracy on the n-back for all Group × Load × Incentive conditions at the incentive visit. As can be seen, the pattern of group and load effects for the incentive task replicated those observed for the baseline task (Figure 2a). Specifically, accuracy declined when working memory manipulation was required, 0-back v. 1- and 2-back, F(1,52) = 80.9, p < .001, d = 1.11; with further decline as WM task demands increased, 1- v. 2-back, F(1,52) = 53, p < .001, d = .96. Children with ADHD had lower accuracy than controls, F(1,52) = 24, p < .001, d = 1.09, and this group difference was greater during the WM manipulation conditions, F(1, 52) = 24.8, p < .001, d = 1.11, than during the 0-back, F(1, 52), = 11.26 p = .001, d = .83, Group × 0-back v. 1- and 2-back, F(1,52) = 7.8, p < .01, d = .57. This group difference in performance did not vary further between the two working memory manipulation conditions, 1- and 2-back, Group × 1- v. 2-back, F(1,52) = .9, p = .34, d = .18.
On average, accuracy was greater during incentive compared to no-incentive conditions, F(1,52) = 88.5, p < .001, d = 1.02. Improvement with incentives was more pronounced during WM manipulation conditions, F(1,52) = 100.2, p < .001, d = 1.17, compared to the 0-back, F(1, 52) = 24.6, p < .001, d = .53, Incentive × 0-back v. 1- and 2-back, F(1,52) = 5.0, p = .03, d = .3. The effect of incentives did not differ between WM manipulation conditions, Incentive × 1- v. 2-back, F < 1, p = .59, d = .11.
Most importantly, incentives led to greater improvement in accuracy among children with ADHD, F(1,52) = 77.1, p <.001, d = 1.28, compared to the TD control group, F(1,52) = 17.9, p < .001, d = 1.1, Group × Incentive F(1,52) = 14.9, p < .001, d = .9. However, improvement with incentives was consistent across load conditions, Group × Incentive × 0- v. 1- and 2-back and Group × Incentive × 1- v. 2-back Fs(1,52) = .68 and 2.3, ps = .41 and .14.
No interactions with group, incentive, or load significantly changed across the two 50-trial repetitions of each condition, all block Fs< 3.1, ps > .08.4
Normalization
On average, accuracy remained lower among the ADHD group during incentive conditions than among the control group during no incentive conditions, group F = 4.5, p < .05, d = .55. However, a marginal Group (ADHD-Incentive v. TD-No-incentive) × 0-back v. 1- and 2-back interaction, F(1,52) = 3.1, p = .08, suggested that incentives normalized average performance during the 0-back, F(1, 52) = 1.5, p = .23 , d = .33, but not during the WM manipulation conditions, F(1,52) = 5.0, p = .03, d = .58, Group × 1- and 2-back, F(1,52) = .4, p = .52.
Study 1 Discussion
In brief, the findings in Study 1 paralleled the hypotheses, replicating working memory deficits among children with ADHD and demonstrating that those deficits were greater when the visual information had to be manipulated (i.e., the 1- and 2-back). Incentives improved performance in both groups but more so among the ADHD group. Study 1 suggested that performance-based incentives markedly attenuated the group differences in working memory but did not fully normalize the process at the group level.
Before discussing these findings or their implications in greater depth, we present Study 2, which examined the separate and combined effects of incentives and stimulants on working memory among children with ADHD. This approach parallels the clinical literature that has established contingency management, stimulant treatment, and their combination as front-line evidence-based interventions for the disorder (American Academy of Pediatrics [AAP], 2011; Fabiano et al., 2009; Pliszka, 2007). Moreover, because several studies demonstrate beneficial effects of methylphenidate on aspects of working memory in ADHD (e.g., Bedard & Tannock, 2008; Bedard, Jain, Johnson, & Tannock, 2007; Bedard, Martinussen, Ickowicz, & Tannock, 2004, Tannock, Ickowicz, & Schachar, 1995), stimulant treatment provides a good benchmark against which to evaluate the emerging data (Shiels et al., 2008; Study 1, above) on performance-based incentives.
In addition, the combination of incentives and stimulants provides an analogue of combination treatment in real-world settings. Though the combination of contingency management and stimulant therapy may produce more improvement than either modality alone (e.g., Carlson et al., 1992; Pelham et al., 1993; 2005; Fabiano et al., 2007) and is a recommended standard of care (AAP, 2011), the analogue of combination treatment is rarely examined (c.f., Epstein et al., 2011a; Solanto et al., 2001) in the otherwise extensive literature (e.g., Nigg et al., 2005; Willcutt et al., 2005) on cognitive processes in ADHD.
Study 2 crossed the incentive manipulation used in Study 1 and of a standard clinical dose of long-acting methylphenidate (Plizska, 2007) in a fully within-subjects design. Because of this medication component, only children with ADHD were included. We predicted that both incentives and stimulants would improve working memory, especially when working memory manipulation and continuous updating was required, and that the combination of incentives and methylphenidate would yield greater improvement than either treatment manipulation alone.
Study 2 Methods
Participants
Participants were 17 children (75% male) between the ages of 9 and 12 years5 who met diagnostic criteria for ADHD–Combined type and all other inclusion and exclusion criteria as described for Study 1 (see Table 2 for sample characteristics). Participants were recruited from those who had completed a previous study of either incentives (Study 1; n = 10) or stimulants (n = 7) on neuro-cognitive processes within the past two years.
Table 2.
Study 2 sample characteristics
| Age in years, mean (SD) | 10.7 (1.0) |
| Gender, % female | 19% |
| Race, % minority | 6% |
| WISC estimated full scale IQ, mean (SD) | 109 (10) |
| WJ Test of achievement, mean (SD) | |
| Letter-word identification | 104 (9) |
| Calculation | 107 (14) |
| Spelling | 103 (11) |
| ADHD Symptoms | |
| Inattentive, DBD-RS mean (SD)* | |
| Parent report | 8 (1.8) |
| Teacher report | 5 (3.5) |
| Hyperactive/Impulsive, DBD-RS mean (SD)* | |
| Parent report | 7 (1.8) |
| Teacher report | 5 (2.9) |
| CBCL Attention Problems subscale t-score (SD) | 73.0 (7) |
| TRF Attention Problems subscale t-score (SD) | 65.1 (10.4) |
| ODD Symptoms | |
| DBD-RS mean (SD)* | |
| Parent report | 4 (2.6) |
| Teacher report | 1 (2.1) |
| CBCL ODD Problems subscale t-score (SD) | 66.1 (8.9) |
| TRF ODD Problems subscale t-score (SD) | 60.9 (8.9) |
| CD Symptoms | |
| DBD-RS mean (SD)* | |
| Parent report | 1 (.9) |
| Teacher report | <1 (12) |
| CBCL CD Problems subscale t-score (SD) | 66.4 (8.3) |
| TRF CD Problems subscale t-score (SD) | 59.8(9.9) |
| Internalizing Symptoms | |
| Child Depression Inventory (CDI) t-score (SD) | 47.7 (5.9) |
| Revised Children's Manifest Anxiety Score (RC-MAS) overall t-score (SD) | 49.3 (10.7) |
Note. These values represent the total number of symptoms endorsed on the DBD-RS (i.e., rated as ‘pretty much’ or ‘very much’).
Procedures
Participants attended a “Research Camp” (as in Study 1) on two consecutive days to allow a fully within-subjects Medication (MPH v. placebo) × Incentive (Incentive v. No-Incentive) design. Medication condition varied between days and incentive condition alternated within day. Medication order and incentive order were counterbalanced between subjects.
Children who were currently taking stimulant medication discontinued medication use for at least 24 hours prior participation. Children were administered active medication or placebo in a double-blind fashion shortly after their 7:30 a.m. arrival (medication order was counterbalanced), and cognitive testing began 90 minutes after administration. During the medication condition, children were administered long-acting OROS methylphenidate (MPH). Dose was selected to provide the nearest commercially available equivalent of 0.3mg/kg t.i.d. of instant release MPH (median OROS dose in MPH equivalents = 0.29mg/kg t.i.d.; SD = 0.02). For two subjects, tolerability concerns (n = 1 stimulant naïve; n = 1 prior clinical dose less than .15mg/kg MPH IR t.i.d.) resulted in use of the next lower available dose which approximated .27 mg/kg MPH t.i.d. of IR MPH. In order to maintain blinding to medication condition, children received identical opaque capsules on each day of testing. Placebo capsules contained micronized methylcellulose.
During “research camp,” children completed the n-back task with an incentive manipulation. This task was identical to the n-back incentive version described in Study 1, except that the fixed 0-back target location was replaced with four possible locations (all corners; randomly assigned). Each child completed the task during one of several possible testing periods throughout the day, and this timing was counterbalanced across children.
Statistical Analyses
Percent accuracy was analyzed with a 2 Medication (placebo vs. MPH) × 2 Incentive (incentive vs. no incentive) × 3 Load (0-back, 1-back, 2-back) repeated measures ANOVA. As in Study 1, planned contrasts examined: 1) performance on the 0-back versus 1-back and 2-back in order to isolate the conditions with a working memory manipulation (i.e., 1-back and 2-back), and 2) 1-back versus 2-back to assess the effects of increasing the load of the working memory manipulation.6. Effect sizes are reported as Cohen's d (1988).
Study 2 Results
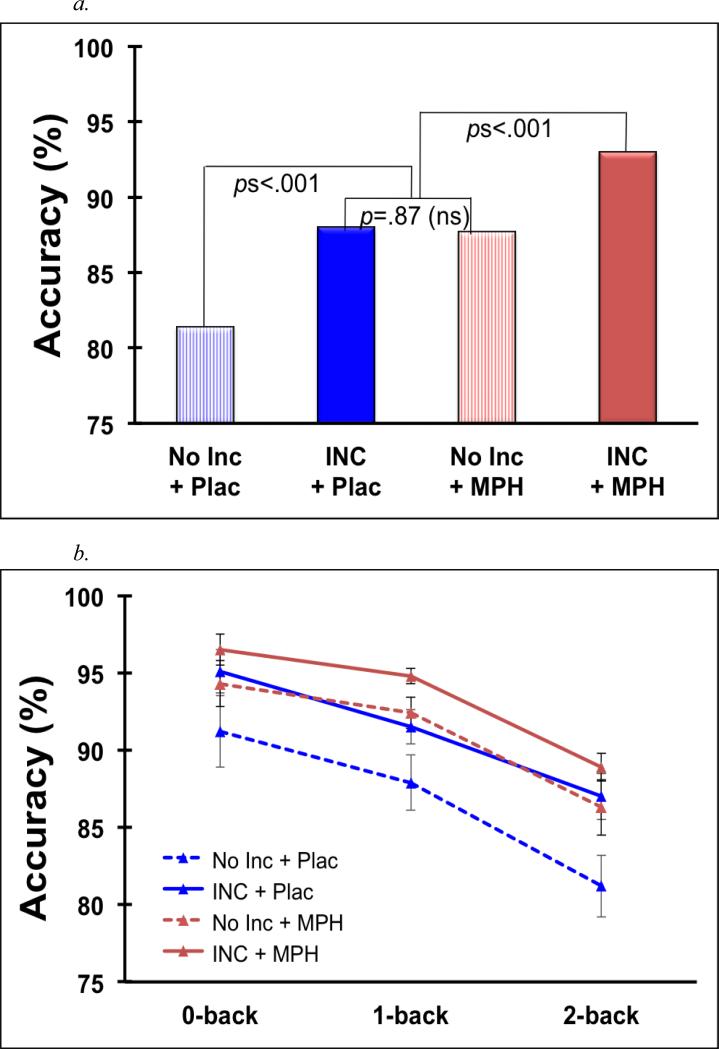
As expected, accuracy (%) significantly declined when working memory manipulation was required, 0-back v. 1- and 2-back, F(1,16) = 26.87, p < .001, d = 1.26, and declined further as the WM manipulation demands increased, 1- v. 2-back, F(1,16) = 10.9, p < .01, d = .8 (see Figure 3a).
Figure 3.
Mean n-back accuracy for all Medication × Incentive × Load conditions in Study 2 (Panel a). Bars are standard error. In Panel b, data are averaged across load to facilitate comparisons among the Medication × Incentive conditions. INC = incentive, MPH = methylphenidate, Plac = placebo.
As observed in Study 1, accuracy was greater under incentive compared to no-incentive conditions, F(1,16) = 33.1, p < .001, d = 1.4, and improvement with incentives was more pronounced during WM manipulation conditions (1- and 2-back), F(1,16) = 31.1, p < .001, d = 1.35, than during the 0-back, F(1,16) = 12.2, p = .003, d = .85, Incentive × 0- v. 1- and 2-back F (1,16) = 4.6, p < .05, d = .68. Also as in Study 1, the incentive effect did not vary between 1- and 2-back conditions, F < 1.
Similar to the observed incentive effects, accuracy improved with MPH compared to placebo, F(1,16) = 22.9, < .001, d = 1.16. The effects of MPH were marginally greater during the WM manipulation conditions, MPH × 1- and 2-back F(1,16) = 23.1, p < .001, d = 1.17, compared to the 0-back, MPH × 0-back F(1, 16) = 7.4, p = .015, d = .66, MPH × 0- v. 1- and 2-back F(1,16) = 3.8, p = .07, d = .47; the amount of improvement with medication did not differ between the 1- and 2-back, F<1.
Notably, the effects of incentives and MPH on accuracy did not interact, Incentive × MPH, Incentive × MPH × 0- vs. 1- and 2-back, and Incentive × MPH × 1- vs. 2-back Fs <1.2, ps > .3. However, given interest in comparing the magnitude of separate and combined incentive and MPH effects, we conducted post-hoc pairwise tests, averaged across load (see Figure 3b). Compared to no treatment, both incentives alone and MPH alone improved accuracy, F = 19.9, ps = .001, .002, ds = 1.04, 0.88. The separate effects of incentives and MPH on accuracy did not differ, F = 19.9, p = .87, d = .04. The combined effect of incentives and MPH resulted in greater accuracy than did either incentives or MPH alone, F = 19.9, ps ≤ .001, ds = 1.02, 1.06. (These same patterns were evident when examining each load individually.)
Discussion
The present work examined the impact of performance-based incentives on visuo-spatial working memory among children with ADHD. Study 1 included a comparison group of typically developing controls and evaluated the degree to which working memory was improved, or even normalized, by incentives. Study 2 examined the separate and combined effects of incentives and a low-to-moderate dose of stimulant medication among an ADHD sample.
Studies 1 and 2 provide clear evidence that incentives improve visuo-spatial working memory in an ADHD sample. These data replicate and extend Shiels and colleagues’ (2008) preliminary finding that incentives enhance task performance when working memory manipulation was required (i.e., backward span). However, n-back task parameters also disentangle working memory load and time on task (a problem with the span task used by Shiels et al.), and demonstrate clear effects of load on task performance. In the present work, incentive effects were stronger in the 1- and 2-back conditions, which require mental manipulation of visuo-spatial information, than in the 0-back condition, which does not. This is broadly consistent with Shiels and colleagues’ (2008) evidence from the spatial span task that incentive effects are stronger when active manipulation and updating of material in working memory is required or, more generally, when task difficulty is moderate-to-high.
Importantly, Study 1 included a comparison sample of typically developing children, and the study replicated the robust working memory deficits commonly identified among children with ADHD (e.g., Martinussen, Hayden Hogg-Johnson, & Tannock, 2005; Nigg, Willcutt, Doyle, & Sonuga- Barke, 2005; Willcutt et al., 2005), a pattern consistent with models implicating WM as a core deficit in the disorder (e.g., Rapport et al., 2008). Paralleling the effect of incentives, this group difference was consistently greater under loads requiring manipulation, compared to the 0-back. This finding is consistent with previous studies demonstrating greater ADHD performance deficits, relative to controls, during larger n-back loads (e.g., Klein et al., 2006; Kobel 2008), though other studies have observed only a general deficit across n-back loads (e.g., Karatekin, Bingham, & White, 2009; Shallice, et al., 2002).
Though the effects of incentives and ADHD group membership are each interesting, the key question addressed by Study 1 was determining the extent to which working memory improvements with incentives varied between groups. As hypothesized, incentives facilitated working memory more among children with ADHD than among controls. This pattern could be interpreted as reflecting a prominent role for motivation over cognition in understanding core deficits in ADHD. However, our normalization analysis suggests this is not that case: incentives reduced the ADHD group performance deficit, but the ADHD group performance remained significantly below that of their TD peers without reward (i.e., incentives did not normalize ADHD group performance). This finding suggests that a core deficit in motivation is not likely to fully account for the working memory deficits commonly observed among clinically referred children with ADHD. That said, incentives markedly attenuated the ADHD deficit in working memory.
A very similar pattern was observed in a recent study by Dovis, Van der Oord, Wiers, and Prins (in press). Dovis et al. examined the effects of motivation on a novel working memory span task among children with and without ADHD. Relative to a feedback-only condition (trial-by-trial auditory and visual stimuli), performance was improved among children with ADHD equally by the addition of 1 or 10 euro rewards for performing “well enough” (in reality, all children were given the reward, regardless of performance) and a videogame-like context. However, none of these enhanced motivation conditions normalized working memory. Performance among controls did not vary across motivational contexts. Though a number of differences between the Dovis et al. study and the present work will be considered below, the two studies generally complement one another and indicate the importance explicitly considering, or even manipulating, the motivational context in which working memory is assessed in ADHD (see also Luman et al., 2005, 2010).
The importance of the motivational context may also be evaluated against stimulant medication, a well-studied and empirically supported treatment for ADHD (Greenhill et al., 2002; Plizska 2007). Extending independent demonstrations that incentives (Dovis et al., in press; Shiels et al., 2008; Study 1) and stimulants (e.g., Bedard & Tannock, 2008; Bedard, Jain, Johnson, & Tannock, 2007) improve aspects of working memory, Study 2 revealed that incentives were just as effective as a moderate dose of methylphenidate in improving working memory among an ADHD sample. Both incentive and methylphenidate effects on working memory were large and easily detected despite the small sample. Moreover, the equivalence of incentives and methylphenidate was not due to a ceiling effect on performance, as the combination of incentives and methylphenidate yielded greater improvement than either alone (see Figure 3b).
We expect that the pattern we observed – robust but equivalent and additive effects of behavioral and pharmacological manipulations on working memory – would be altered by changing the strength of either manipulation. As is typical in the literature examining the cognitive effects of stimulants (Pietrzak et al., 2006), we examined only one active dose of medication. The effects of methylphenidate on spatial working memory can be dose-dependent, though the exact nature of that dose-response function appears to be inconsistent across tasks (e.g., Bedard et al., 2004).
Studies of incentives on cognition are even less likely to examine a dose-response function (c.f., Dovis et al., in press; Luman et al., 2007). The incentive manipulation used in the present work was arguably strong relative to other studies of incentive effects among children with ADHD (see Luman et al., 2005). Our manipulation was guided by theory suggesting that children with ADHD require salient, immediate and consistent reinforcement and may be more likely to discount the value of delayed or more variable reinforcement (e.g., Sagvolden et al., 2005; Tripp & Wickens, 2008; see also review of Luman et al., 2010). Therefore, points were provided on a continuous schedule, with clear visual feedback after each response. Children used their points as currency to shop in an on-site “store” stocked to match their pre-specified preferences. Maximal performance on the 45-minute n-back task yielded $18US in purchasing power.
In contrast, many studies of incentive effects on cognition in ADHD do not provide clear trial-by-trial consequences and feedback, and they often provide modest prizes (e.g., a sheet of stickers, a piece of candy, or a small toy) or prizes of only one value regardless of performance (e.g., Epstein, Langberg, et al., 2011; Tripp & Alsop, 2001; Konrad, Gauggel, Manz, & Schöll, 2000). Dovis et al. (in press) nicely manipulated reward magnitude, comparing working memory performance across 0, 1, and 10 euro reward conditions among children with and without ADHD. The only significant aspect of reward dose was that WM accuracy was better in the 1 and 10 euro conditions than in the 0 euro condition, but only among children with ADHD. The relatively weak differences across incentive doses can be interpreted within the reinforcement framework discussed above. First, all conditions contained trial-by-trial feedback, which is a low magnitude reinforcer – the feedback was consistent, accurate, and immediate. For typically developing children, an incentive effect may only be observed when the baseline condition contains no extrinsic reinforcement (present Study 1); even a modest contingency (e.g., trial-by-trial feedback) may maximize their performance (e.g., Dovis et al., in press).
The dose-response function for incentive effects on working memory among children with ADHD is not yet clear. The present study employed only a “high” dose. In Dovis et al. (in press), the absence of greater improvement with 10 euros compared to 1 euro is difficult to interpret for two reasons. First, unlike feedback, the monetary consequence was all-or-none – participants were told they would earn the full amount or none at all. Perhaps more importantly, the monetary outcome was not actually contingent on performance – all children were given the reward regardless of their performance. This procedure may be methodologically problematic in multi-session experiments. For example, consider a child who completes the 1-euro condition before the 10-euro condition. Even if the 1 euro resulted in sub-maximal motivation, the child would receive the full euro, suggesting that such performance would also be sufficient to earn the 10-euro reward in a later visit. Thus, future work will need to examine true dose-response relationships in order to adequately evaluate reinforcement-based models of ADHD.
Such work is also important for studies comparing medication and motivation effects on cognition. In the small number of studies that include both stimulants and incentives, it is notable that the medication dose is often “optimal” or high (.6 mg/kg per dose), whereas the incentive manipulation is relatively weak (e.g., Epstein, Brinkman, et al., 2011; Solanto et al., 1997). While matching the intensity of incentives and medication is no simple task, failure to do so may bias conclusions about the relative efficacy of medication and behavioral contingencies for improving cognition in ADHD (c.f., Pelham, 1999). In one recent study (Fabiano et al, 2007), a powerful reinforcement condition produced effects in a classroom setting that were comparable to .6 mg/kg MPH per dose. Clear comparative conclusions will require greater attention to dose-response functions.
Still, the nature of “dose” of behavioral contingencies is complex. In addition to reward magnitude, other aspects of the consequence are also important. First, the dopamine transfer deficit model proposed by Tripp and colleagues (e.g., Tripp & Wickens, 2008) hypothesizes that the impact of a reinforcer drops rapidly with even short delays between the behavior and the consequence among children with ADHD, suggesting parametric evaluation of reinforcement delay will be important to consider. Second, the explicit comparison of reinforcement to punishment or response cost is of both theoretical and practical significance (e.g., Quay, 1988), yet working memory studies to date have focused on reinforcement alone (present studies; Shiels et al., 2008) or a mixed reward/punishment context (Dovis et al., in press). Third, the consistency of the consequences in these studies is likely much greater than that observed in more real-world environments, suggesting the effects intermittent consequences (e.g., partial reinforcement; see Douglas & Parry, 1994) merit further examination. Fourth, the emerging literature on incentives, like the literature on stimulant effects, has generally been limited to acute assessments; the stability and durability of such effects on cognition is an important question for future work. Addressing these questions regarding the parameters of behavioral contingencies is critical, not only for informing current theories of ADHD, but also for the translation of laboratory studies to practical application.
In summary, successful completion of many tasks requires active management of information in working memory, and many children with ADHD struggle in this domain. The present studies demonstrate that both stimulant treatment and incentives for better performance each acutely improve visuo-spatial working memory. Though performance-based incentives did not normalize working memory, the ADHD-control difference in performance was roughly cut in half with the present “dose” of reinforcement. The combination of stimulants and incentives yielded better working memory than either alone. These novel data are broadly consistent with treatment guidelines recommending that school-age children with ADHD receive both pharmacotherapy and behavioral intervention (AAP, 2011). In the future, it will be important to test whether stimulant and incentive effects on working memory in the lab predict real-world outcomes in the lives of children with ADHD.
Acknowledgments
We thank Dominica Vito for coordination of the project and Mark Kutgowski for computer programming. This research was supported by grant R01MH069434 to LWH from the National Institute of Mental Health. KS is now at the Kennedy Krieger Institute. WEP and JGW are now at the Center for Children and Families, Florida International University.
Footnotes
Four additional participants were excluded from the sample due to invalid data caused by equipment malfunction (n= 2) or failure to follow task intstructions (n= 2).
Due to a programming error, 23 (10%) of the 224 0-back trial blocks in Study 1 had a target probability of .1, rather than .3. A supplementary analysis excluding the 11 children (4 with ADHD) with one or more of these blocks revealed a nearly identical pattern of results to that obtained for the full sample. Overall, the reduced number of 0-back target stimuli in the subset of the sample did not appear to exert a significant influence on the present data. Nevertheless, the error was corrected prior to Study 2.
Because the order in which incentive and no-incentive conditions are presented can impact performance (see Shiels et al., 2008), we included incentive order as a between-subjects factor. However, despite random assignment, accuracy during the baseline week – in which there were no incentives – varied as a function of incentive order assignment among children with ADHD, F(1, 52) = 4.6, p < .05; Group × Incentive Order F(1,52) = 4.5, p = .039. This pattern suggests some pre-existing difference that occurred by chance but nonetheless precludes clear interpretation of “incentive order” effects. Therefore, incentive order is retained in all models to account for this extraneous variance, but incentive order effects are not discussed.
Supplemental models included FSIQ as a covariate. FSIQ was a statistically significant predictor of overall n-back performance on the baseline day, F(1,51) = 4.2, p<.05, but not on the incentive day, F(1,51)=2.3, p=.14. More importantly, inclusion of FSIQ did not appreciably alter any of the interactions with group and/or incentive in either the baseline or incentive day analyses.
Two additional participants were excluded from the sample due to invalid data resulting from failure to follow task instructions.
Preliminary analyses indicated that neither incentive order nor medication order accounted for significant variance in working memory performance; therefore, to conserve degrees of freedom these factors were removed from subsequent analyses.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- Alderson RM, Rapport MD, Hudek KL, Sarver DE, Kofler MJ. Competing core processes in attention-deficit/hyperactivity disorder (ADHD): do working memory deficiencies underlie behavioral inhibition deficits? Journal of Abnormal Child Psychology. 2010;38(4):497–507. doi: 10.1007/s10802-010-9387-0. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Clinical Practice Guideline: Treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2011;108(4):1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baddeley A. Working memory, thought, and action. Oxford University Press; New York: 2007. [Google Scholar]
- Bedard AC, Jain U, Johnson SH, Tannock R. Effects of methylphenidate on working memory components: influence of measurement. Journal of Child Psychology and Psychiatry. 2007;48(9):872–880. doi: 10.1111/j.1469-7610.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Martinussen R, Ickowicz A, Tannock R. Methylphenidate improves visual-spatial memory in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(3):260–280. doi: 10.1097/00004583-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Tannock R. Anxiety, methylphenidate response, and working memory in children with ADHD. Journal of Attention Disorders. 2008;11(5):546–557. doi: 10.1177/1087054707311213. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Pelham WE, Milich R, Dixon Single and combined effects of methylphenidate and behavior therapy on the classroom performance of children with attention-deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 1992;20(2):213–232. doi: 10.1007/BF00916549. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Dovis S, Van der Oord S, Wiers RW, Prins PJM. Can motivation normalize working memory and task persistence in children with Attention-Deficit/Hyperactivity Disorder? The effects of money and computer-gaming. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-011-9601-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlis AC, Bähne CG, Jacob CP, Herrmann MJ, Fallgatter AJ. Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: a functional near-infrared spectroscopy (fNIRS) study. Journal of Psychiatric Research. 2008;42(13):1060–1067. doi: 10.1016/j.jpsychires.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, Shiels K, Simon JO, Altaye M. Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology. 2011;36(5):1060–1072. doi: 10.1038/npp.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, Brinkman WB, Froehlich T, Simon JO, Altaye M. Evidence for higher reaction time variability in children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25(4):427–41. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Jr., Coles EK, Gnagy EM, Chronis-Tuscano A, O'Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clinical Psychology Review. 2009;29(2):129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Jr., Gnagy EM, Burrows-MacLean L, Coles EK, Chacko A, Robb JA. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Review. 2007;36(2):195–216. [Google Scholar]
- Fabiano GA, Pelham WE, Jr., Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, Onyango AN, Kipp H, Lopez-Williams A, Burrows-Maclean L. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology. 2006;35(3):369–285. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Greenhill L, Beyer DH, Finkleson J, Shaffer D, Biederman J, Conners CK, Gillberg C, Huss M, Jensen P, Kennedy JL, Klein R, Rapoport J, Sagvolden T, Spencer T, Swanson JM, Volkow N. Guidelines and algorithms for the use of methylphenidate in children with attention-deficit/ hyperactivity disorder. Journal of Attention Disorders. 2002;6:89–100. doi: 10.1177/070674370200601s11. [DOI] [PubMed] [Google Scholar]
- Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Iaboni F, Douglas VI, Baker AG. Effects of reward and response costs on Inhibition in ADHD children. Journal of Abnormal Psychology. 1995;104(1):232–240. doi: 10.1037/0021-843X.104.1.232. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory, e-pub ahead of print. 2009:1–19. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Bingham C, White T. Regulation of cognitive resources during an n-back task in youth-onset psychosis and attention-deficit/hyperactivity disorder (ADHD). International Journal of Psychophysiology. 2009;73(3):294–307. doi: 10.1016/j.ijpsycho.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD—A randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kobel M, Bechtel N, Weber P, Specht K, Klarhöfer M, Scheffler K, Opwis K, Penner IK. Effects of methylphenidate on working memory functioning in children with attention deficit/hyperactivity disorder. European Journal of Paediatric Neurology. 2008;13(6):516–523. doi: 10.1016/j.ejpn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS. ADHD and working memory: the impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. Journal of Abnormal Child Psychology. 2010;38(2):149–161. doi: 10.1007/s10802-009-9357-6. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Schöll M. Lack of Inhibition: A Motivational Deficit in Children With Attention Deficit/Hyperactivity Disorder and Children With Traumatic Brain Injury. Child Neuropsychology. 2000;6(4):286–296. doi: 10.1076/chin.6.4.286.3145. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clinical Psychology Review. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. Modulation of response timing in ADHD, effects of reinforcement valence and magnitude. Journal of Abnormal Child Psychology. 2007;36:445–56. doi: 10.1007/s10802-007-9190-8. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neuroscience and Biobehavioral Reviews. 2010;34:744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden D, Hogg-Johnson S, Tannock R. A Meta-Analysis of working memory impairments in children with attention- deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Response inhibition and disruptive behaviors: Toward a multiprocess conception of etiological heterogeneity for ADHD combined type and conduct disorder early-onset type. Annals of the New York Academy of Science. 2003;1008:170–182. doi: 10.1196/annals.1301.018. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57(11):1224–30. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA. Effects of reward and response cost on response inhibition in AD/HD, disruptive, anxious, and normal children. Journal of Abnormal Child Psychology. 1998;26:161–174. doi: 10.1023/a:1022650216978. [DOI] [PubMed] [Google Scholar]
- Pelham WE., Jr. The NIMH multimodal treatment study for attention-deficit hyperactivity disorder: just say yes to drugs alone? Canadian Journal of Psychiatry. 1999;44(10):981–990. doi: 10.1177/070674379904401004. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Burrows-Maclean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, Hoffman MT. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Experimental and Clinical psychopharmacology. 2005;13(2):111–126. doi: 10.1037/1064-1297.13.2.111. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Carlson CL, Sames SE, Vallano G, Dixon MJ, Hoza B. Separate and combined effects of methylphenidate and behavior modification on boys with attention deficit-hyperactivity disorder in the classroom. Journal of Consulting and Clinical Psychology. 1993;61:506–515. doi: 10.1037/0022-006X.61.3.506. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2008;37(1):184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34(3):449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pennington BF. Toward a new neuropsychological model of attention-deficit/hyperactivity disorder: Subtypes and multiple deficits. Biological Psychiatry. 2005;57:1221–1223. doi: 10.1016/j.biopsych.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate- release methylphenidate in children with attention-deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews. 2006;30(8):1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Pliszka S, AACAP Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:894–92. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M, Corkin S. Effects of verbal and non-verbal interference on spatial and object visual working memory. Memory & Cognition. 2005;33(2):203–212. doi: 10.3758/bf03195309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay HC. Attention-deficit disorder and the behavioral inhibition system: The relevance of the neuropsychological theory of Jeffrey A. Gray. In: Bloomingdale LM, Sergeant J, editors. Attention-deficit disorder: Criteria, cognition, intervention. Pergamon Press; New York: 1988. pp. 117–126. [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. Journal of Abnormal Child Psychology. 2008;36(6):825–37. doi: 10.1007/s10802-008-9215-y. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. 9th ed. Western Psychological Services; Los Angeles: 2005. Revised Children's Manifest Anxiety Scale (RCMAS) [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419-368. [DOI] [PubMed] [Google Scholar]
- Saylor CF, Finch AJ, Jr., Spirito A, Bennett B. The Children's Depression Inventory: A systematic evaluation of psychometric properties. Journal of Consulting and Clinical Psychology. 1984;526:955–967. doi: 10.1037//0022-006x.52.6.955. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulkan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Developmental Neuropsychology. 2002;21(1):43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Jr., Lysczek CL, Tannock R, Pelham WE, Jr., Spencer SV, Gangloff BP, Waschbusch DA. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36(6):903–13. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Jr., Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, Jr., Gangloff BP. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2009;17(5):291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response inhibition in children with DSM-IV subtypes of AD/HD and related disruptive disorders: the role of reward. Child Neuropsychology. 2001;7(3):172–189. doi: 10.1076/chin.7.3.172.8746. [DOI] [PubMed] [Google Scholar]
- Slusarek M, Velling S, Bunk D, Eggers C. Motivational effects on inhibitory control in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:355–363. doi: 10.1097/00004583-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Wender EH, Bartell SS. Effects of methylphenidate and behavioral contingencies on sustained attention in attention-deficit hyperactivity disorder: a test of the reward dysfunction hypothesis. J Child Adolescent Psychopharmacology. 1997;7(2):123–136. doi: 10.1089/cap.1997.7.123. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD- A dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130(1-2):29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Swanson JM, McBurnett K, Christian DL, Wigal T. Stimulant medications and the treatment of children with ADHD. Advances in Clinical Child Psychology. 1995;17:265–322. [Google Scholar]
- Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(7):886–896. doi: 10.1097/00004583-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Tripp G, Aslop, Aslop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD). Journal of Child Psychology and Psychiatry. 2001;42(5):691–698. [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Kaplan E, Fein D, Kramer J, Delis D, Morris R, Maerlender A. Manual for the Wechsler Intelligence Scale for Children. 4th Edition. The Psychological Corp.; San Antonio, TX: 2004. Integrated (WISC-IV) [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson® III Test. Riverside Publishing Company; Itasca, IL: 2001. [Google Scholar]
- Willcutt EG, Doyle A, Nigg J, Faraone S, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;5711:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]