Abstract
Concurrent delivery of multiple poorly water-soluble anticancer drugs has been a great challenge due to the toxicities exerted by different surfactants or organic solvents used in solubilizing individual drugs. We previously found that poly(ethylene glycol)-block-poly(D, L-lactic acid) (PEG-b-PLA) micelles can serve as a safe delivery platform for simultaneous delivery of paclitaxel (PTX), 17-allylamino-17-demethoxygeldanamycin (17-AAG), and rapamycin (RAP) to mice. The high tolerance of this polymeric micelle formulation by mice allowed us to investigate the pharmacokinetics of the 3 co-delivered drugs. In this study, it was shown that 3-in-1 PEG-b-PLA micelle delivering high doses of PTX, 17-AAG, and RAP (60, 60, and 30 mg/kg, respectively) significantly increased the values of the area under the plasma concentration-time curves (AUC) of PTX and RAP in mice compared to the drugs delivered individually, while the pharmacokinetic parameters of 17-AAG were similar in both 3-in-1 and single drug-loaded PEG-b-PLA micelle formulations. Moreover, pharmacokinetic study using 2-in-1 micelles indicated that the augmented AUC value of RAP was due to the co-delivery of 17-AAG, while the increase in AUC of PTX was more likely caused by the co-delivery of RAP. In contrast, when 3-in-1 and single drug-loaded PEG-b-PLA micelles were administrated at modest dose (PTX, 17-AAG, and RAP at 10, 10, and 5 mg/kg, respectively), pharmacokinetic differences of individual drugs between 3-in-1 and single drug formulations were eliminated. These results suggest that 3-in-1 PEG-b-PLA micelles can concurrently deliver PTX, 17-AAG, and RAP without changing the pharmacokinetics of each drug at modest doses, but altered pharmacokinetic profiles emerge when drugs are delivered at higher doses.
Keywords: Paclitaxel, 17-Allylamino-17-demethoxygeldanamycin (17-AAG), Rapamycin, PEG-b-PLA micelles, Pharmacokinetics
1. Introduction
An emerging trend in cancer chemotherapy is to combine conventional chemotherapeutic drugs with molecularly targeted drugs, aiming for enhanced antitumor efficacy and acceptable toxicities [1]. As the prototypical inhibitor of heat shock protein 90 (Hsp90), 17-allylamino-17-demethoxygeldanamycin (17-AAG, Tanespimycin) has broad activity attributable to its impact on the litany of Hsp90 client proteins. As several Hsp90 client proteins are involved in cellular responses to cytotoxic chemotherapies, there has been considerable interest in the development of new drug combinations consisting of potent chemotherapeutic agents and 17-AAG. Specifically, combination of 17-AAG with paclitaxel (PTX) or rapamycin (RAP) demonstrated remarkable antitumor efficacy in breast, lung, and ovarian cancers [2–5]. Despite promising in vitro and in vivo antitumor efficacy, the practical use of combination therapy has been hampered by the lack of suitable and safe vehicles for simultaneous delivery of multiple anticancer agents. As many potent anticancer agents are poorly water-soluble, low-molecular-weight surfactants such as Cremophor EL and Polysorbate 80, and organic solvents have been used to enhance their aqueous solubility. However, the use of those solubilizing agents introduces additional safety concerns including vehicle-specific toxicities (e.g. hypersensitivity reactions), potential incompatibility of co-administered drugs, and undesirable alteration of pharmacokinetic profiles [6,7].
We previously demonstrated that PEG-b-PLA micelles can serve as efficient nanocontainers for the three-drug combination, PTX, 17-AAG, and RAP, without the need for Cremophor EL or organic solvents [8]. It was also found that intravenous (i.v.) administration of 3-in-1 PEG-b-PLA micelles carrying PTX, 17-AAG, and RAP could be safely carried out in mice at the maximum tolerated dose (MTD) of PTX [9] and therapeutically relevant doses of 17-AAG and RAP without causing any vehicle related toxicity or animal death. In another study, it was shown that the 3 drug combination simultaneously delivered by PEG-b-PLA micelles at doses of 60, 60, and 30 mg/kg of PTX, 17-AAG, and RAP could effectively inhibit tumor growth in both breast and lung cancer xenograft models [10]. Therefore, pharmacokinetic studies of concurrent delivery of multiple anticancer drugs using 3-in-1 PEG-b-PLA micelles are critical as they provide insights into drug-drug interactions, potential toxicities, as well as mechanisms of enhanced antitumor efficacy.
The aim of this study was to characterize the pharmacokinetic profiles of 1-, 2-, and 3-drug-loaded PEG-b-PLA micelles carrying PTX, 17-AAG, and/or RAP in FVB mice after single i.v. injections at 2 different dose levels: (1) high dose (PTX, 17-AAG, and RAP at 60, 60, and 30 mg/kg, respectively), and (2) moderate dose (PTX, 17-AAG, and RAP at 10, 10, and 5 mg/kg, respectively).
2. Materials and Methods
2.1. Materials
PTX, 17-AAG, and RAP were purchased from LC laboratories (Woburn, MA). 17-amino-17-demethoxygeldanamycin (17-AG, metabolite of 17-AAG) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Methyl p-hydroxybenzate and tert-butyl methyl ether (t-BME) were purchased from Sigma-Aldrich (St. Louis, MO). PEG-b-PLA (Mn of PEG = 4200 g/mol and Mn of PLA = 1900 g/mol, PDI = 1.05) was purchased from Advanced Polymer Materials Inc. (Montreal, Canada). All other reagents were obtained from Fisher Scientific Inc. (Fairlawn, NJ) and were of analytical grade. Blank mouse plasma was obtained from Innovative Research Inc. (Novi, MI).
2.2. Methods
2.2.1. Preparation of drug loaded PEG-b-PLA micelles
3-in-1 PEG-b-PLA micelles solubilizing PTX, 17-AAG, and RAP were prepared and characterized as previously described [8]. To prepare 3-in-1 PEG-b-PLA micelles, 15.0 mg of PTX and 17-AAG each, 7.5 mg of RAP, and 112.5 mg of PEG-b-PLA were dissolved in 5.0 mL of acetonitrile. After dissolving the drugs and polymer, acetonitrile was removed under reduced pressure until the mixture forms a thin film. Pre-warmed 0.9% sodium chloride injection (normal saline, USP) at 60 °C was then added to the film to form micelles. The reconstituted micelle solution was centrifuged at 13,000 rpm for 5 min to remove unencapsulated drugs. The solution was sterilized by filtration using 0.22 μm nylon filter. The final drug concentrations were adjusted to the required concentrations using normal saline before i.v. administration to mice. Single drug-loaded and 2-in-1 PEG-b-PLA micelles were similarly prepared.
2.2.2. Pharmacokinetic study in FVB mice
Pharmacokinetic studies were performed in FVB mice after single i.v. injection of drugs. All animal studies were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee and conducted in accordance with institutional and NIH guidance. Five to six-week-old female FVB albino mice (FVB/NCrl) were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed in ventilated cages with free access to water and food. Seven formulations including single drug-loaded, 2-in-1, and 3-in-1 PEG-b-PLA micelles were tested: Single drug-loaded micelle solutions were injected via tail vein at 60 mg/kg of PTX, 60 mg/kg of 17-AAG, or 30 mg/kg of RAP. Two-in-1 PEG-b-PLA micelles carrying PTX and 17-AAG were injected at 60 mg/kg of both drugs, while 2-in-1 PEG-b-PLA micelles with PTX and RAP were injected at 60 and 30 mg/kg, respectively, and 2-in-1 PEG-b-PLA micelles consisting of RAP and 17-AAG were injected at 30 and 60 mg/kg, respectively. Finally, 3-in-1 PEG-b-PLA micelles with PTX, 17-AAG, and RAP were injected at 60, 60, and 30 mg/kg, respectively. The formulations were injected through the lateral tail vein of mice under restraint using Tailveiner® (TV-150, Braintree Scientific Inc., MA). Blood samples were collected from the jugular vein of mice under isofluorane anesthesia at 0.083, 0.5, 2, 4, and 8 hr after single i.v. administration of each formulation (n = 4–5 for each time point). Collected blood was then transferred to 0.5 mL BD microtainer® tube (BD Biosciences, NJ) and kept on ice, followed by centrifuging at 13,000 rpm for 5 min. Plasma was collected and stored at −70 °C until analysis.
In a separate study, the pharmacokinetic profiles of drugs carried in single drug-loaded and 3-in-1 PEG-b-PLA micelles containing PTX, 17-AAG, and RAP were investigated by injecting at 10 mg/kg of PTX, 10 mg/kg of 17-AAG, and 5 mg/kg of RAP to mice to assess dose-dependent changes in PK parameters. Plasma was collected at 0.083, 0.5, and 2 hr post-injection.
2.2.3. Quantification of drug concentrations in plasma samples by RP-HPLC
A single step liquid-liquid extraction method was developed for extraction of individual drugs and internal standard from plasma samples. Drug concentration was subsequently quantified by reverse-phase HPLC (RP-HPLC) equipped with an UV detector. Briefly, 10 μL of methyl-p-hydroxybenzoate (250 μg/mL in acetonitrile) was added to 100 μL of plasma sample as an internal standard (IS),. 0.5 mL of t-BME was then added and the mixture was vortexed for 2 min. Following centrifugation (13,000 rpm, 5 min), clear supernatant was saved and the residual precipitate was re-extracted with another 0.5 mL of t-BME. Combined supernatants were transferred to microcentrifuge tubes and the solvent was completely evaporated at ambient temperature under a dry-air purge. Dried samples were reconstituted with 150 μL of 50% (v/v) acetonitrile aqueous solution, vortexed for 60 s, and centrifuged at 13,000 rpm, for 5 min. One hundred μL of each sample was injected into reverse-phase HPLC column for drug quantification.
Samples were analyzed on a Shimadzu Prominence HPLC system (Kyoto, Japan), consisting of a LC-20 AT pump, a SIL-20AC HT autosampler, a CTO-20AC column oven, and a SPD-M20A diode array detector. A Zorbax RX-C8 column (4.6 × 250 mm, 5 μm, Agilent Technologies Inc.) was used for the separation of analytes with a linear gradient elution with mobile phase A (deionized water containing 0.1% phosphoric acid) and mobile phase B (acetonitrile) as follows: 50% mobile phase A from 0.0 to 10.0 min, 50% to 80% mobile phase A from 10.1 to 20.0 min, 80% mobile A from 20.1 to 25.0 min, 80% to 50% mobile phase A from 25.1 to 30.0 min. The flow rate was set at 1.0 mL/min. UV absorbance was used to detect PTX, 17-AAG, RAP, and IS at 227, 333, 279, and 280 nm, respectively. Column temperature was maintained at 40 °C during analysis. Each chromatogram was collected and integrated to estimate the peak area of analyte by EZ Chrom® software (version.1.0, Agilent Technologies Inc.). The ratio of peak area of PTX, 17-AAG, or RAP to that of IS was calculated to estimate the drug concentration(s) using a pre-built calibration curve.
2.2.4 Pharmacokinetic data analysis
Major pharmacokinetic parameters were calculated using the noncompartmental analysis module of WINONLIN® (version 5.1, Pharsight Corp.) under sparsely-sampling mode. The area under the plasma concentration curve from time 0 to infinity (AUC0-inf) was calculated as follows: The AUC0-t (AUC from time 0 to the last sampling time) was calculated by the log-linear trapezoidal rule. The AUCt-inf (AUC from the last sampling time to infinity) was estimated by the calculation of Ct (drug concentration at the last sampling time) divided by λz (terminal phase elimination rate constant). Finally, AUC0-inf was calculated by the summation of AUC0-t and AUCt-inf. To calculate mean residence time (MRT), clearance (CL), volume of distribution at the steady state (Vd,ss), and terminal half-life (t1/2) of drugs, the following equations were used:
2.2.5. Statistical analysis
Data are represented as mean ± standard error of the mean (SEM). For statistical analyses, GraphPad prism software (version 5.0) was used. Groups were compared using Student’s t-test or one-way ANOVA with Tukey’s Post Hoc test comparison with a P < 0.05 required to claim a statistically significant difference.
3. Results
3.1 Preparation of drug loaded PEG-b-PLA micelles
The solubility of PTX, 17-AAG, and RAP in PEG-b-PLA micelles in 0.9% NaCl solution is presented in Table 1. 3-in-1 PEG-b-PLA micelles prepared at 8.3 mM (50 mg/mL) of PEG-b-PLA polymer solubilize PTX, 17-AAG, and RAP at 7.2, 7.3, and 3.3 mg/mL, respectively, representing more than a 10–10,000-fold increase in aqueous solubility compared to the intrinsic aqueous solubility of individual drugs. The percentage of total drug loaded in 3-in-1 PEG-b-PLA micelles was 29.5 ± 1.2%. The average particle size of the PEG-b-PLA micelles was 30–40 nm with a narrow particle size distribution (PDI < 0.2) as determined by dynamic light scattering. The PEG-b-PLA micelles were stable for 24 hr at ambient temperature and more than 7 days at 4 °C based on visual appearance, drug loading, and particle size measurements (data not shown).
Table 1.
Summary of single drug-loaded, 2-in-1, and 3-in-1 PEG-b-PLA micelles carrying PTX, 17-AAG, and/or RAP used in the pharmacokinetic study.
| Drug(s) in micelle formulation | Drug solubility (mg/mL) | Drug loading (%) | Total drug loading (%) |
|---|---|---|---|
| PTX | 7.3 ± 0.7 | 12.6 ± 2.5 | 12.6 ± 2.5 |
| 17-AAG | 6.4 ± 1.0 | 10.8 ± 1.5 | 10.8 ± 1.5 |
| RAP | 3.8 ± 0.2 | 6.1 ± 0.1 | 6.1 ± 0.1 |
| PTX + | 6.6 ± 0.5 | 11.7 ± 0.9 | 23.1 ± 1.9 |
| 17-AAG | 6.4 ± 0.5 | 11.4 ± 0.9 | |
| RAP + | 3.1 ± 0.2 | 5.1 ± 0.2 | 16.7 ± 0.2 |
| 17-AAG | 7.2 ± 0.2 | 11.7 ± 0.0 | |
| RAP + | 3.2 ± 0.2 | 5.5 ± 0.2 | 17.2 ± 0.7 |
| PTX | 6.8 ± 0.5 | 11.7 ± 0.6 | |
| RAP + | 3.3 ± 0.2 | 5.4 ± 0.2 | 29.5 ± 1.2 |
| PTX + | 7.2 ± 0.6 | 11.9 ± 0.6 | |
| 17-AAG | 7.3 ± 0.5 | 12.2 ± 0.5 |
3.2 Pharmacokinetics of drugs carried by PEG-b-PLA micelles in FVB mice
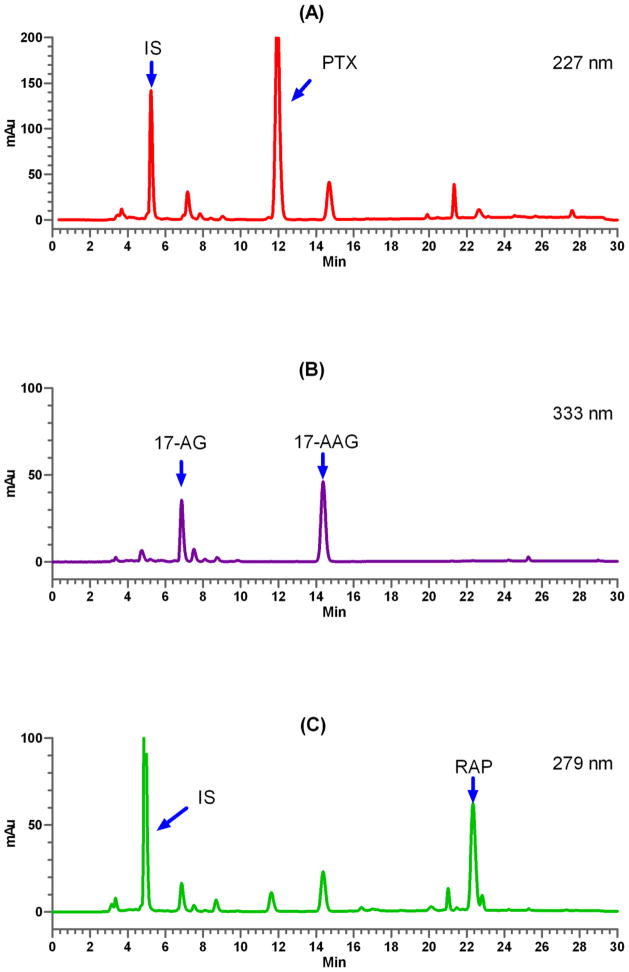
A bioanalytical method was developed to quantify plasma drug concentration(s) of PTX, 17-AAG, and RAP using RP-HPLC equipped with UV detector. The method was fully validated prior to being used for pharmacokinetic study according to ICH guidance [11]. The newly developed assay provides a linear relationship for detection of each drug from 0.2 to 20 μg/mL in mouse plasma samples. All three parent drugs and internal standard could be successfully separated and quantified in the presence of metabolites and endogenous materials from mouse plasma (Fig. 1).
Fig. 1.
Representative chromatograms of drug(s) in mouse plasma at 0.5 h after single i.v. injections of 3-in-1 PEG-b-PLA micelles containing PTX (A) at 60 mg/kg, 17-AAG (B) at 60 mg/kg, and RAP (C) at 30 mg/kg. IS = internal standard.
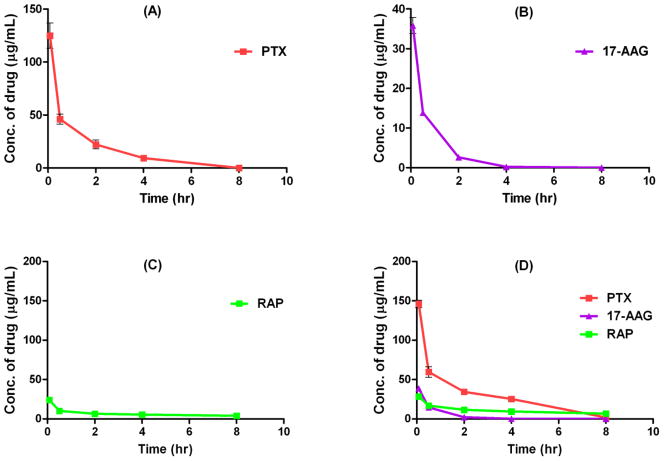
Plasma concentrations of PTX, 17-AAG, or, RAP versus time after single i.v. administration of single drug-loaded, 2-in-1, and 3-in-1 PEG-b-PLA micelles carrying PTX (60 mg/mL), 17-AAG (60 mg/mL), and/or RAP (30 mg/mL) are displayed in Fig. 2 and 3. PTX, 17-AAG, or RAP carried in single drug-loaded micelles showed a typical biexponential curve which can be characterized as a rapid distribution phase (< 0.5 hr), followed by an elimination phase. PTX and 17-AAG were rapidly eliminated from plasma and no drugs were detected at 8 hr post injection, while the plasma concentration of RAP was maintained above 1 μg/mL over the 8 hr time course (Fig. 2A–C). These results conform to previous single drug pharmacokinetic studies [12–14].
Fig. 2.
Plasma drug concentrations versus time after single i.v. injections of single drug-loaded PEG-b-PLA micelles with PTX at 60 mg/kg (A), 17-AAG at 60 mg/kg (B), or RAP at 30 mg/kg (C), and 3-in-1 PEG-b-PLA micelles with PTX, 17-AAG, and RAP at 60, 60, and 30 mg/kg (D) (mean ± SEM, n = 4–5).
Fig. 3.
Plasma drug concentrations versus time after single i.v. injections of 2-in-1 PEG-b-PLA micelles containing PTX and 17-AAG at 60 and 60 mg/kg (A), PTX and RAP at 60 and 30 mg/kg (B), and 17-AAG and RAP at 60 and 30 mg/kg (C). P < 0.05 for the PTX concentration difference between single drug and 2-in-1 PEG-b-PLA micelle administrations at 30 min (#) and 2 h (*) post injection (mean ± SEM, n = 4–5).
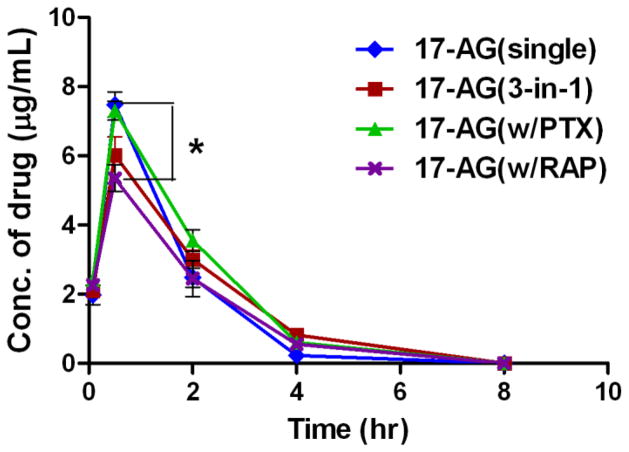
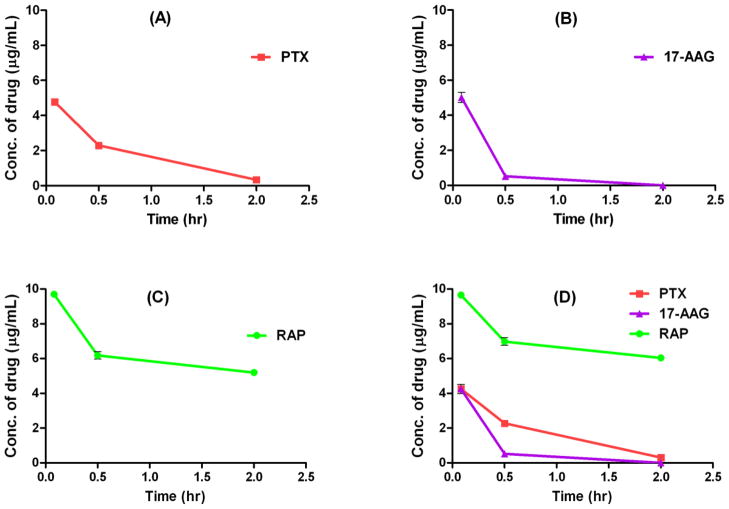
For 3 drug concurrent delivery using 3-in-1 PEG-b-PLA micelles, the plasma concentrations of PTX and RAP increased by 29% and 66%, respectively, at 0.5 hr post injection when compared to those values obtained with single drug-loaded micelles, while the pharmacokinetic profile of 17-AAG in 3-in-1 PEG-b-PLA micelles was almost identical to that of 17-AAG in single drug-loaded micelles (Fig. 2D). To better elucidate potential drug interactions, pharmacokinetic study was carried out using 2-in-1 PEG-b-PLA micelles carrying drug pairs (i.e. PTX with 17-AAG, PTX with RAP, and 17-AAG with RAP) and the results are shown in Fig. 3. The plasma drug concentration-time curves of PTX and 17-AAG were not changed by concurrent delivery of these 2 drugs using 2-in-1 PEG-b-PLA micelles compared to single drug-loaded micelles (Fig. 3A). In contrast, the pharmacokinetic profile of RAP in 2-in-1 micelles containing 17-AAG and RAP (60 and 30 mg/kg, respectively) was significantly altered compared to that of RAP single drug-loaded PEG-b-PLA micelles, while the pharmacokinetic profile of 17-AAG remained similar in this formulation (Fig. 3C). Interestingly, co-delivery of PTX and RAP using 2-in-1 micelles resulted in a transient increase in plasma PTX concentration (at 5 min and 30 min post injection), while the concentrations of PTX at the rest of time points were comparable to those of PTX single drug-loaded PEG-b-PLA micelles (Fig. 3B). In addition, the pharmacokinetics of 17-AG, which is a CYP3A4-mediated metabolite of 17-AAG [15], was similar in all formulations except when 17-AAG was co-delivered with RAP by 2-in-1 PEG-b-PLA micelles (Fig. 4). The formation of 17-AG was slightly reduced at 30 min post injection in that particular formulation (P < 0.05). A comparable pharmacokinetic study was then conducted using single drug-loaded and 3-in-1 PEG-b-PLA micelles carrying clinically more relevant (6-fold lower) doses of PTX, 17-AAG, and RAP at 10, 10, and 5 mg/kg, respectively [18]. Fig. 5 reveals that this more modest dosing eliminated the apparent drug-drug interactions observed with the high-dose multi-drug delivery formulation; the pharmacokinetic profile of each drug in 3-in-1 PEG-b-PLA micelles (10 mg/kg PTX, 10 mg/kg 17-AAG, and 5 mg/kg RAP) closely approximated that of the corresponding drug in the single-drug loaded micelle formulation.
Fig. 4.
Plasma drug concentrations of 17-AG versus time after single i.v. injections of 17-AAG single drug-loaded micelles, 2-in-1 micelles carrying 17-AAG and RAP, 2-in-1 micelles carrying 17-AAG and PTX, and 3-in-1 micelles carrying PTX, 17-AAG, and RAP. * P < 0.05 for the difference between 17-AG (single) and 17-AG (RAP) (mean ± SEM, n = 4–5).
Fig. 5.
Plasma drug concentrations versus time after single i.v. injections of single drug-loaded PEG-b-PLA micelles with PTX at 10 mg/kg (A), 17-AAG at 10 mg/kg (B), or RAP at 5 mg/kg (C), and 3-in-1 PEG-b-PLA micelles with PTX, 17-AAG, and RAP at 10, 10, and 5 mg/kg (D) (mean ± SEM, n = 4–5).
Noncompartmental analysis was performed to calculate the major pharmacokinetic parameters of single drug-loaded, 2-in-1, and 3-in-1 PEG-b-PLA micelles and results are shown in Table 2. The AUC, CL, and t1/2 of PTX single drug-loaded PEG-b-PLA micelles (60 mg/kg PTX) were 148 ± 28 μg*hr/mL, 0.42 ± 0.07 L/hr/kg, and 1.5 ± 0.04 hr, respectively, indicating that PTX was rapidly distributed and removed from plasma. The clearance of PTX in this formulation was larger than the observed value based on Taxol® formulation (0.22 L/hr/kg) but smaller than that of Genexol-PM® formulation (0.77 L/hr/kg) [12]. For 17-AAG single drug-loaded PEG-b-PLA micelles, AUC, CL, and t1/2 of 17-AAG were 28 ± 1 μg*hr/mL, 2.12 ± 0.11 L/hr/kg, and 0.6 ± 0.1 hr, respectively, while the AUC, CL, and t1/2 of RAP as a single drug administration was 101 ± 40 μg*hr/mL, 0.33 ± 0.12 L/hr/kg, and 7.3 ± 3.1 hr, respectively. Recent pharmacological studies showed that 17-AAG formulated with DMSO/egg-phospholipid was cleared rapidly from plasma (CL = 2.0–4.0 L/hr/kg) [15]. Similar level of the elimination rate of 17-AAG (CL = 2.12 L/hr/kg) was also observed in this study using the single drug-loaded PEG-b-PLA micelle formulation.
Table 2.
Pharmacokinetic parameters in mice after i.v. administration of single drug-loaded, 2-in-1, or 3-in-1 PEG-b-PLA micelles containing PTX, 17-AAG, and/or RAP (mean ± SD, n = 4–5).
| Drug(s) | Dose (mg/kg) | AUC (μg*h/mL) | CL (L/h/kg) | MRT (h) | Vd,ss (L/kg) | t1/2 (h) | |
|---|---|---|---|---|---|---|---|
| Single-drug loaded | PTX | 60 | 148 17 ± 27.54 | 0.42 ± 0.07 | 1.76 ± 0.57 | 0.85 ± 0.15 | 1.46 ± 0.04 |
| 17-AAG | 60 | 28.31 ± 1.44 | 2.12 ± 0.11 | 0.58 ± 0.09 | 1.71 ± 0.30 | 0.56 ± 0.10 | |
| RAP | 30 | 100.88 ± 39.76 | 0.33 ± 0.12 | 10.13 ± 4.84 | 3.12 ± 0.25 | 7.36 ± 3.14 | |
|
| |||||||
| 2-in-1 | PTX + | 60 | 171.86 ± 12.11 | 0.35 ± 0.02 | 2.07 ± 0.24 | 0.76 ± 0.19 | 1.49 ± 0.28 |
| 17-AAG | 60 | 36.50 ± 3.59 | 1.66 ± 0.16 | 0.63 ± 0.03 | 1.20 ± 0.19 | 0.50 ± 0.04 | |
| PTX + | 60 | 162.81 ± 17.55 | 0.37 ± 0.03 | 1.42 ± 0.20 | 0.43 ± 0.04 | 0.81 ± 0.10 | |
| RAP | 30 | 95.93 ± 15.14 | 0.32 ± 0.05 | 7.72 ± 2.20 | 2.67 ± 0.42 | 6.02 ± 1.82 | |
| 17-AAG + | 60 | 29.88 ± 1.06 | 2.01 ± 0.07 | 0.60 ± 0.09 | 1.49 ± 0.14 | 0.52 ± 0.05 | |
| RAP | 30 | 144.90 ± 6.44 | 0.21 ± 0.01 | 8.89 ± 0.28 | 1.93 ± 0.08 | 6.45 ± 0.30 | |
|
| |||||||
| 3-in-1 | PTX + | 60 | 245.06 ± 15.16 | 0.25 ± 0.02 | 2.14 ± 0.37 | 0.46 ± 0.07 | 1.31 ± 0.25 |
| 17-AAG + | 60 | 29.72 ± 5.78 | 2.10 ± 0.49 | 0.58 ± 0.07 | 1.86 ± 0.82 | 0.60 ± 0.11 | |
| RAP | 30 | 161.58 ± 23.73 | 0.19 ± 0.03 | 10.55 ± 2.46 | 2.04 ± 0.25 | 7.68 ± 1.90 | |
As seen in Table 2, no significant changes in the pharmacokinetic parameters of 17-AAG could be observed when it was co-delivered with either PTX or RAP in 2-in-1 PEG-b-PLA micelle formulations compared to those of 17-AAG single drug-loaded micelles. In contrast, when PTX or RAP was delivered concurrently with 17-AAG, the AUC value of PTX or RAP increased. Specifically, there were a 1.4-fold increase in the AUC value of RAP and a 1.2-fold increase in the AUC value of PTX relative to those of RAP and PTX, respectively, in single drug-encapsulated micelles. For 3-in-1 PEG-b-PLA micelles, major pharmacokinetic parameters of PTX and RAP were changed compared to those values in single drug-loaded micelles. The AUCs of PTX and RAP in 3-in-1 PEG-b-PLA micelles were 1.7- and 1.6-fold higher than those of single drug-loaded micelles, respectively. However, there was no significant difference in terms of MRT and t1/2 of PTX and RAP in both single drug-loaded and 3-in-1 micelle formulations, indicating that the terminal elimination phase of PTX and RAP were not affected by concurrent delivery of 3 drugs by PEG-b-PLA micelles. For 17-AAG, there was no significant pharmacokinetic variation observed between the single drug-loaded and 3-in-1 PEG-b-PLA micelles.
4. Discussion
Previously, we demonstrated that 3-in-1 PEG-b-PLA micelles carrying PTX, 17-AAG, and RAP at 60, 60 and 30 mg/kg, respectively, could be safely administered to FVB mice, while exhibiting remarkable antitumor efficacy in both breast and lung cancer bearing mouse models [10]. These findings raised questions regarding the pharmacokinetics of the 3 drugs delivered simultaneously by PEG-b-PLA micelles. We hypothesized that the high tolerability of 3-in-1 PEG-b-PLA micelles by mice was due to limited drug-drug interactions implying no alteration in the pharmacokinetic profiles of the 3 drugs in 3-in-1 PEG-b-PLA micelles compared to those of the drugs in single drug-loaded micelles. Considering the relatively narrow therapeutic window of PTX compared to 17-AAG and RAP, it is reasonable to speculate that the tolerability of 3-in-1 PEG-b-PLA micelles reflected minimal changes in PTX pharmacokinetics, i.e. no significant increase in AUC and Cmax, upon co-delivery of the 3 drugs using 3-in-1 PEG-b-PLA micelles.
However, based on the noncompartmental analysis results shown in Table 2, 3-in-1 PEG-b-PLA delivery system resulted in 1.7-fold increase in the AUC of PTX compared to PTX single drug-loaded micelles after i.v. administration with an identical dose of PTX at 60 mg/kg, suggesting the pharmacokinetic profile of PTX was altered when it was delivered by 3-in-1 PEG-b-PLA micelles. Sparreboom et al. reported that it was difficult to correlate the occurrence of acute toxicity with plasma concentration of taxane due to the high clearance and tolerability of chemotherapeutic agents in mouse [16]; therefore, PTX-related toxicity might not be evident with this increased AUC of PTX. The AUC value of RAP was also increased by 1.6-fold when RAP was delivered at 30 mg/kg of RAP using 3-in-1 PEG-b-PLA micelles relative to single drug loaded micelles. A previous study has demonstrated that RAP is tolerated up to 150 mg/kg by mice via single i.v. administration [17] and this may explain the lack of acute toxicity observed even with a 1.6-fold increase in mouse exposure to RAP delivered by 3-in-1 PEG-b-PLA micelles.
The absence of acute toxicity of 3-in-1 PEG-b-PLA micelles may also be further supported by the similar pharmacokinetic profiles of 17-AAG observed in all tested single drug-loaded, 2-in-1, and 3-in-1 PEG-b-PLA micelle treatments (Table 2). The CYP3A4-mediated metabolism of 17-AAG to 17-AG was also evaluated in various formulations to assess the possibility of drug-drug interaction of the co-delivered drugs. As shown in Fig. 4, a slight but statistically significant difference in terms of 17-AAG metabolism was only detected at 30 min post injection when 17-AAG was co-delivered with RAP in 2-in-1 PEG-b-PLA micelles. 17-AAG has shown hepatobiliary toxicity as the dose-limiting toxicity in preclinical studies [18]. The similar pharmacokinetic profiles and minimally modified metabolism of 17-AAG when co-delivered with PTX and RAP using PEG-b-PLA micelles shown in the current study suggest the potential for enhanced hepatic toxicity caused by co-delivery of 3 drugs is low and this has been borne out by the liver function testing in a separate study [10].
The mechanisms for higher animal exposure to PTX and RAP delivered by 3-in-1 PEG-b-PLA micelles are not clear (Fig. 2 and Table 2). Recently, Campone et al. demonstrated that concurrent administration of Everolimus (40-O-(2-hydroxyethyl) derivative of RAP) and PTX, given orally and intravenously, respectively, did not cause any changes in the pharmacokinetic profile of each drug compared to that of single drug delivery [19]. They speculated that the lack of drug-drug interaction might be due to non-overlapping metabolic fate for each drug: PTX is mainly metabolized by CYP2C8, while RAP is only metabolized by CYP3A4. In contrast to this clinical observation, the pharmacokinetic parameters of PTX and RAP were altered when the 2 drugs were concurrently delivered by 2-in-1 PEG-b-PLA micelles in this study (Table 2). The changed pharmacokinetic profiles might be due to partial PTX metabolism by CYP3A4 when PTX and RAP were concurrently delivered using the same administration route (i.v. administration) at high doses (60 mg/mL of PTX and 30 mg/mL of RAP). In addition, marked increased RAP plasma concentration and reduced RAP clearance were probably caused by drug-drug interaction between 17-AAG and RAP, which share the same CYP3A4 metabolic route, when those 2 drugs were delivered by 2-in-1 PEG-b-PLA micelles (Table 2). This drug-drug interaction was further evident by the 30% reduction in the metabolism of 17-AAG to 17-AG at 30 min post injection when 17-AAG was co-delivered with RAP relative to 17-AAG single drug delivery (Fig. 4). Moreover, 17-AAG metabolism was not altered in the presence of PTX in 2 drug delivery, implying that PTX has less influence on the metabolism of 17-AAG. Overall, the results obtained from the 17-AAG metabolism study indicate that 17-AAG may have the highest affinity to CYP3A4 among the 3 drugs, although further in vitro drug-binding study should be carried out to prove this hypothesis. Alternatively, micelle stabilities may impact drug pharmacokinetics. Micelles may be more stable with the incorporation of 2 or 3 hydrophobic drugs than micelles with only 1 drug encapsulated as previously observed [20]. The higher stability of 2-in-1 and 3-in-1 PEG-b-PLA micelles could raise plasma concentrations of the co-delivered drugs especially at early time points after i.v. administrations.
Pharmacokinetic study of drug-loaded PEG-b-PLA micelles at more modest, clinically relevant doses of PTX, 17-AAG, and RAP (10, 10, and 5 mg/kg, respectively) demonstrated no alterations of pharmacokinetic profiles of the drugs, indicating that the drug-drug interactions observed with high-dose 3-drug co-delivery might not be applicable to future translational development. Additional studies including P-glycoprotein efflux and drug biodistribution in tumor-bearing mice will provide additional insights into the previously observed enhanced antitumor efficacy of the 3-in-1 PEG-b-PLA micelle formulation and may prove helpful in designing more effective dosing regimens. Mayer et al. recently reported that the drug ratio in 2 drug combinations as well as sequence of treatment are important for optimizing drug synergy in vitro and in vivo [21, 22]. We previously found that a 5:1 molar ratio of PTX to 17-AAG could exhibit synergistic cytotoxic activity against MCF-7 breast cancer cells. In addition, it was observed in this study that 17-AAG was rapidly cleared from plasma, while PTX and RAP have relatively longer half-lives. Drug biodistribution studies will be critical in determining whether PTX, 17-AAG, and RAP carried in 3 drug-loaded PEG-b-PLA micelles can simultaneously target tumor tissues despite dissimilar pharmacokinetics in plasma.
5. Conclusions
Using a nanoscopic PEG-b-PLA micelle system, we previously proved that a 3 drug combination consisting of PTX, 17-AAG, and RAP at doses of 60, 60, and 30 mg/kg, respectively, was tolerable in mice. The pharmacokinetics of the 3 drugs concurrently delivered by PEG-b-PLA micelles was investigated in this study. Compared to the pharmacokinetic profiles of PTX, 17-AAG, and RAP in single drug-loaded PEG-b-PLA micelle formulations, co-delivery of the 3 drugs at doses of 60, 60, and 30 mg/kg of PTX, 17-AAG, and RAP, respectively, resulted in alteration of pharmacokinetic profiles of PTX and RAP, while the pharmacokinetic profile of 17-AAG was not changed. However, no change in pharmacokinetics compared to drugs in single drug-loaded micelles was observed with 3-in-1 PEG-b-PLA micelles when a more modest dose was administrated (PTX, 17-AAG, and/or RAP at 10, 10, and/or 5 mg/mL, respectively). In light of the far lower doses employed in clinical practice, these data suggest altered pharmacokinetics due to 3-in-1 formulation are unlikely in humans. Moreover, these data provide key guidance in preclinical study design. Additional drug biodistribution studies along with pharmacokinetic-pharmacodynamic studies in tumor-bearing mice are currently underway to optimize the dosing regimen of this 3-in-1 PEG-b-PLA delivery system.
Acknowledgments
The authors wish to thank Zhisheng Jiang and Joan M. Rettig for their assistance with animal studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest. 2008;118:3003–3006. doi: 10.1172/JCI36898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roforth MM, Tan C. Combination of rapamycin and 17-allylamino-17-demethoxygeldanamycin abrogates Akt activation and potentiates mTOR blockade in breast cancer cells. Anticancer Drugs. 2008;19:681–688. doi: 10.1097/CAD.0b013e3283067681. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DM, Lorang D, Chen GA, Stewart JHT, Tabibi E, Schrump DS. Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg. 2001;72:371–378. doi: 10.1016/s0003-4975(01)02787-4. discussion 378–379. [DOI] [PubMed] [Google Scholar]
- 4.Sawai A, Chandarlapaty S, Greulich H, Gonen M, Ye Q, Arteaga CL, Sellers W, Rosen N, Solit DB. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res. 2008;68:589–596. doi: 10.1158/0008-5472.CAN-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sain N, Krishnan B, Ormerod MG, De Rienzo A, Liu WM, Kaye SB, Workman P, Jackman AL. Potentiation of paclitaxel activity by the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin in human ovarian carcinoma cell lines with high levels of activated AKT. Mol Cancer Ther. 2006;5:1197–1208. doi: 10.1158/1535-7163.MCT-05-0445. [DOI] [PubMed] [Google Scholar]
- 6.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139–2144. [PubMed] [Google Scholar]
- 8.Shin HC, Alani AWG, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 Polymeric Micelle Nanocontainer for Poorly Water-Soluble Drugs. Mol Pharm. 2011;8:1257–1265. doi: 10.1021/mp2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, Seo MH. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001;72:192–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 10.Hasenstein JR, Shin HC, Kasmerchak K, Buehler D, Kwon GS, Kozak KR. Anti-tumor activity of triolimus: a novel multi-drug loaded micelle containing paclitaxel, rapamycin and 17-AAG. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidance for Industry Bioanalytical Method Validation. FDA. U.S. Department of Health and Human Services, Center of Drug Evaluation and Research, and Center for Veterinary Medicine; 2001. p. 25. [Google Scholar]
- 12.Kim SC, Yu J, Lee JW, Park ES, Chi SC. Sensitive HPLC method for quantitation of paclitaxel (Genexol in biological samples with application to preclinical pharmacokinetics and biodistribution. J Pharm Biomed Anal. 2005;39:170–176. doi: 10.1016/j.jpba.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Xiong MP, Yanez JA, Kwon GS, Davies NM, Forrest ML. A cremophor-free formulation for tanespimycin (17-AAG) using PEO-b-PDLLA micelles: characterization and pharmacokinetics in rats. J Pharm Sci. 2009;98:1577–1586. doi: 10.1002/jps.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanez JA, Forrest ML, Ohgami Y, Kwon GS, Davies NM. Pharmacometrics and delivery of novel nanoformulated PEG-b-poly(epsilon-caprolactone) micelles of rapamycin. Cancer Chemother Pharmacol. 2008;61:133–144. doi: 10.1007/s00280-007-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egorin MJ, Rosen DM, Wolff JH, Callery PS, Musser SM, Eiseman JL. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res. 1998;58:2385–2396. [PubMed] [Google Scholar]
- 16.Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH. Tissue distribution, metabolism and excretion of paclitaxel in mice. Anticancer Drugs. 1996;7:78–86. doi: 10.1097/00001813-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Center for drug evaluation and research application number: 021083, pharmacological reviews. FDA. U.S. Department of Health and Human Services, Center of Drug Evaluation and Reserach, and Center for Veterinary Medicine; 1999. [Google Scholar]
- 18.Investigator’s brochure for 17-Allylaminogeldanamycin (17-AAG) National. Cancer. Institute; 2004. [Google Scholar]
- 19.Campone M, Levy V, Bourbouloux E, Berton Rigaud D, Bootle D, Dutreix C, Zoellner U, Shand N, Calvo F, Raymond E. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br J Cancer. 2009;100:315–321. doi: 10.1038/sj.bjc.6604851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin HC, Alani AW, Rao DA, Rockich NC, Kwon GS. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J Control Release. 2009;140:294–300. doi: 10.1016/j.jconrel.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 22.Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7:216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]