Summary
Regulatory T (Treg) cells, whose differentiation and function are controlled by X-chromosome-encoded transcription factor Foxp3, are generated in the thymus (tTreg) and extrathymically (peripheral, pTreg) and their deficiency results in fatal autoimmunity. Here we demonstrate that a Foxp3 enhancer, conserved non-coding sequence 1 (CNS1), essential for pTreg, but dispensable for tTreg cell generation, is present only in placental mammals. CNS1 is largely composed of mammalian-wide interspersed repeats (MIR) that have undergone retrotransposition during early mammalian radiation. During pregnancy pTreg cells specific to a model paternal alloantigen were generated in a CNS1-dependent manner and accumulated in the placenta. Furthermore, when mated with allogeneic, but not syngeneic males, CNS1-deficient females showed increased fetal resorption accompanied by increased immune cell infiltration and defective remodeling of spiral arteries. Our results suggest that during evolution a CNS1-dependent mechanism of extrathymic differentiation of Treg cells emerged in placental animals to enforce maternal-fetal tolerance.
Introduction
The benefits of the adaptive immune system of vertebrates, that allows for highly efficient protection against invading pathogens, have come with a substantial trade-off due to overzealous or “unwanted” immune responses and associated inflammation caused by infectious agents, commensal microbiota, autoantigens, and fetal alloantigens during pregnancy in placental animals. Numerous mechanisms operating within the mammalian immune system cooperatively limit deleterious immune responses.
A subset of CD4+ T cells known as regulatory T cells express the X-chromosome encoded transcription factor Foxp3 and suppress inflammatory immune responses against “self” and foreign antigens in a variety of physiological and pathological settings (Littman and Rudensky, 2010). Loss-of-function mutations in Foxp3 result in congenital Treg cell deficiency and severe systemic immunopathology in both mice and humans, which reveal the vital role these cells play in immune homeostasis (Chatila et al., 2000; Brunkow et al., 2001; Wildin et al., 2001; Fontenot et al., 2003). Depletion of Treg cells in normal mice also results in a fatal lympho- and myeloproliferative disorder with widespread inflammatory lesions (Kim et al., 2007). Analysis of CD4+ T cells expressing a functional Foxp3 reporter allele and a Foxp3 reporter null allele showed that Foxp3 is essential for suppressor function of Treg cells (Gavin et al., 2007; Lin et al., 2007).
Recent studies implicated Treg cells in suppression of different types of inflammatory responses during infection, autoimmunity, metabolic inflammation, tissue injury, autoinflammatory responses at barrier sites, and tumor immunity (reviewed in Josefowicz et al., 2012). In the thymus, some thymocytes expressing TCR with a heightened reactivity for “self” antigens up-regulate Foxp3 and differentiate into tTreg cells, whereas pTreg cell generation in the periphery occurs upon stimulation of naïve CD4+ T cells with high affinity cognate TCR ligands in the presence of TGFβ and retinoic acid (Chen et al., 2003; Zheng et al., 2004; Kretschmer et al., 2005; Hall et al., 2011). A recent observation that an intronic Foxp3 enhancer CNS1, that contains Smad3 and RAR (retinoic acid receptor) binding sites, facilitates TGF-β-dependent Foxp3 induction and pTreg cell differentiation, but is dispensable for tTreg generation suggests that biological functions of these two Treg cell subsets are distinct (Zheng et al., 2010). Indeed, in contrast to fatal early onset inflammatory lesions resulting from congenital Treg cell deficiency, selective pTreg cell paucity leads to a rather late onset allergic and asthma-like inflammation in the gut and lung (Josefowicz et al., 2012). Since the principal difference between these two Treg cell subsets is the location and the type of antigen that facilitate their differentiation, tTreg cells are likely responsible for tolerance to self-antigens, whereas pTreg cells restrain immune responses to non-self antigens such as allergens, commensal microbiota, and food.
Pregnancy represents a physiological situation where tolerance to paternal alloantigens is critical for successful reproduction of placental mammals. Treg cells have been suggested to play a role in pregnancy based on their increased numbers in pregnant mice and humans (Somerset et al., 2004). Antibody-mediated depletion of CD25+ Treg cells results in increased resorption of the embryos in allogeneic matings in mice (Aluvihare et al., 2004; Shima et al., 2010) and women with repeated spontaneous abortions and preeclampsia display decreased numbers of CD25+CD4+ Treg cells (Munoz-Suano et al., 2011; Winger and Reed, 2011). These studies left open a question as to whether a role for Treg cells during pregnancy is largely due to their general “house-keeping” role in immune homeostasis and the observed modulation in their numbers is secondary to altered immune balance, or if there is an evolutionary selected mechanism of Treg cell-mediated maternal-fetal tolerance. We hypothesized that given the capacity of pTreg cells to mediate tolerance against non-self antigens, mechanisms supporting their generation arose to mitigate maternal-fetal conflict caused by the immune response to paternal alloantigens in placental mammals. We reasoned that pTreg cell mediated suppression might represent such a mechanism.
In support of this hypothesis, here we show that CNS1 enhancer is present only in eutherian mammals and that CNS1-dependent generation of pTreg cells during allogeneic pregnancy in mice plays an important role by preventing embryo resorption and associated defective spiral artery remodeling with accumulation of activated T cells in the placenta. Our results suggest that extrathymic generation of regulatory T cells emerged during evolution as a means of mitigation of maternal-fetal allogeneic conflict.
Results
CNS1 emerges in placental mammals
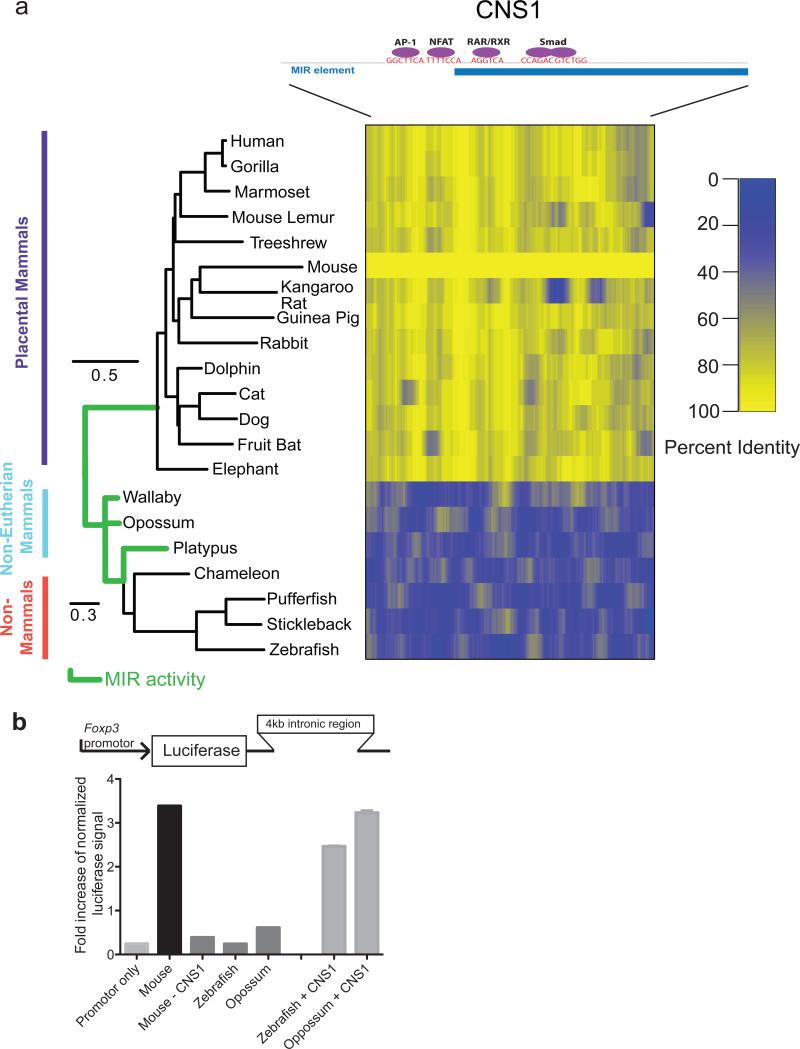
Extrathymic induction of Foxp3 and pTreg differentiation is facilitated by Foxp3 CNS1 enhancer, that contains binding sites for transcription factors activated downstream of three major signaling pathways that have been implicated in this process (Tone et al., 2008; Xu et al., 2010) (Figure 1a). Thus, to test the hypothesis that pTreg differentiation may have been gained during evolution to assist tolerance to the fetus we examined the conservation of the CNS1 element in a variety of vertebrates for which annotated genome sequences are available. While we observed that CNS1 is highly conserved throughout placental mammals, no evidence of a CNS1 sequence homologue was found within 100kb of the transcription start site of any forkhead family member in the monotreme platypus, in marsupials wallaby and opossum, or in non-mammals such as zebrafish (Figure 1a) (Margulies et al., 2007). Importantly, a Foxp3 homologue was previously identified in zebrafish and its forced expression in mouse Foxp3- CD4+ T cells conferred suppressive capacity (Quintana et al., 2010). These results led us to consider that species without identifiable Foxp3 CNS1 sequence homologues lack a proximal regulatory element conferring efficient TGF-β–mediated Foxp3 induction, a key factor in pTreg differentiation. To test this notion we cloned 4kb regions downstream of the promoter of the zebrafish, opossum, and mouse Foxp3 genes (Foxp3-4kb) and evaluated their enhancer activities upon TCR (PMA/ionomycin) and TGF-β induced activation of the Foxp3 promoter in EL-4 cells in a luciferase assay previously used for the assessment of CNS1 enhancer activity (Figure 1b)(Tone et al., 2008). Indeed, only mouse, but not zebrafish or opossum Foxp3-4kb sequence markedly augmented the Foxp3 promoter activity. Furthermore, mouse Foxp3-4kb sequence devoid of CNS1 was lacking enhancer activity whereas incorporation of CNS1 into zebrafish or opossum Foxp3-4kb sequence reconstituted enhancer activity. While the remote possibility remains that in non-eutherians TGF-β can facilitate Foxp3 induction in a species-specific manner, these results support the idea that CNS1-like enhancer activity, a prerequisite for pTreg differentiation, arose in placental mammals.
Figure 1. Foxp3 CNS1 element essential for extrathymic induction of Treg cells is present only in placental mammals.
(A) Schematic of the Foxp3 CNS1 element and binding sites of transcription factors implicated in pTreg cell differentiation (not to scale). Overlap with annotated MIR retrotransposon is indicated. Phylogenetic tree of vertebrates with spatial conservation of CNS1 is shown as percent identity smoothened across a 15 bp window. Scale bars are noted and branches with presumed MIR activity are denoted in green. (B) Luciferase assay of enhancer activity performed in EL-4 cells using Foxp3-4 kB fragments including the first intron of the Foxp3 locus from indicated species inserted downstream of the mouse Foxp3 promoter and luciferase (Luc) gene (schematic above). Error bars represent standard error (n=3).
Interestingly, the majority of the CNS1 element sequence was contained within an annotated SINE retrotransposon of the mammalian-wide interspersed repeat (MIR) family (Figure 1a) suggesting a mechanism for the abrupt emergence of CNS1 in the Foxp3 locus since the MIR family is thought to have been amplified during the Mesozoic era in the course of the radiation of placental mammals, marsupials, and monotremes (Jurka et al., 1995; Smit and Riggs, 1995).
Extrathymic generation of fetal alloantigen-specific Treg cells in pregnancy
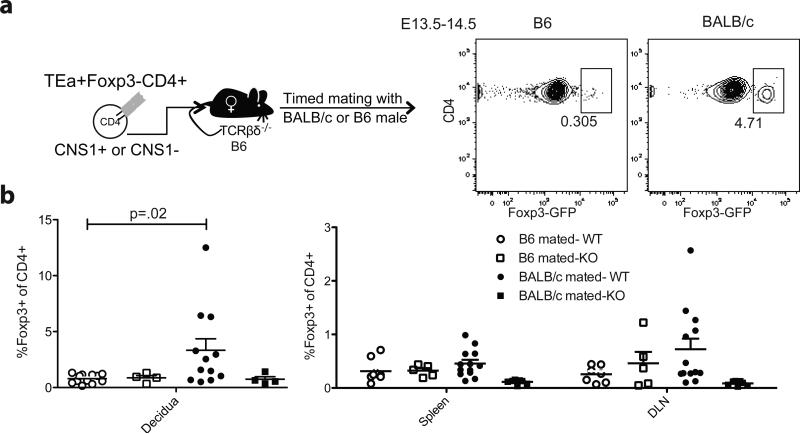
To test whether pTreg cells specific for paternal alloantigen are generated during pregnancy in a CNS1-dependent manner we used CNS1-sufficient and -deficient Foxp3GFP mice expressing transgenic (tg) TEa TCR that recognizes Eα52-68 peptide derived from IEd molecule bound to MHC class II molecule I-Ab (Grubin et al., 1997). This complex is highly expressed in H-2bxd (B6 × BALB/c) F1 mice, yet is absent in either parental strains because B6 do not express peptide donor Eα chain, while BALB/c mice lack the appropriate presenting molecule, I-Ab. FACS purified Foxp3- CD4+ T cells from CNS1-deficient and -sufficient TEa tg Foxp3GFP B6 mice were transferred into T-cell-deficient B6 recipients, which were subsequently mated with BALB/c (H-2d) or B6 (H-2b) male. In this experimental setup TEa cells were able to recognize endogenous Eα52-68:I-Ab complex presented by antigen-presenting cells (APC) of the embryo (H-2dxb) or processed by maternal APC only when recipient B6 females were mated with a BALB/c male (Figure 2a). Induction of Foxp3 in transferred CNS1-sufficient (WT) TEa T cells was observed primarily in the draining lymph node (DLN) and decidua of females mated with BALB/c, but not B6 males. In contrast, no significant induction of Foxp3 was observed upon transfer of CNS1-deficient (KO) TEa T cells (Figure 2b). Although we cannot formally exclude the possibility that under a particular condition extrathymic Treg cells can be generated in the absence of CNS1, these results demonstrate that pTreg cells specific for fetal alloantigens are generated during pregnancy in a CNS1-dependent manner.
Figure 2. CNS1-dependent generation of pTreg cells specific for fetal alloantigen during pregnancy.
(A) Schematic diagram of experimental design with representative flow cytometric analysis of Foxp3 expression in CNS1-sufficient T cells in deciduas of pregnant TCR βδ-/- recipient females. (B) Percent of CD4+ cells that express Foxp3 in indicated tissues on day E13.5-E14.5 of pregnancy in TCR βδ-/- mice transferred with CNS1-sufficient (WT) or -deficient (KO) TEa Foxp3-negative CD4+ T cells and mated with B6 or BALB/c males. Error bars indicate standard error.
pTreg cell paucity results in increased resorption of fetuses
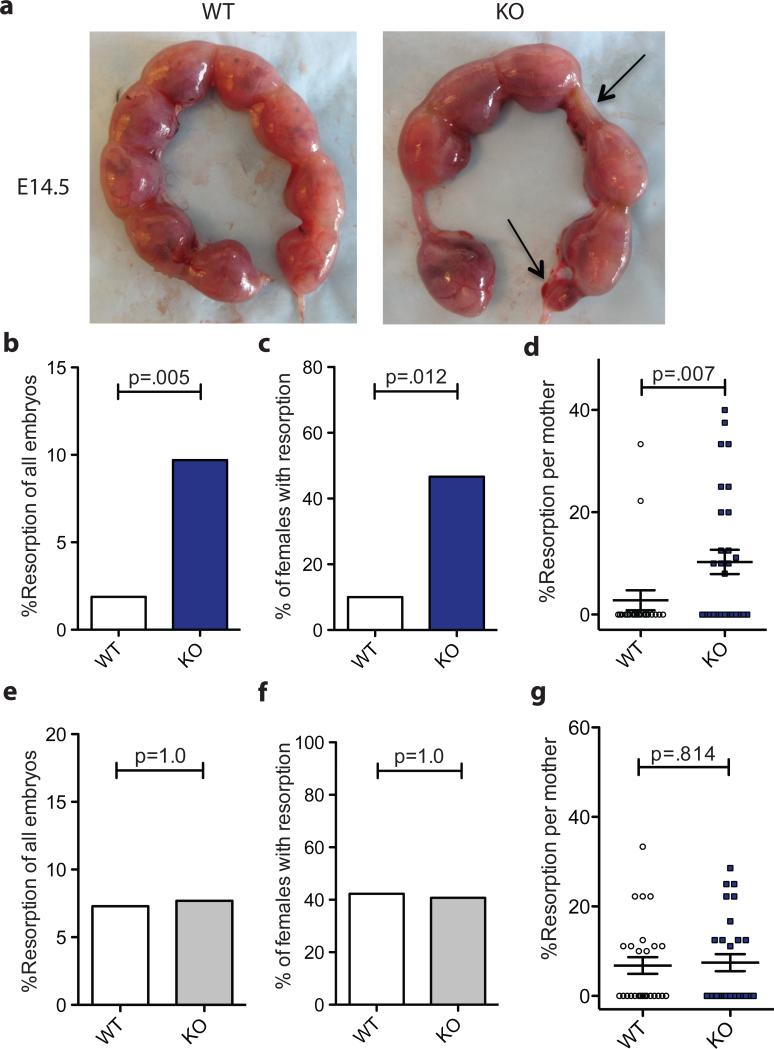
A role for CNS1 and extrathymic generation of Treg cells in maternal-fetal tolerance and fertility was then directly assessed. CNS1-deficient (KO) and -sufficient (WT) B6 females were mated with allogeneic BALB/c males and the embryo resorption was assessed on day E13.5-E14.5 (Figure 3a). Increased resorption was observed in CNS1-deficient females compared with the wild-type littermate controls (Figure 3b). Incidence of resorption and its rate per pregnant female was also increased (Figures 3c-d). Consistent with an effect that can account for evolutionary pressure for the emergence of CNS1-dependent pTreg cell differentiation the number of nonresorbed fetuses was also significantly decreased in CNS1-deficient females (Supplementary Figure 1). Importantly, females impaired in pTreg cell generation did not show increased resorption of embryos sired by B6 males expressing syngeneic MHC allele (Figure 3e-g). The latter observation indicates that CNS1-deficient females are not generally predisposed to spontaneous abortion, but reject embryos expressing mismatched MHC alleles. CNS1-deficient females were always compared to their WT counterparts in identical breedings with syngeneic or allogeneic males to ensure the genetic make-up of the embryos is the same in the two groups compared as different strains of mice can vary in rates of embyo loss due to early fetal death unrelated to immunologic conflicts and since F1 embryos are frequently more robust and survive better.
Figure 3. CNS1 deficiency results in increased resorption of embryos.
(A) Macroscopic evaluation of resorption of allogeneic embryos in uteri of CNS1-sufficient (WT) and –deficient (KO) mice on day E14.5. Arrows indicate resorptions. Representative of 20-30 females is shown. (B) Percent of resorbed embryos in all CNS1-sufficient and -deficient pregnancies with BALB/c males. Two-sided Fisher's exact test was used to assess the significance. (C) Incidence of pregnancies with at least one resorption. Two-sided Fisher's exact test was used to assess the significance. (D) Percent resorption observed in individual mothers with indicated genotype. Error bars indicate standard error. (E) Percent of embryos resorbed in pregnant CNS1-sufficient and -deficient B6 mice mated with B6 males. (F) Incidence of pregnancies with at least one resorption. (G) Percent resorption observed in individual mothers. Error bars indicate standard error. See also Figure S1.
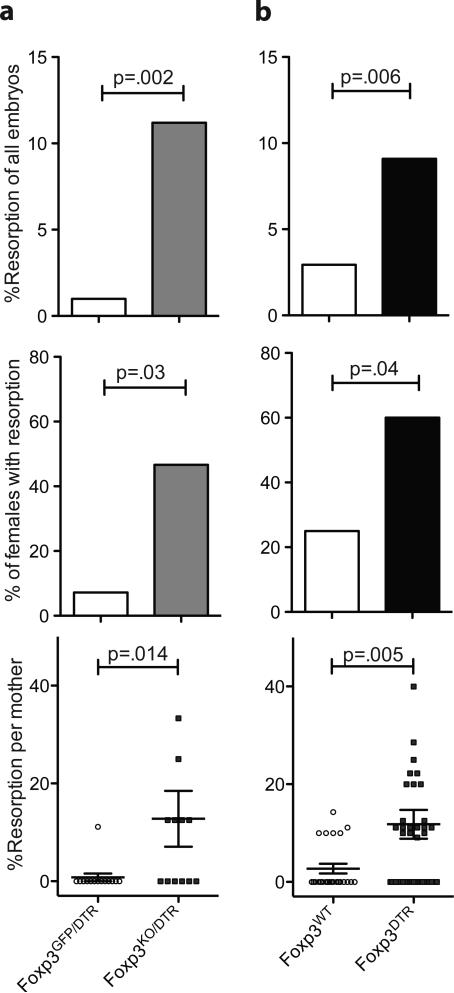
To exclude potential effects of congenital pTreg cell deficiency, we generated heterozygous Foxp3CNS1KO/DTR females containing one CNS1 KO allele and one Foxp3DTR allele. Due to random X chromosome activation half of the cells in a Foxp3CNS1KO/DTR mouse express each allele and are either susceptible to DT-mediated ablation (Foxp3DTR) or unable to induce Foxp3 in the periphery (Foxp3CNS1). Administration of DT allows for depletion of all CNS1-dependent pTreg cells while spares a pool of CNS1-deficient tTreg cells (Supplementary Figure 2). Treatment with DT before and during pregnancy in these mice resulted in increased resorption compared to control heterozygous Foxp3GFP/DTR females where DT ablation leaves CNS1-sufficient Foxp3 (Figure 4a). The observed increases were similar to those observed in intact CNS1-deficient females. Thus, acute pTreg ablation during allogeneic pregnancy results in increased embryo resorption comparable to that observed in CNS1-deficient females.
Figure 4. Acute depletion of pTreg and all Treg cells results in comparable increase in embryo resorption.
(A) Percent of embryos resorbed in pregnant Foxp3GFP/DTR or Foxp3CNS1KO/DTR females treated with DT continuously starting 3 days prior to mating with BALB/c males; incidence of pregnancies with at least one resorption; percent resorption observed in individual mothers. Error bars indicate standard error. (B) Percent of resorbed embryos, incidence of resorption, and percent resorption per mother for wild-type or Foxp3DTR B6 mice mated with BALB/c males and treated with DT on day E5.5 and E7.5. See also Figure S2.
Extrathymically generated Treg cells play a predominant role in maternal-fetal tolerance
Although analysis of allogeneic pregnancy in CNS1-deficient and Foxp3CNS1KO/DTR females suggested that pTreg cells generated in a CNS1-dependent manner prevent “rejection” of MHC-mismatched fetuses, it was possible that their contribution to overall Treg cell-mediated suppression of maternal-fetal allogeneic conflict was relatively minor. To address this question we assessed the effect of essentially complete ablation of Treg cells in pregnant Foxp3DTR B6 females expressing diphtheria toxin receptor under control of the endogenous Foxp3 locus (Kim et al., 2007). Increased resorption of MHC-mismatched embryos was observed in Foxp3DTR B6 females, compared with control B6 mice, when mated with BALB/c males and treated with diphtheria toxin (DT) at day E5.5 and E7.5 (Figure 4b). In contrast to selective pTreg deficiency, DT-mediated combined ablation of pTreg and tTreg cells also increased resorption of syngeneic embryos most likely due to a loss of restraint of T cell reactivity against self-antigens (data not shown). Despite widespread immune mediated inflammation and lympho- and myeloproliferative syndrome in pregnant Foxp3DTR females subjected to “wholesale” Treg ablation, the rates of resorption observed in these mice were similar to those in CNS1-deficient females and ablation of pTreg cells. These findings indicate that pTreg cells play a predominant role in maternal-fetal tolerance.
CNS1-deficient females exhibit signs of inflammation and abnormal spiral artery remodeling
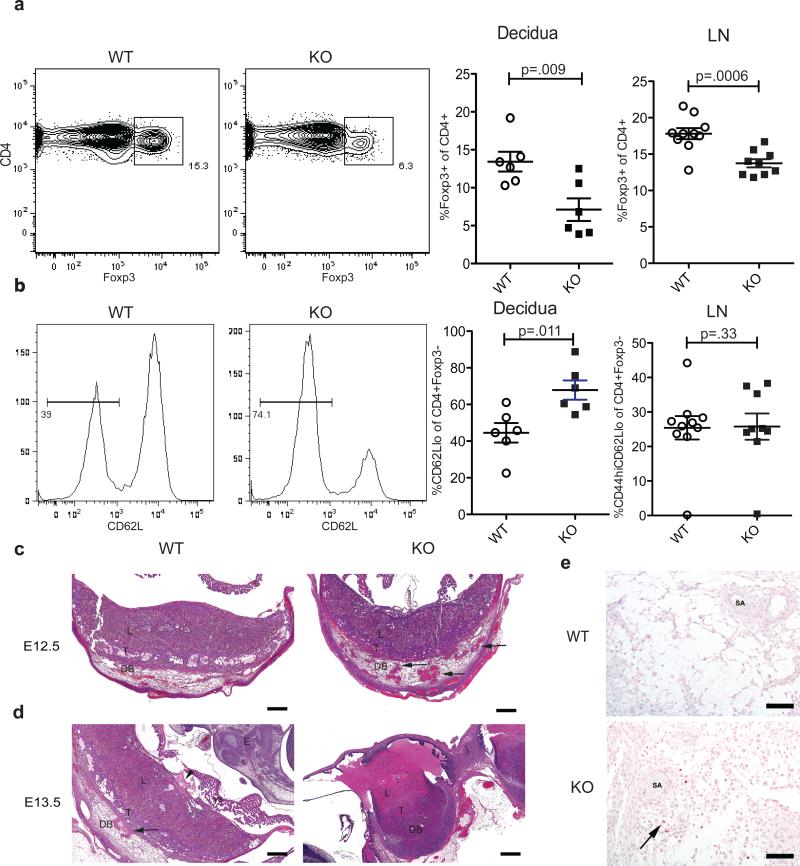
Consistent with the impaired pTreg induction during allogeneic pregnancy in CNS1-deficient females, we observed markedly reduced Treg cell numbers in the decidua in these mice mated with BALB/c males (Figure 5a). Proliferative activity assessed by Ki67 expression was not increased in Treg cell subsets in the decidua and DLN in CNS1-deficient mice in comparison to CNS1-sufficient controls (Supplementary Figure 3). These results suggested that paucity of pTreg cells is not associated with compensatory expansion of tTreg cells in agreement with our recent observation (Josefowicz et al., 2012). The observed decrease in the Treg cell population inversely correlated with the increased presence of activated effector CD62LloCD4+ T cells (Figure 5b), however, no significant changes in effector cytokine production was detected in the DLN or decidua (Supplementary Figure 4). It must be noted that fluid composition and activation state of immune cells in a rapidly changing placental environment could mask potential differences. Histologic examination of the placentas of CNS1-deficient and -sufficient females performed in a blinded fashion showed that the genotype of the dams can be accurately predicted by the morphological status of the decidual spiral arteries; although there was some variability between individual placentas in any one uterus, CNS1-deficient placentas exhibited more prominent clusters of thickened blood vessels as compared to CNS1-sufficient littermate controls (Figure 5c). At day E13.5, placentas of surviving embryos of CNS1-deficient females exhibited early necrosis of spiral arteries and edema, while resorptions were characterized by embryo loss with necrotic labyrinths (Figure 4d). Presumptive early resorption sites exhibited necrosis or thrombosis of decidual vessels and edema in the placentas and embryos (Supplementary Figure 5). Consistent with the immune-mediated pathology, more prominent T cell presence was noted in CNS1-deficient placentas, where single T cells were scattered within all layers of the placenta with clusters prominent in the decidua near spiral arteries (Figure 5e). In contrast, only rare single T cells were observed in CNS1-sufficient placentas and no major changes in the numbers of B cells and macrophages were detected (Supplementary Figure 5). These observations are indicative of an inflammatory pathology associated with allogeneic pregnancy in CNS1-deficient females resembling that of human pregnancy-associated disorders such as preeclampsia (Redman and Sargent, 2005; Renaud et al., 2011).
Figure 5. Decreased Treg cell numbers and histological features of immune-mediated resorption in deciduas of CNS1-deficient female mice.
(A) Representative flow cytometric analysis of Foxp3+ Treg cells in decidua and analysis of decidua and lymph nodes (LN) of CNS1-sufficient (WT) and -deficient (KO) mice mated with BALB/c males and analyzed on day E13.5-E14.5. Error bars indicate standard error (n=8-12). (B) Representative flow cytometric analysis of activated CD62Llo Foxp3-negative CD4+ T cells within the decidua and analysis of decidua and LN. (C) Histopathological evaluation of placentas from WT (left) and CNS1 KO (right) females mated with BALB/c males; low power magnification survey of representative sections of H&E stained placenta. The maternal spiral arteries (SA) are more frequently clustered and prominent in KO placenta at day E12.5 (arrows, upper right panel) (representative of 6-8 mice analyzed per group with 4-10 placental sites each). (D) Analysis of H&E stained sections of the KO placentas at day E13.5; early necrosis of SA (arrow, left) in the decidua (DB) and edema (arrowhead, left) at the chorionic plate. Resorption sites (lower right) shown in the same animal were characterized by loss of embryo and necrotic labyrinths (L) with variable necrosis in the trophoblast (T) layer; embryo (E) and yolk sac (YS) as indicated. Scale bars=500μm. (E) Immunohistochemical staining for CD3 in day E12.5 placentas from CNS1-sufficient (WT) and –deficient (KO) females. CD3+ T cells (brown staining) are more numerous in the KO placentas in the proximity to maternal spiral arteries (SA). Scale bars=100μm. See also Figure S3-5.
Discussion
Over the last decade numerous studies have led to the realization that suppressive function of Treg cells extends far beyond autoimmunity, the originally suggested sphere of their activity. Treg cells have been implicated in control of acute and chronic infections, tissue homeostasis at barrier sites populated by commensal microbiota, allergy, injury response and tissue repair, metabolic syndrome, and cancer (Josefowicz et al., 2012). In this study, we find that the Foxp3 intronic enhancer CNS1, essential for extrathymic differentiation of Treg cells, is present only in eutherian mammals, but not in marsupials or monotremes and that pTreg cell paucity in CNS1-deficient females mated to MHC-mismatched males results in increased spontaneous abortion of embryos. An implication of these observations is that generation of Treg cells in the thymus does not afford adequate protection of the fetus expressing allogeneic MHC alleles from immune mediated attack by maternal T cells. This latter notion is also supported by the comparable extent of embryo resorption associated with acute depletion of pTreg and pan-Treg ablation, i.e. elimination of both pTreg and tTreg cells. This finding implies that extrathymically generated Treg cells serve as the predominant subset mitigating maternal-fetal allogeneic conflict. We would propose that once in place, extrathymic generation of Treg cells, primarily driven by the pressure to enforce maternal fetal tolerance, likely assumed additional functions including control of responses to non-self antigens leading to allergy and asthma and to commensal organisms in the gut (Lathrop et al., 2011; Josefowicz et al., 2012).
Although pTreg cell deficiency in CNS1-deficient mice resulted in significantly increased resorption of fetuses during allogeneic pregnancy, its penetrance was incomplete. This was a rather expected result likely due to multiple mechanisms that operate during pregnancy to limit encounter of maternal alloreactive T cells with, or their response to, fetal alloantigens. These mechanisms include, but are not limited to, inactivation of immune cells by tryptophan deprivation by indoleamine 2,3-dioxygenases (Munn et al., 1998), Fas-Fas ligand mediated apoptosis of activated alloreactive T cells (Hunt et al., 1997), expression of immunosuppressive mediators such as TGF-ß and galectin-1 (Simpson et al., 2002; Blois et al., 2007), entrapment of endometrial dendritic cells (Collins et al., 2009), limited expression of MHC molecules on trophoblasts (Erlebacher et al., 2007), and increased expression of inhibitory B7 family members (PD-L1, B7H3, B7H4) (reviewed in Petroff and Perchellet, 2010). It seems reasonable to suggest that pTreg cell mediated suppression enforces maternal-fetal tolerance not single-handedly, but jointly with other numerous immunomodulatory mechanisms.
Additional factors, which could influence the degree of immune mediated resorption associated with pTreg cell or pan-Treg cell deficiency include the genetic background, microbial status, and stress exposure. It is likely that in the absence of pTreg cells infection may result in a more severe pregnancy disruption. It must be also noted that the three week-long gestation period in mice is relatively short; extrathymic generation of Treg cells may play a more pronounced role in maternal-fetal tolerance in mammals with longer gestation times where there would be higher probability of the encounter of alloreactive T cells of the mother with paternally encoded alloantigens and for the immune response to develop.
The aforementioned possible influences affecting severity of pregnancy disruption and differences in experimental design might account for a varying degree of embryo resorption observed in our experiments and in previous reports employing adoptive T cell transfers and CD25 antibody mediated Treg cell depletion in lymphopenic or lymphoreplete mice (Aluvihare et al., 2004; Shima et al., 2010). However, we have not encountered pervasive fetal death observed upon continuous DT-mediated ablation of Treg cells starting at mid-gestation daily which was likely due to secondary effects of the poor health condition of the mother (Rowe et al., 2011).
Our demonstration of a key role of extrathymic generation of Treg cells in maternal-fetal tolerance substantially adds to previous studies demonstrating the general importance of Treg cells in control of maternal immune responses to the allogeneic fetus in mice. In humans, Treg cells are present in increased numbers during pregnancy in the blood and the decidua (Heikkinen et al., 2004; Somerset et al., 2004). Decreases in Treg cells have been associated with frequent human pregnancy disorders including preeclampsia and repeated spontaneous abortions (Arruvito et al., 2009; Darmochwal-Kolarz et al., 2012). The histological features of allogeneic pregnancy in pTreg cell-deficient females were redolent of abnormal spiral artery remodeling associated with pre-eclampsia and other complications of pregnancy in humans and accompanying increased local inflammation (Redman and Sargent, 2005; Avagliano et al., 2011; Renaud et al., 2011). These observations raise an intriguing possibility of a link between these conditions and impaired pTreg generation or function. Our study suggests that the reduced Treg accumulation and resulting pathology may be partially due to defective peripheral induction of Treg cells to paternal antigens and potential therapies could be developed to address this defect.
The analysis of CNS1 sequence conservation suggests that this enhancer was gained during evolution of eutherian mammals. CNS1 contains binding sites for transcription factors downstream of three major signaling pathway required for pTreg generation and its deletion results in a selective impairment of this differentiation process (Zheng et al., 2010). Thus, the introduction of this several hundred base pair-long DNA sequence into the Foxp3 locus could have been sufficient to enable the differentiation of pTreg cells. This line of reasoning implies that the increased interaction between the mother and fetus during gestation necessitated a mechanism of acquired active tolerance afforded by peripheral generation of regulatory T cells. Consistent with this idea, diminished litter size observed in allogeneic pregnancy in CNS1-deficient vs. –sufficient females suggested that pTreg generation afforded a reproductive advantage.
The process of insertion of CNS1 sequence into the first intron of the Foxp3 locus appears to have occurred via a MIR family retrotransposon activity during the Mesozoic era at a time overlapping with the evolution of placental mammals. We found that in the mouse genome MIR elements resembling CNS1 are enriched for SMAD and RXR binding sites (data not shown) suggesting that these elements may have endowed TGF-β and retinoic acid response capacity to Foxp3 and other genes in agreement with the idea of exaptation where portions of transposable elements acquire a function that serves their host (Brosius and Gould, 1992). These elements can then confer novel signaling pathway responsiveness to existing genes, thereby augmenting their function. A mechanism of retrotransposon-mediated exaptation affecting the structure or regulation of pre-existing genes upon introduction of novel exons or enhancers has been previously reported (Bejerano et al., 2006; Mikkelsen et al., 2007). We suggest that acquisition of CNS1 supporting extrathymic Treg cell generation in eutherian mammals represents a novel example of retrotransposition-mediated innovation in regulation of gene expression where a distinct biological purpose and novel functionality associated with the CNS1 enhancer is implicit of the potential evolutionary pressure underlying its conservation.
It is noteworthy in this regard that the emergence of chorioallantoic placenta, which allowed for viviparity in therian mammals, was assisted by appropriation of several retroviral genes including retrotransposon-derived Peg10 and Peg11/Rtl1 and syncitin-A and –B originating from the envelope protein of a defective retrovirus. These genes are essential for normal function of placenta and trophoblast fusion, respectively (Mi et al., 2000; Ono et al., 2006; Sekita et al., 2008; Dupressoir et al., 2011). Taken together, these results and our findings suggest that in addition to facilitating placentation during mammalian evolution retrotransposon-mediated innovation helped to alleviate immune conflict associated with this acquisition.
In conclusion, we suggest that the mechanism of extrathymic differentiation of Treg cells may have been gained during evolution to reinforce tolerance to paternal alloantigens presented by the fetus during the increasingly long gestation period in placental mammals. This adaptation was realized with the aid of the Foxp3 CNS1 enhancer responsible for induction of Foxp3 in peripheral CD4+ Foxp3- T cells, which likely emerged upon capture of a MIR retrotransposon containing TGF-β and retinoic acid receptor response elements. A role of extrathymically generated Treg cells in maternal-fetal tolerance may provide an important insight into potential clinical complications of human pregnancies.
Experimental Procedures
Mouse strains and timed matings
Foxp3GFP, Foxp3DTR, TEa, and CNS1-deficient mice on a B6 background were previously described (Grubin et al., 1997; Fontenot et al., 2005; Kim et al., 2007; Zheng et al., 2010). TCRβδ-deficient B6 mice were purchased from Jackson Laboratory and maintained as a homozygous colony. All the mice were bred and housed in the specific pathogen-free animal facility at the Memorial Sloan-Kettering Cancer Center and used in accordance with institutional guidelines. Diphtheria toxin (DT) (Sigma) was administered twice i.p. as indicated.
Timed matings
1 or 2 female mice were setup in the afternoon with individual males. Females were checked daily for the presence of a vaginal plug in the mornings and plugged females were separated from males; the day of plug detection was considered day E0.5. Plugged females were analyzed for resorbed fetuses at E14.5 and the resorption was always analyzed in 3 different ways to confirm significance (see Statistical analysis).
Adoptive cell transfers
TEa CD4+Foxp3-negative (GFP-) cells were purified using an Aria2 cell sorter (BD Biosciences) after enrichment of CD4 cells using Dynal CD4 magnetic beads according to manufacturer instructions (Invitrogen). 4 × 106 cells were injected i.v. into TCRβδ-deficient B6 females and the recipient mice were time mated with B6 or BALB/c males. Pregnant mice were analyzed on day E13.5.
CNS1 sequence analysis
The Foxp3 locus, including the 100kb flanking sequence 5' and 3' of the Foxp3 gene, was found in each species by ENSEMBL annotations. The most CNS1-like sequence in all species was determined by scanning for mouse CNS1 across the Foxp3 locus using global-local alignment. For scoring alignments, there was no penalty for opening gaps, -1 for extending gaps or mismatched nucleotides, and +1 for matched nucleotides. All of CNS1 was aligned to a moving window twice the size of CNS1 and the window was moved 50% of the size of CNS1. Genome-wide phylogenies were downloaded from http://hgdownload.cse.ucsc.edu/goldenPath/hg19/phyloP46way/vertebrate.mod and branch lengths were scaled to the number of substitutions per site(Nikolaev et al., 2007; Pollard et al., 2010). Phylogenetic tree was unrooted and the root was arbitrarily selected. The tree had two scales since the distance between non-eutherians is much larger than non-eutherians.
Luciferase assays
Foxp3 luciferase expression constructs were generated using Infusion cloning system (Clontech) and verified by restriction digests and sequencing. 5 × 106 EL4-LAF cells were mixed with 5 μg of indicated vector and 0.8 μg of pRL-TK control vector in complete RPMI with 20% fetal bovine serum (FBS) and electroporation was performed using a Biorad electroporator (300V, 1000 μF). Cells were rested for 15 minutes and then incubated in complete RPMI supplemented with 10% FBS for 1 hour before addition of PMA, ionomycin, and TGFβ (250 ng/ml, 25 ng/ml, and 4 ng/ml, respectively). After 18-24 hours cells were lysed and dual luciferase activity was measured according to manufacturer instructions (Promega). Firefly luciferase activity levels were normalized to Renilla luciferase and the resulting normalized values with stimulation were divided by those without to determine the stimulation dependent enhancement of promoter activity.
Flow cytometry
Single-cell suspensions from lymph nodes and spleen were prepared by mechanical disruption after dissection. Deciduas were isolated by careful dissection from the uterus and separation of the yolk sac containing the fetus followed by mechanical disruption. Fluorophore-conjugated antibodies were purchased from BD-Biosciences and eBioscience. Intracellular Foxp3 staining was performed using Foxp3 mouse Treg cell staining kit (eBioscience). Stained cells were analyzed using an LSRII flow cytometer (BD Biosciences) and data were analyzed using FlowJo software (Treestar).
Histological analyses
Hematoxylin-, eosin- and periodic acid Schiff-stained placental-fetal units from day E12.5 to 13.5 were examined histologically for qualitative changes to morphologically characterize early resorptions. Changes evaluated included clustered and thickened decidual spiral arteries, necrosis, thrombosis, edema, and inflammation in any placental layer and necrotic placenta sites that lacked embryos (resorptions). For each uterus, all intact placental sites were evaluated individually and scored for viability (resorptions) and prominence of clustered spiral arteries, necrosis in the metrial gland and necrosis and inflammation in the trophoblast layer. A total of 6 WT uteri with 60 gestational sites and 7 KO uteri with 51 sites were examined.
Immunohistochemical methods
Placental-embryo units were stained with antibodies against CD3 (T cells; Clone CD3-12, AbD Serotec), F4/80 (macrophages; CALTAG), and CD31 (blood vessels; Abcam). Appropriate isotype controls and Bond Polymer Refine (DAB) detection kit including peroxide block, polymer and hematoxylin (Leica) were used. Slides were qualitatively examined for signal detected by DAB staining (brown). Images were captured with Nikon Eclipse 80i with CFI plan apo-objectives and Nikon Digital Sight DS-Fi1 12 mega pixel camera and Nikon Basic Elements Software (Nikon). Raw images were edited for brightness in Adobe Photoshop Elements.
Statistical analysis
Unless otherwise noted statistical analysis was performed using the unpaired two-tailed Student's t-test with Welch's correction for unequal variances for individual biological replicates in Prism (GraphPad). The Fisher's exact test was used to assess significance of the ratios of healthy and resorbed fetuses. This method assumes that each individual fetus represents an independent event due to physical separation of individual embryos from mother's alloreactive T cells and likely probabilistic factors affecting alloreactive T cell activation in the placenta. In support of the choice of the Fisher's exact test as the adequate way to analyze the data in question, we very rarely observe large numbers of resorbed embryos in a given female or strings of resorbing embryos, i.e. frequent resorptions of neighboring embryos. These observations suggested that resorption of each fetus seems to be an independent event with a considerable stochastic component. Nevertheless, to exclude the potential concern of non-independence, we also performed the Fisher's exact test on the incidence of mother's having any resorption event. Lastly, the Student's t-test was also used to compare the percent resorption of the fetuses in individual females. Non-parametric tests were also significant but p values are not shown.
Supplementary Material
Research Highlights.
CNS1, a Foxp3 enhancer driving extrathymic Treg generation, appears in eutherians
CNS1 facilitates accumulation of fetal alloantigen-specific Treg cells in decidua
Increased fetal resorption during allogeneic pregnancy of CNS1-deficient females
Acknowledgements
We would like to thank J. Gerard, Y. Liang, D. Canner, M. Samstein and the UW Histology and Imaging Core for technical assistance; Z. Williams for helpful discussion; S. Gelber for review of histology; P. Samollow and J. Vandeberg for providing opossum genomic DNA. R.M.S. was supported by NIH DK091968 and MSTP grant GM07739. A.Y.R. is an investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Arruvito L, Billordo A, Capucchio M, Prada ME, Fainboim L. IL-6 trans- signaling and the frequency of CD4+FOXP3+ cells in women with reproductive failure. J. Reprod. Immunol. 2009;82:158–165. doi: 10.1016/j.jri.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Avagliano L, Bulfamante GP, Morabito A, Marconi AM. Abnormal spiral artery remodelling in the decidual segment during pregnancy: from histology to clinical correlation. J. Clin. Pathol. 2011;64:1064–1068. doi: 10.1136/jclinpath-2011-200092. [DOI] [PubMed] [Google Scholar]
- Bejerano G, Lowe CB, Ahituv N, King B, Siepel A, Salama SR, Rubin EM, Kent WJ, Haussler D. A distal enhancer and an ultraconserved exon are derived from a novel retroposon. Nature. 2006;441:87–90. doi: 10.1038/nature04696. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Brosius J, Gould SJ. On “genomenclature”: a comprehensive (and respectful) taxonomy for pseudogenes and other “junk DNA”. Proc Natl Acad Sci U S A. 1992;89:10706–10710. doi: 10.1073/pnas.89.22.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged- helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei K-J, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MK, Tay C-S, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012;93:75–81. doi: 10.1016/j.jri.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Dupressoir A, Vernochet C, Harper F, Guégan J, Dessen P, Pierron G, Heidmann T. A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast. Proc Natl Acad Sci U S A. 2011;108:E1164–73. doi: 10.1073/pnas.1112304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen J, Möttönen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol. 2004;136:373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JS, Vassmer D, Ferguson TA, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158:4122–4128. [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J, Zietkiewicz E, Labuda D. Ubiquitous mammalian-wide interspersed repeats (MIRs) are molecular fossils from the mesozoic era. Nucleic Acids Res. 1995;23:170–175. doi: 10.1093/nar/23.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, Boehmer, von H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C-W, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh C-S. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Margulies EH, Cooper GM, Asimenos G, Thomas DJ, Dewey CN, Siepel A, Birney E, Keefe D, Schwartz AS, Hou M, et al. Analyses of deep mammalian sequence alignments and constraint predictions for 1% of the human genome. Genome Res. 2007;17:760–774. doi: 10.1101/gr.6034307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, Duke S, Garber M, Gentles AJ, Goodstadt L, Heger A, et al. Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature. 2007;447:167–177. doi: 10.1038/nature05805. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- Nikolaev S, Montoya-Burgos JI, Margulies EH, NISC Comparative Sequencing Program. Rougemont J, Nyffeler B, Antonarakis SE. Early history of mammals is elucidated with the ENCODE multiple species sequencing data. PLoS Genet. 2007;3:e2. doi: 10.1371/journal.pgen.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono R, Nakamura K, Inoue K, Naruse M, Usami T, Wakisaka-Saito N, Hino T, Suzuki-Migishima R, Ogonuki N, Miki H, et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat Genet. 2006;38:101–106. doi: 10.1038/ng1699. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Perchellet A. B7 family molecules as regulators of the maternal immune system in pregnancy. Am. J. Reprod. Immunol. 2010;63:506–519. doi: 10.1111/j.1600-0897.2010.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20:110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Iglesias AH, Farez MF, Caccamo M, Burns EJ, Kassam N, Oukka M, Weiner HL. Adaptive autoimmunity and Foxp3-based immunoregulation in zebrafish. PLoS ONE. 2010;5:e9478. doi: 10.1371/journal.pone.0009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Renaud SJ, Cotechini T, Quirt JS, Macdonald-Goodfellow SK, Othman M, Graham CH. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186:1799–1808. doi: 10.4049/jimmunol.1002679. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekita Y, Wagatsuma H, Nakamura K, Ono R, Kagami M, Wakisaka N, Hino T, Suzuki-Migishima R, Kohda T, Ogura A, et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat Genet. 2008;40:243–248. doi: 10.1038/ng.2007.51. [DOI] [PubMed] [Google Scholar]
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Simpson H, Robson SC, Bulmer JN, Barber A, Lyall F. Transforming growth factor beta expression in human placenta and placental bed during early pregnancy. Placenta. 2002;23:44–58. doi: 10.1053/plac.2001.0746. [DOI] [PubMed] [Google Scholar]
- Smit AF, Riggs AD. MIRs are classic, tRNA-derived SINEs that amplified before the mammalian radiation. Nucleic Acids Res. 1995;23:98–102. doi: 10.1093/nar/23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- Wildin R, Ramsdell F, Peake J, Faravelli F, Casanova J, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Winger EE, Reed JL. Low circulating CD4(+) CD25(+) Foxp3(+) T regulatory cell levels predict miscarriage risk in newly pregnant women with a history of failure. Am. J. Reprod. Immunol. 2011;66:320–328. doi: 10.1111/j.1600-0897.2011.00992.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity. 2010;33:313–325. doi: 10.1016/j.immuni.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.