Abstract
SAP, an adaptor molecule that recruits Fyn to the SLAM-family of immunomodulatory receptors, is mutated in X-linked lymphoproliferative disease. CD4+ T cells from SAP-deficient mice have defective TCR-induced IL-4 production and impaired T cell-mediated help for germinal center formation; however, the downstream intermediates contributing to these defects remain unclear. We previously found that SAP-deficient CD4+ T cells exhibit decreased PKC-θ recruitment upon TCR stimulation. We demonstrate here using GST-pulldowns and co-immunoprecipitation studies that SAP constitutively associates with PKC-θ in T cells. SAP-PKC-θ interactions required R78 of SAP, a residue previously implicated in Fyn recruitment, yet SAP’s interactions with PKC-θ occurred independent of phosphotyrosine binding and Fyn. Overexpression of SAP in T cells increased and sustained PKC-θ recruitment to the immune synapse and elevated IL-4 production in response to TCR plus SLAM-mediated stimulation. Moreover, PKC-θ, like SAP, was required for SLAM-mediated increases in IL-4 production and conversely, membrane-targeted PKC-θ mutants rescued IL-4 expression in SAP−/− CD4+ T cells, providing genetic evidence that PKC-θ is a critical component of SLAM/SAP-mediated pathways that influence TCR-driven IL-4 production.
Keywords: TH2 Differentiation, IL-4, Protein Kinases, T Cell Receptor, SLAM receptors
Introduction
Regulation of CD4+ T helper cell cytokine production plays a critical role in facilitating proper immune homeostasis and developing immune responses to infectious agents. In particular, T helper 2 (TH2) cytokines are important for barrier responses to the environment and immune responses to certain extracellular parasites. Abnormal activation of TH2 responses can be associated with pathological states of hypersensitivity, including asthma (1). Cytokines produced by TH2 cells, including IL-4, IL-5 and IL-10, also help regulate immunoglobulin isotype switching and specific types of antibody responses. Recently, IL-4 expression has also been shown to be expressed by follicular T helper (TFH) cells, a CD4+ T cell population found in germinal centers and required for their formation (2–4). Understanding the signaling pathways involved in the regulation of IL-4 is therefore of interest both for understanding immune homeostasis as well as for the potential development of therapeutic approaches to TH2-mediated diseases.
X-linked lymphoproliferative disease (XLP) is a rare genetic disorder characterized by fulminant infectious mononucleosis, hyperactivation of immune cells, dysgammaglobulinemia and lymphoma. Although immune dysregulation in this disorder is usually triggered by infection with Epstein-Barr virus (EBV), hypogammaglobulinemia and other immune irregularities can develop in the absence of EBV infection, suggesting a more basic immune dysfunction associated with this disease (5). The gene that is mutated in most cases of XLP, Sh2d1a, encodes a 14 kDa protein known as SAP (SLAM-Associated Protein), which consists almost entirely of a single SH2 domain. SAP binds to a sequence termed an immunoreceptor tyrosine-based switch motif, TxYxxV/I in the intracellular tail of the costimulatory molecule SLAM (Signaling Lymphocyte Activation Molecule, CD150) and related receptors, including 2B4 (CD244), Ly9 (CD229), Ly108 (NK, T, B cell antigen, NTB-A human), CD84 and CD319 (CD2-like receptor activating cytotoxic cells, CRACC) (6). SAP has been found to be essential for signaling downstream of SLAM, 2B4 and Ly108 in T and NK cells (7–10). Binding of SAP to SLAM promotes the recruitment and activation of the tyrosine kinase Fyn, which binds to a region of SAP centered on the R78 residue and subsequently phosphorylates SLAM, leading to the recruitment of SHIP, Docking protein (Dok)1 and Dok2 (9, 11). Other evidence has implicated the protein tyrosine phosphastases SHP-1 and 2, as well as PAK-Interacting exchange factor (PIX), non-catalytic region of tyrosine kinase (NCK)1 and Protein Kinase C-theta (PKC-θ) in signaling downstream of SLAM and SAP (7, 11–14). Nonetheless, besides Fyn it has not been conclusively shown that any of these signaling intermediates are required for downstream function of SLAM in T cells.
We have previously reported that SLAM engagement enhanced T cell receptor (TCR) regulation of IL-4 in a SAP-dependent manner in murine CD4+ T cells (5, 7). Additionally, both SAP−/− and SLAM−/− CD4+ T cells exhibit defects in TCR-driven TH2 cytokine production (5, 7, 15–18). Nonetheless, in response to exogenous IL-4, which can polarize CD4+ T cells along a TH2 differentiation pathway, SAP−/− T cells do produce TH2 cytokines, suggesting that SAP is not required for responses to cytokines. SAP is also required for CD4+ T cell-mediated help for B cells required for germinal center formation and long-term humoral immunity (19–24). We have recently found SAP-deficient T cells show specific defects in adhesion to B cells (24) as well as decreased numbers of TFH cells associated with reduced IL-21 production (25, 26), all defects that are likely to contribute to this phenotype (24).
Interestingly, data examining the R78A mutant of SAP, which severely impairs Fyn recruitment, suggest that the signaling pathways downstream of SAP for IL-4 regulation and germinal center formation may be distinct; germinal center generation is rescued by this mutant, while TH2 cytokine production is not (19, 27). However, which downstream signaling intermediates contribute to the different effects of SAP on T cell function remains unclear. We have previously reported that SLAM-SAP signaling contributes to the stable and prolonged recruitment of PKC-θ to the immune synapse upon TCR stimulation (7). Intriguingly, T cells from PKC-θ−/− mice have been reported to have TH2 defects (28, 29).
To further understand the signaling pathways involving SAP, we examined the involvement of PKC-θ in SAP-mediated signal transduction. We demonstrate that SAP constitutively associates with PKC-θ in T cells via an interaction that is dependent on arginine 78 of SAP, a residue previously shown to be required for SAP-mediated regulation of IL-4 production (7, 16). Mice over-expressing SAP displayed enhanced and sustained PKC-θ recruitment that was associated with increased IL-4 production in response to TCR and SLAM ligation. Moreover, PKC-θ−/− CD4+ T cells, like those from SAP−/− mice, had impaired IL-4 production and failed to increase IL-4 production in response to SLAM. Conversely, expression of constitutively activated membrane-targeted PKC-θ constructs markedly improved IL-4 production in both SAP−/− and WT CD4+ T cells. Together, these data provide evidence that PKC-θ is a critical intermediate in pathways involved in SAP-mediated IL-4 production and that altered PKC-θ regulation directly contributes to the IL-4 defect in observed in SAP-deficient CD4+ T cells. Moreover, these data further define the SAP-dependent signaling pathways required for IL-4 expression that are distinct from those required for T:B cell-mediated adhesion and germinal center formation.
Materials and Methods
Mouse strains
SAP−/− mice (15) backcrossed to C57BL/6J for 10 generations. SAP transgenic mice were generated by subcloning a myc-tagged human SAP cDNA into the vector p29Δ2(sal-), containing the T cell-specific CD2 promoter and enhancer (kindly donated by P. Love), and microinjecting the transgene fragment into CD1 pronuclei. Genotyping of founders was done by Southern blotting. Founders were bred to C57BL/6 mice and subsequent genotyping performed by PCR with the primers: 5’ TGG GGC TTT CAG GCA GAC ATC 3’ and 5’ GGA GCA CAT CAG AAG GGC TG 3’. Expression of human SAP was confirmed using Western blot. Fyn−/−, AND and OT-II TCR transgenic mice purchased from Jackson Laboratory. B10.BR mice were purchased from Taconic. PKC-θ−/− (30) and cells from PKC-θ−/−AND mice backcrossed to C57Bl/6 for 15 generations (31) were kindly provided by D. Littman and M. Dustin, respectively. Mice were maintained in sterile microisolator cages on autoclaved water and food, according to institutional guidelines.
Antibodies and reagents
Antibodies and reagents were from the following sources: anti-mouse SAP was previously described (15); anti-GFP (Roche), anti-PKC-θ, anti-Fyn, anti-Lck, anti-SAP (Santa Cruz); anti-myc and anti-human SAP (Cell Signaling); anti-phosphotyrosine 4G10 (Upstate), anti-TCR, anti-CD28, anti-CD3, (BD PharMingen); anti-SLAM (Biolegend); anti-rabbit HRP (Chemicon International); anti-mouse HRP (Roche); Alexa Fluor 594 phalloidin; anti-rabbit rhodamine (Jackson ImmunoResearch Labs), anti-mouse Alexa 568 (Invitrogen), ICAM2-Fc and SLAM-Fc (R&D). PCC88–104 was purchased from SynPep and OVA323–339 was purchased from ANASpec.
Cell lines, Constructs, Transfection and Transduction
The P13.9 fibroblast cell line expressing I-Ek, CD80, and ICAM (32) as well as the SLAM-expressing variant were described previously (7, 19). Jurkat-E6 cells were grown in RPMI-1640 supplemented with 5% FBS, 5% FCS, and 4 mM glutamine. The pEBG mammalian glutathione S-transferase (GST) fusion vector and pEBG-SLAM (cytoplasmic tail) were previously described (33). Jurkat-E6 cells were transiently transfected via electroporation with 10 µg of plasmid in 0.4-mm cuvettes (315 V, 10.8 ms, BTX-850). SAP cDNA was cloned from C57BL/6 mouse thymocytes, the R55L and R78A mutants generated by site directed mutagenesis (Stratagene), and subcloned into glutathione S-transferase (GST)-expression and green fluorescent protein (GFP)-expression vectors. To examine SAP-PKC-θ interactions in vivo, CD4+ T cells were activated with anti-CD3 plus anti-CD28 for 72 h, rested in IL-2 (10 U/ml) for 48 h and nucleofected with 4µg of GFP-SAP, GFP-SAP(R78A) or GFP-SAP(R55L) by Amaxa nucleofection (Amaxa) as previously described (24). Constitutively active PKC-θ was generated by site directed mutagenesis of the Arg-145 and Arg-146 in the pseudokinase domain to Ile and Trp, respectively (34). Myr-PKC-θ was constructed by fusing the catalytic domain of PKC-θ with the NH2-terminal seven amino acids from Lck (35). PKC-θ cDNAs were subcloned into the retroviral vector MIGR containing an IRES-GFP marker. CD4+ T cells were retrovirally reconstituted with vector control (Migr), Myr-PKC-θ or PKC-θ KA in the presence of blocking cytokine antibodies as described (7).
Immunoprecipitation, in vitro binding assays and western blots
Cells were cultured in starvation medium at 37°C for 60–90 min for peripheral T cells or 90–120 min for thymocytes. Thymocytes and peripheral T cells were stimulated with either biotinylated anti-CD3 (5µg/ml) and cross-linked with streptavidin (5µg/ml) or with pervanadate for the indicated times. Pervanadate (PV) was freshly prepared by mixing 1 M solutions of vanadate and H2O2 in phosphate-buffered saline to give a 0.5 M solution of pervanadate, then diluted into cells at the indicated final concentration. For co-immunoprecipitations, 5×107 cells were lysed in 50 mM Tris pH 8.0, 1% NP-40, 2 mM EDTA supplemented with protease and phosphatase inhibitors. Lysates were precleared, immunoprecipitated overnight with the indicated antibodies and immune complexes captured with protein-A agarose or anti-mouse agarose (Santa Cruz). GST fusion proteins were produced in BL21 bacteria and purified on agarose-glutathione beads. In vitro binding assays were performed using lysates from 5×107 thymocytes or 2×107 peripheral T cells with 10µg of GST fusion proteins. Protein complexes were resolved on SDS-PAGE gels and transferred to PVDF or nitrocellulose membranes for immunoblotting with the indicated antibodies.
Isolation, Differentiation and Cytokine Production
To isolate splenic DCs, spleens were digested with Liberase CI (Roche) and purified with CD11c microbeads (Miltenyi Biotec). Polyclonal B cells were isolated by CD43 B cell isolation kit (Miltenyi Biotec) and stimulated with LPS (ALEXIS Biochemicals). CD4+ T cells from were purified with CD4 isolation kits (Miltenyi Biotec), sorted for CD62LhiCD44lo naïve cells and stimulated 24–72 h with either anti-CD3 plus anti-CD28 or peptide pulsed APCs, and supernatants analyzed for cytokines via ELISA (R&D). T cell differentiation was as described (7, 15). AND CD4+ T cells were stimulated with p13.9 (APC) or SLAM expressing variant pulsed with PCC peptide for 72 h, changed into media with 10U/ml IL-2 for 24 h and restimulated with PMA (3ng/ml) and ionomycin (1µg/ml) for intracellular cytokine analysis as described (7). Flow cytometric data analysis was performed using Flojo (TreeStar).
Northern Blot and Real-Time PCR Analysis
RNA was isolated with Trizol (Invitrogen). Northern blots with 8–10µg of RNA were hybridized to a GATA-3 cDNA probe and normalized to β2M. Real-time PCR and primers and probes were previously described (7).
Conjugate Assay and Immunofluorescence
Latex beads (Interfacial Dynamics) were coated with anti-TCR (H57) or anti-H2Kb in PBS for 1 h at 37°C. Beads were spun, resuspended in PBS, and anti-CD28, ICAM2-Fc or SLAM-Fc added to a portion for 1 h. Beads were washed in PBS/3% BSA, resuspended in complete RPMI 1640 and stored at 4°C. T cell:bead conjugates were prepared and scored as described (36). Data are presented as the average percentage of cells with polarized signaling molecules per conjugate scored for at least 3 separate experiments ± SEM. For T-APC conjugates, eight-chambered coverglasses (LabTek) were cleaned by treatment with 1 M HCl, 70% ethanol for 30 min and dried at 60°C for 30 min. Chambers were treated with a 0.01% w/v poly-L-lysine solution (Sigma) overnight, drained, washed extensively with PBS and stored at 4°C. Cell conjugates were formed with 2×105 LPS-activated peptide pulsed B cells and 1×105 antigen-specific CD4+ T cells. CD4+ T cells and B cells were centrifuged (5 min 500 rpm, 4oC), incubated at 37°C for 15–60 min, plated onto poly-L-lysine−coated wells (37°C for 10 min), and were fixed for 20 min at 25°C with 3% paraformaldehyde in PBS. After fixation, coversglasses were quenched with NH4Cl and permeabilized in 0.3% Triton X-100 for 3 min. Blocking and antibody incubations were performed in PBS/0.05% saponin/0.25% fish skin gelatin. Specimens were mounted in Prolong Gold antifade reagent with DAPI (Invitrogen). Conjugates were analyzed with a Zeiss Fluorescence Axioskop2 microscope with IPLab imaging software. Images were cropped as necessary by using Adobe Photoshop CS3.
Results
SAP associates with PKC-θ
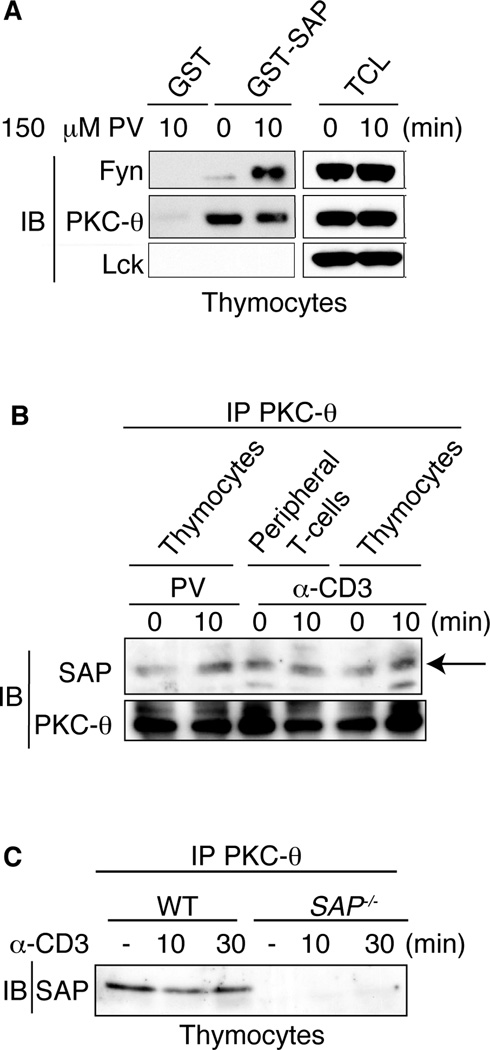
We have previously found that T cells from SAP−/− mice show decreased recruitment of PKC-θ to the site of TCR stimulation (7). To determine whether SAP associates with PKC-θ, we generated a glutathione S-transferase (GST)-SAP fusion protein. Thymocytes and peripheral T cells were either left untreated or treated with pervanadate (PV), a potent tyrosine phosphastase inhibitor that increases protein tyrosine phosphorylation, to assess SAP interactions following cellular activation. In agreement with the known inducible interaction of SAP and Fyn upon SLAM engagement, GST-SAP pulled down Fyn from T cell lysates following PV treatment (Figure 1A). In contrast, we were unable to detect an interaction between the GST-SAP fusion protein and Lck, despite a previous report demonstrating an interaction between Lck and SAP by yeast two-hybrid and GST pull down analyses (37). While the differences between our results are not clear, this discrepancy may reflect in vitro assay disparities or differences in the cell type and activation status of cells examined. Interestingly, the GST-SAP fusion protein also pulled down PKC-θ. The interaction of SAP with PKC-θ appeared to be independent of increased tyrosine phosphorylation, since it occurred in lysates from both untreated and PV stimulated thymocytes and peripheral cells (Figure 1A and data not shown). Thus, SAP can associate with PKC-θ in lysates from both stimulated and unstimulated T cells.
FIGURE 1.
SAP constitutively associates with PKC-θ. A, Thymocytes were either untreated or treated with 150 µM pervanadate (PV) for 10 min, lysed, precleared and GST or GST-SAP fusion proteins incubated with cell lysates. GST pull downs were immunoblotted for Lck, Fyn and PKC-θ. TCL: Total cell lysates. B, Thymocytes and peripheral T cells were stimulated with either anti-CD3 or PV for 10 min, lysed, immunoprecipitated for PKC-θ, then immunoblotted for SAP and PKC-θ. Arrow indicates band corresponding to SAP. C, Thymocytes from WT and SAP−/− mice were stimulated with anti-CD3 and immunoprecipitated as in B. A protein corresponding to SAP immunoprecipitated with PKC-θ in WT cells only.
To evaluate whether SAP and PKC-θ interact in vivo, PKC-θ was immunoprecipitated from resting or activated thymocytes and mature peripheral T cells and the precipitated proteins were immunoblotted for SAP. Consistent with our GST-SAP pulldown assays, SAP associated with PKC-θ in both thymocytes and mature T cells that were either untreated or treated with anti-CD3 or PV, conditions that increase phosphorylation of both PKC-θ and SLAM (Figure 1B and C; data not shown). As expected, SAP protein was not detected when PKC-θ was immunoprecipitated from SAP-deficient T cells, confirming the identification of SAP in these co-immunoprecipitations (Figure 1C). These data indicate a constitutive SAP-PKC-θ association independent of tyrosine phosphorylation.
In reciprocal experiments, we were not able to detect PKC-θ in SAP immunoprecipitates from WT cells due to inefficient immunoprecipitation with our murine SAP antibody. However, we were able to detect PKC-θ co-immunoprecipitating with SAP in both resting and activated thymocytes from transgenic mice that over-express human SAP (see below) using an anti-human SAP (Supplemental Figure 1).
SAP R78 residue is critical for PKC-θ association
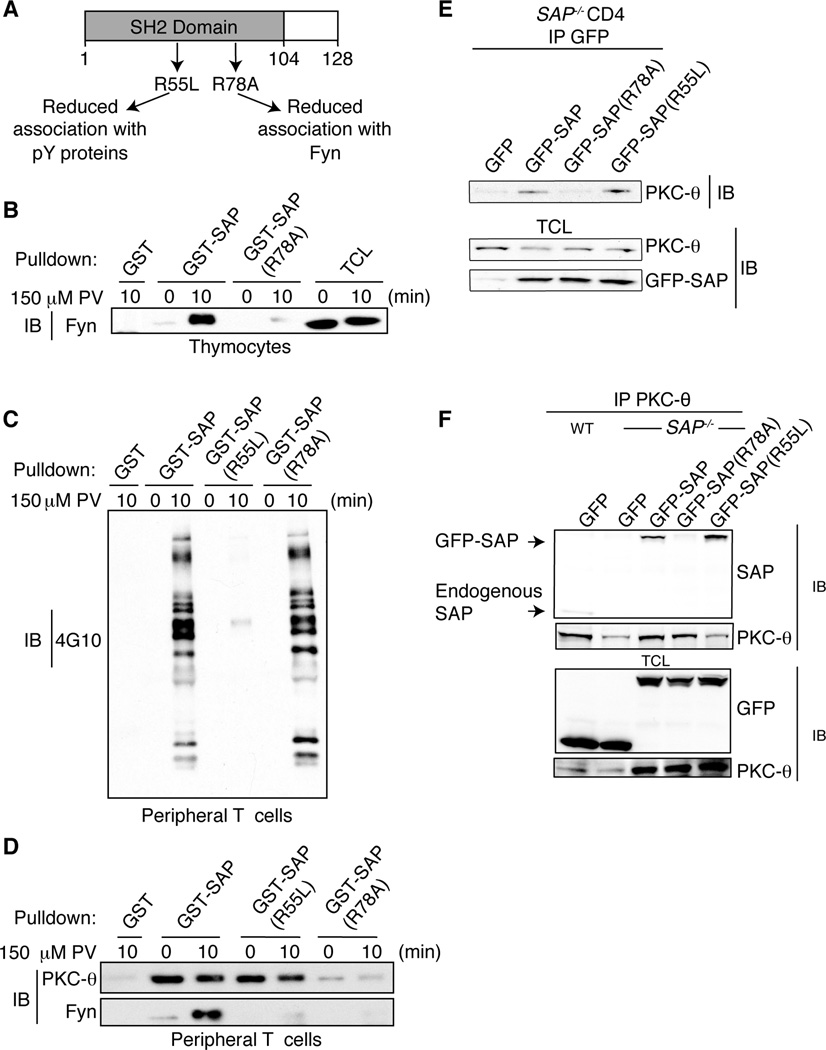
To explore which region of SAP is required for PKC-θ binding, we generated mutant GST-SAP fusion proteins (Figure 2A and Supplemental Figure 2). Arginine 78 of SAP has previously been reported to be required for mediating the interaction between SAP and the SH3 domains of Fyn, PIX and NCK1 (12, 13, 38, 39). Mutating arginine 78 of SAP to alanine [SAP(R78A)] greatly diminishes the interaction of SAP with Fyn following engagement of SLAM-related receptors on T and NK cells (38–40). We also observed a large reduction in Fyn binding to the GST-SAP(R78A) protein compared with the WT GST-SAP construct, although some residual Fyn binding to GST-SAP(R78A) could be detected (Figure 2B). Consistent with the findings that the R78A mutant still retains phosphotyrosine binding and does not affect SAP binding to SLAM-related receptors, (38–40) GST-SAP(R78A) still bound many tyrosine-phosphorylated proteins (Figure 2C). However, GST-SAP(R78A) exhibited impaired binding to PKC-θ in lysates from both untreated and PV stimulated T cells (Figure 2D). Thus, efficient interaction of SAP with PKC-θ requires the R78 motif of SAP.
FIGURE 2.
SAP R78 residue is critical for PKC-θ association. A, SAP protein with indicated mutants. GST proteins are shown in Supplemental Figure 2. B–C, Peripheral T cells or thymocytes were stimulated with 150 µM PV and lysed. GST, GST-SAP, GST-SAP(R78A) and GST-SAP(R55L) fusion proteins were incubated with cell lysates and pull downs immunoblotted for the presence of (B) Fyn or (C) phosphotyrosine containing proteins with 4G10. D, Peripheral T cells stimulated with 150 µM PV, lysed, incubated with GST fusion proteins and pull downs immunoblotted for Fyn and PKC-θ. E–F, GFP, GFP-SAP, GFP-SAP(R78A) or GFP-SAP(R55L) were expressed in WT and SAP−/− CD4+ T cells. E, PKC-θ co-immunoprecipitated with GFP-SAP and GFP-SAP(R55L). F, GFP-SAP and GFP-SAP(R55L) but not R78A co-immunoprecipitated with PKC-θ. TCL: Total cell lysates.
SAP R55L is a mutation found in some XLP patients that affects SH2 phosphotyrosine binding yet still generates a stable SAP protein (Supplemental Figure 2); the GST-SAP(R55L) mutant failed to bind most tyrosine phosphorylated proteins (Figure 2C). The GST-SAP(R55L) mutant also exhibits reduced Fyn binding (Figure 2D and (41)), (although the extent of this reduction was more variable), consistent with the inducible formation of a SLAM-SAP-Fyn complex (40). In contrast, GST-SAP(R55L) was able to pull-down PKC-θ from lysates of untreated or PV stimulated cells at similar levels as GST-SAP. Thus, the SAP-PKC-θ interaction does not require the ability of SAP to bind tyrosine phosphorylated proteins via its SH2 domain.
To ascertain the requirements for SAP/PKC-θ interactions in vivo, activated SAP-deficient CD4+ T cells were transfected with green fluorescent protein (GFP)-SAP fusion constructs (Supplemental Figure 3 for transfection efficiency) and lysates were immunoprecipitated with either anti-PKC-θ or anti-GFP. PKC-θ only co-immunoprecipitated efficiently with GFP-SAP and GFP-SAP(R55L) (Figure 2E). Moreover, in reciprocal immunoprecipitations, only GFP-SAP and GFP-SAP(R55L), but not GFP-SAP(R78A), were able to co-immunoprecipitate efficiently with PKC-θ when expressed in SAP-deficient CD4+ T cells (Figure 2F). Thus, the constitutive interaction of SAP with PKC-θ in vivo requires R78.
Fyn is not required for SAP binding to PKC-θ
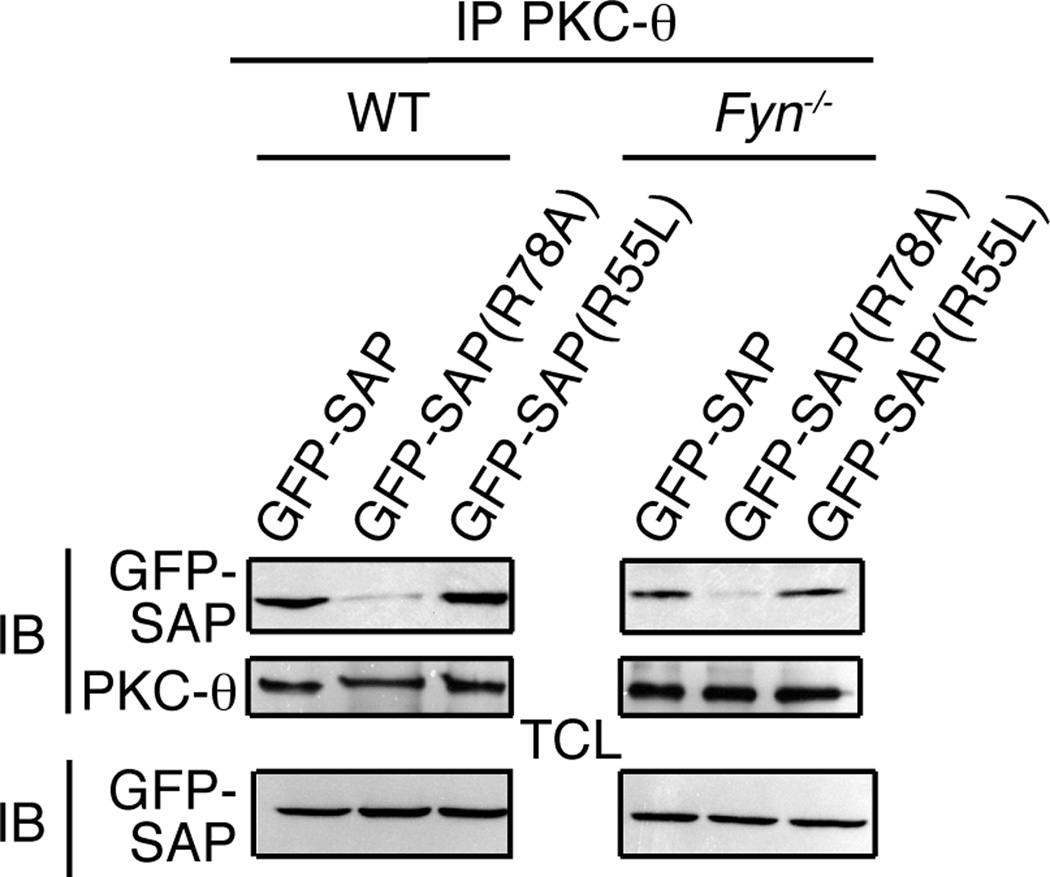
Fyn is thought to be the major tyrosine kinase responsible for SLAM family receptor signal transduction via SAP, which recruits Fyn via an interaction mediated by R78 (38, 39). To evaluate if the SAP/PKC-θ interaction required Fyn, WT or Fyn−/− thymocytes were stimulated and lysates assessed for binding to GST-SAP. PKC-θ was pulled down with GST-SAP in lysates from both WT and Fyn−/− thymocytes, under both resting and stimulation (PV or anti-CD3 treated) conditions (Supplemental Figure 4). To further examine this issue, activated CD4+ T cells from Fyn−/− mice were transfected with GFP-SAP fusion constructs. PKC-θ immunoprecipitated with reconstituted GFP-SAP and GFP-SAP(R55L) in both WT and Fyn−/− CD4+ T cells, but coimmunoprecipitated much less well with GFP-SAP(R78A) (Figure 3). Although the GFP-SAP constructs are expressed at higher levels than the endogenous SAP protein, these data suggest that SAP does not require Fyn to interact with PKC-θ.
FIGURE 3.
SAP associates with PKC-θ in the absence of Fyn. GFP-SAP, GFP-SAP(R78A) or GFP-SAP(R55L) constructs were expressed in WT and Fyn−/− CD4+ T cells. PKC-θ was immunoprecipitated and immunoblotted for GFP and PKC-θ. Samples from WT and Fyn−/− T cells are from the same exposure of the same blot.
Overexpression of SAP enhances PKC-θ recruitment
Following T cell stimulation, PKC-θ translocates to the site of contact between the T cell and APC where it plays a major role in activation of NF-κB, JNK and NFAT (42). We have previously found that SAP−/− CD4+ T cells showed reduced recruitment of PKC-θ to either peptide pulsed APCs or to anti-TCR coated beads (7). To assess the impact of SAP overexpression on PKC-θ localization, we examined T cells from mice that overexpress SAP from a CD2 promoter driven construct. Thymocytes and peripheral T cells isolated from these animals exhibited increased expression of SAP protein (Figure 4A and Supplemental Figure 5A). Overexpression of SAP did not alter T or NKT cell development nor expression of the TCR (Supplemental Figure 5B, 5C and data not shown).
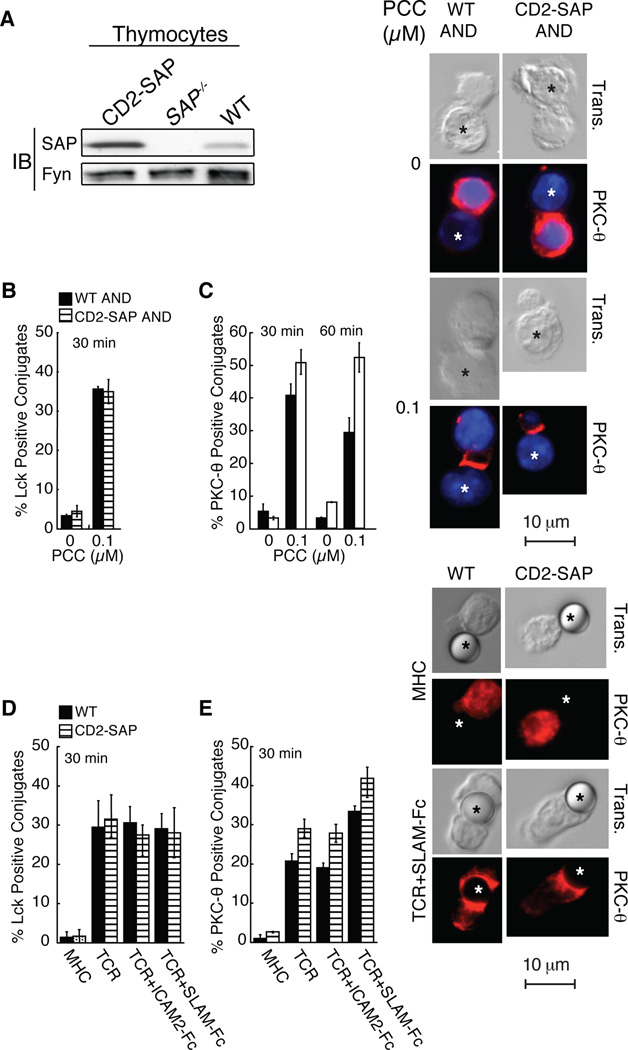
FIGURE 4.
CD2-SAP transgenic CD4+ T cells have increased PKC-θ recruitment to the site of TCR engagement. A, SAP protein expression. Thymocyte lysates were immunoblotted for SAP and Fyn. B–C, AND CD4+ T cell blasts were permitted to conjugate to LPS-activated peptide pulsed B cells, then fixed and stained. Graphs represent the percentage of cells scoring positive for polarized (B) Lck or (C) PKC-θ (representative example shown), n=3, scoring a minimum of 30 T: B cell conjugates (* denotes B cell). D–E, WT and CD2-SAP CD4+ T cell blasts were incubated with latex beads coated with either anti-MHC (0.1µg/ml) or anti-TCR (0.1µg/ml) ± ICAM2-Fc (3µg/ml) or SLAM-Fc (3µg/ml) at 37°C and stained for (D) Lck or (E) PKC-θ (representative example shown). Graphs represent the average percentage of cells scoring positive for enhanced staining at the site of T cell-bead interface, n=3 scoring a minimum of 30 bead conjugates each (* denotes bead).
To assess the level and kinetics of PKC-θ recruitment to the site of TCR stimulation, AND TCR transgenic CD4+ T cell blasts (specific for a peptide from pigeon cytochrome c (PCC)) were stimulated with PCC peptide pulsed LPS-activated B cells. Both WT and CD2-SAP CD4+ T cells displayed similar recruitment of Lck suggesting that these cells were equivalently stimulated and polarized (Figure 4B). However, CD2-SAP AND CD4+ T cells showed enhanced as well as sustained recruitment of PKC-θ to the site of APC contact compared to WT AND cells (Figure 4C). Similar results were observed when naïve CD4+ T cells were stimulated with peptide pulsed APCs or antibody coated beads (Supplemental Figure 6). Thus, the level of SAP expression correlates with the extent of PKC-θ recruitment, consistent with the idea that SAP potentiates PKC-θ recruitment following T cell activation.
SLAM is expressed at low levels on naïve CD4+ T cells and markedly upregulated upon T cell activation (19, 25). To evaluate the role of SLAM in PKC-θ recruitment, a T cell-bead conjugate assay was implemented. When CD4+ T cell blasts were stimulated with beads coated with anti-TCR, CD2-SAP cells exhibited an increase in the percentage of cells with PKC-θ recruited to the site of stimulation. However, when beads were coated with anti-TCR and SLAM-Fc, both WT and CD2-SAP CD4+ T cells demonstrated increased PKC-θ recruitment compared to anti-TCR coated beads (Figure 4D–E). Thus, SLAM engagement enhances recruitment of PKC-θ to the site of stimulation.
A SLAM/SAP/PKC-θ complex forms upon activation
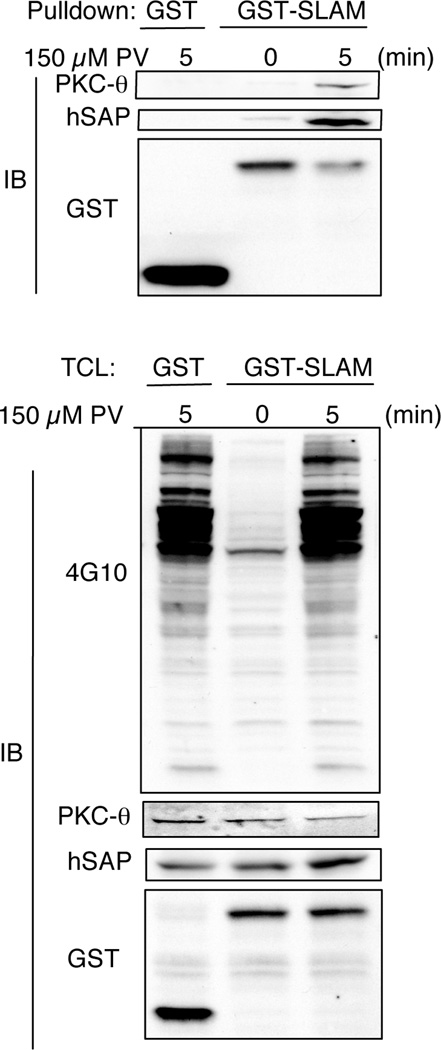
The observation that SLAM engagement increased PKC-θ recruitment to the site of T cell stimulation suggests a SAP/PKC-θ complex could associate with SLAM in T cells. To address this possibility, mammalian expression vectors in which GST was fused to the cytoplasmic tail of human SLAM were expressed in Jurkat T cells. As previously demonstrated, a small amount of SAP bound SLAM in resting cells (9), which was enhanced following pervanadate treatment. Consistent with the increase in SAP-SLAM association, PKC-θ could be detected as part of the SLAM-SAP complex following T cell activation (Figure 5). Taken together, our data suggest that SLAM engagement can help recruit PKC-θ via a ternary SLAM/SAP/PKC-θ complex following T cell activation.
FIGURE 5.
Formation of a ternary SLAM/SAP/PKC-θ complex. Jurkat E6 cells were transiently transfected with GST or GST-SLAM (cytoplamsic tail). Cells were stimulated with 150 µM PV, lysed and GST pull downs immunonblotted for PKC-θ, hSAP and GST. TCL: Total cell lysates.
Elevated IL-4 production from CD2-SAP CD4+ T cells
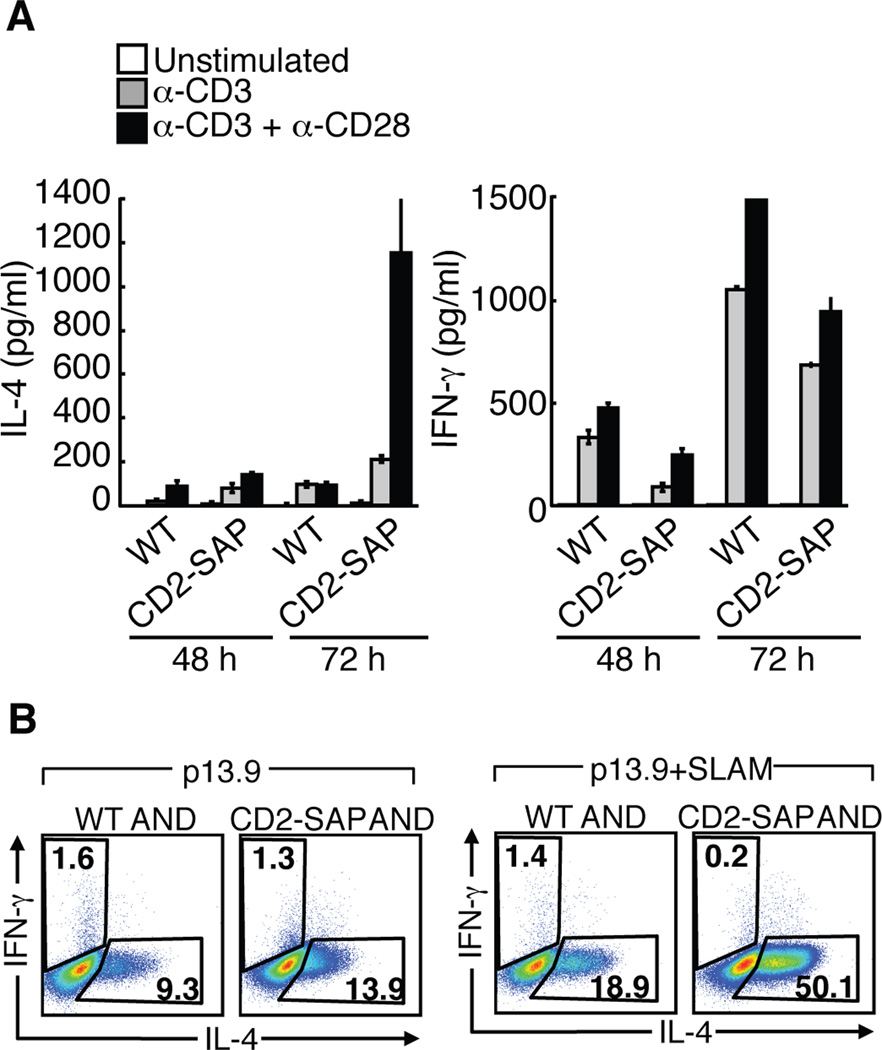
SAP−/− CD4+ T cells demonstrate impaired IL-4 production and GATA-3 expression following T cell activation, yet can produce TH2 cytokines normally in the presence of exogenous IL-4 (7, 15, 16, 18). In contrast to SAP−/− CD4+ T cells, we found that stimulation of naïve CD4+ T cells from CD2-SAP transgenic mice led to increased IL-4 and decreased IFN-γ production (Figure 6A). Similar patterns of cytokine production were observed in an independently derived transgenic line overexpressing SAP (39). Furthermore, CD2-SAP transgenic CD4+ T cells exhibited increased expression of GATA-3, as detected both via real-time PCR and northern blot analysis (Supplemental Figure 5D and E) while not affecting expression of c-Maf and T-bet. Thus, overexpression of SAP, which increases PKC-θ recruitment, leads to increased IL-4 production.
FIGURE 6.
CD2-SAP transgenic CD4+ T cells have elevated IL-4 production in response to TCR and SLAM engagement. A, Cytokines detected via ELISA from negatively selected sorted CD62LhiCD44lo naïve CD4+ T cells stimulated with anti-CD3 and anti-CD28 for 48 and 72 h. B, Naïve WT AND and CD2-SAP AND CD4+ T cells were stimulated with 10 mM PCC pulsed APC, p13.9 or the SLAM expressing variant for 72 h, rested in media with IL-2 for 24 h, then restimulated for intracellular cytokine analyses. Cytokine analyses are representative of 3 or more independent experiments.
We have previously shown that SLAM engagement enhanced IL-4 production in a SAP-dependent manner ((7) and Figure 7B). To investigate the effects of SAP overexpression on IL-4 expression in response to TCR and SLAM activation, naïve WT and CD2-SAP AND CD4+ T cells were stimulated with PCC peptide pulsed p13.9 fibroblast antigen presenting cell (APC) line, which expresses I-Ek, ICAM-1, and B7-1. Stimulation with low peptide dose preferentially leads to IL-4 production (43–45). Under these conditions, an increased percentage of CD2-SAP CD4+ T cells produced IL-4 (Figure 6B). We then stimulated cells with a variant of the p13.9 cell line that expresses SLAM (p13.9-SLAM). Since SLAM interacts homotypically, SLAM expression on the APC line will engage SLAM on the T cells. Strikingly, an even higher percentage of the CD2-SAP CD4+ T cells produced IL-4 compared to WT cells when SLAM was present on the APC (Figure 6B). Thus, overexpression of SAP potentiates the effects of SLAM on IL-4 production, suggesting that SAP expression may be a limiting factor coordinating the TCR and SLAM-mediated signaling pathways contributing to IL-4 regulation.
FIGURE 7.
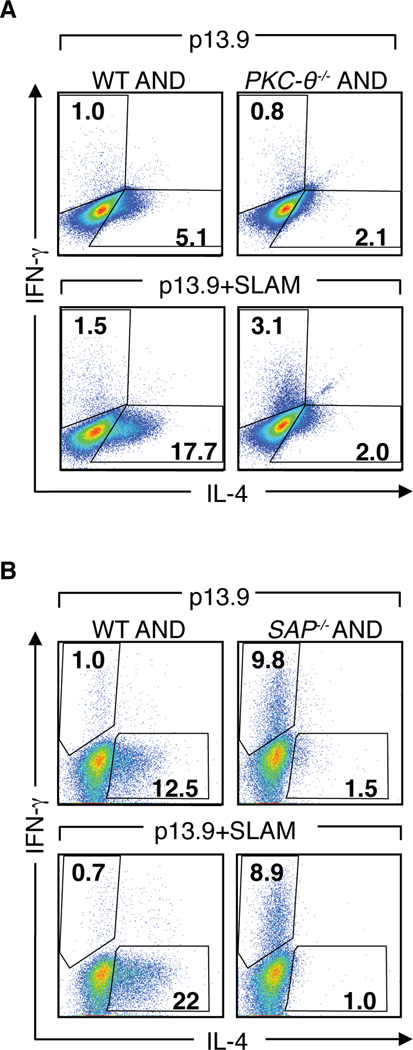
PKC-θ−/− CD4+ T cells do not increase IL-4 production in response to SLAM. A, WT and PKC-θ−/− AND CD4+ T cells were stimulated with 10 mM PCC pulsed p13.9 ± SLAM APCs for 3 days, expanded in media with IL-2 for 24 h, then restimulated for intracellular cytokine analyses. B, WT and SAP−/− AND CD4+ T cells were evaluated as in (A). Cytokine analyses are representative of 3 or more independent experiments.
PKC-θ recruitment is required for IL-4 production in SAP-deficient CD4+ T cells
To determine whether the interaction of SAP with PKC-θ is functionally important, we evaluated whether PKC-θ was required for SLAM-mediated increases in IL-4 production. Naïve PKC-θ−/− AND CD4+ T cells stimulated with varying concentrations of PCC pulsed p13.9 cells gave rise to fewer IL-4 producing cells compared to WT AND CD4+ T cells (Figure 7A), consistent with previous reports that PKC-θ−/− mice exhibit TH2 defects. Strikingly, stimulation in the presence of SLAM did not increase the percentage of PKC-θ−/− AND CD4+ T cells producing IL-4, similar to observations with SAP−/− AND cells (Figure 7A and B). These results indicate that PKC-θ is required for IL-4 production not only in response to TCR stimulation, but also in response to SLAM-mediated pathways.
Activated PKC-θ rescues IL-4 production in SAP-deficient CD4+ T cells
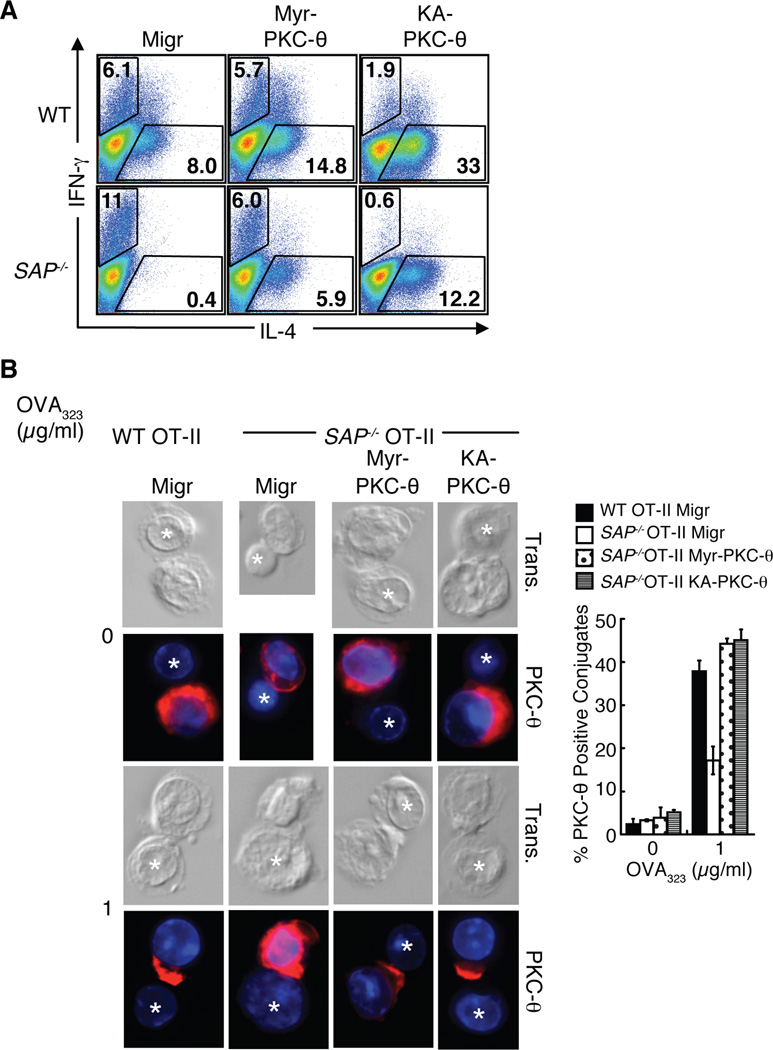
To address whether rescue of PKC-θ recruitment to the site of TCR engagement improves IL-4 production in SAP−/− CD4+ T cells, we generated bicistronic GFP retroviral vectors that expressed either of two activating mutants, KA-PKC-θ and Myr-PKC-θ, which target PKC-θ to the plasma membrane (details in methods section). Expression of either of these activated constructs improved proliferation as well as IL-2 and IL-4 production by PKC-θ−/− CD4+ T cells (Supplemental Figure 7A and data not shown). Expression of these PKC-θ mutants increased IL-4 production in WT CD4+ T cells, supporting the important role of PKC-θ in regulation of IL-4 (Figure 8A and Supplemental Figure 7B). Notably, expression of either the activating PKC-θ mutants in SAP−/− CD4+ T cells distinctly improved IL-4 expression and even reduced IFN-γ production as compared to the vector control infected cells (Figure 8A and Supplemental Figure 7B). To examine the effect of these mutants on PKC-θ localization, antigen specific OT-II CD4+ T cells were retrovirally transduced, sorted based on GFP expression and restimulated with OVA323 peptide pulsed LPS-activated B cells (see Supplementary Figure 7C for GFP expression). Strikingly, expression of either Myr-PKC-θ or KA-PKC-θ in SAP−/− OT-II CD4+ T cells markedly improved PKC-θ recruitment to the immune synapse (Figure 8B). Thus, constitutive activation and recruitment of PKC-θ rescues the defect in IL-4 production in SAP−/− CD4+ T cells, providing support that PKC-θ is a critical mediator of cytokine regulation downstream of SAP.
FIGURE 8.
Activated PKC-θ improves IL-4 production from SAP−/− CD4+ T cells. A, Negatively selected sorted naïve WT and SAP−/− CD4+ T cells were stimulated in the presence of blocking cytokine antibodies for 24 h and infected with the indicated retrovirus. Viable CD4+ cells were isolated and restimulated for intracellular cytokine analysis of CD4+GFP+ cells. B, OT-II CD4+ T cells were stimulated with OVA323 pulsed dendritic cells for 24 h and infected with indicated retrovirus. Viable, sorted GFP+ OT-II CD4+ T cells were permitted to conjugate to LPS-activated OVA323 pulsed B cells for 30 min, fixed and stained for PKC-θ. Graphs represent the percentage of cells scoring positive for polarized PKC-θ (n=3, scoring a minimum of 30 T: B cell conjugates).
Discussion
We present data that a SAP-PKC-θ signaling module plays a critical role in signal transduction pathways leading to IL-4 expression after T cell activation. Our data suggest that defects in IL-4 production from SAP-deficient cells result in-part from an inability of these cells to recruit and sustain sufficient PKC-θ to the site of contact between the T cell and APC. Our data further argues that SLAM-mediated signaling helps activate this pathway. Consistent with this interpretation, we observed that: 1) SAP overexpression augmented PKC-θ recruitment and increased IL-4 production, which was accentuated when APCs expressed high levels of SLAM; and 2) PKC-θ was required for SLAM-mediated increases in IL-4 production. These results therefore suggest that SLAM engagement contributes to the regulation of IL-4 production in response to T cell activation via a SLAM-SAP-PKC-θ-mediated pathway. The observation that SAP−/− T cells show decreased IL-4 production in response to TCR stimulation argues that these SAP-mediated pathways influence TCR-induced IL-4 production and that SAP expression may be a limiting factor in this process. Our observations provide evidence of cross-talk between TCR and SLAM-mediated pathways, consistent with data suggesting that TCR signal transduction influences SLAM phosphorylation (46) and SAP recruitment to NTB-A (47).
SAP was originally identified as an adaptor molecule involved in the intracellular signaling pathways elicited through SLAM. SAP binds to a tyrosine-based motif in SLAM-related receptors that allows the formation of an inducible SLAM/SAP/Fyn ternary complex mediated via binding of the SH3 domain of Fyn to residues surrounding R78 of SAP. Formation of this complex allows Fyn to phosphorylate tyrosine residues in the cytoplasmic domain of SLAM, which then provide docking sites for recruitment of downsteam signaling molecules including SHIP, Dok1 and 2 (9, 38, 39). We have previously demonstrated that retroviral re-expression of SAP, but not the SAP R78A mutant, rescued IL-4 production from SAP−/− CD4+ T cells (7); similar findings were observed in a SAP R78A knock-in mouse (16). These results were initially interpreted as implicating Fyn in IL-4 regulation downstream of SAP. However, the effects of Fyn on IL-4 expression are complex. Although, anti-CD3 stimulation of Fyn−/− T cells resulted in reduced TCR-mediated activation, proliferation and cytokine production relative to WT T cells (7, 16, 48–50), other groups have observed elevated proliferation and cytokine production when Fyn−/− T cells were stimulated in the presence of APCs (49, 51, 52). However, Fyn functions downstream of multiple receptors including, but not limited to, TCR and SLAM family members, and the effects of Fyn deletion may vary depending on the context of the assay and the cells examined. Fyn is also required for NKT cell development and interpretation of experiments using whole splenocytes may be complicated (53). Moreover, although Fyn is required for initial TCR-mediated activation (7, 48, 50, 51, 54, 55), Fyn is also involved in a negative feedback loop regulating the activity of Lck in CD8+ T cells (51). In light of our observations that R78 is required for a SAP-PKC-θ interaction and PKC-θ is essential for SLAM-mediated increases in IL-4 production, our data suggest that PKC-θ is a critical component of the SLAM/SAP-mediated signaling pathways regulating IL-4 production. Although several signaling molecules have been implicated downstream of SAP, to our knowledge only Fyn has been shown to be required for functional outcomes of SLAM-mediated signaling in T cells. Our data here provides the first genetic evidence that another signaling molecule, PKC-θ, is also important for pathways downstream of SLAM and SAP in primary T cells.
We demonstrate here that SAP can be found associated with PKC-θ in primary T cells. PKC-θ association was not dependent on the ability of SAP to bind to phosphotyrosine containing sequences, as the SAP(R55L) mutant could still associate with PKC-θ. Furthermore, an association between SAP and PKC-θ could be detected even in resting T cells. It is therefore of interest that the SAP(R78A) mutant shows impaired PKC-θ association. Recently, the R78 residue of SAP was found to interact with the PIX SH3 domain and participate in a complex with Cdc42. Although the SAP-PIX-Cdc42 complex was not competed by a Fyn peptide, a requirement for Fyn was not specifically examined in vivo (12). Our experiments revealed that SAP interacts with PKC-θ in Fyn−/− T cells. These results are consistent with our observations that SAP-PKC-θ interactions occur prior to T cell activation and do not require the ability of SAP to bind phosphotyrosine residues (or SLAM). Thus, it is possible that SAP either directly interacts with PKC-θ via SAP R78 or that SAP is part of complexes containing multiple proteins that are mediated by R78 interactions independently of Fyn. Whether different pools of SAP bind Fyn and PKC-θ or whether there are temporal differences in the recruitment of these molecules to SLAM family members remains to be investigated. However, since the SAP-PKC-θ association appears to occur independent of T cell activation, it is possible that SLAM engagement potentiates recruitment of a pool of PKC-θ that is constitutively associated with SAP to the synapse. It is of note that although PKC-θ is recruited to the immune synapse, the mechanism of how it specifically localizes remains poorly understood. How SAP helps to recruit and sustain PKC-θ at the site of contact between T cells and APCs and whether SAP and SLAM family members are critical components of PKC-θ synapse localization remain important questions.
Whether SLAM is the only or primary SLAM family member that contributes to IL-4 regulation also remains an open question. Recent data suggest that SLAM is specifically required for IL-4 expression in TFH cells (56). However, T cells from mice containing mutations of a number of individual SLAM family members exhibit defects in IL-4 production, although less severe than SAP−/− T cells (17, 57, 58). It is therefore possible that multiple SLAM family members contribute to the regulation of TCR-driven IL-4 production, similar to recent findings on SLAM family members in NKT cell development (59) and on CD4+ T cell: B cell conjugate-pairing (25). It should be noted that although SAP is required for NKT cell development, variation in NKT cells is unlikely to account for the TH2 defects in SAP-deficient T cells, since reduced IL-4 production is observed with antigen-specific TCR transgenic systems and from sorted naïve CD4+ T cells (7). Additionally, purified CD4+ T cells from the CD2-SAP mice produce more IL-4, despite similar numbers of NKT cell numbers relative to WT mice (data not shown).
Expression of the SAP(R78A) mutant, which does not bind PKC-θ, does rescue the ability of SAP−/− T cells to adhere to B cells and promote germinal center formation (19, 24, 25, 27) suggesting that a SAP/PKC-θ pathway is not involved in this aspect of humoral responses. However, while PKC-θ does not appear to be entirely essential for germinal center formation and the development of antigen specific antibodies ((60) and data not shown), PKC-θ−/− mice exhibit reduced serum IgG titres, particularly IgG1 titers post-immunization (unpublished data). This defect may reflect the effects of impaired T cell survival (61–64). Alternatively, reduced serum IgG1 could also result from impaired IL-4 production by TFH cells (2–4). This hypothesis is supported by recent data from Crotty and colleagues who described a phenotypically distinct germinal center TFH subset that produced IL-4 during a viral infection. This unique population of TFH cells was dependent on SLAM expression for the production of IL-4, even though SLAM expression did not affect germinal center formation (56). Our findings help define SAP-dependent signaling pathways that influence IL-4 expression and strengthen the concept that there are distinct mechanisms for SAP-dependent signal transduction: IL-4 production appears more dependent on interactions with proteins via R78 including PKC-θ, while T:B cell conjugation and germinal center formation may be dependent on other pathways that affect cell adhesion (24, 25). What are the critical SAP-dependent, but R78-independent signaling pathways regulating T:B cell adhesion, which are likely to be initiated by different SLAM family members (25) remains an important question for investigation.
The signals that regulate IL-4 production in response to TCR stimulation involve multiple signaling pathways and components. Although roles for Ca2+/NFAT and MAPK/AP-1-mediated pathways have been well documented, the contribution of other TCR-mediated signaling pathways have been less well defined. How SLAM family members influence and integrate these signaling events therefore is of importance. It is therefore of interest that gene-targeted animals have demonstrated roles for both PKC-θ and Vav1 (which is required for PKC-θ localization) in TH2 differentiation (28, 29, 65, 66). Our data support a growing body of experiments contending that PKC-θ-mediated pathways are important for TCR-driven IL-4 production, but furthermore, provide evidence that SLAM/SAP-mediated pathways may be critical components integrating the activation of these signaling pathways.
Supplementary Material
Acknowledgements
We would like to thank R. Handon and A. Venegas for invaluable technical assistance and L. Samelson for critical reading of this manuscript. Funding was provided by the intramural programs of NHGRI and NCI and the Pharmacology Research Associate Training (PRAT) program (to JZW).
Footnotes
The authors have no conflicting financial interests.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinhardt RL, Liang H-E, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 6.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, Terhorst C. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 7.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates TH2 differentiation and PKC-θ-mediated activation of NF-κB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Relouzat F, Roncagalli R, Aoukaty A, Tan R, Latour S, Veillette A. Molecular dissection of 2B4 signaling: implications for signal transduction by SLAM-related receptors. Mol Cell Biol. 2004;24:5144–5156. doi: 10.1128/MCB.24.12.5144-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latour S, Gish G, Helgason CD, Humphreis RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat Immunol. 2001;2:681–690. doi: 10.1038/90615. [DOI] [PubMed] [Google Scholar]
- 10.Zhong M-C, Veillette A. Control of T lymphocyte signaling by Ly108, a signaling lymphocytic activation molecule receptor implicated in autoimmunity. J Biol Chem. 2008;283:19255–19264. doi: 10.1074/jbc.M800209200. [DOI] [PubMed] [Google Scholar]
- 11.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 12.Gu C, Tangye SG, Sun X, Luo Y, Lin Z, Wu J. The X-linked lymphoproliferative disease gene product SAP associates with PAK-interacting exchange factor and participates in T cell activation. Proc Natl Acad Sci USA. 2006;103:14447–14452. doi: 10.1073/pnas.0606624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Schibli D, Li SS-C. The XLP syndrome protein SAP interacts with SH3 proteins to regulate T cell signaling and proliferation. Cell Signal. 2008;21:111–119. doi: 10.1016/j.cellsig.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, Schaik Sv, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, Vries JED, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 15.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson D, Shi X, Zhang S, Wang H, Nemer M, Ono N, Ohno S, Yanagi Y, Veillette A. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to src-related kinase FynT in TH2 cytokine regulation. Immunity. 2004;21:707–717. doi: 10.1016/j.immuni.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Satoskar A, Faubion W, Howie D, Okamoto S, Feske S, Gullo C, Clarke K, Sosa MR, Sharpe AH, Terhorst C. The cell surface receptor SLAM controls T cell and macrophage functions. J Exp Med. 2004;199:1255–1264. doi: 10.1084/jem.20031835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, Sosa MR, Edwards MJ, Borrow P, Satoskar AR, Sharpe AH, Biron CA, Terhorst C. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–413. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 19.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 21.Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, Peng SL. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200:261–266. doi: 10.1084/jem.20040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morra M, Barrington RA, Abadia-Molina AC, Okamoto S, Julien A, Gullo C, Kalsy A, Edwards MJ, Chen G, Spolski R, Leonard WJ, Huber BT, Borrow P, Biron CA, Satoskar AR, Carroll MC, Terhorst C. Defective B cell responses in the absence of SH2D1A. Proc Natl Acad Sci USA. 2005;102:4819–4823. doi: 10.1073/pnas.0408681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veillette A, Zhang S, Shi X, Dong Z, Davidson D, Zhong M-C. SAP expression in T cells, not in B cells, is required for humoral immunity. Proc Natl Acad Sci USA. 2008;105:1273–1278. doi: 10.1073/pnas.0710698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlies germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007;178:817–828. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- 28.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C θ is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protien kinase Cθ. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 31.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, Sheetz MP, Littman DR, Dustin ML. Opposing effects of PKCθ and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 32.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 33.Lewis J, Eiben LJ, Nelson DL, Cohen JI, Nichols KE, Ochs HD, Notarangelo LD, Duckett CS. Distinct interactions of the X-linked lymphoproliferative syndrome gene product SAP with cytoplasmic domains of members of the CD2 receptor family. Clinical Immunol. 2001;100:15–23. doi: 10.1006/clim.2001.5035. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Graham C, Parravicini V, Brown MJ, Rivera J, Shaw S. Protein kinase C θ is expressed in mast cells and is functionally involved in Fcε receptor I signaling. J Leuk Biol. 2001;69:831–840. [PubMed] [Google Scholar]
- 35.Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, Stipdonk MJB, Altman A. Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 36.Finkelstein LD, Shimizu Y, Schwartzberg PL. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J Immunol. 2005;175:5923–5930. doi: 10.4049/jimmunol.175.9.5923. [DOI] [PubMed] [Google Scholar]
- 37.Simarro M, Lanyi A, Howie D, Poy F, Bruggeman J, Choi M, Sumegi J, Eck MJ, Terhorst C. SAP increases FynT kinase activity and is required for phosphorylation of SLAM and Ly9. Int Immunol. 2004;16:727–736. doi: 10.1093/intimm/dxh074. [DOI] [PubMed] [Google Scholar]
- 38.Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 39.Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 40.Chen R, Latour S, Shi X, Veillette A. Association between SAP and FynT: inducible SH3 domain-mediated interaction controlled by engagement of the SLAM receptor. Mol Cell Biol. 2006;26:5559–5568. doi: 10.1128/MCB.00357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Iosef C, Jia CYH, Gkourasas T, Han VKM, Li SS-C. Disease-causing SAP mutants are defective in ligand binding and protein folding. Biochem. 2003;42:14885–14892. doi: 10.1021/bi034798l. [DOI] [PubMed] [Google Scholar]
- 42.Marsland BJ, Kopf M. T-cell fate and function: PKC-θ and beyond. Trends Immunol. 2008;29:179–185. doi: 10.1016/j.it.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Constant S, Pfeiffer C, Woodard A, Passqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differences of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-αβ-transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine enviroment. J Exp Med. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howie D, Simarro M, Sayos J, Guirado M, Sancho J, Terhorst C. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP and CD150-mediated costimulation. Blood. 2002;99:957–965. doi: 10.1182/blood.v99.3.957. [DOI] [PubMed] [Google Scholar]
- 47.Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, Hoff Jv, Dhar D, Nichols KE, Filipovich AH, Su HC, Bleesing JJ, Lenardo MJ. SAP augments proximal T cell receptor signal strength necessary for restimulation-induced apoptosis of activated T lymphocytes. J Clin Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1993;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 49.Mamchak AA, Sullivan BM, Hou B, Lee LM, Gilden JK, Krummel MF, Locksley RM, DeFranco AL. Normal development and activation but altered cytokine production of Fyn-deficient CD4+ T cells. J Immunol. 2008;181:5374–5385. doi: 10.4049/jimmunol.181.8.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein PL, Lee H-M, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1993;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 51.Filby A, Seddon B, Kleczkowska J, Salmond R, Tomlinson P, Smida M, Lindquist JA, Schraven B, Zamoyska R. Fyn regulates the duration of TCR engagement needed for commitment to the effector function. J Immunol. 2007;179:4635–4644. doi: 10.4049/jimmunol.179.7.4635. [DOI] [PubMed] [Google Scholar]
- 52.Tamura T, Igarashi O, Hino A, Yamane H, Aizawa S, Kato T, Nariuchi H. Impairment in the expression and activity of fyn during differentiation of naive CD4+ T cells into the Th2 subset. J Immunol. 2001;167:1962–1969. doi: 10.4049/jimmunol.167.4.1962. [DOI] [PubMed] [Google Scholar]
- 53.Gadue P, Morton N, Stein PL. The src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filipp D, Zhang J, Leung BL, Shaw A, Levin SD, Veillette A, Julius M. Regulation of Fyn through translocation of activated Lck into lipid rafts. J Exp Med. 2003;197:1221–1227. doi: 10.1084/jem.20022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugie K, Jeon M-S, Grey HM. Activation of naive CD4 T cells by anti-CD3 reveals an important role for Fyn in Lck-mediated signaling. Proc Natl Acad Sci USA. 2004;101:14859–14864. doi: 10.1073/pnas.0406168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, DiToro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cells require SLAM (CD150) for IL-4 production. J Immunol. 2010 doi: 10.4049/jimmunol.0903505. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graham DB, Bell MP, McCausland MM, Huntoon CJ, van Deursen J, Faubion WA, Crotty S, McKean DJ. Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice. J Immunol. 2006;176:291–300. doi: 10.4049/jimmunol.176.1.291. [DOI] [PubMed] [Google Scholar]
- 58.Howie D, Laroux FS, Morra M, Satoskar AR, Rosas LE, Faubion WA, Julien A, Rietdijk S, Coyle AJ, Fraser C, Terhorst C. Cutting edge: the SLAM family receptor Ly108 controls T cell and neutrophil functions. J Immunol. 2005;174:5931–5935. doi: 10.4049/jimmunol.174.10.5931. [DOI] [PubMed] [Google Scholar]
- 59.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berg-Brown NN, Gronski MA, Jones RG, Elford AR, Deenick EK, Odermatt B, Littman DR, Ohashi PS. PKCθ signals activation versus tolerance in vivo. J Exp Med. 2004;199:743–752. doi: 10.1084/jem.20031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manicassamy S, Gupta S, Huang Z, Sun Z. Protein kinase C-θ-mediated signals enhance CD4+ T cell survival by up-regulating Bcl-xL. J Immunol. 2006;176:6709–6717. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- 62.Manicassamy S, Sun Z. The critical role of protein kinase C-θ in fas/fas ligand-mediated apoptosis. J Immunol. 2007;178:312–319. doi: 10.4049/jimmunol.178.1.312. [DOI] [PubMed] [Google Scholar]
- 63.Saibil SD, Jones RG, Deenick EK, Liadis N, Elford AR, Vainberg MG, Baerg H, Woodgett JR, Gerondakis S, Ohashi PS. CD4+ and CD8+ T cell survival is regulated differentially by protein kinase Cθ, c-rel and protein kinase B. J Immunol. 2007;178:2932–2939. doi: 10.4049/jimmunol.178.5.2932. [DOI] [PubMed] [Google Scholar]
- 64.Villalba M, Bushway p, Altman A. Protein kinase C-θ mediates a selective T cell survival signal via phosphorylation of BAD. J Immunol. 2001;166:5955–5963. doi: 10.4049/jimmunol.166.10.5955. [DOI] [PubMed] [Google Scholar]
- 65.Hehner SP, Li-Weber M, Giaisi M, Droge W, Krammer PH, Schmitz ML. Vav synergizes with protein kinase Cθ to mediate IL-4 gene expression in response to CD28 costimulation in T cells. J Immunol. 2000;164:3829–3836. doi: 10.4049/jimmunol.164.7.3829. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka Y, So T, Lebedeva S, Croft M, Altman A. Impaired IL-4 and c-Maf expression and enhanced Th1-cell development in Vav-deficient mice. Blood. 2005;106:1286–1295. doi: 10.1182/blood-2004-10-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.