Abstract
This study employed visually evoked event-related potential (ERP) methodology to examine temporal patterns of structural and higher-level face processing in birth and foster/adoptive mothers viewing pictures of their children. Fourteen birth mothers and 14 foster/adoptive mothers engaged in a computerized task in which they viewed facial pictures of their own children, and of familiar and unfamiliar children and adults. All mothers, regardless of type, showed ERP patterns suggestive of increased attention allocation to their own children’s faces compared to other child and adult faces beginning as early as 100–150 ms after stimulus onset and lasting for several hundred milliseconds. These data are in line with a parallel processing model that posits the involvement of several brain regions in simultaneously encoding the structural features of faces as well as their emotional and personal significance. Additionally, late positive ERP patterns associated with greater allocation of attention predicted mothers’ perceptions of the parent–child relationship as positive and influential to their children’s psychological development. These findings suggest the potential utility of using ERP components to index maternal processes.
Keywords: Event-related brain potential, Face processing, Attention allocation
The mother–child bond plays an ethologically important role in promoting a child’s survival and healthy development. As infants of many species are born completely dependent upon their mothers, the absence of a mother’s attention would likely result in perilous circumstances for her infant. For example, a rat mother that does not adequately attend to her pups would fail to protect them from predators or to provide them with nourishment. Similarly, a human mother who does not attend to her infant might fail to protect the infant from unnecessary harm or to provide the nurturance necessary to promote the infant’s welfare. Delineating the neurobiological mechanisms underlying maternal attention may result in a better understanding of the nature of the mother–child bond under both adaptive and maladaptive circumstances.
Whereas olfactory stimuli are the most salient social cues for many non-human mothers, visual information gleaned from facial features appears to be especially salient for humans (Zebrowitz, 2006). Facial features provide the means to recognize familiar vs. unfamiliar individuals and to ascertain valuable information such as age, race, gender, and emotional state. The perception of faces also evokes emotionally laden, affiliative memories and plays a facilitative role in forming and maintaining human affiliations (Depue and Morrone-Strupinsky, 2005). Given the social utility of faces, there are several neurological mechanisms in place to facilitate the rapid processing of facial information, including the identification of emotional or motivational significance of face stimuli (for reviews see Adolphs, 2002 and Vuilleumier and Pourtois, 2007).
Event related brain potentials (ERPs) provide a non-invasive, temporally sensitive method of studying the neural mechanisms subserving face processing. The advantage of using ERPs, characterized by their polarity and position along the waveform, is that they offer the ability to examine stimulus processing at different functional stages. In the context of the mother–child relationship, the point at which a mother’s brain selectively responds to her child’s face may have implications for her ability to take meaningful action towards her child under various circumstances and therefore may prove instrumental to the child’s survival and healthy development.
One classic model of face processing posits the orchestration of several brain regions operating simultaneously to encode structural features of a face, as well as to extrapolate higher-level meaning and significance from face stimuli (Bruce and Young, 1986). Extensive research in this area has linked ERP components emerging at varying points along the waveform to these functionally unique but related brain processes. For example, ERP components that peak at frontocentral recording sites relatively early in the waveform, such as N1, P2, and N2, have shown increased positivity to affective face stimuli compared to neutral faces (Eimer and Holmes, 2007). Although these ERP components have also been associated with non-emotional aspects of stimulus processing when studied in different contexts (Dien et al., 2004), their positive shift following affective face stimuli has been attributed to the rapid detection of emotional content of faces (Eimer and Holmes, 2007). In fact, some evidence suggests such processing may occur pre-attentively, responding to emotional content even before conscious awareness (see Kiss and Eimer (2008)). Occurring almost simultaneously with the frontocentral P2 is the N170 component, a negative deflection typically observed at parietal and temporal electrode sites. The N170 has been shown to discriminate between face and non-face stimuli, but has not shown modulation by facial emotion (Eimer and Holmes, 2007). Some argue that the N170 reflects activity of face-specific areas of the visual cortex, namely the fusiform gyrus (Eimer and Holmes, 2007; Haxby et al., 2000). In contrast to N170, the later peaking P3 and LPP components, like the early frontocentral positive shift in N1, P2, and N2, show enhanced positivity to emotional compared to neutral faces (cf. Eimer and Holmes, 2007). However, unlike these earlier components, the effect on P3 and LPP is more broadly distributed and manifests at frontal and parietal electrode sites, suggesting recruitment of different neural mechanisms across time during affective face processing (Eimer and Holmes, 2007). The enhanced P3 and LPP to affective faces may therefore reflect further, perhaps higher-level evaluation of the emotional content of faces. Together, the early and late enhanced positivities observed in the context of affective face processing (Eimer and Holmes, 2007) may reflect an ethologically valuable facility to selectively attend to the emotional significance of face stimuli. Similar to affective facial expression, face stimuli that represent an affiliate or loved one, even if presented with a neutral expression, will possess some degree of emotional significance and so may also recruit increased neural activity (Palermo and Rhodes, 2007). Indeed, recent findings show that the P3 component, for example, is enhanced during viewing of pictures of friends and relatives compared to newly learned faces (Bobes et al., 2007). Broadly speaking, these ERP modulations may reflect what Schupp et al. (2004) refer to as motivated attention, or “[attention to]… emotionally arousing stimuli [that] activate the brain’s motivational circuits…” (p. 594). Thus, whereas early frontocentral and later distributed enhanced positivities to emotional faces seem to reflect significance processing, as proposed by Bruce and Young, the N170 appears to index the structural encoding mechanism.
To our knowledge, there are no studies that have examined visually evoked ERP correlates of attention allocation to one’s children. In fact, very little work in general has explored neurological mechanisms involved in the processing of stimuli relating to one’s children. One study reported increased attention and arousal in postpartum mothers to their own infants’ cry vs. a neutral word stimulus compared to non-mothers, as indexed by a frontocentral negative deflection occurring 100 ms after stimulus onset (i.e., the auditory N1 ERP component; Purhonen et al., 2001). Similarly, one fMRI study reported high levels of activation in prefrontal regions and areas associated with motivation and reward including the ventral tegmental area, retrorubral field, and nucleus accumbens when mothers listened to infant cries (Lorberbaum et al., 2002). Other researchers have studied maternal attentional processes using paradigms in which mothers viewed facial pictures of their children. Leibenluft et al. (2004) showed that when mothers viewed pictures of their own children compared to pictures of other children they exhibited increased activation in the right amygdala and left anterior insula, both associated with responses to emotionally significant stimuli. In another fMRI study, postpartum parents viewing own and other infant pictures exhibited increased activation in the anterior cingulate, thalamus, amygdala, putamen, and insula, particularly in parents demonstrating high sensitivity to their children as determined by observational coding of parent–child interactions (Swain et al., 2007).
Considering the dearth of research examining neural correlates of attentional processes in human mothers and the ability of ERPs to reveal temporally sensitive cognitive and emotional processes, the primary aim of the current study was to examine ERPs while mothers passively viewed pictures of their children and other, familiar and unfamiliar, children and adults. In so far as frontocentral and parietal positive shifts reflect enhanced processing of emotionally significant stimuli, we hypothesized that mothers would show more positive N1, P2, N2, P3, and LPP responses to their own children compared to familiar and unfamiliar children and adults (cf. Eimer and Holmes, 2007). Modulation of the N170 component was not expected, as it is mainly associated with the structural encoding of facial stimuli (Eimer and Holmes, 2007).
In order to help rule out the possibility that ERP patterns were the result of greater familiarity of faces and not their emotional significance per se, we compared ERPs generated in response to familiar vs. unfamiliar faces of children and adults. Like others (e.g., Bobes et al., 2007) we chose to control for familiarity by including newly learned faces as opposed to faces of acquaintances in order to prevent varying degrees of familiarity between subjects caused by differences in the amount of time spent with acquaintances.
Another goal of the study was to investigate possible differences in ERP patterns between mothers who had given birth to their child and surrogate mothers who had not given birth to the child in their care (i.e., birth mothers vs. foster/adoptive mothers). This comparison is interesting given two competing perspectives: that birth mothers attend to their children’s needs to a greater degree than foster/adoptive mothers vs. that foster/adoptive mothers are more attentive to their children’s needs. Evidence favoring birth mother advantages suggests that the hormonal changes that occur during pregnancy and the period of mother–child bonding immediately following birth critically influence subsequent maternal behavior (reviewed in Numan and Insel (2003)). In addition, some evolutional theorists maintain that because foster/adoptive parents and their children do not share biological ties that they invest fewer resources in their children than do birth parents (Hamilton et al., 2007). To our knowledge the only empirical research in support of this perspective was conducted by a group of anthropologists who reported decreased parental investment in stepfathers of non-kin children (Anderson, 2005; Anderson et al., 1999a; Anderson et al., 1999b). In contrast, a different theory asserts that adoptive parents show greater investment than birth parents because of a motivation to compensate for not being their children’s natural parents (Hamilton et al., 2007). Indeed, foster/adoptive parents typically must complete rigorous training regimens and are often considered to have a unique, altruistic motivation to care for children, and in some cases to reverse the negative consequences of their children’s early adversity. Hamilton et al. (2007) compared birth and adoptive parents on a number of variables and reported that adoptive parents allocated more resources (i.e., economic, cultural, social, interactional) than birth parents but attributed some of these effects to adoptive parents’ older age, higher education, and greater socioeconomic status. We considered our comparison of birth and foster/adoptive parents to be important, but entertained both possibilities and thus made no specific hypotheses regarding differences in ERP responses to their own children vs. other children and adults.
We also sought to investigate possible differences in mothers’ ERP patterns as a function of individual differences in caregivers’ perceived relationships with their children, specifically caregivers’ perception of their relationships as positive (i.e., Acceptance), permanent (i.e., Commitment), and important to the child’s emotional and psychological development (Awareness of Influence; Bates and Dozier, 1998). These constructs may be useful indicators of the quality of the parent–child relationship. For example, caregiver commitment has been shown to predict relationship stability in foster parent–child dyads over a two-year period (Dozier and Lindhiem, 2006). Further, greater Awareness of Influence scores corresponded to greater caregiver sensitivity as determined by observational coding during a parent–child interactive play assessment (Bates and Dozier, 2002). In the current study, we hypothesized that mothers with higher scores on these relational indices would show more positive N1, P2, N2, P3, and LPP components (i.e., greater positive components [P2, P3, LPP] and smaller negative components [N1, N2]) than mothers with lower scores when presented with pictures of their own children compared to pictures of other children. We did not expect a significant relation between TIMB scores and the N170 component.
Finally, we were interested in comparing ERPs observed while mothers viewed faces of unfamiliar children vs. unfamiliar adults. Based on Leibenluft and colleagues’ (2004) study in which stronger responses in brain areas associated with face processing, attention, and empathy were seen when mothers viewed pictures of children in general compared to adults, perhaps reflecting an enhanced sensitivity to child vs. adult stimuli in mothers, we hypothesized that the experience of parenting and/or an innate sensitivity in mothers to children would lead to more positive ERP responses to unfamiliar children than to unfamiliar adults. In addition, we considered the possibility of finding differences between birth and foster/adoptive mothers in response to stimuli of children in general. We speculated that perhaps individuals who choose to foster or adopt non-kin children as their own are unique in that they possess a greater sensitivity to child than to adult cues. We made no specific hypotheses in either direction, however.
The current study engaged birth and foster/adoptive mothers in a computer task eliciting ERPs indicative of stimulus processing across time. Primary aims were: (a) to determine if mothers’ generation of ERPs differed as a function of whether they were presented with their own children’s faces or familiar or unfamiliar children or adult faces; (b) to explore possible differences between birth mothers’ and foster/adoptive mothers’ generation of ERPs; (c) to determine if mothers’ generation of ERPs was associated with measures of mothers’ perceived relationship with their children; and (d) to compare ERP components recorded while mothers viewed faces of unfamiliar children vs. unfamiliar adults.
1. Method
1.1. Participants
The sample included 28 mothers (14 birth mothers and 14 foster/adoptive mothers) of children between the ages of 1.6 and 4.7 years (M = 2.7, SD = .9). Mothers were 18–55 years of age (M = 36.61, SD = 8.26). Ten foster/adoptive mothers and two birth mothers were part of a larger study examining the efficacy of an intervention for infants in foster care. Mothers were classified as Caucasian (67.9%), African American (21.4%), Hispanic (3.6%), Asian (3.6%) or Biracial (3.6%). Annual household income ranged from $10,000 to 150,000 (M = $63,687, SD = $40,246). Birth mothers and foster/adoptive mothers did not differ significantly with regard to race, years of education completed, or annual household income. Child gender did not significantly differ between groups. However, there were statistically significant age differences by parent type and child type with foster/adoptive mothers (M = 39.93, SD = 7.84) older than birth mothers (M = 33.29, SD = 7.51, t(26) = −2.29, p = .030), and birth children (M = 3.2, SD = 0.93) older than foster/adoptive children (M = 2.21, SD = 0.55, t(26) = 3.41, p = .002). In addition, child race significantly differed between groups, with more of the foster/adoptive children classified as African American, χ2 (3, N = 28) = 9.33, p = .025.
1.2. Stimuli and measures
1.2.1. Face stimuli
Digital pictures were taken with a 7.2 megapixel Sony Cyber-shot camera and uploaded into Adobe Photoshop CS Version 8.0. Pictures were cropped to 800 × 800 pixels (8 in. × 8 in.) so that the head, neck, and shoulders occupied the majority (i.e., about 90%) of the frame. Pictures were adjusted for visual clarity using the Auto Levels command. The space surrounding the head, neck, and shoulders was filled in with the color black. The edges of the figure touching the black background were softened with the blur tool. Pictures were then de-saturated to a gray scale. Pictures (8 in. × 8 in.) given to subjects to become familiar with during the seven-day familiarity task were printed on Kodak matte photo paper.
1.2.2. This Is My Baby (TIMB) interview
The TIMB (Bates and Dozier, 1998) is a semi-structured interview coded for three dimensions reflecting a mother’s relationship with her child (i.e., Acceptance, Commitment, and Awareness of Influence). For example, parents are asked how they think their relationship with their child is affecting him right now and how it will affect him in the long term, what they want for their child now and in the future, and how much they would miss their child if she ever had to leave their care. Each dimension of the TIMB is quantified using a five-point Likert scale (1 = lowest, 5 = highest). The Acceptance Scale measures a mother’s perception of her child and her relationship with her child as positive and rewarding. Commitment scores reflect the degree to which a mother is emotionally invested in her child and thinks of her relationship with her child as permanent and enduring. The Awareness of Influence Scale measures the extent to which a mother perceives her relationship with her child as influencing psychological and emotional aspects of her child and the child’s future. The TIMB scales have demonstrated convergent validity with other relational measures, as well as predictive validity to parent–child relationship outcomes (see Bates and Dozier, 2002 and Lindhiem and Dozier, 2007 for examples). Inter-rater reliability on the Acceptance, Commitment, and Awareness of Influence Scales were .89, .90, and .84, respectively.
1.3. Procedures
Initial contact with mothers occurred over the telephone. Mothers interested in the study agreed to learn more about the study during a home visit. Mothers still interested during the home visit provided written consent, using a form approved by the University of Delaware Human Subjects Review Board, and completed a demographics questionnaire and the This Is My Baby (TIMB; Bates and Dozier, 1998) interview, recorded for future coding. Subjects received a packet including two unfamiliar black and white photographs: a facial picture of a child, matched to their own child by age, sex, and race, and a facial picture of a female adult, matched to their own child by race. Female adult faces were used to minimize any effect gender might have on mothers’ response to same-age adults vs. children. The facial pictures were chosen from a face database that was established prior to the study. Subjects in the current study were instructed to complete seven daily questionnaires asking questions about each face such as how familiar each face appeared to them. The function of this task was to familiarize subjects with the faces. During the home visit, several digital facial pictures were taken of the subjects’ children. One of the pictures was later chosen based on visual clarity, formatted to 800 × 800 pixels, and de-saturated in order to match the pictures in the face database. At the end of the home visit, the EEG lab sessions were scheduled and subjects received $20 compensation.
1.3.1. Computer task
The computer task took place in the EEG laboratory approximately seven days after the initial home visit. The completed packets were obtained and mothers were instructed to identify the two faces that were included in the packet on a recognition test in which 42 child and adult faces were presented together on a single page. All of the subjects correctly identified the two faces, demonstrating visual familiarity with the child and adult faces. After a brief introduction to the laboratory, sensors were attached. Subjects were told that five faces would appear a number of times in random order on the computer screen: their own child, a familiar and unfamiliar child, and a familiar and unfamiliar adult. Faces were presented for a total of 120 trials, each face appearing on a computer for 1000 ms using Presentation software (Neurobehavioral Systems Inc.). Child faces (i.e., own child, familiar child, unfamiliar child) were presented 20 times each. Given that adult faces had one fewer categories (i.e., familiar adult, unfamiliar adult) and to avoid the confound of an oddball effect, adult faces were presented 30 times each. This imbalance was not expected to significantly influence the signal/noise ratio. Upon completion of the task, subjects were compensated $25.
1.3.2. Psychophysiological recording and data reduction
Two tin 9 mm cup disk electrodes (Med-Associates) were attached on the left and right mastoids (M1 and M2, respectively). Two tin miniature electrodes (Med-Associates) were attached 1 cm above and below subjects’ left eyes to record the electrooculogram (EOG). A clip electrode functioning as a ground was attached to subjects’ left ears. Recordings were taken from frontal (Fz), frontocentral (FCz), central (Cz), and parietal (Pz) areas along the midline using an ECI electrocap. All electrode impedances were below 10 kΩ and the data from all channels were recorded using a Grass Model 7D polygraph with Grass Model 7P1F preamplifiers (bandpass = .016–35 Hz). During the recording, all activity was referenced to Cz, then re-referenced to average mastoids offline.
Bioelectric signals were digitized on a laboratory microcomputer using VPM software (Cook, 1999). The EEG was sampled at 200 Hz. Data collection began 500 ms prior to picture presentation and continued 1000 ms after picture onset. The EEG data for each trial were corrected for vertical EOG artifact using a procedure by which the relation (i.e., propagation factor) between the EOG channel and each EEG channel is estimated using least-squares regression and then used to subtract the scaled EOG values from the raw EEG data (Gratton et al., 1993).
If there was evidence of excessive physiological artifact (i.e., 25 ms of invariant analog data on any channel or A/D values on any channel equaling that converter’s minimum or maximum values), trials were rejected and excluded from subsequent analyses. The mean number of trials rejected per subject was 2.21. Single trial EEG data were lowpass filtered at 20 Hz with a 51-weight FIR digital filter. Stimulus-locked ERPs were averaged separately for each type of face stimulus.
The N1, N170, P2, N2, P3, and LPP components were defined as the average amplitudes within time windows of 100–150, 150–185, 185–240, 240–340, 350–525, and 550–725 ms, respectively, following face presentation. A baseline equal to the average activity in a 200 ms window prior to picture onset was subtracted from each data point.
1.4. Statistical methods
Analyses of ERP components were limited to electrode sites in which they were largest. Except for the N170 component, which was only observed at Pz, mixed model analyses of variance (ANOVAs) determined which electrode sites contained the largest N1, P2, N2, P3, and LPP components. After electrode sites were identified for each component, single site ERP data were analyzed using a mixed model ANOVA with caregiver type (birth vs. foster/adoptive) as the between-subject variable, face type (own child vs. familiar child vs. unfamiliar child vs. familiar adult vs. unfamiliar adult) as the within-subject factor, and average amplitude ERPs in microvolts as the dependent variable. Greenhouse-Geisser correction was applied to p-values when sphericity could not be assumed. Eight pairwise post-hoc comparisons (own child vs. familiar child, unfamiliar child, familiar adult, and unfamiliar adult, unfamiliar child vs. unfamiliar adult, familiar child vs. familiar adult, familiar child vs. unfamiliar child, and familiar adult vs. unfamiliar adult) were conducted using Hochberg (1988) modified Step-Up Bonferonni procedure. Partial eta squared values are reported to demonstrate the size of effects in ANOVA models, where .05 represents a small effect, .1 represents a medium effect, and .2 represents a large effect (Cohen, 1969). Possible relations between ERP responses to mothers’ own children and mothers scores on the TIMB while controlling for ERP responses to all other faces were explored using linear regression analyses with average ERP responses to own child, familiar child, unfamiliar child, familiar adult, and unfamiliar adult as independent variables and TIMB scale scores as dependent variables in separate analyses.
2. Results
2.1. ERP components
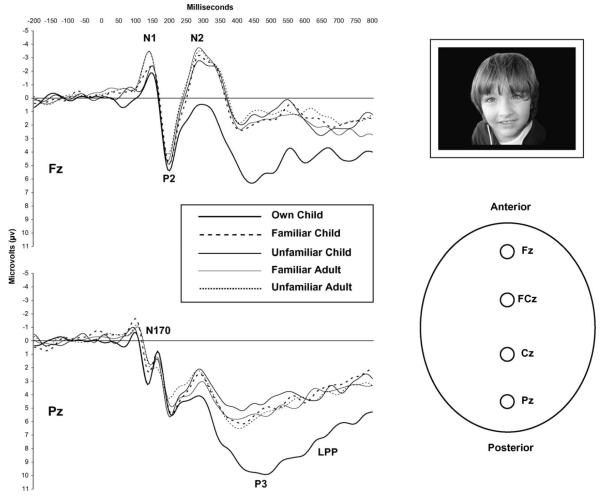
Raw waveforms from all electrode sites are presented in Fig. 1. Analyses of ERP components N1, N170, P2, N2, P3, and LPP are presented below.
Fig. 1.
ERP raw waveforms at Fz, FCz, Cz, and Pz.
2.1.1. N1
A mixed model ANOVA revealed a main effect for electrode site, F(3,312) = 21.65, p < .001, = .45. Hochberg post-hoc tests indicated that average amplitude N1s were significantly larger (i.e., more negative) at electrode site Fz than other sites, thus additional analyses were conducted using measures from Fz only. At electrode site Fz, a mixed model ANOVA revealed a main effect for face type, F(4,104) = 3.52, p = .010, = .12. Average amplitude N1s were significantly more positive (i.e., smaller) in response to mothers’ own children compared to familiar and unfamiliar adult face pictures per Hochberg post-hoc tests. In addition, average amplitude N1s were significantly more positive in response to unfamiliar child face pictures than to unfamiliar adult face pictures. No other comparisons were significant. There was no main effect for caregiver type, F(1,26) = 2.51, p = .125, = .09, and no face type x caregiver type interaction, F(4,104) = 1.17, p = .327, = .04.
2.1.2. N170
Analyses were conducted using measures from electrode site Pz only, as the N170 component was not observed at other electrode sites and therefore could not be adequately measured, which is in accord with other studies (e.g., Caharel et al., 2002). There was a main effect for caregiver type, F(1,26) = 5.7, p = .025, = .18, such that foster/adoptive mothers exhibited more negative (i.e., larger) N170s than birth mothers. There was no main effect for face type, F(4,104) = 1.65, p = .168, = .06, and no face type x caregiver type interaction, F(4,104) = 1.08, p = .370, = .04.
2.1.3. P2
Mixed model ANOVA revealed a main effect for electrode site, F(3,312) = 16.4, p < .001, = .39. Hochberg post-hoc tests indicated that average amplitude P2 s were significantly larger at Pz than at all other sites, thus additional analyses were conducted using measures from Pz only. At electrode site Pz, a mixed model ANOVA revealed no main effect for face type, F(4,104) = 1.05, p = .388, = .04, no main effect for caregiver type, F(1,26) = 1.06, p = .314, = .04, and no face type x caregiver type interaction, F(4,104) = .532, p = .712, = .02.
2.1.4. N2
A mixed model ANOVA revealed a main effect for electrode site, F(3,312) = 50.57, p < .001, = .66. Hochberg post-hoc tests indicated that average amplitude N2s were significantly larger at electrode site Fz than at all other sites, thus additional analyses were conducted using measures from Fz only. At Fz, a mixed model ANOVA revealed a main effect for face type, F(4,104) = 10.73, p < .001, = .29. Average amplitude N2s were significantly more positive (i.e., smaller) in response to mothers’ own children compared to all other pictures per Hochberg post-hoc tests. In addition, there was a main effect for caregiver type, F(1,26) = 4.73, p = .039, = .15, such that foster/adoptive parents exhibited more positive N2s than birth parents. There was no face type x caregiver type interaction, F(4,104) = 1.54, p = .197, = .06.
2.1.5. P3
A mixed model ANOVA revealed a main effect for electrode site, F(3,312) = 54.1, p < .001, = .68. Hochberg post-hoc tests indicated that average amplitude P3s were significantly larger at electrode site Pz than at all other sites, thus additional analyses were conducted using measures from Pz only. At Pz, a mixed model ANOVA revealed a main effect for face type, F(4,104) = 12.6, p < .001, = .33. Average amplitude P3s were significantly more positive (i.e., larger) in response to mothers’ own children compared to all other pictures per Hochberg post-hoc tests. There was no main effect for caregiver type, F(1,26) = 1.3, p = .170, = .07, and no face type x caregiver type interaction, F(4,104) = 1.8, p = .134, = .07.
2.1.6. LPP
A mixed model ANOVA revealed a main effect for electrode site, F(3,312) = 20.46, p < .001, = .44. Hochberg post-hoc tests indicated that average amplitude LPPs were significantly larger at electrode site Pz than at all other sites, thus additional analyses were conducted using measures from Pz only. At Pz, a mixed model ANOVA revealed a main effect for face type, F(4,104) = 15.2, p < .001, = .37. Average amplitude LPPs were significantly more positive (i.e., larger) in response to mothers’ own children compared to all other pictures per Hochberg post-hoc tests. There was no main effect for caregiver type, F(1,26) = 1.01, p = .323, = .04, and no face type x caregiver type interaction, F(4,104) = 1.2, p = .314, = .04.
2.2. This is My Baby interview (TIMB)
Birth and foster/adoptive mothers’ scores did not significantly differ on the TIMB Awareness of Influence Scale, t(26) = −.26, p = .795, Acceptance Scale, t(26) = −.61, p = .548, or Commitment Scale, t(26) = 1.55, p = .134.
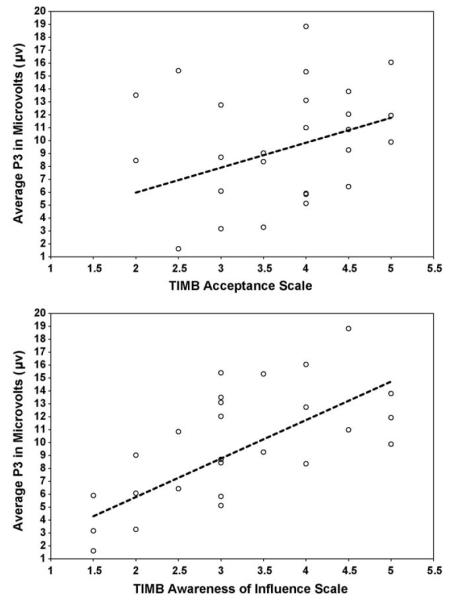
Analyses to examine the relations between ERP components and TIMB Scales were only conducted for those components that significantly differed between own child faces and other faces, namely the P3, LPP, N1, and N2 components. Controlling for P3 responses to all familiar and unfamiliar children and adults, a linear regression analysis revealed significant positive relations between P3 responses to own child faces and mothers’ scores on the TIMB Awareness of Influence Scale, b = .86, t(22) = 3.77, p = .001 (see top of Fig. 2), and mothers’ scores on the TIMB Acceptance Scale, b = .72, t(22) = 2.75, p = .012 (see bottom of Fig. 2), controlling for multiple comparisons using the Hochberg procedure. There was no significant relation between P3 responses to own child faces and mothers’ scores on the TIMB Commitment Scale, b = −.09, t(22) = −.34, p = .741. Controlling for LPP responses to all familiar and unfamiliar children and adults, a linear regression analysis revealed a significant positive relation between LPP responses to own child faces and mothers’ scores on the TIMB Awareness of Influence Scale, b = .57, t(22) = 2.9, p = .009, after controlling for multiple comparisons using the Hochberg procedure. A positive relation between LPP responses to own child faces and mothers’ scores on the TIMB Acceptance Scale, b = .49, t(22) = 2.1, p = .048, was not significant after implementing the Hochberg procedure. There was no significant relation between LPP responses to own child faces and mothers’ scores on the TIMB Commitment Scale, b = .26, t(22) = .98, p = .337. In addition, there were no significant relations between average N1 and N2 responses to own child faces at Fz and mothers’ scores on the TIMB Awareness of Influence Scale, b = −.05, t(22) = −.21, p = .835, and, b = .5, t(22) = 1.34, p = .194, respectively, the TIMB Acceptance Scale, b = −.64, t(22) = −.30, p = .764, and, b = .84, t(22) = 2.32, p = .030, respectively, and the TIMB Commitment Scale, b = −.04, t(22) = −.18, p = .863, and, b = .53, t(22) = 1.6, p = .125, respectively, using the Hochberg procedure.
Fig. 2.
TIMB Scale and P3 scatterplots.
Lastly, we examined any contribution of child age and caregiver age, both variables that significantly differed between birth and foster/adoptive parents, to comparisons of parent type. Simple correlations were obtained between caregiver age, child age and each of the N170, N2, P2, P3, and LPP components, averaged across stimuli and none of the correlations were statistically significant.
3. Discussion
The results of the current study demonstrate an increased positivity at early and late points along the ERP waveform suggesting neurological processes that can detect the emotional significance of face stimuli as early as 100–200 ms after stimulus onset and lasting for several hundred milliseconds. This is consistent with face processing studies reviewed by Eimer and Holmes (2007) demonstrating increased positivity to face pictures that reveal greater emotional expression. Our results complement other non-ERP studies reporting increased attention and arousal in human parents presented with pictures of their children (e.g.,Leibenluft et al., 2004; Swain et al., 2007) and offer preliminary information regarding the onset of attention allocation to personally significant faces.
Early in the waveform, during the N1 component, mothers exhibited significantly more positivity in response to pictures of their own children compared to familiar and unfamiliar adult pictures. Additionally, mothers exhibited more positive N1 s in response to unfamiliar child pictures compared to unfamiliar adult pictures, a finding that complements research suggesting enhanced sensitivity in mothers to unfamiliar child vs. adult facial stimuli (Leibenluft et al., 2004) and to unfamiliar infant cries vs. white noise (Lorberbaum et al., 2002). There was no difference in N1 s in response to familiar child vs. familiar adult pictures. One interpretation of these findings suggests rapid, but crude, processes that utilize facial features and recognition to distinguish between significant and non-significant face stimuli. More sophisticated, higher-level processing may be necessary to distinguish own child vs. other child faces due to the congruity of their structural facial features, and familiar child vs. familiar adult faces given that both are familiar. Unlike adult faces, own child faces possessed child facial features and were familiar. Likewise, unfamiliar child and unfamiliar adult pictures were both unfamiliar and unique in terms of their facial features. Thus, the greater contrast between these particular comparisons may have facilitated more accurate distinctions at this early, rudimentary stage. This rapid but crude process may relate to the subcortical ‘low road’ root proposed by Le Doux (1996) that identifies stimuli as significant based on rudimentary information.
The N170 component was not modulated by face type. As Eimer and Holmes (2007) review of the literature suggests, “The N170 component is assumed to reflect the pre-categorical perceptual encoding of faces in face-specific ventral visual areas, which provides structural representations that are utilized by subsequent face recognition stages” (p. 21). We also failed to find significant face type differences in P2 responses, which is at odds with some other studies demonstrating affective face modulation (Eimer and Holmes, 2007). While this may be due to differences in recording sites (we did not employ high-density recording), it is also possible that the functional significance of P2 is more closely linked with the structural encoding processes indexed by the N170 component. Indeed, some have argued that the P2 and N170 be considered the same component (e.g., Campanella et al., 2002) as both the N170 and P2 are modulated in nearly identical ways following structural face manipulations (Campanella et al., 2002).
Starting around 240 ms post-stimulus onset and continuing beyond 500 ms, during the N2, P3, and LPP components, mothers exhibited significantly more positivity in response to pictures of their own children compared to pictures of familiar and unfamiliar children and adults. These findings likely reflect a robust shift towards attending to the personal significance of face stimuli following higher-level, neocortical processing of face stimuli. In a recent review, the N2 has been discussed as entailing three functionally distinct subcomponents, two anterior and one posterior (Folstein and Petten, 2008). The differences in stimulus elicited N2 we observed might be described as the anterior subcomponent proposed to reflect aspects of cognitive control, or the processing of feedback that informs or facilitates one’s actions (Folstein and Petten, 2008). For example, studies have demonstrated an enhanced N2 when subjects must inhibit an anticipated response to a stimulus, such as in the No-Go paradigm (Bruin and Wijers, 2002). However, the N2’s association with cognitive control processes typically manifests as an enhanced negativity to non-target stimuli, indexing the degree to which one must inhibit a response. For example, non-target stimuli possessing target characteristics would elicit larger N2 s (Azizian et al., 2006). Thus, the larger N2s observed in response to other children and adults compared to own children in the current study might reflect the inhibition of action due to a mother’s anticipation of her own child’s picture. She might designate her own child as the target stimulus even though no overt action is expected.
An alternative explanation follows that the N2 component is overlapped by a robust emotional positivity to own child faces occurring during the same time period and persisting into the P3 and LPP (Kiss and Eimer, 2008). In the latter case, the positivity elicited by affective stimuli later in the waveform, during the N2, P3, and LPP is thought to reflect top-down control processes involved in the regulation of emotional content (Eimer and Holmes, 2007). One proposed contributor, especially with regards to the P3, involves the locus coeruleus – norepinephrine arousal system (LC-NE; Aston-Jones and Cohen, 2005; Nieuwenhuis et al., 2005). The locus coeruleus is a structure that is activated by dopaminergic projections from limbic areas and that sends norepinephrine to cortical and other subcortical regions to facilitate attention and action to emotionally or motivationally significant events (Aston-Jones and Cohen, 2005). According to the Adaptive Gain Theory, arousal and attention are modulated by tonic and phasic activity of locus coeruleus neurons (Aston-Jones and Cohen, 2005). When tonic activity is low, an individual is non-alert or inattentive, whereas when tonic activity is high, an individual is hyper-alert or distractible (Aston-Jones and Cohen, 2005). Phasic activity of locus coeruleus neurons acts as an attentional filter, inhibiting neuronal firing to non-specific stimuli and shifting attention to specific relevant stimuli (Aston-Jones and Cohen, 2005). Phasic activity is driven by other systems (e.g., orbitofrontal cortex, anterior cingulate cortex, limbic system) that communicate whether a stimulus is meaningful and whether one should act (e.g., approach, avoid) on the stimulus (Aston-Jones and Cohen). Thus, emotional or personally significant stimuli, like one’s child, may give rise to phasic activity, reallocating attentional resources towards further processing the stimulus.
In sum, the enhanced positivities observed throughout the ERP waveform in response to own child compared to other face pictures and the findings regarding N170 and P2 are in line with a parallel processing model that posits the involvement of several brain regions in simultaneously encoding the structural features of faces as well as their emotional and personal significance (Bruce and Young, 1986).
The positive relations found between P3 and LPP responses to mothers’ own children controlling for other faces and TIMB Awareness of Influence Scale scores and Acceptance Scale scores provide some support for the validity of the P3 and LPP to index maternal attentional processes. The TIMB Awareness of Influence Scale indicates the extent to which mothers acknowledge the influence that the mother–child relationship has on their children’s emotional and psychological development and future (Bates and Dozier, 1998). Mothers high on this index are more cognizant of their infants’ need for nurturance and thus might demonstrate a greater awareness of subtle infant social cues or bids for attention. The TIMB Acceptance Scale reflects the degree to which parents associate positive feelings with their relationship with their children and express taking pleasure in parenting their children. Mothers high on this index relish their role as parent and experience particular gratification when attending to their children. Thus, these parents may be particularly attentive to stimuli associated with their children. The Commitment Scale reflects the degree to which parents are emotionally invested in their children and regard their relationship with their child as permanent and enduring. We were surprised that Commitment scores were not significantly related to P3 and LPP responses. However, perhaps the Awareness of Influence and Acceptance scales are better indices of mothers’ responsiveness to their children’s social cues, whereas the Commitment Scale may indicate mothers’ expectation of the longevity of the relationship but not so much their attention to their children. In addition, earlier ERP components exhibiting face type modulatory effects failed to reveal significant associations with relational indices. Since the positivity observed at frontal sites likely reflects earlier, more rudimentary emotional processes (e.g., detection of emotional content) than those reflected by late positive potentials (Eimer and Holmes, 2007), it seems plausible that mothers’ perception of the parent–child relationship would not influence these earlier components. More specifically, the P3 and LPP is associated with later processing stages dedicated to more elaborative processing for the purposes of updating working memory and creating long-term memory traces (Donchin and Coles, 1988; Nieuwenhuis et al., 2005).
The fact that both birth mothers and foster/adoptive mothers showed similar positivity in response to their own children’s faces compared to other faces suggests that electrophysiological indicators of cognition and emotion are similarly modulated in mothers viewing pictures of their own children, whether biologically related or not. Thus, greater attention allocation to one’s children in mothers, as indexed by these ERPs, is not specific to biologically related mother–child dyads but also exists in the context of foster care and adoption. Understanding these processes in surrogate parents is important considering the documented benefits of having a nurturing caregiver, as well as the consequences of experiencing inadequate caregiving. For example, some benefits include more positive self-representations among young children with foster parents showing greater parental acceptance (Ackerman and Dozier, 2005), more behavioral problems among older foster children with greater detachment from their foster parents (Leathers, 2002), fewer internalizing symptoms among traumatized children with greater perceived support from their caregivers, even those with a genetic predisposition for depression (Kaufman et al., 2006; Kaufman et al., 2004), and greater improvement of externalizing symptoms among traumatized children whose parents co-participate in a trauma-focused treatment for posttraumatic stress disorder (Cohen et al., 2006). Further, gaining a better understanding of the associations between the foster parent–child relationship and child outcomes might help to enhance child welfare interventions.
We found no ERP differences between unrelated familiar and unfamiliar face pictures. However, the issue of controlling for familiarity was a limitation. Whereas we, consistent with methodology of Bobes et al. (2007), decided to familiarize mothers with faces by repeated exposure, others have used faces of relatives or friends to control for familiarity (e.g., Leibenluft et al., 2004). Neither approach fully addresses the fact that familiarity to mothers’ own children will always exceed that of control faces because of the greater amount of time spent with their children. However, we familiarized mothers with new faces to minimize variability in how personally relevant and emotionally significant familiar faces were to subjects. If we had used pictures of acquaintances we would have had little control over how long subjects had known acquaintances and how much time they had spent together, and thus would have had little control over the degree of familiarity of acquaintances.
Another limitation stems from our neglecting to systematically collect data on the number of birth children had by foster/adoptive mothers. Though we are unaware of research suggesting that foster/adoptive mothers’ experience raising birth children is predictive of their relationship with their foster/adopted children or information processing differences, this variable may be important to examine in future research.
It is important to note that the ERP patterns observed in the current study are not specific to the mother–child relationship. Rather, as Bobes et al. (2007) demonstrated, for example, the P3 differentiates stimuli representing unknown individuals from known acquaintances and relatives and so appears to index other personally relevant social stimuli as well. Perhaps differences in ERP responses to social stimuli correspond to the personal significance or strength of relationships and can be used to delineate the hierarchical structure of individuals’ social network. This was not explored in the current study but suggests a direction for future research.
We found significant caregiver type differences in the overall magnitude of the N170, N2, and LPP. Foster/adoptive mothers had a mean N170 that was greater than the mean for the birth mothers. In contrast, foster/adoptive mothers had a more positive (i.e., smaller) N2. These overall differences (main effects) were not expected and do not readily lend themselves to interpretation given our interaction-focused hypotheses.
4. Summary
As the field of social neuroscience advances (Cacioppo et al., 2007), translational research on the neurobiology of human social relationships may profit from ERP methodology. Studying brain activity using ERPs is non-invasive and cost-effective, providing data on emotional and cognitive processes with excellent temporal resolution, an attribute that can compliment research relying exclusively on fMRI. The current study used ERPs to elucidate attentional processes in mothers viewing their children. Results supported the initial hypothesis that mothers viewing pictures of their own children vs. other children and adults would exhibit ERP components reflecting greater attention allocation to mothers’ own children. These processes were recruited very quickly for own child faces and in a similar manner for both birth mothers and foster/adoptive mothers. In addition, positive relations found between P3 and LPP responses to mothers’ own children and measures of mothers’ perception of the mother–child relationship as positive and as influential to the emotional and psychological development of their children strengthens the validity of using ERP components to index maternal processes.
References
- Ackerman JP, Dozier M. The influence of foster parent investment on children’s representations of self and attachment figures. Journal of Applied Developmental Psychology. 2005;26:507–520. [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Anderson KG. Relatedness and investment in children in South Africa. Human Nature. 2005;16:1–31. doi: 10.1007/s12110-005-1005-4. Special Issue: Kin Investment. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Kaplan H, Lam D, Lancaster J. Paternal care by genetic fathers and stepfathers. II. Reports by Xhosa high school students. Evolution and Human Behavior. 1999a;20:433–451. [Google Scholar]
- Anderson KG, Kaplan H, Lancaster J. Paternal care by genetic fathers and stepfathers. I. Reports from Albuquerque men. Evolution and Human Behavior. 1999b;20:405–431. [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual review of neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Azizian A, Freitas AL, Parvaz MA, Squires NK. Beware misleading cues: perceptual similarity modulates the N2/P3 complex. Psychophysiology. 2006;43:253–260. doi: 10.1111/j.1469-8986.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- Bates B, Dozier M. “This Is My Baby” Coding Manual. University of Delaware; Newark: 1998. Unpublished manuscript. [Google Scholar]
- Bates BC, Dozier M. The importance of maternal state of mind regarding attachment and infant age at placement to foster mothers’ representations of their foster infants [Empirical Study] Infant Mental Health Journal. 2002;23:417–431. [Google Scholar]
- Bobes MA, Quinonez I, Perez J, Leon I, Valdes-Sosa M. Brain potentials reflect access to visual and emotional memories for faces. Biological Psychology. 2007;75:146–153. doi: 10.1016/j.biopsycho.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Bruin KJ, Wijers AA. Inhibition, response mode, and stimulus probability: a comparative event-related potential study. Clinical Neurophysiology. 2002;113:1172–1182. doi: 10.1016/s1388-2457(02)00141-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Amaral DG, Blanchard JJ, Cameron JL, Carter CS, Crews D, et al. Social neuroscience: progress and implications for mental health. Perspectives on Psychological Science. 2007;2:99–123. doi: 10.1111/j.1745-6916.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- Caharel S, Poiroux S, Bernard C, Thibaut F, Lalonde R, Rebai M. ERPs associated with familiarity and degree of familiarity during face recognition. International Journal of Neuroscience. 2002;112:1499–1512. doi: 10.1080/00207450290158368. [DOI] [PubMed] [Google Scholar]
- Campanella S, Quinet P, Bruyer R, Crommelinck M, Guerit JM. Categorical perception of happiness and fear facial expressions: an ERP study. Journal of cognitive neuroscience. 2002;14:210–227. doi: 10.1162/089892902317236858. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Academic Press; New York: 1969. [Google Scholar]
- Cohen JA, Mannarino AB, Deblinger E. Treating Trauma and Traumatic Grief in Children and Adolescents. Guilford Press; New York: 2006. [Google Scholar]
- Cook EW., III . VPM Reference Manual. Alabama; Birmingham: 1999. [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–395. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MG. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–427. [Google Scholar]
- Dozier M, Lindhiem O. This is my baby: differences among foster parents’ commitment to their young children. Child Maltreatment. 2006;11:338–345. doi: 10.1177/1077559506291263. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Petten CV. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1993;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hamilton L, Cheng S, Powell B. Adoptive parents adaptive parents: evaluating the importance of biological ties for parental investment. American Sociological Review. 2007;72:95–116. [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Grasso D, Lipschitz DS, Houshyar S, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Houshyar S, Lipschitz DS, Krystal J, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Eimer M. ERPs reveal subliminal processing of fearful faces. Psychophysiology. 2008;45:318–326. doi: 10.1111/j.1469-8986.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers SJ. Foster children’s behavioral disturbance and detachment from caregivers and community institutions. Children and Youth Services Review. 2002;24:239–268. [Google Scholar]
- Le Doux JE. The Emotional Brain. Simon and Schuster; New York: 1996. [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lindhiem O, Dozier M. Caregiver commitment to foster children: the role of child behavior. Child Abuse & Neglect. 2007;31:361–374. doi: 10.1016/j.chiabu.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel T. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Purhonen M, Kilpelainen-Lees R, Paakkonen A, Ypparila H, Lehtonen J, Karhu J. Effects of maternity on auditory event-related potentials to human sound. Neuroreport: For Rapid Communication of Neuroscience Research. 2001;12:2975–2979. doi: 10.1097/00001756-200109170-00044. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: motivated attention. Cognition & Emotion. 2004;18:593–611. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman DE, Hoyt E, Kang H, et al. Baby stimuli activate postpartum parent brains: studies over time, gender, experience and psychology. Paper presented at the Biennial Meeting of the Society for Research in Child Development; Boston, M.A.. 2007. [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA. Finally, faces find favor. Social Cognition. 2006;24:657–701. [Google Scholar]