Abstract
The pradimicin family of antibiotics is attracting attention due to its anti-infective properties and as a model for understanding the requirements for carbohydrate recognition by small molecules. Members of the pradimicin family are unique among natural products in their ability to bind sugars in a Ca2+-dependent manner, but the oligomerization to insoluble aggregates that occurs upon Ca2+ binding has prevented detailed characterization of their carbohydrate specificity and biologically relevant form. Here we take advantage of the water solubility of pradimicin S (PRM-S), a sulfated glucose-containing analog of pradimicin A (PRM-A), and show by NMR spectroscopy and analytical ultracentrifugation that at biologically relevant concentrations, PRM-S binds Ca2+ to form a tetrameric species that selectively binds and engulfs the trisaccharide Manα1-3(Manα1-6)Man over mannose or mannobiose. In functional HIV-1 entry assays, IC50 values of 2-4 μM for PRM-S corrrelate with the concentrations at which oligomerization occurs as well as the affinities with which PRM-S binds the HIV surface envelope glycoprotein gp120. Together these data reveal the biologically active form of PRM-S, provide an explanation for previous speculations that PRM-A may contain a second mannose binding site, and expand our understanding of the characteristics that can engender a small molecule with the ability to function as a carbohydrate receptor.
Pradimicin S (1, PRM-S)1,2 is a water-soluble, sulfated analog of pradimicin A (2, PRM-A),3 an antibiotic that exhibits in vivo antifungal activity, and is an inhibitor of HIV-14 and hepatitis C virus (HCV) entry.5 In addition to their potent anti-infective activities, PRM-S and PRM-A have attracted much interest because they represent rare examples of non-peptidic, small molecules with lectin-like activity.6-8 PRM-A has been reported to oligomerize into large insoluble aggregates and to bind d-mannose with mM equilibrium dissociation constants (KD’s) in a Ca2+-dependent manner.9 Although a high-resolution structure of a pradimicin-carbohydrate complex would provide unmatched insight into carbohydrate recognition by a small organic molecule, the propensity of all pradimicins to form solid aggregates has thwarted such efforts. The increased solubility of PRM-S over other pradimicin family members provided us with an opportunity to study pradimicin assembly and carbohydrate recognition under physiological conditions, and determine how these properties relate to their biological activity. To accomplish this, we used an integrated approach employing analytical ultracentrifugation and NMR spectroscopy coupled with cell-based neutralization assays to characterize the Ca2+-bound oligomerization of PRM-S; determine its carbohydrate specificity, and identify carbohydrate atoms involved in PRM-S binding; and correlate the physical properties of PRM-S with its inhibitory activity in functional HIV-1 neutralization assays. We found that IC50 values coincide with conditions at which oligomerization occurs and the affinities with which PRM-S binds the HIV surface envelope glycoprotein gp120.
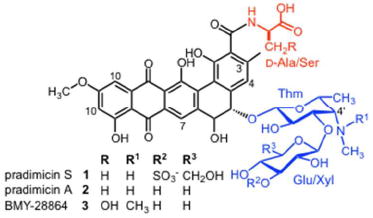
The chemical structure of PRM-S is composed of a benzo[a]naphthacenequinone aglycon, a d-Ala amino acid, and the disaccharide 3’-O-sulfo-β-d-glucosyl-β-d-thomosamine. In the presence of Ca2+, pradimicins have been shown to form insoluble, solid aggregates; however, physical studies to date have been performed primarily with mM samples of pradimicins,9,10 concentrations that exceed by orders of magnitude those at which their biological activities occur. To determine whether PRM-S is able to form discrete oligomeric structures at biologically relevant concentrations and in the presence of Ca2+, we used analytical ultracentrifugation on a series of samples where the concentration of PRM-S ranged from approximately 10 to 40 μM (details appear in the Supporting Information). The sedimentation velocity data in Fig. 1A show that in the absence of Ca2+, PRM-S exists as a single monodisperse species whose sedimentation coefficient (0.41 S) corresponds to a molecular mass of 950 ± 15 g mol-1, in excellent agreement with the molecular weight of PRM-S (948.9 g mol-1). When excess Ca2+ is added to the solutions, a series of discrete species that sediment significantly faster than the monomer is observed (Fig. 1B). At the lowest loading concentration (9.5 μM), only two species are observed: the slowest sedimenting and most abundant species at 0.67 S has a best-fit molar mass of 3350 ± 60 g mol-1, while the minor species at 1.32 S is significantly larger and has an estimated mass of 9300 ± 290 g mol-1 (SI, Fig. S1). The ultracentrifugation data show that higher molecular weight species become more prominent as the concentration of PRM-S is increased (Fig. 1B), but they comprise a very small component of the solution at low micromolar concentrations (SI, Fig. S1). (Indeed, we were unable to detect the presence of large particles by dynamic light scattering; data not shown.) Together, these data demonstrate that even in the presence of Ca2+, PRM-S can form discrete, soluble oligomers in solution at low micromolar concentrations.
FIGURE 1.
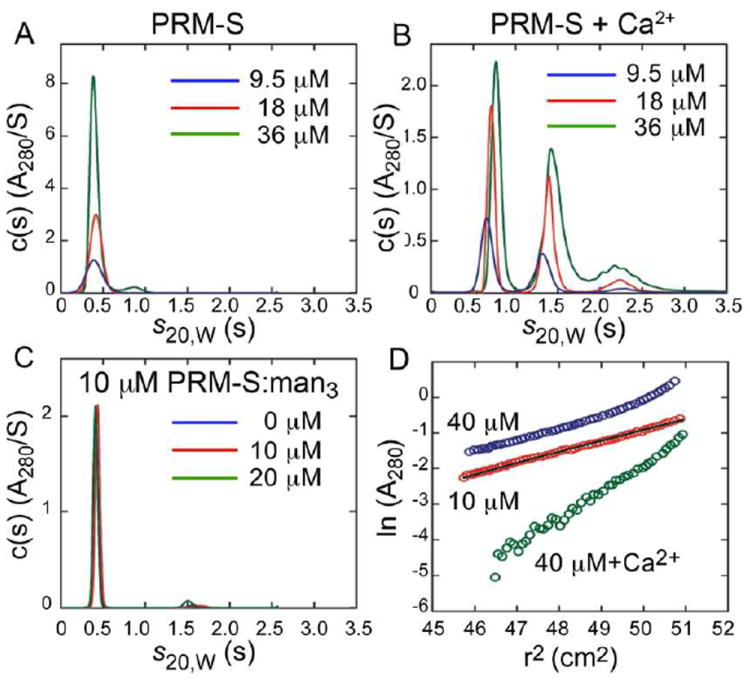
Characterization of PRM-S by analytical ultracentrifugation. Panels A-C show sedimentation velocity data of PRM-S in (A) the absence and (B) the presence of 5 mM CaCl2 at loading concentrations of 9.5 (blue), 18 (red) and 36 μM (green); (C) sedimentation velocity data of 10 μM PRM-S in the presence of 0 (blue), 10 (red) and 20 μM (green) mannotriose. Best fits of the data in the absence of Ca2+ and absence of Ca2+/presence of mannotriose yield a molecular mass of 950 ± 15 g mol-1 corresponding to a PRM-S monomer. In the presence of CaCl2 (B), discrete higher molecular weight PRM-S species are observed, the most prominent of which corresponds to a PRM-S oligomer with a molecular mass of 3350 ± 60 g mol-1. (D) Sedimentation equilbrium data of 10 μM (red) and 40 μM (blue) PRM-S in the absence of Ca2+, and 10 μM PRM-S in the presence of 5 mM CaCl2 (green). The superposed black line shows the expected slope for monomeric PRM-S. Data collected at 60,000 rpm are plotted in terms of the natural log of the absorbance against the radius-squared (r2) to illustrate the degree of PRM-S association.
To determine whether, in the absence of Ca2+, the carbohydrate ligands alone can induce PRM-S association, sedimentation velocity data were measured on solutions containing only PRM-S and Manα1-3(Manα1-6)Man (see below). As seen in Fig. 1C, the data are comparable to those of PRM-S in the absence of Ca2+ demonstrating that carbohydrate alone does not induce PRM-S oligomerization. Last, to confirm the observations made by sedimentation velocity data, sedimentation equilibrium experiments were carried out on PRM-S solutions in the absence or presence of 5 mM CaCl2 (Fig. 1D). Best fits of the data confirm that apo-PRM-S and Ca2+-bound PRM-S are present as a monodisperse monomer and oligomer, respectively (SI).
We took advantage of the water solubility of PRM-S by using 1H NMR to further investigate the individual effects of Ca2+, carbohydrate, and PRM-S concentration on the oligomerization of PRM-S under physiological conditions. 1H NMR spectra were recorded on samples containing 5, 10, 20 and 50 μM PRM-S in D2O (pH 6.85). As seen in Fig. S2 (SI), each spectrum showed the presence of sharp 1H signals for PRM-S indicating the existence of a single low molecular weight species and overall absence of aggregation under these conditions, consistent with the analytical ultracentrifugation data. By comparison, in aqueous solutions of PRM-A prepared at similar concentrations and under identical conditions, we observed immediate formation of insoluble aggregates that precipitated from solution, and the 1H NMR spectra of those samples consisted solely of broadened peaks (SI Fig. S3).
PRM-S and PRM-A were recently shown to be HIV-1 entry inhibitors that act at the early stage of virus infection, presumably through interactions with oligomannosides present on the viral envelope glycoprotein gp120.11 We sought to determine the carbohydrate specificity of PRM-S using Saturation Transfer Difference (STD) NMR12 combined with a fragment-based approach employing di– and trisaccharide components of high mannose oligosaccharides (Fig. S4, SI). STD NMR is a powerful technique that is routinely used to define precise binding epitopes on small molecule ligands by way of NMR relaxation. The method requires samples comprising a large molecular weight receptor (~10 kDa or larger) in the presence of an excess of ligand (~50-100–fold). Mindful of the ultracentrifugation data, we recorded STD NMR spectra of synthetic fragments of oligomannose in the presence of 50 μM PRM-S and Ca2+, conditions at which oligomeric species larger than 10 kDa are present (Fig. 1B). As seen in the STD NMR spectrum in Fig. 2A, in the presence of PRM-S–Ca2+ we observed remarkably strong enhancements for the trisaccharide core Manα1-3(Manα1-6)Man (mannotriose) with approximately equal intensities for the majority of the protons present (SI, Table S1). Although spectral overlap did not allow us to integrate individual peaks of the mannotriose difference spectrum, the degree of enhancements indicate that the trisaccharide is nearly completely surrounded by the pradimicin oligomer. Significantly reduced binding was observed for the terminal disaccharide Manα(1-2)Man (Fig. 2B), and binding to Man-O-Me was too weak to be detected by solution NMR (Fig. 2C). No binding was observed to the core structure GlcNAc2 (Fig. S4, SI). Thus, PRM-S shows remarkable specificity for the mannotriose core present of N-linked oligomannosides.
FIGURE 2.
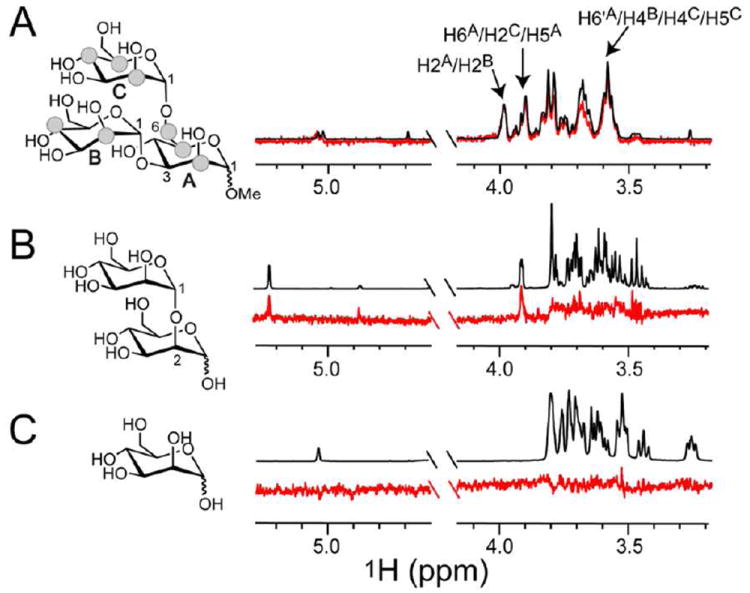
Carbohydrate specificity of PRM-S by STD NMR. Spectra were recorded on samples containing 50 μM PRM-S, 1 mM CaCl2, and 1 mM (A) Manα1-3(Manα1-6)Man, (B) Manα(1-2)Man, or (C) Man-O-Me with identical acquisition parameters (SI). Difference spectra (red) are superimposed (A) or shown below (B,C) their corresponding reference spectrum (black). Strong enhancements are observed for virtually all protons of mannotriose. Signals showing the strongest enhancements and used for normalization are labeled and depicted as grey spheres. Enhancements for mannobiose are weak in comparison, and binding to mannose could not be detected.
The surface envelope glycoprotein of HIV, gp120, is distinguished by its dense display of complex carbohydrates, where an abundance of high mannose oligosaccharides is present.13,14 The carbohydrate-binding properties of the pradimicins engender these small molecules with the ability to inhibit HIV-1 entry through carbohydrate-mediated interactions with the viral envelope glycopro tein gp120. As with many carbohydrate-mediated HIV-1 entry inhibitors, the mode of binding of PRM-S to gp120 has not been defined, nor has the effect of oligomerization on antiviral activity. To examine the kinetics of binding of PRM-S to gp120 we used surface Plasmon resonance where soluble gp120 (HIV-1 strain HXB2) was immobilized to the surface, and serial dilutions of PRM-S in the presence and absence of Ca2+ were analyzed for binding. To obtain excellent quality data, it was necessary to determine empirically the amount of gp120 to be immobilized onto the surface (details provided in the SI). As seen in Fig. 3A, the data fit well to a heterogeneous ligand model with KD values of 2 and 32 μM (SI, Table S2). In the absence of Ca2+, we did not observe binding to gp120 at any of the concentrations tested.
FIGURE 3.
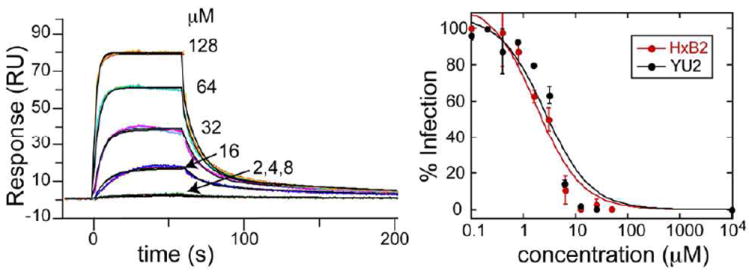
PRM-S binds HIV-1 gp120 and inhibits HIV-1 entry. (A) Binding of PRM-S to gp120 in the presence of Ca2+ measured by surface Plasmon resonance. (B) Inhibition curves for PRM-S toward HIV-1 strains HxB2 and YU2 obtained from HIV-1 infectivity assays. IC50 values are on the order of 2-4 μM, consistent with the KD values of pradS/Ca2+ binding to gp120.
To characterize the active form of PRM-S that is capable of inhibiting viral entry, we tested our characterized PRM-S solutions in single round HIV-1 infectivity assays15 using two different HIV-1 strains (including the CXCR4-using strain HXB2 and the CCR5-using strain YU2, SI). As seen in Fig. 3B, PRM-S gave similar dose-response curves for both HIV-1 strains, with IC50 values around 2-4 μM, comparable to values reported for other pradimicins toward HIV-1.4,11 Our surface Plasmon resonance results showed clearly that in the absence of Ca2+, PRM-S is unable to bind gp120 in vitro. Because of the cellular requirement for Ca2+ and the duration of the neutralization assay (72 hr), it is not possible to perform an analogous Ca2+-free HIV-1 entry assay. However, our combined data indicate that during neutralization, PRM-S exists as a discrete monodisperse species when binding to virus-associated gp120, and the IC50 values for inhibitory activity correlate with concentrations at which Ca2+-dependent tetramer formation is observed.
In this study we present the first detailed characterizaion of a pradimicin in solution at biologically relevant concentrations. By using an integrated approach that included NMR, analytical ultracentrifugation, surface Plasmon resonance and viral neutralization assays, we conclusively demonstrated that PRM-S is able to form discrete Ca2+-dependent oligomers at low micromolar concentrations, a characteristic that has yet to be demonstrated for other pradimicins. Further, these PRM-S:Ca2+ complexes show extremely high selectivity for the mannotriose core structure Manα1-6(Manα1-3)Man that is present in all high mannose oligosaccharides. Interestingly and in contrast to observations for PRM-A, PRM-S binding to O-methyl mannoside was so weak as to be virtually undectable by STD NMR, a technique that readily detects weak carbohydrate-receptor interactions having mM KD values.12,16,17 On this note, others have speculated that a second mannose-binding site may be present in the pradimicins,9,18 and this would be consistent with the stronger binding observed here for mannotriose over mannose. A major difference among the pradimicins is the identity and number of sugar units present in each of the natural products. Thus, it is possible that the presence of the 3-sulfo-glucosyl unit unique to PRM-S accounts for its specificity toward mannotriose. By employing an integrated approach to the study of other pradimicin-carbohydrate complexes, we are optimistic that we will learn more about the assembly and carbohydrate recognition that occurs within this family of antibiotics. In closing, these results expand our understanding of small molecule-based carbohydrate receptors, and reveal new structural features that may augment design of synthetic carbohydrate receptors.
Supplementary Material
Acknowledgments
C. I. D.-I. is a recipient of an Intramural AIDS Research Fellow award, Office of the Director, NIH.
Funding Sources
This work was supported in part by the Intramural Research Program of the National Institutes of Health (NIDDK), and KU Leuven, PF-10/18 (J.B.).
ABBREVIATIONS
- STD NMR
Saturation Transfer Difference NMR
- Man3 or mannotriose
Manα1-3(Manα1-6)Man
- mannobiose
Manα(1-2)Man
References
- 1.Saitoh K, Tenmyo O, Yamamoto S, Furumai T, Oki T. J Antibiot (Tokyo) 1993;46:580. doi: 10.7164/antibiotics.46.580. [DOI] [PubMed] [Google Scholar]
- 2.Saitoh K, Tsuno T, Kakushima M, Hatori M, Furumai T, Oki T. J Antibiot (Tokyo) 1993;46:406. doi: 10.7164/antibiotics.46.406. [DOI] [PubMed] [Google Scholar]
- 3.Oki T, Konishi M, Tomatsu K, Tomita K, Saitoh K, Tsunakawa M, Nishio M, Miyaki T, Kawaguchi H. J Antibiot (Tokyo) 1988;41:1701. doi: 10.7164/antibiotics.41.1701. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe A, Nakashima H, Yoshida O, Yamamoto N, Tenmyo O, Oki T. J Antibiot (Tokyo) 1988;41:1708. doi: 10.7164/antibiotics.41.1708. [DOI] [PubMed] [Google Scholar]
- 5.Bertaux C, Daelemans D, Meertens L, Cormier EG, Reinus JF, Peumans WJ, Van Damme EJ, Igarashi Y, Oki T, Schols D, Dragic T, Balzarini J. Virology. 2007;366:40. doi: 10.1016/j.virol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Davis AP, James TD. In: Functional Synthetic Receptors. Schrader T, Hamilton AD, editors. Wiley-VCH; Weinheim: 2005. [Google Scholar]
- 7.Mazik M. RSC Advances. 2012;2:2630. [Google Scholar]
- 8.Ueki T, Numata K, Sawada Y, Nakajima T, Fukagawa Y, Oki T. J Antibiot (Tokyo) 1993;46:149. doi: 10.7164/antibiotics.46.149. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Doi T, Masuda Y, Takegoshi K, Igarashi Y, Ito Y. J Am Chem Soc. 2011;133:17485. doi: 10.1021/ja207816h. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa Y, Masuda Y, Yamada K, Doi T, Takegoshi K, Igarashi Y, Ito Y. Angew Chem Int Ed Engl. 2011;50:6084. doi: 10.1002/anie.201007775. [DOI] [PubMed] [Google Scholar]
- 11.Balzarini J, Francois KO, Van Laethem K, Hoorelbeke B, Renders M, Auwerx J, Liekens S, Oki T, Igarashi Y, Schols D. Antimicrob Agents Chemother. 2010;54:1425. doi: 10.1128/AAC.01347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer M, Meyer B. Angew Chem Int Ed. 1999;38:1784. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1784::AID-ANIE1784>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, Burton DR, Crispin M, Scanlan CN. Proc Natl Acad Sci U S A. 2010;107:13800. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go EP, Chang Q, Liao HX, Sutherland LL, Alam SM, Haynes BF, Desaire H. J Proteome Res. 2009;8:4231. doi: 10.1021/pr9002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. J Virol. 2005;79:10108. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansman GS, Shahzad-Ul-Hussan S, McLellan JS, Chuang GY, Georgiev I, Shimoike T, Katayama K, Bewley CA, Kwong PD. J Virol. 2012;86:284. doi: 10.1128/JVI.05909-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O’Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Nature. 2011;480:336. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujikawa K, Tsukamoto Y, Oki T, Lee YC. Glycobiology. 1998;8:407. doi: 10.1093/glycob/8.4.407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


