Abstract
The development of methods to achieve efficient reprogramming of human cells while avoiding the permanent presence of reprogramming transgenes represents a critical step towards the use of induced pluripotent stem cells (iPSC) for clinical purposes, such as disease modeling or reconstituting therapies. While several methods exist for generating iPSC free of reprogramming transgenes from mouse cells or neonatal normal human tissues, a sufficiently efficient reprogramming system is still needed in order to achieve the widespread derivation of disease-specific iPSC from humans with inherited or degenerative diseases. Here we report the use of a humanized version of a single lentiviral ‘stem cell cassette’ vector in order to accomplish efficient reprogramming of normal or diseased skin fibroblasts obtained from humans of virtually any age. Simultaneous transfer of either 3 or 4 reprogramming factors into human target cells using this single vector allows derivation of human iPSC containing a single excisable viral integration, that upon removal generates human iPSC free of integrated transgenes. As a proof of principle, here we apply this strategy to generate >100 lung disease-specific iPSC lines from individuals with a variety of diseases affecting the epithelial, endothelial, or interstitial compartments of the lung, including cystic fibrosis, alpha-1 antitrypsin deficiency-related emphysema, scleroderma (SSc), and sickle cell disease. Moreover, we demonstrate that human iPSC generated with this approach have the ability to robustly differentiate into definitive endoderm in vitro, the developmental precursor tissue of lung epithelia.
Keywords: Human induced pluripotent stem cells (iPSC), reprogramming, human excisable single lentiviral vector, stem cell cassette, endoderm, lung disease-specific iPSC, cystic fibrosis, emphysema, alpha-1 antitrypsin deficiency, sickle cell, scleroderma
INTRODUCTION
The reprogramming of post-natal cells by defined transcription factors has allowed the derivation of induced pluripotent stem cells (iPSC) with similar functional and molecular phenotypic characteristics to embryonic stem cells (ESC)1–4. This seminal advance has considerable implications for the field of regenerative medicine and suggests the prospect of generating autologous pluripotent stem cells from easily accessible human tissues, such as skin biopsies, hair follicles, or peripheral blood5–10. The original method for deriving iPSC as described by Yamanaka and colleagues, employed 4 integrating retroviral vectors to deliver the four ‘reprogramming transcription factors’, Oct4, Sox2, Klf4, and c-Myc into mouse fibroblasts in order to generate approximately 150 iPSC clones per 8×105 transduced target cells, indicating a reprogramming efficiency of 0.01%3. It has since become clear that combinations of alternative genes or chemicals can be used to substitute for some of the original 4 reprogramming factors, modifying the number of viral vectors required, in some cases at the expense of reprogramming efficiency7, 9, 11, 12. More recently, derivation of iPSC with non-integrating vectors, plasmid transfection or even direct protein delivery has been achieved, although with exceedingly low efficiencies that prevent reliable application for reprogramming disease-specific adult human somatic cells13–17.
Regardless of the method used, somatic cells from humans appear to be more difficult to reprogram than murine cells7. Moreover, it is becoming clear that the development of methods to achieve efficient reprogramming of cells from adult humans with disease while avoiding the permanent presence of the reprogramming transgenes, represents a critical step towards the use of this technology for clinical purposes18–20. Importantly, such methodology should allow for the reliable and consistent reprogramming of human somatic cells, regardless of the age or disease state of the individual from whom they are derived.
Recently, we reported the use of a single lentiviral ‘stem cell cassette’ (STEMCCA) encoding all 4 reprogramming factors, Oct4, Sox2, Klf4, and c-Myc in a single polycistronic vector21. By combining all reprogramming transgenes in a single cassette, STEMCCA accomplished reprogramming of post-natal mouse fibroblasts with high efficiency and allowed the derivation of mouse iPSC containing a single viral integration. Most recently, we generated an excisable version of STEMCCA based on Cre/loxP technology that allowed for the derivation of murine iPSC free of exogenous transgenes22. Here we report the use of a humanized version of the single lentiviral ‘stem cell cassette’ vector flanked by loxP sites (hSTEMCCA-loxP) in order to achieve highly efficient reprogramming of normal or diseased post-natal human skin fibroblasts. Simultaneous transfer of either 3 or 4 reprogramming factors into human target cells using this single vector allows derivation of human iPSC containing a single excisable viral integration, that upon removal generates human iPSC free of integrated transgenes. In contrast to previously described methods, the high efficiency of reprogramming with this reagent allows minute quantities of viral vector to be used for reprogramming normal and disease-specific somatic cells taken from humans of virtually any age. As a proof of principle, here we apply this strategy to generate >100 of the first known lung disease-specific iPSC lines from individuals with diseases affecting the epithelial, endothelial, or interstitial compartments of the lung.
MATERIALS AND METHODS
Vector Design and Construction
The humanized hSTEMCCA lentiviral vector was constructed by adapting the previously published mouse pHAGE2-EF1α-STEMCCA vector21 as follows: First, human cDNAs encoding the transcription factors OCT4, KLF4, SOX2, and cMYC were amplified using the following primers: hOCT4 5′ NotI (5′TTT TGC GGC CGC CAT GGC GGG ACA CCT GGC TTC GG-3′); hOCT4 F2A 3′ (5′-CCT GCA AGT TTC AGC AAA TCA AAG TTT AAT GTC TG CTT TAC TGG CGC ACC CGA ACC CGA GTT TGA ATG CAT GGG AGA GCC CAG AGT GGT G-3′); hKLF4 F2A 5′ (5′-GCA GAC ATT AAA CTT TGA TTT GCT GAA ACT TGC AGG TG ATG TAG AG TCA AAT CCA GGT CCA ATG GCT GTC AGC GAC GCG CTG CTC CCA TC-3′); hKLF4 3′ BamHI (5′TGT TGG ATC CTT AAA AAT GCC TCT TCA TGT GTA AGG CG-3′); hSOX2 5′ NdeI (5′-TTT AGT GCA TAT GAT GTA CAA CAT GAT GGA GAC GG AGC TG-3′); hSOX2 P2A 3′-(5′-TTC TCT TCG ACA TCC CCT GCT TGT TTC AAC AGG GA GAA GTT AGT GGC TCC GCT TCC GGA CAT GTG TGA GAG GGG CAG TGT GCC GTT AAT G-3′); hcMYC P2A 5′ (5′-GCC ACT AAC TTC TCC CTG TTG AAA CAA GCA GGG GA TGT CGA AGA GAA TCC CGG GCC AAT GCC CCT CAA CGT TAG CTT CAC CAA CAG GAA C-3′); hcMYC 3′ AccI (5′-TTT AGC AGT GGT ACG TCG ACT TAC GCA CAA GAG TTC CGT AGC TGT TC-3′). Cloning of amplified products into the pHAGE2 vector was performed as described21.
Next, the loxP sequence flanked by AscI restriction enzyme sites was constructed by annealing together the following complementary oligonucleotides: 5′-CGC GCA GGT ACC ATA ACT TCG TAT AAT GTA TGC TAT ACG AAG TTA TGG – 3′ and 5′-CGC GCC ATA ACT TCG TAT AGC ATA CAT TAT ACG AAG TTA TGG TAC CTG – 3′. The resulting double-stranded DNA fragment was inserted in the deleted U3 portion of the pHAGE2 lentiviral 3′LTR by ligation of cohesive compatible ends into the vector’s AscI restriction site. Cloning of mCherry was performed as previously described22.
Lentiviruses were produced in 293T packaging cells by 5 plasmid co-transfection and were concentrated by ultracentrifugation as previously described21, 22. Viral titers were calculated based on Southern blots of genomic DNA (gDNA) from FG293 cells transduced with defined volumes of concentrated viral supernatants in order to determine transducing units per mL (TU/mL). On average, viral titers of ~1×108TU/mL were employed for reprogramming experiments.
For transient Cre expression, the plasmid pHAGE2-EF1α-Cre-IRES-PuroR was constructed by ligating the Cre cDNA into compatible NotI-BamHI restriction sites downstream of the EF1αpromoter of the pHAGE2 backbone. The PuroR cDNA was ligated downstream of the IRES element and upstream of a WPRE sequence using NdeI-ClaI sites.
Skin biopsies and expansion of human dermal fibroblasts
Individuals with alpha-1 antitrypsin (AAT) deficiency due to inheritance of two Z alleles of the AAT protease inhibitor (PiZZ), individuals with cystic fibrosis caused by inheritance of homozygous ΔF508 CFTR mutations, or individuals with systemic sclerosis (SSc) underwent 6mm full thickness skin punch biopsies from the arm. All procedures were approved by the Institutional Review Board (IRB) of the Boston University Medical Campus or the University of Vermont College of Medicine, and informed consent was documented from all individuals. De-identified skin samples were digested overnight at 37°C with 0.25% Collagenase I (Worthington-biochem.com; CLS-1) and 0.05% DNAse I, (Sigma) in high glucose DMEM containing 20% fetal bovine serum (FBS). Cell suspensions cultured in T75 plates were split 1:3 when 80% confluent, in order to obtain outgrowth of dermal fibroblasts. Reprogramming was typically initiated on passage 3 or 4.
Human Fibroblast Reprogramming and Characterization of iPSC
1×105 human fibroblasts were plated in DMEM with 10%FBS on a gelatin-coated 35mm plastic tissue culture dish. The next day polybrene was added to the media (5 ug/mL) and the cells were infected with hSTEMCCA-loxP lentiviruses at a multiplicity of infection (MOI)=0.1, 1, or 10 where indicated in the text. On day 2, the media was changed to serum-free ‘iPSC media’ (see below), and on day 6 the entire well was trypsinized and passed at a 1:16 split by plating onto two 10cm gelatin-coated culture dishes which had been pre-seeded the day before with mitomycin C-inactivated mouse embryonic fibroblast (MEF) feeder cells. iPSC colonies were mechanically isolated 30 days post-infection with the 4 factor hSTEMCCA-loxP or 45 days post-infection with the 3 factor hSTEMCCA-RedLight-loxP based on morphology and expanded on MEF feeders in iPSC media. For 3 factor reprogramming, where indicated, GSK3 inhibitor (Bio) (EMD Biosciences, 361550; 10μM) was added to the culture media on days 7–30 of reprogramming. Reprogramming efficiency was calculated by dividing the number of total colonies obtained on day 30 by the number of starting input fibroblasts. To avoid miscalculating efficiency as can occur when counting the progeny of passaged cells, efficiency was determined from separate experiments in which the input fibroblasts were not passaged onto feeders prior to colony counting.
Candidate iPSC clones were characterized based on staining for expression of alkaline phosphatase (Alkaline Phophatase Substrate Kit I, Vector Laboratories, SK – 5100) or immunostaining of 4% paraformaldehyde-fixed cell colonies with antibodies against SSEA-4, TRA1-60, and TRA1-81 (ES Cell Characterization Kit, Millipore, SCR001). Primary antibodies were detected with secondary Alexa Fluor 488-conjugated, goat anti-mouse IgG or IgM (Invitrogen, A10680). In addition, the number of hSTEMCCA lentiviral integrations was determined by Southern blot of gDNA digested with BamHI and probed for the WPRE element as previously published21. RT-PCR was performed as previously described21.
In order to evaluate the degree of DNA methylation of the human NANOG promoter, gDNA extracts of each indicated sample underwent bisulfite conversion using the EpiTect Bisulfite Kit (Qiagen). Quantitative methylation analyses of 6 CpG islands in the proximal NANOG promoter were performed via pyrosequencing by EpigenDx Inc (Worcester, MA) using the ADS502/Human NANOG promoter assay, spanning positions −565 to −431 relative to the NANOG ATG start site.
For teratoma formation assays, 6 wells of a 6 well plate of iPSC colonies were harvested with Collagenase IV and resuspended in 140μl of DMEM/F12. Immediately prior to injection, 60μl of Matrigel (BD Biosciences) was added to the cell suspension at 4°C, and the resulting mixture was injected sub-dermally between the scapulae of each anesthetized SCID–Beige mouse (Charles River, strain 250). Resulting tumors were harvested at 6–8 weeks after injection, fixed in 4% paraformaldehyde, and paraffin tissue sections were prepared and stained with hematoxylin and eosin according to standard methods.
Tissue culture maintenance of undifferentiated iPSC
Reprogrammed cells were propagated in ‘iPSC Media’ consisting of Dulbecco’s Modified Eagle’s medium (DMEM) F12 (Sigma-Aldrich) with 20% KnockOut Serum Replacement (Invitrogen), 1 mM of non-animal L-glutamine (Sigma-Aldrich), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), 1% non-essential amino acid solution (Invitrogen), and 10 ng/mL of FGF2 (Invitrogen). Culture dishes were coated with sterile gelatin (Millipore) before use. The cells were cultured on a feeder layer of mitomycin-C (Fisher) treated mouse embryonic fibroblasts (MEFs), and were incubated at 37°C at 5% CO2. iPSC and hESC lines were typically passaged approximately every five days at a one-to-three split ratio. Collagenase IV (Invitrogen) was used to loosen the cells from the dish before mechanically scraping to pass. The cells were maintained in the undifferentiated state by scraping off differentiated cells with a glass pipette or alternatively by mechanical passage of individual colonies of undifferentiated cells.
Quantitative RT-PCR
Total RNA was isolated from cells with the RNeasy micro kit (Qiagen) and treated with RNase-free DNase (Qiagen). 100ng to 1μg RNA was reverse transcribed into cDNA using random hexamers with Superscript III Reverse Transcriptase (Invitrogen). Real-time quantitative PCR was performed in triplicate for all samples using the LightCycler 480 Real-Time PCR System (Roche) with LightCycler 480 SYBR Green I Master (Roche). A 10 fold dilution series of human gDNA ranging from 10ng to 10pg per reaction was used to evaluate the efficiency of the PCR and calculate the copy number of each gene relative to the house keeping gene Cyclophilin. Calculated expression levels for each indicated gene were then reported as number of molecules of RNA for that gene per number of molecules of Cyclophilin, following a similar method previously described using mouse ESC23. Given this approach, all primers were designed not to cross introns; the oligonucleotide sequences for primers were as follows: Cyclophilin F-GAA GAG TGC GAT CAA GAA CCC ATG AC, R-GTC TCT CCT CCT TCT CCT CCT ATC TTT ACT T; DNMT3B F-TAC AGA CGT GTG CAG TTG TAG GCA, R-GTG CAG ACT CCA GCC CTT GTA TTT; NANOG-CCT GAA GAC GTG TGA AGA TGA G, R-GCT GAT TAG GCT CCA ACC ATA C; OCT4 F-AAC CTG GAG TTT GTG CCA GGG TTT, R-TGA ACT TCA CCT TCC CTC CAA CCA; SOX2 F-AGA AGA GGA GAG AGA AAG AAA GGG AGA GA, R-GAG AGA GGC AAA CTG GAA TCA GGA TCA AA; SOX17-AGG AAA TCC TCA GAC TCC TGG GTT, R-CCC AAA CTG TTC AAG TGG CAG ACA; FOXA2 F- GCA TTC CCA ATC TTG ACA CGG TGA; R-GCC CTT GCA GCC AGA ATA CAC ATT; SOX7 F-TGG AGG TTG CAG TGA GCT GAG ATT G; R- TGC ATG AAG TGG GCA TGT GTC TCT; HNF4A F-TCC AAC CCA ACC TCA TCC TCC TTC TT; R- TCC TCT CCA CTC CAA GTT CCT GTT.
Flow Cytometry
iPSC were dissociated by incubation with trypsin for 2–4 minutes and stained for the following cell surface antigens: anti-human CD117-allophycocyanin, anti-human CXCR4-phycoerythrin (Invitrogen), anti-human TRA 1-81-phycoerythrin (Biolegend), and anti-human SSEA4–Alexa647 (Biolegend). Intracellular staining was performed as follows: Cells were fixed in 1.6% paraformaldehyde for 20 minutes at 37°C, washed, then permeabilized and stained in 1× saponin buffer (Biolegend). The permeabilized cells were stained with anti-human FOXA1 (Santa Cruz sc-101058) and anti-human OCT4 (Santa Cruz sc-5279), followed by goat anti-mouse IgG2a-DyLight488 (Jackson ImmunoResearch) and goat anti-mouse IgG2b-DyLight649 (Jackson ImmunoResearch). Cells were analyzed on a FACSCantos II flow cytometer (Becton Dickenson) and analysis was performed using FlowJo software (Tree Star Inc.).
Excision of hSTEMCCA
The transfection of human iPSC colonies for the excision of viral sequences was performed using the Hela Monster transfection reagent (Mirus) according to manufacturer’s instructions. Briefly, 30% confluent 35mm tissue culture wells of iPSC colonies growing on puromycin resistant MEFs were exposed to media containing 2μg of pHAGE2-Cre-IRES-PuroR plasmid DNA along with 6μl of transfection reagent and 3μl of the Monster reagent. The media was changed the next day and puromycin selection (1.2 μg/ml) was begun 24 hours following the initial transfection and lasted for 48 hours. The reemergence of 10–50 colonies was noted within 1 week, and 5 colonies from each well were picked on day 11–14 for passaging. PCR of gDNA extracted from each subclone was performed to screen for excision of the hSTEMCCA vector using the following primers and conditions: cMYC F5’-GGA ACT CTT GTG CGT AAG TCG ATA G-3′; WPRE R5′-GGA GGC GGC CCA AAG GGA GAT CCG-3′; 95°C × 3min; followed by 33 cycles of 94°C × 30s, 60°C × 30s, and 72°C × 1min; followed by a single cycle of 72°C × 5 min. Vector excision was then confirmed by Southern blotting of BamHI digested gDNA, probed against WPRE as above.
Directed Differentiation of Human iPSC into Definitive Endoderm
Cells were differentiated in a serum-free media base developed for mouse ES cell differentiation24. Briefly, iPSC were plated on Matrigel (Becton Dickinson) in iPSC media to deplete feeder cells prior to differentiation. iPSC were trypsinized for 1–2 minutes with cold 0.25% trypsin/EDTA then scraped off the dish to form cell clusters (5–50 cells per cluster). To induce differentiation, cells were first cultured for 24 hours in serum-free media with the addition of human BMP4 at 10 ng/ml (R&D systems). The next day to induce differentiation towards definitive endoderm, the cells were changed to media containing Activin A at 100 ng/ml (R&D systems), FGF2 2.5 ng/ml, and BMP4 0.5 ng/ml. The cultures were fed fresh media on day 4 and analyzed for differentiation on day 6. As a negative control, parallel aliquots of cells in each experiment were differentiated into extra-embryonic endoderm. This was performed by addition of only BMP4 10ng/ml in serum-free differentiation media with iPSC plated in adherent cultures on Matrigel and analyzed on day 6.
RESULTS
Design of a human STEMCCA vector for the efficient generation of human iPSC
Using a similar strategy to that employed to generate the mouse STEMCCA vector21, 22 we constructed a single lentiviral vector expressing a constitutive polycistronic message encoding the four human transcription factors, OCT4, KLF4, SOX2 and cMYC (Fig. 1A). In addition, we inserted a loxP site at the 3′ LTR of the vector, for future Cre-mediated excision, and named this vector hSTEMCCA-loxP. A combination of 2A peptide and IRES elements allowed for the production of the four individual transcription factors. The simultaneous expression of all 4 factors resulted in a high efficiency of reprogramming human cells, as evidenced by the large number of putative iPSC colonies obtained after infection of human foreskin fibroblasts (HFF) with concentrated hSTEMCCA-loxP viral particles at a multiplicity of infection (MOI) of 1 (Fig. 1B). The efficiency of reprogramming using our vector was ~1%, however, this result varied with viral MOI. For example, increasing MOIs >10 resulted in declining reprogramming efficiencies and observable death of HFF during the first week post infection, suggesting some toxicity associated with either viral transduction or transgene overexpression. The dynamics of reprogramming with the hSTEMCCA-loxP vector was evident morphologically by microscopy beginning 6–8 days post transduction, followed by formation of early colonies 12–15 days post transduction. Outgrowth of mature candidate iPSC colonies appeared at 25 days, which were picked at 30 days after initial infection. Using this method >70% of picked colonies expanded in culture after passaging with a morphology indistinguishable from control H9 human ESC (Fig. 1B). Passaged colonies expressed a broad panel of ‘pluripotent marker genes’ by RT-PCR and immunostaining, including GDF3, NANOG, hTERT, REX1, TRA-1-81, TRA-1-60, and SSEA-4 (Fig. 1C and D). G banding analysis of a representative clone (Fig. 1E) revealed a normal 46XY karyotype with no apparent chromosomal abnormalities.
Figure 1. Human iPSC generation using a humanized, floxed single lentiviral stem cell cassette (hSTEMCCA-loxP).
A) Vector schematic illustrating the polycistronic lentiviral backbone encoding either 4 reprogramming factors or 3 factors plus mCherry. A loxP site inserted in the viral 3′LTR is duplicated to the 5′LTR during viral infection and reverse transcription. The resulting floxed vector integrated in the host mammalian genome can then be excised upon exposure to Cre recombinase. LTR=long terminal repeats. dU3=deleted U3 region of viral LTR. EF1α=Elongation Factor 1 alpha constitutive promoter. WPRE=Woodchuck Hepatitis Virus Post-transcriptional Regulatory Element. B) Representative micrographs of human foreskin fibroblasts (HFF) in culture (left panel) and reprogrammed human iPSC colonies (right panel). Multiple alkaline phosphatase (AP) positive colonies are observed 30 days after reprogramming 50,000 human fibroblasts with the hSTEMCCA-loxP virus. C and D) Characterization of four independent iPSC clones, generated with either 4 factor or 3 factor hSTEMCCA-loxP, showing expression by RT-PCR (C) and immunostaining (D) of typical pluripotent stem cell markers. E) Representative normal 46XY karyotype of HFF-derived iPSC clone. F) Southern blot of BamHI digested gDNA from representative HFF-derived iPSC clones, probed against WPRE, demonstrates a single viral integration in all clones. HFF=human foreskin fibroblasts. hESC=H9 human embryonic stem cells. Bars= 200 μm (B); 250 μm (D).
iPSC generated with the hSTEMCCA-loxP vector contain a single excisable viral integrant
An important attribute of a human reprogramming vector should be its ability to reliably produce iPSC clones containing a single viral integration that could be simply excised allowing the generation of iPSC free of exogenous transgenes. While this approach has been achieved previously with a piggyBAC transposon-transposase method19, 25 in mouse embryonic fibroblasts, the method has yet to be successfully applied for generating transgene-free human iPSC. Importantly, an excisable multi-vector lentiviral system has been utilized to reprogram fibroblasts from individuals with Parkinson’s disease at low efficiency18, however, multiple integrated copies of the 4 dox-inducible vectors, along with additional integrated copies of RTTA-expressing vectors were required. In order to achieve reprogramming of human cells with a single viral integration, we reasoned that reducing the number of infectious particles would increase the chance of obtaining single copy integrations for reprogramming. Surprisingly, as evidenced by Southern blot analysis of genomic DNA, we observed that regardless of the MOI (ranging from 0.1–10), we consistently obtained a high percentage (94%+/−9%; avg+/−SD) of iPSC colonies containing a single viral integration (Fig. 1F and 3). This suggests that a specific range of reprogramming transgene expression, in this case depending on the number of viral copies per cell, must be obtained in order for a transduced cell to complete reprogramming to the point of colony expansion post-picking.
Figure 3. Generation of disease-specific iPSC.
A) Human fibroblasts in culture and their reprogrammed iPSC progeny, derived from individuals with alpha-1 antitrypsin deficiency, cystic fibrosis, sickle cell disease, and scleroderma (SSc). B and C) characterization of lung disease-specific cystic fibrosis iPSC by RT-PCR (B), immunostaining (C), and real time qRT-PCR (D) demonstrating expression of stem cell markers, quantified as molecules of RNA of the indicated gene per molecules of RNA of the housekeeping gene, Cyclophilin. H1, H9, HES2= 3 control lines of human embryonic stem cells. CL=clone #. DF508= ΔF508 homozygous cystic fibrosis iPSC clones. E) Southern blot of BamHI digested gDNA demonstrating 18 of 20 individual iPSC clones (made from 4 individuals with either inherited AAT deficiency or CF) have been reprogrammed with a single integrated vector copy. Arrowheads indicate lanes with clones reprogrammed with 2 vector copies as well as a known 2 copy clone (far right lane). Blot has been probed against WPRE sequence of the lentiviral vector backbone. hiPS=human induced pluripotent stem cell. Clone # represents human volunteer (100–102) followed by clone number after hyphen (e.g. 100-2). PiZZ=homozygous Z alleles of the alpha-1 antitrypsin (AAT) protease inhibitor (Pi). Bars=200 μm.
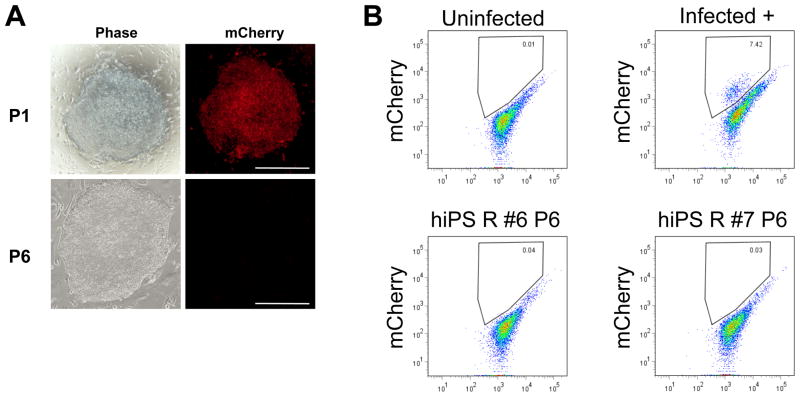
We have previously reported the use in mice of a modified version of the floxed STEMCCA vector where cMyc is replaced with the red fluorescent reporter mCherry (STEMCCA-RedLight-loxP), allowing monitoring in real time of Cre/loxP-mediated vector excision during the derivation of transgene-free murine iPSC. Because the transgene cMYC has also been shown to be dispensable for reprogramming human cells26, we generated a humanized version of the STEMCCA-RedLight-loxP vector by similarly replacing cMYC with mCherry in the hSTEMCCA vector. As in previous reports, the absence of cMYC diminished the overall efficiency of reprogramming and extended the time required to detect reprogrammed colonies to at least 6 weeks post infection (data not shown). We found 3 factor reprogramming with this approach to be unreliable, however, as dermal fibroblasts obtained from two different adults failed to produce any colonies even after 8 weeks post infection with hSTEMCCA-RedLight-loxP despite >90% transduction efficiency monitored by mCherry transduction. In contrast, the same fibroblasts from these 2 individuals yielded >100 colonies within 4 weeks of reprogramming with the 4 factor hSTEMCCA-loxP vector. Consequently, we used a known GSK3 inhibitor (BIO) that has been suggested to enhance the efficiency of reprogramming27. Indeed, the presence of BIO allowed for the generation of iPSC using the hSTEMCCA-RedLight-loxP vector with an efficiency of approximately 0.01%. However, we noted the mCherry fluorochrome, which was easily visible during the first 3 weeks of reprogramming, became undetectable by fluorescence microscopy in 7 out of 7 picked iPSC clones, within two passages (Fig. 2A). Absence of mCherry expression following reprogramming of human cells was confirmed by FACS, suggesting some degree of silencing of the lentiviral vector (Fig. 2B), a result that sharply contrasted with our previous observation that the STEMCCA-RedLight-loxP vector is not silenced in mouse iPSC22. As a result of the observation that lentiviral silencing followed human reprogramming, the application of the mCherry-containing vector to visually monitor vector cre-excision in human cells was not possible. Thus, in all subsequent studies we elected to employ only the 4 factor hSTEMCCA-loxP vector.
Figure 2. Human 3 factor reprogramming with hSTEMCCA-RedLight-loxP vector.
A) Flourescence microscopy shows mCherry fluorescence in an early passage (P1) iPSC clone, which becomes undetectable in later passages (P6). B) Flow cytometry of uninfected control hESC as well as hESC 72 hours after hSTEMCCA-RedLight-loxP lentiviral infection (infected+), compared to two individual late passage (P6) iPSC clones (hiPS R #6 and #7) showing absence of detectable mCherry gene expression. Bars=200 μm.
Generation of human lung disease specific iPSC
We sought to apply our reprogramming vector for the generation of disease-specific iPSC free of reprogramming transgenes. We chose to derive iPSC from individuals with a variety of lung diseases, since there is a significant lack of conventional therapies or human model systems for many inherited or degenerative lung diseases. Pluripotent stem cells or their differentiated progeny have been proposed as attractive candidates for reconstituting injured lung tissues in vivo or modeling lung disease pathogenesis in vitro28, however, to date no lung-disease specific iPSC have been available. Hence we sought to reprogram fibroblasts taken from humans with diseases affecting the three broad cell lineages of the adult lung: lung epithelium, endothelium, and interstitium. Fibroblasts were obtained from humans with either of the two most common inherited lung diseases: cystic fibrosis (CF; which affects the airway epithelium) or Alpha-1-Antitrypsin (AAT) deficiency-related emphysema (which affects the lung interstitium and epithelium29). In addition, we obtained fibroblasts from individuals with systemic diseases known to frequently affect the lung: sickle cell disease (which results in pulmonary endothelial injury and pulmonary arterial hypertension30) and systemic sclerosis (scleroderma or SSc; which frequently leads to pulmonary arterial hypertension as well as interstitial pneumonitis31). We focused on reprogramming dermal fibroblasts we obtained from 6mm skin punch biopsies taken from recruited volunteers with either inherited PiZZ phenotype AAT deficiency, CF (homozygous ΔF508 mutant CFTR genotype), or Ssc. In some cases we obtained additional banked, frozen CF or AAT deficient fibroblasts from the Coriell Cell Repositories. Using the same strategy detailed above, the hSTEMCCA-loxP vector allowed efficient generation of iPSC clones from all samples (Fig. 3A), regardless of the age of the individual from which the cells originated (Table I). As with normal HFF-derived iPSC, the disease-specific iPSC we generated expressed the full complement of stem cell marker genes by RT-PCR, qRT-PCR, and immunostaining (Fig. 3B, C and D). In addition, the majority of clones had been reprogrammed with a single integrated vector copy as evidenced by Southern blot (Fig. 3E), and G-banding analysis revealed a normal karyotype (Fig. 5E). The range of reprogramming efficiency in these experiments ranged from 0.1–1.5% without significant correlation of efficiency with age, gender, or disease type (Table I and data not shown). Finally to functionally assess pluripotency of the disease-specific iPS cells generated with this method, we sub-dermally transplanted three representative iPS cell lines into immunodeficient mice and found the cells gave rise to teratomas comprised of differentiated tissues characteristic of the three primary germ layers, ectoderm, mesoderm, and endoderm (Fig. 4).
Table 1.
Human iPSC lines derived from somatic parental cell lines of varying disease types using one of two reprogramming vectors
| Disease Type | Parental Cell Line | Source | Sex | Age | Diseased Allele(s) | Vector | Number of iPSC Lines |
|---|---|---|---|---|---|---|---|
| Wild-type | HFF | ATCC, CRL2097 | M | Neonatal | N/A | EF1α-hSTEMCCA-loxP | 8 |
| EF1α-hSTEMCCA-RedLight-loxP | 11 | ||||||
| Emphysema (AAT deficient) | Dermal Fibroblast | Patient biopsies (4) | F | 64 yo | PiZZ | EF1α-hSTEMCCA-loxP | 15 |
| 61 yo | 15 | ||||||
| 57 yo | 15 | ||||||
| 47 yo | 20 | ||||||
| Liver Fibroblast | Coriell, GM11423 | M | 16 wk | 9 | |||
| Cystic Fibrosis | Fibroblast | Coriell, GM1012A | M | 8yo | Homozygous ΔF508 | EF1α-hSTEMCCA-loxP | 15 |
| Dermal Fibroblast | Patient biopsies (4) | F | 21 yo | 10 | |||
| F | 31 yo | 10 | |||||
| M | 33 yo | 4 | |||||
| M | 29 yo | 2 | |||||
| Scleroderma | Dermal Fibroblast | Patient biopsy | F | 47 yo | Unknown | EF1α-hSTEMCCA-loxP | 3 |
| Sickle cell | Fibroblast | Coriell, GM02340 | F | 20 yo | SS | EF1α-hSTEMCCA-loxP | 26 |
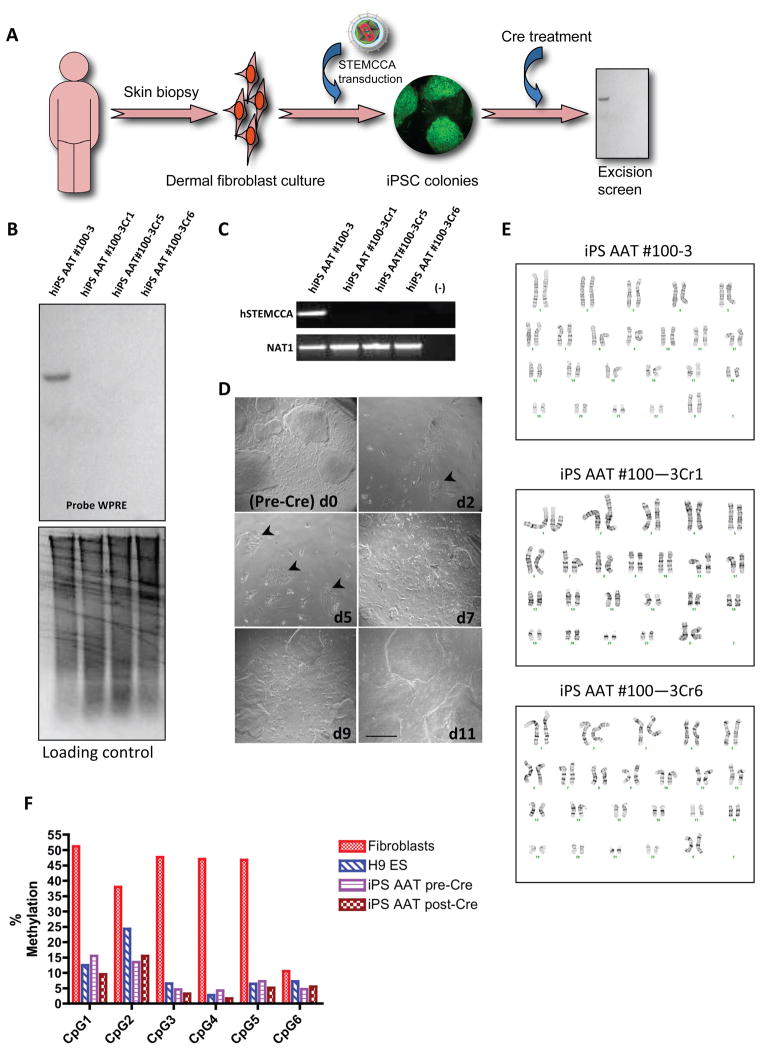
Figure 5. Cre-mediated excision of hSTEMCCA-loxP to generate transgene-free lung disease-specific iPSC.
A) Schematic summarizing experimental approach for generating transgene-free iPSC. B) Southern blot of BamHI digested gDNA probed against the lentiviral WPRE fragment (top), demonstrating a representative parental iPSC line (AAT #100-3) and successful Cre-mediated excision of the single copy hSTEMCCA vector in 3 iPSC subclones (AAT #100-3-Cr1, -Cr5, and –Cr6). Equal DNA loading in each lane is evident by ethidium bromide (bottom) staining of the gel prior to transfer. C) PCR of gDNA confirming vector excision of the clones shown in B. D) Micrographs of iPSC showing colony morphologies after transfection of Cre-IRES-PuroR plasmid and 48 hours of antibiotic selection of excised colonies, at different time points after selection. Arrowheads denote emerging iPSC colonies. E) Karyotypic stability (46XX) by G-banding analysis of iPSC clone AAT #100-3 both before Cre-mediated vector excision, as well as in the subclones after vector excision. F) Pyrosequencing analysis of bisulfite-treated gDNA provides quantitation of methylation of the human NANOG promoter across 6 CpG islands in dermal fibroblasts prior to reprogramming, as well as in the AAT iPSC clone before and after Cre-mediated excision (pre-Cre vs. post-Cre) of the hSTEMCCA vector. H9 ES=control human embryonic stem cells. Bar=200 μm.
Figure 4. Functional in vivo pluripotency of disease-specific human iPSC assessed by teratoma formation assay.
Transplantation of a representative cystic fibrosis-specific iPSC (clone 202) into immunodeficient mice gave rise to a teratoma containing differentiated lineages representing the three primary germ layers, mesoderm, ectoderm, and endoderm. A) low power magnification (4×) of hematoxylin and eosin-stained tissue section of teratoma, illustrating formation of multiple glandular and cystic structures. B) Mesodermal differentiation demonstrated by bone formation (Bn) and ectoderm differentiation demonstrated by pigmented neuroepithelium (arrow), 40× magnification. C) Cartilage formation (*) indicative of mesodermal differentiation. D, E) glandular (endodermal) epithelium. F) pigmented ectodermal differentiation reminiscent of retinal pigmented epithelium (arrow). Bars= 200 μm (A), 100 μm (B,C,E,F), and 50 μm (D).
Generation of transgene-free human iPSC by Cre/loxP mediated excision
Next, we sought to excise the single vector copy in order to generate lung-disease specific iPSC free of reprogramming transgenes. We have previously shown that constitutive expression of reprogramming transgenes interferes with mouse iPSC differentiation into lineages of all three primary germ layers22. Moreover, aberrant expression of some or all of the reprogramming factors could lead to tumorigenesis2 in vivo and may affect global gene expression18. For these reasons, we aimed at generating iPSC free of reprogramming transgenes based on Cre/loxP-mediated excision of the reprogramming cassette (See schematic Fig. 5A). During normal lentiviral reverse transcription, the loxP site present in the deleted U3 region of the vector LTR is duplicated to the 5′ LTR, resulting in a floxed version of the hSTEMCCA (Fig. 1A). In order to achieve excision, we selected iPSC clones containing a single integration and performed transient transfection with a plasmid expressing Cre and a puromycin resistance gene (Cre-IRES-PuroR). The cells were then exposed to puromycin antibiotic selection for 48 hours, followed by a 1–2 week recovery period prior to picking any surviving colonies (Fig. 5D). Employing this method we were able to recover transgene free subclones within 2 weeks of transfection, as demonstrated by both PCR and Southern blotting (Fig. 5B, C). In three repeated experiments, 5 subclones were derived from each of 10 disease-specific iPSC lines (50 subclones total; CF and AAT deficient) following Cre exposure. Deletion of the hSTEMCCA vector was found in 100% of these screened subclones (Fig. 5 and Supplemental Fig. 1). In subsequent experiments, occasionally we found iPSC lines (n=2 to date) that were resistant to successful Cre-mediated excision for unclear reasons. Our inclusion in the Cre-IRES-PuroR plasmid of a WPRE sequence allowed us to exclude the presence of any residual integrated STEMCCA or Cre-IRES-PuroR events in the successfully excised clones, using a Southern blot probe able to detect this element common to both vectors (Fig. 5). To further exclude the possibility of integration of the Cre-IRES-PuroR plasmid in the excised clones, we demonstrated the return of puromycin susceptibility in all subclones two weeks after picking by re-exposure to puromycin antibiotic for 48 hours with resultant death of >99% of cells.
Following Cre-mediated excision, we assessed the self-renewal capacity, stability of epigenetic reprogramming, and karyotypic stability of the transgene free iPSC as follows: AAT deficient iPSC (pre- and post-vector excision) were maintained in continuous culture for 3 months (equivalent to passage 20) and karyotypes were evaluated by G-banding analyses (Fig. 5E). Even after this prolonged time in culture, the iPSC showed stability of normal karyotype, stable colony morphology, and stable expression of stem cell marker genes (SSEA4, TRA-1-81, and TRA-1-60 by FACS and immunostaining; SOX2, DNMT3B, OCT4, NANOG, and REX1 by quantitative RT-PCR; Fig. 5 and supplemental Fig. 2). As expected, quantitative RT-PCR evaluation of hSTEMCCA transgene mRNA expression revealed removal of all traces of transgene expression in all subclones after Cre-mediated excision of the hSTEMCCA vector (supplemental Fig. 3).
To assess stable reprogramming of the epigenetic state of the NANOG promoter in disease-specific iPS cells before vs. after vector excision, we quantified the degree of DNA methylation of 6 CpG islands in the NANOG promoter by pyrosequencing. We found 5 of 6 CpG islands evaluated were mostly methylated in dermal fibroblasts prior to reprogramming, but 6 of 6 islands were unmethylated in iPSC both before and after vector excision, similar to control H9 ESC (Fig. 5F).
Differentiation of iPSC into definitive endoderm, the developmental precursor lineage of lung and liver epithelia
The lung epithelium develops in the embryo from multipotent definitive endoderm progenitors of the anterior foregut32. Only recently have protocols been developed for the reliable derivation of definitive endoderm progenitors from ESC33–35. Deriving definitive endoderm from pluripotent stem cells generated from AAT deficient individuals is also a pre-requisite for obtaining endoderm-derived hepatic cells, an important goal since hepatocytes are the main secretors of circulating AAT protein in mammals. Hence, we attempted to direct the differentiation of our iPSC towards definitive endoderm, the direct precursor population of lung and liver epithelium. As shown in Figure 6A and B, using a previously described method for activating nodal/activin signaling33, 34, iPSC were differentiated in serum-free culture conditions into definitive endoderm cells expressing the endodermal transcriptional regulators, FOXA1, FOXA2, SOX17, and HNF4A, and the cell surface markers CXCR4 and CD117. Induction of endodermal markers was accompanied by expression of the known nodal target CER136 together with loss of expression of the pluripotent markers SSEA3 and OCT4 (Supplemental Fig. 4 and data not shown). To confirm directed differentiation into definitive endoderm rather than extra-embryonic/visceral endoderm we assessed expression levels of the early extra-embryonic endodermal marker, SOX7, and found no evidence of induction of this lineage. In contrast, SOX7 expression was robustly induced in negative control aliquots of cells differentiated in parallel in conditions designed to induce extra-embryonic endoderm (in the presence of BMP4 alone). The efficiency of definitive endodermal differentiation of the iPSC (70–80% by day 6; Fig. 6) was robust in disease-specific iPSC both before or after vector excision, a finding of particular importance if iPSC are to be used to generate endodermal progenitors for lung and liver disease modeling or future clinical use.
Figure 6. Directed differentiation of disease-specific iPSC into definitive endoderm after activin stimulation.
A) Representative undifferentiated iPSC clone from an individual with alpha-1 antitrypsin (AAT) deficiency, assessed both before (AAT pre-Cre) and after (AAT post-Cre) excision of the hSTEMCCA reprogramming vector. Left panel shows FACS dot plot analyses of SSEA4 and TRA 1-81 cell surface markers. Right panel shows FACS analysis after intracellular staining for the stem cell marker, OCT4 (black line) vs. isotype control staining (grey histogram). B and C) Directed differentiation of the iPSC clones shown in A, in cultures designed to induce definitive endoderm vs. extra-embryonic endoderm (ExE) over 6 days. FACS (B) was used to quantify induction of the definitive endodermal markers CXCR4, CD117 (C-KIT), and FOXA1. Grey histogram indicates undifferentiated cells on day 0 (d0). Quantitative RT-PCR (C) shows induction of endodermal transcriptional regulators FOXA2, SOX17, and HNF4A on day 6 (d6), or the extra-embryonic marker SOX7. Gene expression levels are represented as molecules of RNA of the indicated gene per molecules of RNA of the housekeeping gene, Cyclophilin.
DISCUSSION
Our results demonstrate a complete platform for reliably reprogramming disease-specific somatic cells from fresh or banked clinical samples into iPSC that are free of reprogramming transgenes. The reliability of the platform rests on the efficiency of the hSTEMCCA-loxP vector to obtain hundreds of putative iPSC clones from a single starting 35mm plate of human dermal fibroblasts. The vast majority of clones obtained with this method contain a single integrated vector copy, which can be excised to obtain transgene-free iPSC clones. We have illustrated how this method may be widely applied for clinical studies by recruiting volunteers and producing >100 of the first known iPSC relevant for the study of a variety of lung diseases (summarized in Table 1). At this point the entire process from skin biopsy to banking transgene-free iPSC requires approximately 3–4 months in a protocol that will need further optimization and high-throughput adaptation if it is to be properly applied for clinical trials or epidemiologic and genome wide association studies (GWAS) of disease.
Although several methods have been published previously for deriving iPSC free of any reprogramming transgenes, most methods to date have been employed either to reprogram only murine cells or to reprogram only neonatal normal human cells with an efficiency too low to ensure reliable reprogramming of valuable clinical disease-specific samples. In contrast we show that similar to our original description in the mouse system, we can consistently and reliably reprogram human somatic cells with high efficiency by using a single polycistronic excisable lentivirus expressing the four transcription factors, OCT4, KLF4, SOX2 and cMYC. The high efficiency of reprogramming likely derives in part from favorable stoichiometry of the four reprogramming transcription factors when expressed in this particular order by a polycistronic system, as suggested by previous publications14. More importantly, reprogramming with our system can be achieved regardless of the age or disease state of the donor individual. We believe that these are important practical considerations that will have a significant impact on the use of this technology in the clinical arena.
Several teams have noted that vector-based reprogramming is often ‘incomplete’ resulting in a significant number of ‘partially reprogrammed’ iPSC clones37, 38, which can be distinguished from fully reprogrammed clones on the basis of marker gene expression37. We found all clones generated with the hSTEMCCA-loxP vector expressed a broad complement of ‘stem cell markers’ including those previously reported to be absent in ‘partially reprogrammed’ clones, such as TRA-1-60, REX1 and DNMT3B37. We speculate that the majority of those clones reported as partially reprogrammed in other studies may have arisen from cells that either did not receive all reprogramming factors or expressed the factors with stoichiometries or expression levels that did not allow for complete reprogramming. Since most clones generated with the hSTEMCCA-loxP vector received a single copy of all 4 reprogramming factors, partial reprogramming may be minimized, potentially explaining why our results contrast with those of prior studies using multi-vector approaches.
It is often claimed that iPSC will be utilized for regenerative medicine applications, either as in vitro models of disease, or as in vivo vehicles for gene or cell therapies. In order for this promise to be realized, iPSC will need to be generated from individuals with inherited or degenerative diseases and differentiated into the relevant cell lineages required to model or treat those diseases. While we predict there will be variability in the differentiation capacity of each iPSC clone generated by any method, we have demonstrated that iPSC derived with hSTEMCCA-loxP have robust potential to differentiate into definitive endoderm, the developmental precursor lineage of lung epithelium. In contrast to our previous studies in mouse iPSC, where excision of the STEMCCA vector clearly improved endodermal differentiation capacity21, human iPSC appeared to differentiate efficiently to endoderm, both before or after vector excision, as quantified by the percentage of cells expressing CXCR4, CD117, or FOXA1; however quantitative RT-PCR suggested improved expression of some endodermal marker genes following vector excision (Fig. 6B and C). Further assessment of the differentiation capacity of many more clones pre vs. post vector excision, however, will be required in order to assess whether any significant effect on differentiation potential occurs following excision of the reprogramming vector in human iPSC.
For the two most common inherited lung diseases, cystic fibrosis and AAT deficiency-related emphysema, disease-specific iPSC are particularly promising research tools. Both diseases result from single gene defects that typically cause misfolding of CFTR or AAT proteins in lung or liver epithelial cells, respectively. Primary cells from individuals with these diseases are difficult to expand in culture for studies of disease pathogenesis, drug therapy, or targeted correction of their diseased loci. Hence the prospect of deriving an inexhaustible supply of disease-specific lung and liver epithelia from iPSC is attractive for modeling or treating these diseases.
It should be emphasized that clinical application of iPSC for therapy will need to take into account the benefit to risk ratio of employing this novel stem cell population with known potential to differentiate into any cell type as well as capacity for tumorigenesis in vivo. While we have minimized tumorigenic risk by removing exogenous transgenes, it should be noted, however, that ~200 bp of an inactive viral LTR remains in the host genome after excision, and hence the theoretical risk of insertional mutagenesis that may arise from genomically integrated exogenous DNA is not completely eliminated. While the statistical probability is minimal that a single integration event can induce gene disruption and dysregulation, this minimal risk could be further reduced in the future by targeting the STEMCCA into a safe genomic locus, as has been described39. Alternatively, improvement in the efficiency of reported reprogramming methods that do not modify the host genome would allow the future derivation of clinically-relevant disease-specific iPSC while avoiding the need for integrating sequences.
Supplementary Material
Acknowledgments
While this manuscript was under review Dr. Marie-France Demierre, one of the authors, died suddenly and unexpectedly at the age of 43. This work is dedicated to her memory. Dr. Demierre, a Professor of Dermatology at Boston University School of Medicine and Director of the Skin Oncology Program in Dermatology at Boston Medical Center, leaves a legacy of research publications, trainees, and patients who continue to benefit from her life’s work in academic medicine and dermatologic oncology.
The authors wish to thank Drs. Maria Trojanowska and Andrea Bujor for assistance with dermal fibroblast culture techniques, Dr. Laertis Ikonomou and Dr. Gary Stein of the University of Massachusetts Human Stem Cell Bank for fruitful discussions, and Dr. Andrew Wilson for assistance with volunteer recruitment. DNK and GM are supported by NIH PO1 HL047049-16A1, 1RC2HL101535-01, and 1R01 HL095993-01, a grant from the Cystic Fibrosis Foundation, and an ARC award from the Evans Center for Interdisciplinary Research at Boston University.
Footnotes
Author Contributions
AS, JCJ, CAS, CCF, JAM, and LY: collection and assembly of data, data analysis and interpretation; AO, AGS, and BWS: collection and assembly of data; RL, DJW, MFD: supply reagents; DLF, PG: conception and design, data analysis and interpretation; GJM: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; GM and DNK: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
The authors have no conflicts of interest to declare.
References
- 1.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 5.Staerk J, Dawlaty MM, Gao Q, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 7.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 10.Novak A, Shtrichman R, Germanguz I, Segev H, Zeevi-Levin N, Fishman B, Mandel Y, Daniel L, Kotton DN, Mostoslavsky G, Binah O, Itskovitz-Eldor J. Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicle using a single excisable lentivirus. Stem Cells. 2010 doi: 10.1089/cell.2010.0027. [DOI] [PubMed] [Google Scholar]
- 11.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 12.Han J, Yuan P, Yang H, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 15.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heng JC, Feng B, Han J, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6:167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Kaji K, Norrby K, Paca A, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soldner F, Hockemeyer D, Beard C, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Sommer CA, Stadtfeld M, Murphy GJ, et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer CA, Sommer AG, Longmire TA, et al. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells. 2010;28:64–74. doi: 10.1002/stem.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nostro MC, Cheng X, Keller GM, et al. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gadue P, Huber TL, Paddison PJ, et al. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusa K, Rad R, Takeda J, et al. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 27.Silva J, Barrandon O, Nichols J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss DJ, Kolls JK, Ortiz LA, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies JC, Alton EW. Airway gene therapy. Adv Genet. 2005;54:291–314. doi: 10.1016/S0065-2660(05)54012-4. [DOI] [PubMed] [Google Scholar]
- 30.Klings ES, Wyszynski DF, Nolan VG, et al. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173:1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch DA. Lung disease related to collagen vascular disease. J Thorac Imaging. 2009;24:299–309. doi: 10.1097/RTI.0b013e3181c1acec. [DOI] [PubMed] [Google Scholar]
- 32.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 33.Gouon-Evans V, Boussemart L, Gadue P, et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- 34.Kubo A, Shinozaki K, Shannon JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 35.D’Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M, Saijoh Y, Perea-Gomez A, et al. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- 37.Chan EM, Ratanasirintrawoot S, Park IH, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 38.Sridharan R, Tchieu J, Mason MJ, et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadtfeld M, Maherali N, Borkent M, et al. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods. 2010;7:53–55. doi: 10.1038/nmeth.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.