Abstract
The rewarding value of female sexual stimuli develops across puberty, as sexually-naïve adult, but not prepubertal, male hamsters show a conditioned place preference (CPP) for both vaginal secretions and a receptive female. Similarly, only adults show an endogenous testosterone surge when they encounter vaginal secretions. Testosterone by itself can condition a place preference in male rodents. Therefore, Experiment 1 assessed whether the endogenous testosterone surge elicited by vaginal secretions is necessary to show a CPP. Both gonad-intact and gonadectomized, testosterone-treated adult males showed a CPP for vaginal secretions, indicating that the rewarding value of this social cue is independent of an endogenous testosterone surge. However, organizational effects of pubertal testosterone could be necessary for adolescent development of social reward, as pubertal testosterone organizes adult-typical expression of sexual behavior. To investigate this possibility, in Experiment 2, sexually-naïve prepubertal and adult male hamsters were gonadectomized and received testosterone-filled capsules four weeks later. Testing began after two weeks of testosterone replacement. Adult males showed a CPP for both vaginal secretions and a receptive female, whether or not they experienced pubertal testosterone. Thus, the acquisition of positive valence of sexual stimuli is not organized by pubertal testosterone. Taken together, the ability of female sexual stimuli to serve as an unconditioned reward to adult male hamsters is independent of the chemosensory-induced endogenous testosterone surge and also organizational effects of pubertal testosterone. Instead, sexual reward may be dependent either on activational effects of testosterone or gonadal hormone-independent mechanisms.
Keywords: conditioned place preference, puberty, social reward, pheromone, vaginal secretions, testosterone, sexual behavior
Introduction
Maturation of social information processing is a universal feature of mammalian adolescence that maximizes the probability of appropriate and successful social interactions in adulthood (Dodge, 1993, Nelson et al., 2005). This phenomenon is exemplified in male Syrian hamsters by the adolescent maturation of behavioral responses to vaginal secretions, which contain female pheromones required for successful mating (Murphy and Schneider, 1970). For example, sexually naïve adult male hamsters show an unconditioned attraction to vaginal secretions that is not seen in prepubertal males (Johnston and Coplin, 1979). In addition, sexually naïve adult male hamsters show a conditioned place preference (CPP) to both vaginal secretions alone and sexual interactions with a female, whereas prepubertal males do not show a CPP to vaginal secretions, indicating that this chemosensory stimulus is unconditionally rewarding to adult, but not prepubertal, males (Bell et al., 2010, unpublished data). Thus, neural processing of vaginal secretions changes over the course of pubertal development such that they acquire positive valence, even without sexual experience. The mechanisms underlying this change in social reward are unknown.
One possible mediator is testosterone, which is produced by adult but not prepubertal males. Indeed, vaginal secretions induce a surge of testosterone within 30–60 minutes of exposure in sexually naïve adult male hamsters, but this neuroendocrine response does not occur in prepubertal males (Macrides et al., 1974, Pfeiffer and Johnston, 1992, Romeo et al., 1998). Testosterone is intrinsically rewarding to adult male hamsters and rats (Alexander et al., 1994, Packard et al., 1997, Wood, 2004, Wood et al., 2004), and one intriguing possibility is that the rewarding value of female chemosensory stimuli is mediated by the endogenous rise of testosterone elicited by them. If so, then the absence of this surge in prepubertal males could explain their inability to form a CPP to this social stimulus.
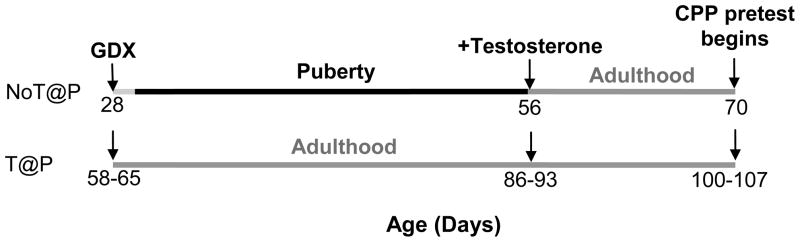
Alternatively, elevated testosterone during puberty may organize the neural circuitry responsible for evaluating the social relevance of vaginal secretions and sexual interactions with a receptive female. Using an experimental model that can distinguish organizational from activational effects of pubertal testosterone, we have shown that during puberty, testosterone organizes neural circuits underlying male sexual behavior (Schulz et al., 2004, Schulz and Sisk, 2006). In this model, male hamsters are deprived of testicular hormones, either during the normal time of puberty (via castration before 28 days of age; NoT@P), or for an equivalent amount of time in adulthood (via castration after 56 days of age; T@P), and testosterone is replaced in adulthood. Although adult testosterone treatment is sufficient to activate sexual behavior in NoT@P males, these animals still show aberrant patterns in the expression of sexual behavior, such as high levels of ectopic mounts even after sexual experience and low levels of intromissions, mounts, and ejaculations relative to T@P males (Schulz et al., 2004, Schulz and Sisk, 2006). In addition, testosterone replacement restricted to the normal period of puberty is sufficient to normalize adult sexual behavior in males gonadectomized at postnatal day 10 (Schulz et al., 2009). Thus, pubertal testosterone (or a metabolite) organizes the adolescent brain to enhance behavioral enactment in adulthood. Because NoT@P males do mate with females, although not proficiently, we infer that the social information contained in vaginal secretions is appropriately processed, at least to the extent that male sexual behavior is activated. However, the behavioral deficits observed in NoT@P males could be related to deficits in sexual reward.
The present studies were therefore designed to investigate two potential roles of endogenous testosterone in mediating social reward in adulthood. Experiment 1 determined whether the acute testosterone surge induced by vaginal secretions is necessary for adult males to show a CPP, and Experiment 2 determined whether the ability of adult males to show a CPP to vaginal secretions or a receptive female is the result of organizational effects of pubertal testosterone.
Methods
Animals
Sexually naïve male Syrian hamsters were ordered from Harlan Sprague-Dawley Laboratories (Madison, WI) and individually housed upon arrival in clear polycarbonate cages (30.5 × 10.2× 20.3 cm) with ad libitum access to food and water in a 14:10 light/dark cycle (lights out at 1300 h). Sixty ovariectomized (OVX) adult female Syrian hamsters were used as stimulus animals. All animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals and protocols were approved by the Michigan State University Institutional Animal Care and Use Committee.
Animal treatments for Experiment 1: Is the vaginal secretion-induced testosterone surge necessary for adult males to show a CPP?
Thirty-one adult male hamsters (56–70 days old; P56–70) were used in this experiment. Ten males were gonadectomized (GDX) and given two subcutaneous testosterone-filled capsules (13 mm and 5 mm of testosterone with 4 mm of sealing glue on both ends; inner diameter 1.98 mm; outer diameter 3.18 mm). These males all received pairings of the stimulus (vaginal secretions; stimulus-paired) with a specific chamber in a CPP paradigm (described below). The remaining 21 males were left gonad intact and were either stimulus-paired or controls (no stimulus pairings) in the CPP paradigm. For all hamsters, the CPP procedure began between P64–P78, one week after GDX and testosterone treatment in one group.
Animal treatments for Experiment 2: Is pubertal testosterone necessary for adult males to show a CPP for vaginal secretions or a receptive female?
Experiments 2a and 2b tested two different stimuli (vaginal secretions or receptive female, described below), but were of identical design (Figure 1). Specifically, 21 prepubertal (P28; NoT@P) and 21 adult (P58–65; T@P) male hamsters were GDX, while 22 young adult (P47–62) males remained gonad intact. Four weeks after GDX, the NoT@P and T@P males received two subcutaneous testosterone-filled capsules as described in Expt 1. After two weeks of testosterone replacement, or continued development in gonad-intact animals, the CPP procedure began. NoT@P and T@P males served as stimulus-paired subjects, while intact males served as no-stimulus-paired controls.
Figure 1.
Experimental Design. Prepubertal (P28; NoT@P) and adult (P58–63; T@P) male hamsters were gonadectomized (GDX) such that animals experienced adolescent development either without or with endogenous testosterone, respectively. Four weeks later in adulthood, all NoT@P and T@P males received testosterone-filled capsules two weeks before CPP testing began.
Stimulus Preparation
Behavioral receptivity was induced in 60 OVX females by an injection of estradiol benzoate (10 μg in 0.05 mL sesame oil, subcutaneous) and progesterone (500 μg in 0.1 mL sesame oil, subcutaneous) 52 hours and 4–5 hours, respectively, prior to use either for collection of vaginal secretions or as a stimulus female. For Experiment 1 and 2a, an hour before conditioning sessions began, vaginal secretions were collected from 30 hormonally primed females by vaginal palpation and mixed together to total approximately 500μl. For Experiment 2b, each female was tested for receptivity 30 minutes before conditioning sessions began by placing a non-experimental, sexually experienced male from our colony into her home-cage until she displayed lordosis, at which time the male was immediately removed. Only females who showed behavioral receptivity were used in conditioning sessions.
Conditioned Place Preference (CPP) Apparatus
CPP testing occurred in an apparatus with three distinct compartments (Med Associates, St. Albans, VT). The middle compartment (12×21×21 cm) was gray with a smooth Plexiglas floor and was connected to the two outer compartments (28×21×21 cm) by manually controlled sliding doors. One outer compartment was white, with metal grid flooring. Fresh pine pellets were placed in the waste pan beneath the floor before each conditioning session. The other outer compartment was black, with black scalloped solid Plexiglas flooring, and scented with a 2% glacial acetic acid solution swabbed along the top of the walls and ceiling before each conditioning session. Time spent in each compartment was recorded using MED-PC software connected to infrared photobeams spaced at 5-cm intervals along the bottom of the apparatus. Prior work with Syrian hamsters has demonstrated that CPP is successful both in the light and dark phases of the daily light/dark cycle, as long as training and testing occur at the same time across days (Ralph et al., 2002). We have found this to be the case in our laboratory as well [unpublished observations]. Therefore, in order to complete each experiment with just one cohort of animals, control animals underwent conditioning and testing under normal white light during the late phase of their light phase. All conditioning and tests with stimulus-paired animals were conducted under dim red light 1 hour into the dark phase. The Pretest (described below) for control hamsters occurred between 1000 and 1200 h and the Pretest for stimulus-paired hamsters occurred between 1400 and 1800 h. Testing and conditioning sessions were arranged so that for a single animal, sessions occurred at the same time each day +/− 40 minutes.
Pretest
An initial place preference test, here called the Pretest, was used to determine each hamster’s initial compartment preference. Following a 5-minute habituation period in the middle gray compartment, the doors were raised and the hamster was able to move freely throughout the apparatus for 15 minutes. The outer compartment in which the hamster spent the most time was defined as the initially preferred compartment. If a male did not enter both compartments at least 5 times during the Pretest, then he was excluded from the experiment.
Conditioning (adapted from Bell et al., 2010)
Following the Pretest, males received a series of conditioning sessions, with one session per day across consecutive days. No Stimulus (NoS) or Stimulus (S; vaginal secretions or receptive female) conditioning sessions took place on alternating days, beginning with the NoS conditioning session. NoS conditioning sessions were in the initially preferred compartment and S conditioning sessions were in the initially non-preferred compartment. Control animals were never exposed to the stimulus, and were placed in either the initially preferred or non-preferred compartment on alternating days. The control animals were used to confirm that preference scores do not change over the period of exposure to the two different compartments in the absence of conditioning. This biased assignment approach to CPP has been used previously for both sexual and drug rewards (Paredes and Alonso, 1997, Pierman et al., 2006, Dominguez-Salazar et al., 2008, Camacho et al., 2009, Meerts and Clark, 2009, Bell et al., 2010). The CPP apparatus was cleaned thoroughly with 25% ethanol following each Test and conditioning session between animals, and 75% ethanol after each S day.
Experiment 1 and 2a conditioning: CPP for vaginal secretions
Conditioning sessions were each 30 minutes long and held on 10 consecutive days. Of the 500 μl of vaginal secretions, approximately 15 μl were applied to water-moistened cotton gauze packed into a 2-ml Eppendorf tube, one tube for each male, and the remaining ~200 μl were mixed with 1.5 ml of mineral oil. Stimulus-paired males were removed from their home cages and a metal spatula was used to apply approximately 50 μl of either blank (NoS) or vaginal secretion-containing (S) mineral oil directly onto their noses immediately before they were placed into the initially preferred or non-preferred compartment, respectively. Either a clean or vaginal secretion-containing Eppendorf tube was taped to the top of the back wall of each respective compartment, out of reach of the male. The purpose of the two modes of vaginal secretions delivery was to ensure exposure to both volatile and non-volatile components, as both are important and potentially have different roles in male sexual behavior (as discussed in Bell et al., 2010). Control animals received blank oil and empty tubes on all sessions.
Experiment 2b conditioning: CPP for receptive female
Conditioning sessions were each 20 minutes long and held on 6 consecutive days. Stimulus-paired males were placed alone (NoS) or with a receptive female (S) into the initially preferred or non-preferred compartment, respectively. Males were paired with a different stimulus female for each S conditioning session. Behavior was observed to ensure that all males mated with the females throughout the S conditioning sessions, but was not quantified due to visibility limitations inherent in CPP apparatus design. Control animals were alone on all sessions.
Tests for CPP
Twenty-four hours after the last conditioning session, males were tested for their place preference following the same procedure used for the Pretest (Test 1). Two weeks later, without further training, all males were tested again (Test 2). Test 2 was used to determine if the males would maintain the stimulus-induced CPP for up to 2 weeks (Experiment 1 and 2), and if pubertal testosterone played a role in the maintenance (Experiment 2).
Plasma testosterone concentration
Twenty-four hours after Test 1, the males were put under isoflurane anesthesia and blood was collected via survival cardiac puncture. Twenty-four hours after Test 2, the males were given an overdose of sodium pentobarbital (150 mg/kg) and blood was collected via terminal cardiac puncture. Plasma testosterone concentrations were determined by radioimmunoassay. Duplicate 50-μl samples were analyzed within a single assay per experiment using the Coat-A-Count Total testosterone Kit (Diagnostic Products, Los Angeles, CA). For Experiment 1, the minimum detectable concentration for the assay was 0.1 ng/ml of testosterone and the intra-assay coefficient of variance was 4.1%. For Experiment 2, the minimum detectable concentration for the assay was 0.1 ng/ml of testosterone and the intra-assay coefficient of variance was 7.6%.
Statistical Analysis (adapted from (Bell et al., 2010))
To assess whether the stimuli induced a CPP, data from the Pretests and Tests were used to calculate a preference score, defined as time in the stimulus-paired compartment/(time in stimulus-paired compartment + time in no-stimulus compartment), and a difference score, defined as the time in the no-stimulus compartment–time in the stimulus-paired compartment. A repeated-measures ANOVA using a Geisser-Greenhouse correction was used to determine if there was a significant change in preference and difference scores between Pretest, Test 1, and Test 2 within each group of males with the alpha level set at p < 0.05. If a significant difference was revealed by the ANOVA within a specific group, post-hoc paired t-tests were used to evaluate the change in preference and difference scores between the Pretest and Tests within that group with the alpha level set at p < 0.05. The group sample sizes varied between and within experiments due to animals either not meeting the Pretest criteria (n = 3), loss of testosterone capsule (n = 3), or death from unknown causes between Test 1 and 2 (n = 2). Thus, there were 7–11 males/group for Experiment 1, 9–12 males/group for Experiment 2a, and 10 males/group for Experiment 2b.
Results
Testosterone concentrations
All males used in Experiments 1 and 2 had circulating testosterone concentrations within normal adult male physiological range at both Test 1 and Test 2 (Table 1).
Table 1.
concetration of circulating plasma testosterone(ng/ml) per group of each experiment taken 24 hours after test 1and test 2
| Plasma Testosterone (ng/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Experiment 1
|
Experiment 2a
|
Experiment 2b
|
|||||||
| Control
|
Intact
|
GDX+T
|
Control
|
T@P
|
NoT@P
|
Control
|
T@P
|
NoT@P
|
|
| Test 1 | 2.37 ± 0.87 | 2.3 ± 0.75 | 2.77 ± 1.18 | 3.17 ± 0.43 | 2.26 ± 0.51 | 2.46 ± 0.65 | 2.52 ± 0.75 | 3.24 ± 1.38 | 2.86 ± 0.94 |
| Test 2 | 2.91 ± 0.92 | 1.85 ± 0.78 | 2.11 ± 0.75 | 2.29 ± 0.80 | 2.03 ± 0.53 | 2.47 ± 0.37 | 3.16 ± 1.50 | 2.26 ± 0.49 | 2.01 ± 0.49 |
Experiment 1: The vaginal secretion-induced testosterone surge is not necessary for adult males to show a CPP
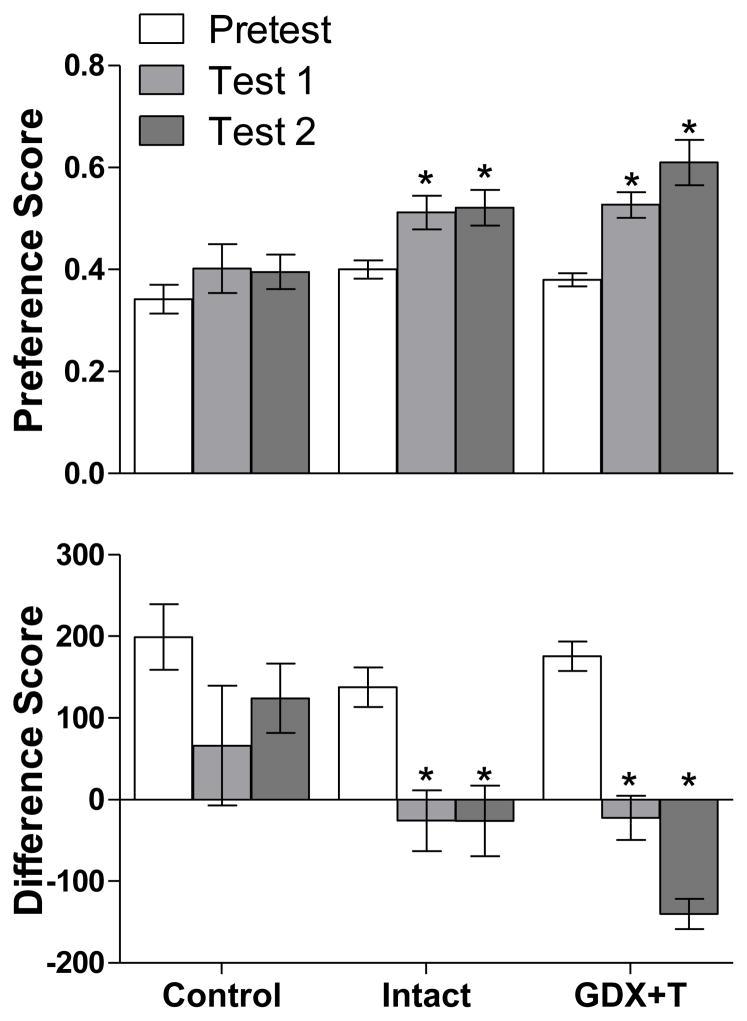
As shown in Figure 2, a repeated measures ANOVA revealed a significant change in preference and difference scores between tests for both intact [F(1, 9) = 5.11, p = 0.042; F(1, 10) = 7.43, p = 0.014, respectively] and GDX+T [F(1, 10) = 14.85, p = 0.002; F(1, 10) = 22.01, p = 0.001, respectively] males, but not controls [F(2, 19) = 0.83, p = 0.452; F(2, 19) = 1.27, p = 0.302, respectively]. Follow-up paired t-tests for intact and GDX+T males revealed a significant increase in preference score and decrease in difference score between Pretest and Test 1 for both intact [t(9) = −3.374, p = .008; t(9) = 3.987, p = .003, respectively] and GDX+T [t(8) = −6.872, p = .000; t(8) = 8.411, p = .000, respectively] males. Similarly, paired t-tests revealed a significant increase in preference score and decrease in difference score between Pretest and Test 2 for both intact [t(7) = −2.782, p = .027; t(7) = 3.168, p = .016, respectively] and GDX+T [t(7) = −5.040, p = .001; t(7) = 6.123, p = .000, respectively] males. There was no significant difference between Test 1 and Test 2 preference or difference scores within either group.
Figure 2.
Mean (±SEM) preference score and difference score on Pretest, Test 1, and Test 2 demonstrate that both Intact (n = 10 for Pretest and Test 1, n = 8 for Test 2) and GDX+T (n = 9 for Pretest and Test 1, n = 8 for Test 2) males showed a CPP for vaginal secretions. There was no difference in either score between Pretest, Test 1, and Test 2 for the intact, no-stimulus-paired controls (n = 11). *indicates a significant change in preference and difference score between the Pretest and Tests within a group, p < 0.05.
Experiment 2a: The presence of pubertal testosterone is not necessary for adult males to show a CPP for vaginal secretions
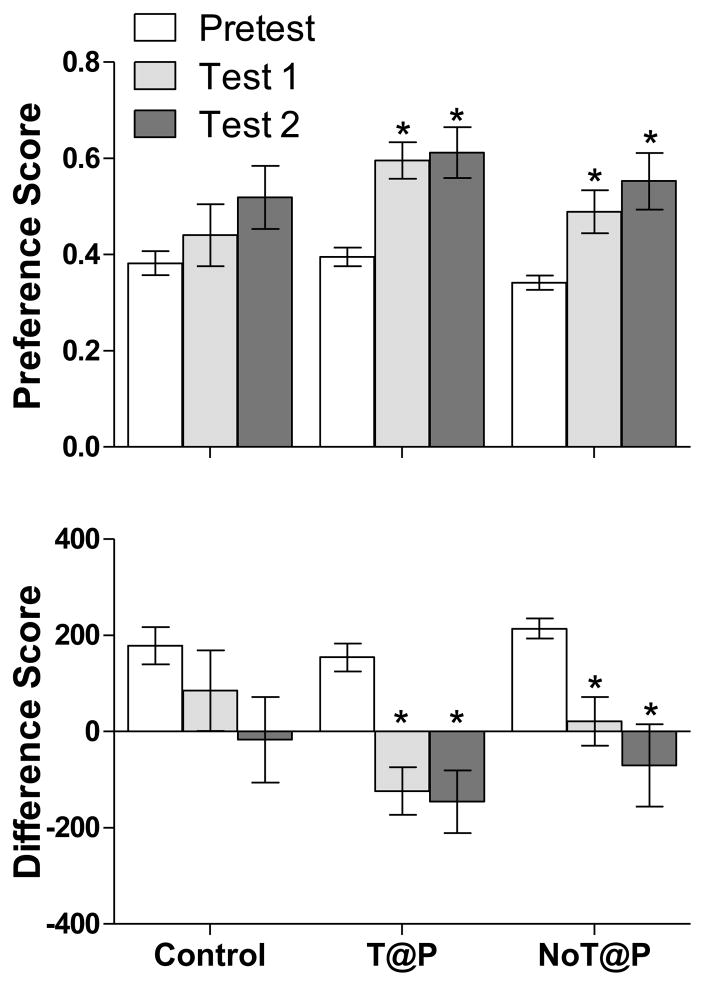
As shown in Figure 3, a repeated measures ANOVA revealed a significant change in preference and difference scores between tests for both T@P [F(2, 14) = 14.61, p = 0.001; F(2, 13) = 19.10, p = 0.000, respectively] and NoT@P [F(2, 18) = 9.30, p = 0.002; F(1, 15) = 7.83, p = 0.008, respectively] males, but not controls [F(2, 17) = 2.45, p = 0.124; F(2, 18) = 2.63, p = 0.108, respectively]. Follow-up paired t-tests for T@P and NoT@P males revealed a significant increase in preference score and decrease in difference score between Pretest and Test 1 for both T@P [t(8) = −4.653, p = .002; t(8) = 5.053, p = .001, respectively] and NoT@P [t(10) = −3.468, p = .006; t(10) = 3.598, p = .005, respectively] males. Similarly, paired t-tests revealed a significant increase in preference score and decrease in difference score between Pretest and Test 2 for both T@P [t(8) = −4.159, p = .001; t(8) = 4.623, p = .002, respectively] and NoT@P [t(10) = −3.674, p = .004; t(10) = 3.121, p = .011, respectively] males. There was no significant difference between Test 1 and Test 2 preference or difference scores within either group.
Figure 3.
Mean (±SEM) preference score and difference score on Pretest, Test 1, and Test 2 demonstrate that both T@P (n = 9) and NoT@P (n = 11) males showed a CPP for vaginal secretions. There was no difference in either score between Pretest, Test 1, and Test 2 for the intact, no-stimulus-paired controls (n = 12). *indicates a significant change in preference and difference score between the Pretest and Tests within a group, p < 0.05.
Experiment 2b: The presence of pubertal testosterone is not necessary for adult males to show a CPP for a receptive female
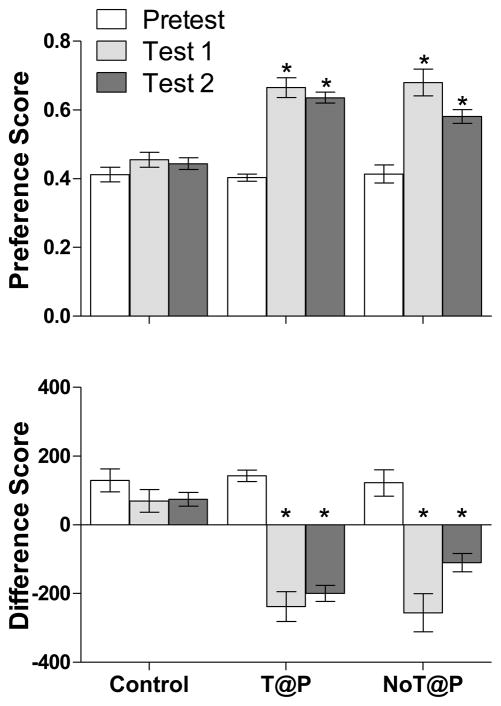
As shown in Figure 4, a repeated measures ANOVA revealed a significant change in preference and difference scores between tests for both T@P [F(1, 12) = 54.22, p = 0.000; F(1, 12) = 48.45, p = 0.000, respectively] and NoT@P [F(2, 17) = 17.20, p = 0.000; F(2, 17) = 17.44, p = 0.000, respectively] males, but not controls [F(2, 15) = 1.32, p = 0.290; F(2, 15) = 1.34, p = 0.287, respectively]. Follow-up paired t-tests for T@P and NoT@P males revealed a significant increase in preference score and decrease in difference score between Pretest and Test 1 for both T@P [t(9) = −7.287, p = .000; t(9) = 6.880, p = .000, respectively] and NoT@P [t(9) = −5.562, p = .000; t(9) = 5.496, p = .000, respectively] males, but not controls. Similarly, paired t-tests revealed a significant increase in preference score and decrease in difference score between Pretest and Test 2 for both T@P [t(9) = −11.747, p = .000; t(9) = 10.644, p = .000, respectively] and NoT@P males [t(9) = −4.011, p = .003; t(9) = 3.937, p = .003, respectively]. There was no significant difference between Test 1 and Test 2 preference or difference scores within either group.
Figure 4.
Mean (±SEM) preference score and difference score on Pretest, Test 1, and Test 2 demonstrate that both T@P (n = 10) and NoT@P (n = 10) males showed a CPP for a receptive female. There was no difference in either score between Pretest, Test 1, and Test 2 for the intact, no-stimulus-paired controls (n = 10). *indicates a significant change in preference and difference score between the Pretest and Tests within a group, p < 0.05.
Discussion
These experiments demonstrate that social reward derived from female sexual stimuli does not depend on either an endogenous surge of testosterone in response to the social sensory experience, or on organizational effects of pubertal testosterone. In Experiment 1, testosterone-treated gonadectomized adult male hamsters formed a CPP to vaginal secretions, even though they could not have elicited a testosterone surge in these males. Similarly, in Experiment 2, both NoT@P and T@P males formed a CPP to vaginal secretions and sexual interactions with a female, indicating that the presence of endogenous testosterone during puberty is not a requirement for the evaluation of vaginal secretions or sexual behavior as rewarding. Thus, it appears that adult social reward is not mediated by rewarding properties of testosterone per se, nor is adolescent maturation of social reward organized by testosterone.
Because testosterone is inherently rewarding (Wood, 2004, Wood et al., 2004), it seemed plausible that the testosterone surge induced by vaginal secretions could mediate the rewarding value associated with them, but this was not found to be the case. The stimulus-induced increase in testosterone is observed 30–60 minutes after the introduction of vaginal secretions (Macrides et al., 1974, Pfeiffer and Johnston, 1992, Romeo et al., 1998). Therefore, the time lapse between the chemosensory experience and the surge in testosterone may preclude a psychological association of vaginal secretions with testosterone reward. Male hamsters are dependent on neural processing of vaginal secretions in order to mate, and therefore it may be disadvantageous for the rewarding value of vaginal secretions to be tightly coupled with a physiological response (i.e. testosterone surge) that does not occur relatively soon after the chemosensory experience.
It is noteworthy that pubertal testosterone does not organize the acquisition of positive valence of female sexual stimuli, considering the importance of pubertal testosterone to the expression of adult-typical male sexual behavior (Schulz et al., 2004, Schulz and Sisk, 2006). NoT@P males show low levels of mounts, intromissions, and ejaculations, as well as consistently high numbers of ectopic mounts after sexual experience, compared to T@P males. These data, together with those from the present experiments, suggest that pubertal testosterone organizes the neural circuitry involving sexual proficiency, but not motivation to mate. Indeed, sexual motivation and sexual performance are regulated by different neural circuitries (as reviewed in Becker, 2009), and may be differentially influenced by pubertal hormones. The dichotomy between sexual motivation and sexual proficiency is paralleled in social information processing theory, which distinguishes the appropriate perception and interpretation of social stimuli from the appropriate enactment of a response through behavior (Dodge, 1993). Our results suggest that organizational effects of pubertal testosterone are necessary for proficient enactment of behavioral responses to vaginal secretions and a receptive female (i.e., sexual behavior), but not for the perception of vaginal secretions or sexual behavior as rewarding. The independence from organization by pubertal hormones of early stage enactment of sexual behavior may be beneficial in preventing potential hormonal disturbances during adolescence from completely abolishing reproductive success in adulthood.
To further investigate the involvement of testosterone in the perception of social reward, we tested the persistency of the positive valence assigned to female sexual stimuli. In both of the current experiments, all stimulus-paired males maintained a CPP for vaginal secretions and a receptive female after two weeks even with no further conditioning. These data indicate that associative learning about vaginal secretions or a receptive female persists for some time regardless of whether a testosterone surge or pubertal testosterone is present. Thus, while deficits in learning to modify copulatory behavior exist in NoT@P males, their atypical sexual behavior compared to T@P and intact males is not the result of insufficient long-term associations between sexual behavior and reward. The persistent and unconditioned reinforcing properties of vaginal secretions in sexually-naïve males demonstrate the strong saliency of these specific natural rewards, which are necessary for the expression of sexual behavior in male hamsters. Although maintenance of drug-induced CPPs have been demonstrated to last up to 12 weeks in rats (Mueller and Stewart, 2000, Mueller et al., 2002), this is the first known report of sexual stimuli-induced CPPs persisting for at least two weeks.
The adolescent acquisition of social reward may be dependent on activational effects of testosterone and/or a developmentally timed hormone-independent process, and this question remains to be investigated. However, it is currently unknown whether circulating testosterone is even necessary for adult male hamsters to find vaginal secretions rewarding. Indeed, it seems likely that it is necessary because castrated adult male hamsters do not mate with a receptive female or show attraction to vaginal secretions (Gregory et al., 1975, Wood and Newman, 1995). In addition, testosterone may be sufficient to activate a positive valence in prepubertal males. For example, prepubertal hamsters that are given adult levels of testosterone show attraction to vaginal secretions and increased anogenital investigation of a receptive female, although they do not mate with a receptive female (Johnston and Coplin, 1979, Meek et al., 1997, Schulz and Sisk, 2006). These data suggest that testosterone facilitates sexual/social reward via activational effects on reward circuitry, and this possibility will be the focus of future research.
In conclusion, the current study sought to investigate the role of endogenous testosterone in the differential behavioral responses to vaginal secretions and a receptive female between prepubertal and adult male hamsters. The data revealed that the positive valence associated with vaginal secretions in adults is independent of the chemosensory-evoked surge of testosterone in adulthood. Additionally, the ability of adult males to show a CPP to vaginal secretions and a receptive female does not depend on organizational effects of pubertal testosterone. These data, in conjunction with previous reports (Schulz et al., 2004, Schulz et al., 2006), demonstrate that adolescent maturation of social cognition involves both pubertal hormone–dependent and hormone independent mechanisms. This dichotomy provides insight into the role of pubertal hormones in the adolescent remodeling of neural circuitry underlying social behaviors.
Highlights.
Endogenous testosterone surge in adulthood is not required for sexual reward
Sexual reward does not depend on organizational effects of pubertal testosterone
Conditioned place preference for female sexual stimuli persists up to two weeks
Acknowledgments
This work was supported by National Institutes of Health grant R01-MH068764 to C.L.S. Many thanks to Margaret Mohr, Dr. Heather Molenda-Figueira, Dr. Sarah Meerts, Jane Venier, Rayson Figueira, Jennifer La, Genevieve Trombly, Dana Gradl, Allison Melkonian, Robyn Weston, and Alice Hoffman for their contributions to data collection and comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: implications for the biological basis of sexual motivation. Behavioral neuroscience. 1994;108:424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: a novel mechanism? Hormones and behavior. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Meerts SH, Sisk CL. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Hormones and behavior. 2010;58:410–414. doi: 10.1016/j.yhbeh.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho FJ, Portillo W, Quintero-Enriquez O, Paredes RG. Reward value of intromissions and morphine in male rats evaluated by conditioned place preference. Physiology & behavior. 2009;98:602–607. doi: 10.1016/j.physbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Dodge KA. Social-cognitive mechanisms in the development of conduct disorder and depression. Annual review of psychology. 1993;44:559–584. doi: 10.1146/annurev.ps.44.020193.003015. [DOI] [PubMed] [Google Scholar]
- Dominguez-Salazar E, Camacho FJ, Paredes RG. Perinatal inhibition of aromatization enhances the reward value of sex. Behavioral neuroscience. 2008;122:855–860. doi: 10.1037/0735-7044.122.4.855. [DOI] [PubMed] [Google Scholar]
- Gregory E, Engel K, Pfaff D. Male hamster preference for odors of female hamster vaginal discharges: studies of experiential and hormonal determinants. Journal of comparative and physiological psychology. 1975;89:442–446. doi: 10.1037/h0077043. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Coplin B. Development of responses to vaginal secretion and other substances in golden hamsters. Behavioral and neural biology. 1979;25:473–489. doi: 10.1016/s0163-1047(79)90242-5. [DOI] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Fernandez F, D’Angelo W. Effects of exposure to vaginal odor and receptive females on plasma testosterone in the male hamster. Neuroendocrinology. 1974;15:355–364. doi: 10.1159/000122326. [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Hormones and behavior. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Conditioned place preference for mating is preserved in rats with pelvic nerve transection. Behavioral neuroscience. 2009;123:539–546. doi: 10.1037/a0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behavioural brain research. 2002;136:389–397. doi: 10.1016/s0166-4328(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behavioural brain research. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science (New York, NY. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behavioral neuroscience. 1997;111:219–224. doi: 10.1037//0735-7044.111.1.219. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behavioral neuroscience. 1997;111:123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Socially stimulated androgen surges in male hamsters: the roles of vaginal secretions, behavioral interactions, and housing conditions. Hormones and behavior. 1992;26:283–293. doi: 10.1016/0018-506x(92)90048-z. [DOI] [PubMed] [Google Scholar]
- Pierman S, Tirelli E, Douhard Q, Baum MJ, Bakker J. Male aromatase knockout mice acquire a conditioned place preference for cocaine but not for contact with an estrous female. Behavioural brain research. 2006;174:64–69. doi: 10.1016/j.bbr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Ko CH, Antoniadis EA, Seco P, Irani F, Presta C, McDonald RJ. The significance of circadian phase for performance on a reward-based learning task in hamsters. Behavioural brain research. 2002;136:179–184. doi: 10.1016/s0166-4328(02)00131-6. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Parfitt DB, Richardson HN, Sisk CL. Pheromones elicit equivalent levels of Fos-immunoreactivity in prepubertal and adult male Syrian hamsters. Hormones and behavior. 1998;34:48–55. doi: 10.1006/hbeh.1998.1463. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Hormones and behavior. 2006;50:477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and behavior. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and cellular endocrinology. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiology & behavior. 2004;83:279–289. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology. 2004;171:298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J Neurosci. 1995;15:7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]