Abstract
In Con8 rat mammary epithelial tumor cells, indirect immunofluorescence revealed that Sgk (Serum- and Glucocorticoid-regulated kinase) and Erk/MAPK (Extracellular signal-Regulated protein kinase/Mitogen Activated protein kinase) co-localized to the nucleus in serum treated cells and to the cytoplasmic compartment in cells treated with the synthetic glucocorticoid dexamethasone. Moreover, the subcellular distribution of the importin-alpha nuclear transport protein was similarly regulated in a signal dependent manner. In vitro GST-pull down assays revealed the direct interaction of importin-alpha with either Sgk or Erk/MAPK, while RNA interference knockdown of importin-alpha expression disrupted the localization of both Sgk and Erk into the nucleus of serum-treated cells. Wild type or kinase dead forms of Sgk co-immunoprecipitated with Erk/MAPK from either serum- or dexamethasone-treated mammary tumor cells, suggesting the existence of a protein complex containing both kinases. In serum treated cells, nucleus residing Sgk and Erk/MAPK were both hyperphosphorylated, indicative of their active states, whereas, in dexamethasone treated cells Erk/MAPK, but not Sgk, was in its inactive hypophosphorylated state. Treatment with a MEK inhibitor, which inactivates Erk/MAPK, caused the relocalization of both Sgk and ERK to the cytoplasm. We therefore propose that the signal dependent co-localization of Sgk and Erk/MAPK mediated by importin-alpha represents a new pathway of signal integration between steroid and serum/growth factor regulated pathways.
Introduction
The coordinate regulation and intracellular communication between steroid receptor transcriptional signaling and tyrosine-kinase growth factor receptor activated phosphorylation cascades is critical for the physiological control of cellular function [1-10]. Glucocorticoids, one class of steroid hormones, elicit their responses through an intracellular steroid receptor complex that functions in the nucleus either as a potent ligand-activated transcriptional stimulator or repressor of gene expression [1, 9-16]. In glucocorticoid-responsive cells, convergence points between glucocorticoid receptor responsive pathways and signaling by growth factor receptor activated Erk/MAPK (Extracellular signal-regulated protein kinase/mitogen activated protein kinase) plays an important role in regulating cellular responses to both types of hormonal stimuli. Cross talk between these signaling pathways has been shown to occur at several distinct levels of cellular regulation [17]. One well characterized mechanism of signal integration is the targeting of expression and function of a select group of transcription factors, such as c-Jun and c-Fos, that are common down-stream components of both pathways [4, 5, 14, 18, 19]. These interactions can lead to either the potentiation or attenuation of gene expression depending on availability of tissue specific transcription factors and co-regulators as well as the presence of other extracellular stimuli.
Glucocorticoid receptors can be targeted directly by Erk/MAPK signaling. For example, Erk/MAPK has been shown to directly phosphorylate the glucocorticoid receptor [20-22] and the estrogen receptor [23, 24] at sites conserved between the two receptors, although the cellular function of this modification has yet to be established. Several studies have uncovered evidence that Erk/MAPK signaling can modify glucocorticoid receptor responsiveness, such as promoting glucocorticoid resistance in immune cells [25], as well as attenuating the glucocorticoid induced transactivation of gene expression in human epidermal keratinocytes [26]. In a complementary manner, in some systems glucocorticoid receptor signaling can regulate components of the growth factor-mediated signaling pathways that are upstream of Erk/MAPK and ultimately control Erk/MAPK activity [27-31]. For example, glucocorticoids alter the expression of a variety of growth factors and/or their cognate receptors in several different cell types [32, 33]. Glucocorticoids also attenuate the expression, localization or modification of certain intracellular cell signaling components [34-40] and have been shown, in one case, to cause the long-term down-regulation of Erk/MAPK transcripts [40]. Several studies have established that glucocorticoids can induce expression of MAPK phosphatase-1, a negative regulator of Erk/MAPK signaling [41].
To define additional layers of signal integration between glucocorticoid receptor signaling events and Erk/MAPK function, we have utilized the Con8 rat mammary epithelial tumor cell line [42, 43]. This cell line is derived from the hormone-responsive DMBA-induced 13762NF rat mammary adenocarcinoma [44]. Glucocorticoid hormones strongly suppress the growth of these mammary tumor cells by causing a G1 block in cell cycle progression [45] and induce certain differentiated properties, such as the formation of tight junctions [46-49]. In contrast, serum stimulation or exposure to transforming growth factor-alpha [32], which acts through epidermal growth factor (EGF) receptor-mediated phosphorylation cascades [50], induces cell cycle progression [46] and abrogates the glucocorticoid stimulation of tight junction formation [51]. The opposing proliferative effects of glucocorticoids and serum/growth factor stimulation suggest that the cellular utilization of specific glucocorticoid regulated gene products may be linked to Erk/MAPK.
One candidate glucocorticoid regulated gene is the serum and glucocorticoid-inducible protein kinase, Sgk, which we cloned from the Con8 rat mammary tumor cells as a novel transcriptionally-regulated serine/threonine protein kinase [52, 53]. Sgk is approximately 45-55% homologous to the catalytic domains of Akt/PKB, protein kinase A, protein kinase C-zeta and the rat p70S6K/p85S6K kinases (MW), and is enzymatically activated by the phosphoinositide 3-kinase pathway through PDK1-mediated phosphorylation [54]. We have documented that a biologically significant feature of Sgk is that its transcription, enzymatic activity and subcellular localization are regulated in stimulus-dependent and tissue-specific manner [52, 53, 55-58]. Transcription of the sgk gene is rapidly and strongly stimulated by either glucocorticoids or serum [52, 53], which is due to specific signal regulated elements in the promoter, such as a functional glucocorticoid response element [58]. In serum-stimulated cells, Sgk shuttles between the nucleus and cytoplasmic compartment in synchrony with the cell cycle; a hyper-phosphorylated form of Sgk is recruited to the nucleus of S and G2 phase cells [56]. In contrast, Sgk remains exclusively localized to the cytoplasmic compartment in glucocorticoid G1 arrested cells or after hyperosmotic stress [55, 56]. Analogous to Sgk, in several systems Erk 1 and Erk 2 have been shown to translocate to the nucleus in response to growth factor stimulation but can reside in the cytoplasm in certain quiescent cells [59-61]. In this study, we demonstrate that Sgk and Erk/MAPK co-localize to either the nucleus or the cytoplasmic compartment in a signal dependent manner that is mediated by their interactions with importin-alpha, a component of the nuclear transport machinery. We propose that the coordinate subcellular localization of Sgk and Erk/MAPK represents a new level of signal integration between glucocorticoid receptor and growth factor-dependent cell signaling events.
Materials and Methods
Cell culture conditions
Rodent Con8 mammary epithelial tumor cells were routinely cultured on standard tissue culture plates in DMEM/F-12 (Cambrex, Walkersville, MD) supplemented with 10% calf serum (Cambrex, Walkersville, MD) and penicillin/streptomycin (Cambrex, Walkersville, MD), and maintained at 37°C and 5% CO2 as described [42, 43, 56]. The cells were incubated with serum-free medium prior to their treatment with the indicated concentrations of serum and/or 1 μM dexamethasone and/or 1 mM hydroxyurea for the indicated times. The HEK-293 fibroblast cells were cultured in DMEM media supplemented with 10% fetal calf serum and antibiotics (Cambrex, Walkersville, MD).
Western blot analysis
Soluble whole cell extracts (20-50 μg protein) were electrophoretically resolved by SDS-PAGE, and the proteins were transferred to Nitran Plus membranes (Schleicher & Schuell, Keene, NH). The membrane was probed with a 1:5,000 dilution of affinity-purified polyclonal rabbit anti-Sgk antibodies, 1:500 dilution of monoclonal mouse anti-Erk1/Erk2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), 1:100 dilution of polyclonal rabbit anti-active MAPK antibodies (Promega, Madison, WI), 1:1000 dilution of polyclonal goat anti-importin-alpha antibodies (Santa Cruz Biotechnology), or 1:1000 dilution of polyclonal goat anti-actin antibodies (Santa Cruz Biotechnology) in 50 mM Tris, pH 8.0, 150 mM NaCl, 0.05% Tween 20 with 1% nonfat dry milk. The generation, purification, and characterization of the affinity-purified rabbit polyclonal antibodies to Sgk was described previously [56]. The secondary antibodies were goat anti-rabbit, goat anti-mouse, or rabbit anti-goat IgG HRP-conjugated antibodies (BioRad, Richmond, CA). The secondary antibodies were used at a dilution of 1:1,000. The western blots were developed by using the Renaissance developing kit (NEN, Boston, MA) and exposed to X-ray film.
Indirect immunofluorescence microscopy for Sgk and Erk/MAPK localization
Con8 cells were cultured on 8-well Lab-Tek Permanox slides (Nalgene Nunc International, Naperville, IL) or on sterile cover slides and grown to 30% confluency before the indicated combinations of serum and/or dexamethasone were added for the indicated time frame. Cell confluency prior to fixation did not exceed 60%. For indirect immunofluorescence microscopy, cells were washed with PBS, fixed for 15 minutes in 3.7% formaldehyde/0.1% glutaraldehyde, rinsed with PBS, and permeabilized with 50% methanol/50% acetone for 1 min. Following a rinse in PBS, the cells were preabsorbed for 5 minutes in PBS containing 4% normal goat serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Cells were incubated with a 1:300 dilution of affinity-purified rabbit polyclonal anti-Sgk antibody for 1-2 hours at 25°C. After 5 washes with PBS, the cells were treated for 5 minutes with PBS containing 4% normal goat serum. The cells were incubated with a 1:300 dilution of anti-rabbit FITC-conjugated secondary antibody (Cappel Research Products, Durham, NC) in PBS and then incubated for 30 minutes at 25°C. Cells were washed 5 times with PBS and mounted with 50% glycerol, 50 mM Tris (pH 8.0) containing 4 mg/ml n-propyl gallate, and examined on a Nikon Optiphot fluorescence microscope. Images were captured using Adobe Photoshop 3.0.5 (Adobe Systems, Inc., Mountain View, CA) and a Sony DKC-5000 digital camera. Nonspecific fluorescence was determined by incubation with the secondary antibody alone and shown to be negligible. Due to the use of an affinity-purified antibody, preimmune serum showed a relatively higher background and was not used as a negative control.
Double-labeling for Sgk and Erk1/MAPK localization utilized the above procedures with the following modifications. Erk 1 and Erk 2 were detected using a monoclonal antibody directed against amino acids 324-345 of Erk1 that also recognizes Erk2 (Zymed, San Francisco, CA). To recognize the Erk/MAPK monoclonal antibody (red fluorescent staining), a 1:300 dilution of an anti-mouse rhodamine-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was added in PBS and incubated at 25°C for 30 minutes.
Immunofluorescence microscopy for importin-alpha transfected cells
Con8 mammary epithelial cells were plated at low confluency (~30%) on 2-well Lab-Tek Permanox slides (Nalgene Nunc International, Naperville, IL) and transiently transfected with full length importin-alpha encoding construct (pCMV-HA-FLIa) using the lipofectamine procedure as detailed in the previous sections. Cells were serum starved for 36 hours and subsequently treated with 10% calf serum or with 1 μM dexamethasone for 15 hours. For indirect immunoflorescence microscopy, cells were washed with PBS, fixed for 20 minutes in 3.7% paraformaldehyde, rinsed in PBS, followed by permeabilization with methanol/acetone (1:1) for 2 minutes at −20°C. Cells were blocked in 5% non fat dry milk in PBS for 15 minutes, and incubated with affinity purified, anti-Sgk antibodies at 1:150 dilution, in combination with 1:1000 dilution of murine anti-HA monoclonal antibodies (Babco, Richmond, CA) for 1-2 hours at room temperature on a rocking platform. Slides were washed five times in PBS, blocked in 5% milk solution as mentioned above for 15 minutes, and then incubated with a 1:150 dilution of anti-rabbit florescein isothiocyanite-conjugated secondary antibody (Mol. Probes Inc., Eugene OR) and Texas red-conjugated goat anti-mouse secondary antibody (Mol.Probes Inc., Eugene OR), also at 1:150 dilution for 1 hour, at room temperature. Slides were washed three times in 5% milk solution, rinsed in PBS in the last wash and mounted with Vectashield mounting medium (Vector Laboratories Inc.), and cells viewed at 63× magnification with an oil objective lens using Zeiss Axiophot optics equipped with a camera and UV illumination through a fluorescein isothiocyanate (FITC) and texas-red filter respectively.
Generation of wild type, nuclear localization signal-containing, and kinase dead sgk expression vectors
The pcDNA3 mammalian expression vector (Invitrogen, Carlsbad, CA) with the full length Sgk cDNA was utilized for the ectoptic expression of Sgk in cell culture. Standard PCR techniques were used starting with the full length wild type rat sgk cDNA [53]. The final expression vector contains an Asp718/XbaI insert which consists of the full cDNA of sgk fused in frame to a poly-8-His tag at the C-terminus. To generate this chimeric construct with the full length sgk gene in-frame with the poly-His tag and with compatible restriction sites for the vector’s cloning sites, the two PCR primers used were 5′--AGGTACCGCCACCATGACCGTCA AAACCGAGGCTG--3′, which contains an Asp718 site and the first codons of the sgk sequence and 5′--TTCTAGATCAATGATGATGATGATGATGATGATGGAGGAAG GAGTCCATAGGAGGG--3′, which contains the XbaI site, the Stop-codon, eight His-residues and the final amino acids of the Sgk C-terminus.
The pcDNA3 NLS-sgk-His expression vector was generated by fusing the nuclear localization signal (NLS) of the SV40 large T antigen [62] in frame to the C-terminus of the full length sgk cDNA, followed in frame by eight His-residues. The PCR primers used were 5′--AGGTACCGCCACCATGACCGTCAAAACCGAGGCTG--3′, which contains an Asp718 site and the first codons of the sgk sequence and 5′--TTCTAGATCAATGATGATGATGATGATGATGATGGAACTTTACCTTCCTCTTCTT CTTTGGGAGGAAGGAGTCCATAGG--3′. This second primer contains a XbaI site, a stop-codon, eight His-residues, the nuclear localization signal (NLS), which is specified as the amino acid sequence FKVKRKKKP, and the last amino acids of the Sgk C-terminus. The PCR-generated inserts in both vectors were fully sequenced to confirm the fidelity of the PCR reactions and ligations.
To generate a kinase-dead mutant, a lysine-to-methionine mutation was introduced at K127, which is a critical lysine in the ATP binding site. For this purpose, the following primer was designed: 5′–TCTTCTGCAAAACCATGACGGCCATAGAA– 3′. The underlined residue harbors the point mutation that converted the wild type reverse codon AAA (lysine) into ATG (methionine). This primer was used with a 5′ bac primer, which was used to amplify the 5′ end of the vector cloning sites. The fragment was then used in a second round of PCR with a 3′ bac primer to amplify the 3′ end of the vector cloning sites. The full length kinase dead mutant was then digested with NcoI/BamHI for directional cloning into a NcoI/BamHI-digested pVL1392 His vector to insert sgk in-frame with C-terminal poly-His tag. This construct was digested with Bsu36I and inserted into Bsu36I-digested pcDNA3 expression vector.
Co-Immunoprecipitation Assays
Expression vectors encoding either wild-type sgk gene or the kinase dead sgk gene were transiently transfected into mammary tumor cells. Five hours post-transfection, cells were treated with combinations of 10% serum and/or 1μM dexamethasone. Twenty-four hours after transfection, the cells were harvested, and Erk1/Erk2 were immunoprecipitated from soluble cell extracts for 12 hours at 4°C using Protein G beads and monoclonal antibodies that recognize both Erk1 and Erk2 or actin antibodies as a control. The immunoprecipitates were washed with a gradually increasing concentration of 0.3-0.6 M KCl. The Erk1/Erk2 immune complex was resolved by SDS-PAGE, and the blotted proteins were probed with affinity-purified anti-Sgk antibodies at a final dilution of 1:1000, followed by incubation with HRP-conjugated goat anti-rabbit secondary antibodies at 1:1000 dilution.
Plasmid constructs for in vitro GST pull down assay
The expression plasmids encoding the full length epitope-tagged Sgk was constructed by standard PCR cloning methods. The sgk cDNA fragment containing a 5′ EcoR1 and 3′ Xho1 restrictions sites were subcloned into pCMV-5 vector designed to contain a N-terminal HA epitope tag. Expression plasmids for HA-erk2 and HA-Jnk proteins were kindly provided by G.S. Martin (Dept. of Biochemistry, U.C. Berkeley, Berkeley CA 94720) and J.S Gutkind (Molecular signaling unit, National Institute of Dental Research NIH, Bethesda, MD 20892) and have been described previously. The full length mouse importin-alpha cDNA subcloned into pGEX-3X (forming GST-FLIa) was a kind gift from Marian Waterman (Univ. of California, Irvine, CA). The truncated importin-alpha containing the carboxy terminal 106 amino acid (a.a. 423 –529) was subcloned into EcoR1/Xho1 sites within the GST vector pGEX-4T1 (Amersham Pharmacia Biotech) to yield the GST-TIa plasmid. The GST-importin-alpha fusion proteins were isolated from the bacterial strain AB1899 cells transformed with purified GST-FLIa and GST-TIa expression plasmids. Briefly, bacteria was initially grown at 37°C for two hours (O.D.=0.5-0.7) and subsequently induced with 0.1 mM IPTG (isopropyl1-thio-beta-D-galactpyranoside) for 6 hours at 37°C. Cells were lysed using the French Press (three times) in lysis buffer (PBS containing 0.05% Tween 2 mM EDTA, 1 mM DTT and 0.1% beta mercapto-ethanol). The GST-importin-alpha fusion proteins were purified on glutathoine agarose beads (Pharmacia) as we described previously [57].
In vitro GST-pull down assays in using cell extracts containing exogenously expressed HA-Sgk, HA-Erk2/MAPK and HA-Jnk proteins
Transient transfections containing 10 μg of relevant expression plasmid (HA-sgk, HA-erk2 and HA-Jnk) in combination with 10 μg of filler DNA were carried out in sub-confluent (~70%) HEK-293 fibroblast cells as previously described [63]. Control cells received empty vector alone (20 μg) corresponding to the respective transfected cDNAs. Forty-eight hours post-transfection, cells were lysed in HEGMN buffer (25 mM Hepes, 100 mM KCl, 12.5 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 0.1% Nonidet P-40, pH 7.9), centrifuged for 15 minutes at 14,000 × g, and supernatant fractions recovered. Recombinant GST-FLIa or GST-TIa immobilized on glutathoine-sepharose beads were incubated with the respective cell supernates expressing HA-Sgk, HA-Erk2/MAPK or HA-Jnk proteins, overnight at 4°C on a nutator. The beads were washed three times in the wash buffer (200 mM NaCl, 0.2% Tween 20, 10 mM Tris pH 7.5 and 0.5% non-fat dry milk). After removing the supernatant fractions in the final wash, the samples were resuspended in 25 μl of 2× SDS sample buffer, boiled for 5 minutes, and the proteins retained on the beads resolved by (7.8%) SDS-PAGE. Proteins were transferred to nitrocellulose membranes and probed for the presence of HA-tagged proteins using monoclonal anti-HA antibodies. The monoclonal anti-HA antibodies (MMS-101Rclone, Babco, Richmond, CA) were diluted 1:1000 in 1% non fat dry milk containing wash buffer and incubated overnight at 4°C. The goat anti-mouse secondary antibody, (Bio-Rad, Hercules CA) was used at a dilution 1:10,000 in 3% non fat dry milk containing wash buffer the Western blots were developed using the Renaissance developing kit (NEN, Boston MA) and exposed to X-ray film.
RNA interference
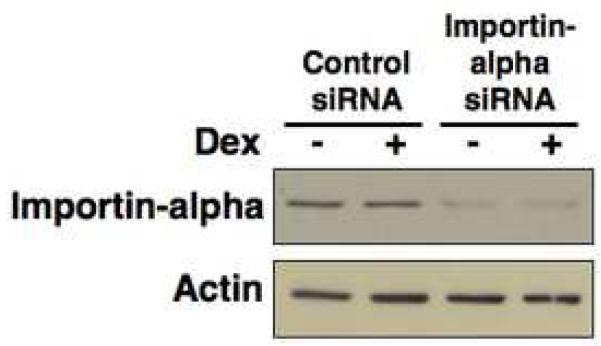
Con8 cells were reverse transfected with 150 ng of HP GenomeWide siRNA for importin-alpha or non-specific control siRNA with HiPerFect transfection reagent at the time of plating according to Qiagen’s protocol. Cells were treated with 1 uM dexamethasone for 24 hours the day after transfection. Western blots probing for importin-alpha and actin and indirect immunofluorescence microscopy for Sgk and Erk were performed as described above.
Results
Stimulus-regulated co-localization of Sgk and MAPK
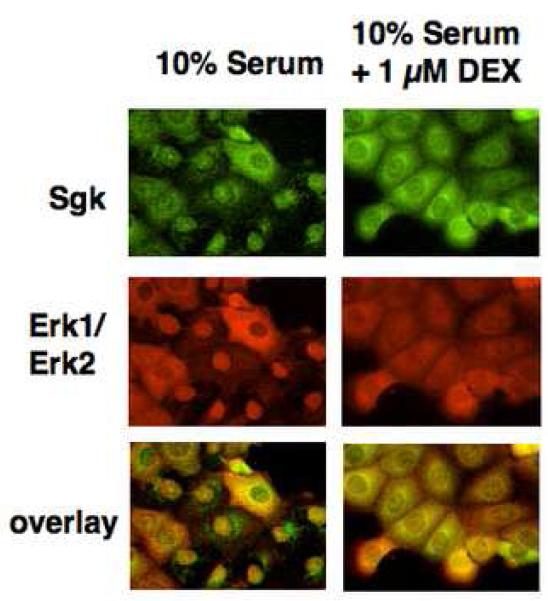
Indirect immunofluorescence microscopy was used to determine whether endogenous Sgk and Erk/MAPK co-localize to the nucleus and the cytoplasm in the same cell type in a signal dependent manner. Con8 mammary epithelial tumor cells were treated with 10% serum in the presence or absence of glucocorticoids for 24 hours, and the fixed cells co-stained for Sgk and Erk/MAPK using the corresponding primary antibodies and distinct secondary antibodies to examine their subcellular distribution. As shown in Fig. 1 (left panels), in the majority of serum treated cells both Sgk and Erk/MAPK co-localized primarily in the nucleus, whereas in the small subset of serum-treated cells that express Sgk in the cytoplasm, Erk/MAPK likewise distributes to this compartment. Strikingly, treatment with 1 μM dexamethasone, a synthetic glucocorticoid, caused both protein kinases to distribute to the cytoplasmic compartment (Fig. 1, upper and middle right panels). Erk/MAPK co-localized with Sgk in virtually every cell examined in both serum-treated and glucocorticoid-treated cells as shown by the orange color observed after merging the green (Sgk staining) and red (Erk/MAPK staining) fluorescence signals (see overlay in the bottom right panels of Fig. 1).
Fig. 1.
Effects of serum and glucocorticoids on the co-localization of Sgk and Erk/MAPK. Con8 mammary tumor cells were treated with 10% serum in the presence or absence of 1μM dexamethasone for 24 hours. The subcellular distribution of Sgk was examined by indirect immunofluorescence microscopy using affinity-purified rabbit polyclonal antibodies to Sgk followed by FITC-conjugated goat anti-rabbit secondary antibodies (green fluorescent staining). Erk1/Erk2 were detected using monoclonal antibodies that specifically recognized both MAPK family members followed by a rhodamine-conjugated anti-mouse secondary antibody employed to selectively visualize this protein kinase (red fluorescent staining). The orange color in the lower panels show the overlap of the green FITC staining and the red rhodamine signal.
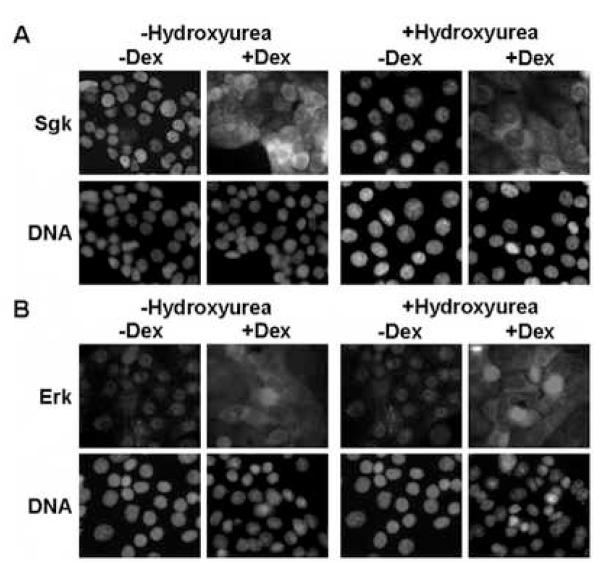
We previously established that treatment of Con8 mammary tumor cells with dexamethasone causes a G1 cell cycle arrest [45]. Therefore, it was important to distinguish whether the signal dependent subcellular distribution of Sgk and Erk was a specific response to glucocorticoids and serum, or whether the co-localization was an indirect consequence of the growth state of the cells. To examine this possibility, indirect immunofluorescence was used to characterize the signal dependent localization of Sgk and Erk in cells treated with or without 1 mM hydroxyurea, a DNA synthesis inhibitor that causes a G1 cell cycle arrest. As shown in Fig. 2, hydroxyurea had no effect on the nuclear co-localization of Sgk and Erk (Fig 2A and Fig 2B, left panels) in serum treated cells not incubate with dexamethasone (−Dex). Similarly, both Sgk and Erk resided in the cytoplasmic compartment in dexamethasone treated cells either in the presence or absence of hydroxyurea (Fig 2A and Fig 2B, right panels). These data demonstrate that the serum- and glucocorticoid-dependent subcellular distribution of Sgk and Erk is a specific signal dependent response.
Fig. 2.
Effects of hydroxyurea on the signal-dependent colocalization of Sgk and Erk/MAPK. Con8 cells were treated with 10% serum in the presence or absence of 1 mM hydroxyurea for 24 hours to induce a cell cycle arrest prior to treatment with 1 μM dexamethasone (Dex) for 24 hours. Panel A: The subcellular distribution of Sgk was examined by indirect immunofluorescence microscopy using affinity-purified rabbit polyclonal antibodies to Sgk followed by FITC-conjugated goat anti-rabbit secondary antibodies. Panel B: Erk1/Erk2 were detected using monoclonal antibodies that specifically recognized both MAPK family members followed by a rhodamine-conjugated anti-rabbit secondary antibody employed to selectively visualize this protein kinase. DNA was stained to visualize the nucleus using DAPI.
Stimulus-dependent subcellular distribution of Importin-alpha
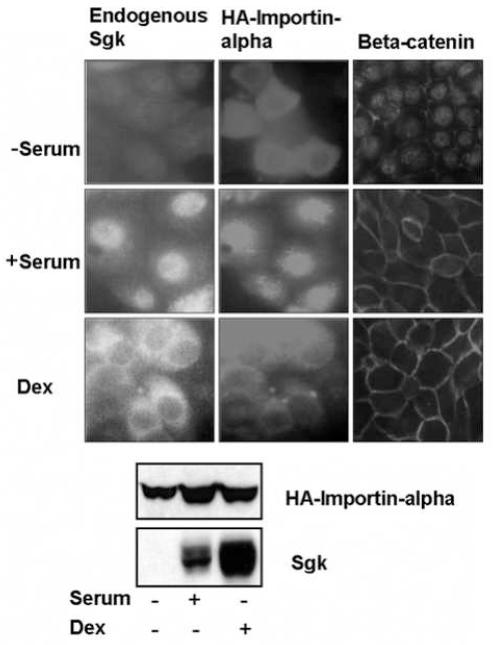
We have previously shown that Sgk directly binds to the importin-alpha nuclear import protein through a specific nuclear localization signal within Sgk [57]. Because the subcellular distribution of Sgk is regulated in a signal dependent manner, the specific interactions between Sgk and importin-alpha suggested that the nuclear/cytoplasmic localization of importin-alpha might be similarly regulated by serum and/or glucocorticoid treatment. Therefore, to test this possibility, low confluency Con8 cells were transiently transfected with an expression plasmid encoding HA-importin-alpha. Following 36 hours of serum starvation, cells were stimulated with 10% calf serum, treated with 1 μM dexamethasone for 15 hours, or maintained in serum-free media. As shown in Fig. 3 (Top panels) in the absence of stimuli, the exogenous HA-tagged importin-alpha remained dispersed throughout the cytoplasm. Sgk is not expressed under these conditions, whereas, consistent with our previous work [64], control immunofluorescence showed that beta-catenin localized mostly to the nucleus in the serum-free conditions. Serum stimulation resulted in the nuclear localization of HA-importin-alpha, which was congruent with the nuclear staining pattern of endogenous Sgk, whereas, beta-catenin localized primarily to the cell periphery (Fig. 3, middle panels). In contrast, exogenous importin-alpha resided primarily in the cytoplasmic compartment in dexamethasone treated cells, similar to the predominantly cytoplasmic staining of endogenous Sgk in cells exposed to glucocorticoids (Fig. 3, lower panels). Under these conditions, beta-catenin remained localized to the cell periphery.
Fig. 3.
Stimulus-dependent localization of importin-alpha and endogenous Sgk. Low confluent (30%) mammary epithelial cells, grown on 2 well lab-tek slides were transfected with expression vectors encoding full length importin-alpha (HA-FLIa) using lipofectamine. The cells were serum starved for 36 hours, and then maintained without serum (−serum) or treated with either 10% calf serum (+ serum) or with 1 μM dexamethasone (Dex) for 15 hours. The localization of HA-importin-alpha (middle panels) and of endogenous Sgk (left panels) and beta-catenin (right panels) was examined by indirect immunoflorescence microscopy as described in the Materials and Methods section. Protein expression of HA-importin-alpha and endogenous Sgk under each of treatment conditions used for localization studies was evaluated by immunoblotting with anti-HA or anti-Sgk antibodies respectively.
Western blots using anti-HA antibodies demonstrated that the level of expression of the epitope tagged exogenous importin-alpha (HA-importin-alpha) remained approximately the same under all three conditions (Fig. 3, blots). As expected, in the absence of any stimuli, no Sgk protein is produced, while serum or dexamethasone induced a high level of endogenous Sgk protein. Taken together, our results suggest that the signal dependent localization of importin-alpha may play a role in the subcellular co-distribution of Sgk and Erk/MAPK.
In vitro binding of Sgk and Erk2/MAPK to the recombinant full length and truncated importin-alpha nuclear import protein
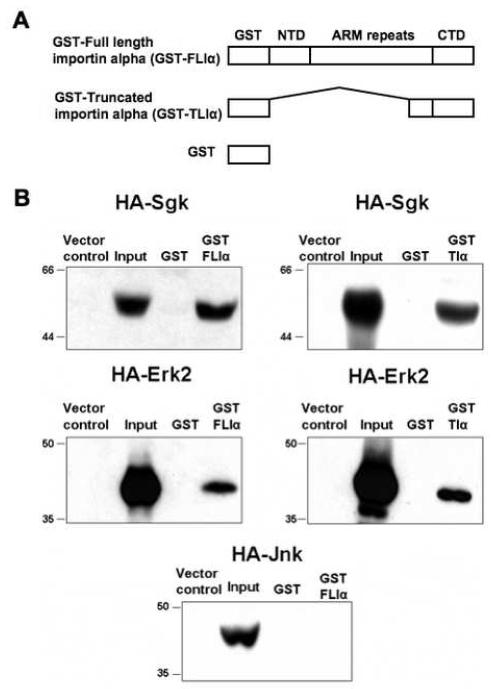
As an initial step to elucidate the mechanistic basis of the co-localization of Sgk and Erk/MAPK, GST-pull down binding assays were employed to compare the in vitro binding of Sgk and Erk/MAPK to importin-alpha. Importin-alpha is a 529 amino acid, 58 kDa protein that contains a hydrophobic amino terminus, a conserved central hydrophilic region punctuated with 8-10 degenerate arm repeats and a short hydrophilic carboxy terminus that binds the nuclear localization sequences (NLS) in cargo proteins [65]. Recombinant glutathione-S-transferase (GST) fusion proteins were constructed with the full length importin-alpha sequences (GST-FLIα), or with a truncated importin-alpha encoding the carboxy terminal 106 amino acids that encompasses approximately half of the eighth arm repeat and the entire ninth repeat extending up to the carboxy terminal region (GST-TIα). The GST protein alone was utilized as a negative control for the binding assay (Fig. 4A).
Fig. 4.
Interaction of ectopically expressed HA-Erk2 and HA-Sgk to recombinant full length and truncated importin-alpha. Panel A: GST-FLIa (full length importin-alpha) fusion protein is composed of a GST epitope tag, amino-terminal domain (NTD), Arm repeat containing domain, and a carboxy-terminal domain (CTD). GST-Tia (truncated importin-alpha) is composed of GST linked to the carboxy-terminal 106 amino acids (amino acids 423-529). GST alone was used as a negative control. Panel B: Hek-293 cells were transiently transfected with expression plasmids encoding either wild-type HA epitope tagged Erk2 (HA-Erk2) or Sgk (WT-HA-Sgk) or HA-Jnk or vector alone, and 48 hours post-transfection cell lysates were prepared as described in the Methods section. GST protein alone or GST-FLIa or GST-TIa was incubated with the indicated lysates containing exogenously expressed wild-type HA-tagged Sgk, or HA-Erk2, or HA-Jnk. The proteins bound to the Glutathione bead-bound GST or GST-fusion proteins were separated on SDS-PAGE and immunoblotted with anti-HA antibodies to detect bound proteins. Inputs denote 10% of the extract and lysates prepared from vector transfected samples served as controls as shown in each panel. Molecular weight markers are shown on the left side of each panel. Experiments were repeated three times with similar results.
Each of the GST fusion proteins, as well as the GST control protein were immobilized on glutathione-sepharose beads, and incubated with cell extracts from transfected human embryonic Hek293 fibroblasts containing ectopically expressed HA-epitope tagged Sgk (HA-Sgk), Erk2 (HA-Erk2), or Jun-N-terminal kinase (HA-Jnk). Expression of each protein kinase from their corresponding expression vectors in transfected cells (Input) relative to cells transfected with the empty vector controls was confirmed by anti-HA immunoblotting (Fig. 4B, left lanes in all blots). The retention of epitope-tagged proteins on the GST fusion protein loaded beads was determined by fractionation in SDS-PAGE and individual kinases detected by anti-HA Western analysis. Ectopically expressed HA-Erk2 or Sgk associated with both the full-length (GST-FLIα) and the truncated (GST-TIα) importin-alpha proteins (Fig. 4B). The carboxy-terminal 106 amino acids of importin-alpha appear to be sufficient for binding to both Erk2/MAPK and Sgk. No detectable binding of either protein kinase was observed with the control GST protein. Jnk, which is highly related to Erk/MAPK, has been shown to shuttle between the cytoplasm and nucleus in several cell types [66]. Strikingly, no binding was evident between the full length importin-alpha and ectopically expressed HA-Jnk in the cell extracts (Fig. 4B, lower blot), thereby establishing the specificity of the importin-alpha interactions with Erk/MAPK. Taken together, these results suggest a role for importin-alpha in the stimulus-dependent nuclear import of a subset of protein kinases.
RNAi knock down of importin-alpha disrupts the nuclear localization of Sgk and Erk
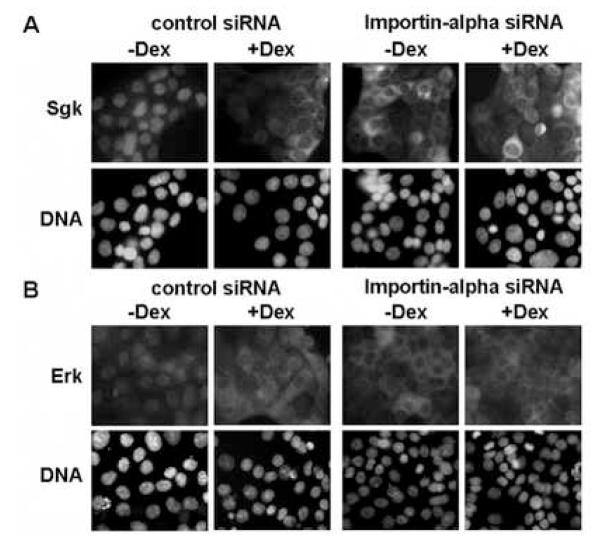
To determine if importin-alpha is responsible for the signal-dependent localization of Sgk and Erk, importin-alpha transcripts were knocked down using RNA interference (RNAi). Western blot analysis demonstrated that expression of importin-alpha specific small interfering RNA (siRNA) in dexamethasone treated or untreated cells significantly reduced production of importin-alpha protein, whereas, expression of control siRNA had no effect on importin-alpha levels (Fig. 5). No changes were observed in actin protein levels in either condition. In a parallel set of experiments, indirect immunofluorescence was used to characterize the subcellular distribution of Sgk and Erk in glucocorticoid treated and untreated cells (cultured in serum) in cells expressing either importin-alpha specific or control siRNA. As shown in Fig. 6, knock down of importin-alpha prevented the nuclear localization of both Sgk and Erk in serum-treated cells (Fig 6A and Fig 6B, −Dex right hand panels), whereas, both protein kinases resided in the cytoplasmic compartment in the presence of dexamethasone (Fig 6A and Fig 6B, + Dex right hand panels). As shown in the left hand panels of Fig 6A and Fig 6B, the control siRNA had no effect on the signal dependent co-localization of Sgk and Erk. Taken together, these functional data demonstrate that importin-alpha is responsible for the stimulus-dependent nuclear localization of Sgk and Erk, and that the cytoplasmic localization of both protein kinases in the glucocorticoid treated cells appears to result from the loss of importin-alpha function.
Fig. 5.
Knockdown of importin-alpha using RNA interference. Con8 cells were reverse transfected with 150 nM siRNA specific to importin-alpha or 150 nM of nonspecific (control) siRNA at the time of plating. Cells were then treated with or without 1 uM dexamethasone for 24 hours. Cell lysates were separated on SDS-PAGE and immunoblotted with anti-importin-alpha or actin specific antibodies as described in the Materials and Methods section.
Fig. 6.
Effects of RNAi knock down of importin-alpha on the localization of Sgk and ERK. Con8 cells were reverse transfected with 150 nM siRNA specific to importin-alpha or 150 nM of nonspecific (control) siRNA at the time of plating. Cells cultured in 10% serum were then treated with or without 1 uM dexamethasone (Dex) for 24 hours. Panel A: The subcellular distribution of Sgk was examined by indirect immunofluorescence microscopy using affinity-purified rabbit polyclonal antibodies to Sgk followed by FITC-conjugated goat anti-rabbit secondary antibodies. Panel B: Erk1/Erk2 were detected using monoclonal antibodies that specifically recognized both MAPK family members followed by a rhodamine-conjugated anti-rabbit secondary antibody employed to selectively visualize this protein kinase. DNA was stained to visualize the nucleus using DAPI.
Co-immunoprecipitation of Sgk with Erk/MAPK
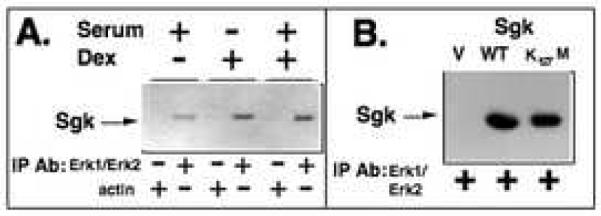
Co-immunoprecipitations were employed to test whether Sgk and Erk/MAPK associate in a common protein complex that could conceivably coordinate their stimulus-regulated co-localization. Wild type Sgk was ectopically expressed by transient transfection into the mammary tumor cells, which were then treated with 10% serum and/or 1 μM dexamethasone, in which both proteins either reside in the nucleus or in the cytoplasm. Twenty-four hours after transfection, Erk/MAPK was immunoprecipitated from soluble cell extracts using an Erk1/Erk2-specific monoclonal antibody. The immunoprecipitates were electrophoretically fractionated and the blotted proteins were probed with affinity-purified polyclonal anti-Sgk antibodies. As shown in Fig. 7A, Sgk co-immunoprecipitated with Erk/MAPK under all three cell culture conditions suggesting that their cellular interaction occurs in both the nuclear and cytoplasmic compartments. Western blot analysis of the control immunoprecipitations, which utilized an actin-specific monoclonal antibody, failed to detect Sgk, demonstrating the specificity of the Sgk co-immunoprecipitation with Erk/MAPK.
Fig. 7.
Co-immunoprecipitation of Sgk with Erk/MAPK. Panel A: Cells were transiently transfected with a wild-type Sgk expression vector and then treated with the indicated combinations of 10% serum and/or 1 μM dexamethasone for 24 hours. Cell extracts were immunoprecipitated with antibodies directed against either Erk1/Erk2 or to actin, the immunoprecipitated material electrophoretically fractionated and probed for the presence of Sgk. Panel B: Cells were transfected with a wild-type (WT) or the kinase dead (K127M) forms of Sgk or with an empty expression vector (V). Erk1/Erk2 was immunoprecipitated from the cell extracts, electrophoretically fractionated and western blots probed for the presence of Sgk.
In a second set of co-immunoprecipitations, serum-treated Con8 cells were transiently transfected with expression vectors encoding either the wild type (WT) Sgk or a kinase dead form of Sgk [63] in which the lysine at position 127 was mutated to methionine (designated K127M). As a negative control, another transfection was carried out with an empty expression vector (V). Erk/MAPK was immunoprecipitated from the cell extracts, and a western blot of the final immunoprecipitates was probed for Sgk. As shown in Fig. 7B, approximately the same levels of both the wild type and kinase dead forms of Sgk were recovered in the Erk/MAPK immunoprecipitates. Thus, Sgk kinase activity is not required to detect Sgk in the Erk/MAPK immune complex. In vitro binding assays failed to detect binding of either endogenous Sgk or exogenous Sgk with Erk/MAPK (data not shown), suggesting that Sgk and Erk/MAPK do not directly interact with each other. The presence of Sgk protein in Erk/MAPK immune complex formed with anti-Erk1/Erk2 antibodies, in combination with the importin-alpha GST pull down assays implicate importin-alpha in tethering Sgk and MAPK into the same protein complex.
Analysis of Sgk and Erk/MAPK phosphorylated forms in serum stimulated or glucocorticoid treated cells
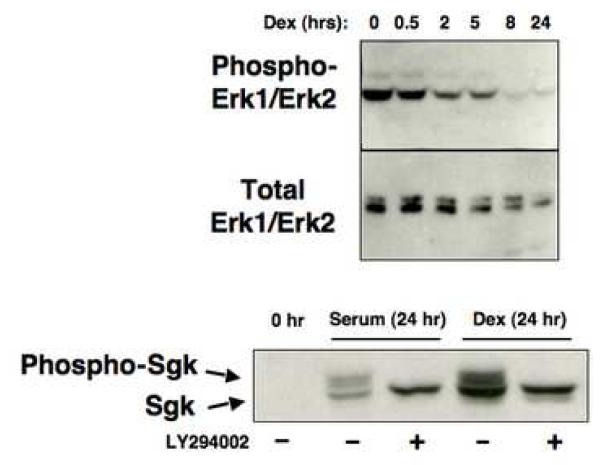
The nuclear localization of several serine/threonine protein kinases that shuttle between the nucleus and cytoplasmic compartment has been coupled to their phosphorylation states [67-69]. To determine whether Erk/MAPK phosphorylation is regulated by serum and/or glucocorticoids in a manner that correlates with its subcellular distribution, Con8 cells were grown in complete medium supplemented with 10% fetal bovine serum, then treated with 1 μM dexamethasone for different durations over a 24 hour time course of steroid treatment. Whole cell extracts were electrophoretically fractionated, and western blots probed either with monoclonal antibodies directed against amino acids 324-345 of Erk1/MAPK that recognize the Erk1 and Erk2 members of the MAPK family or with antibodies that selectively recognize the dually-phosphorylated active form of Erk/MAPK (Zymed, San Francisco, CA). Dexamethasone treatment caused a rapid and nearly complete reduction in the level of dually phosphorylated active Erk/MAPK even though the cells were continuously exposed to serum (Fig. 8, top blot). A significant reduction in expression of the dually-phosphorylated Erk/MAPK was observed as early as 2 hours after the addition of dexamethasone. Using myelin basic protein (MBP) as a substrate, the Erk/MAPK immunoprecipitated from dexamethasone-treated cells displayed a significantly reduced ability to phosphorylate MBP in vitro as compared to the Erk/MAPK immunoprecipitated from serum-stimulated cells (data not shown). During this same time course, the total level of Erk1-Erk2/MAPK protein remained relatively constant during the first 2 hours of dexamethasone treatment and exhibited only a modest decline by 24 hours in glucocorticoids (Fig. 8 middle blot).
Fig. 8.
Effects of serum and glucocorticoids on production of the hyperphosphorylated forms of Sgk and Erk/MAPK. Serum-treated cells were incubated with 1 μM dexamethasone and at the indicated time points, total cell extracts were electrophoretically fractionated. Western blots were probed for production of activated MAPK (top panel) or total Erk1/Erk2 (middle panel) using the appropriate antibodies. Cells were treated with 10% serum or 1 μM dexamethasone (Dex) in presence or absence of 50 μM LY294002, which inhibits PI 3-kinase activity, for 24 hours. A control culture was maintained in the absence of either stimuli (0 hr). Cell extracts were electrophoretically fractionated and probed for Sgk using affinity purified anti-Sgk antibodies. The hyperphosphorylated and hypophosphorylated Sgk species are designated with arrows.
In contrast to the stimulus-dependent changes in Erk/MAPK phosphorylation, the hyperphosphorylated form of Sgk is produced in serum stimulated or in dexamethasone treated cells. Western blots of total cell extracts showed that slower-migrating species that correspond to the hyperphosphorylated forms of Sgk [54, 55] are produced in mammary tumor cells treated for 24 hours with either 10% fetal bovine serum or 1 μM dexamethasone (Fig. 8, lower blot). No Sgk was detected in serum-starved cells, and after 24 hours the level of Sgk produced in dexamethasone-treated cells is generally greater than that observed after serum stimulation. Sgk is phosphorylated and activated by the PI3-kinase dependent pathway through PDK1 [54]. To test whether this pathway is functional in both serum-stimulated or dexamethasone treated conditions, cells were treated with each stimulus in the presence of the PI3 kinase inhibitor LY294002 and the resulting Sgk proteins analyzed by western blots. As also shown in Fig. 8 (lower blot), only the faster migrating Sgk protein band can be observed in the presence of LY294002, confirming that the higher molecular weight Sgk proteins represent forms of this kinase that are phosphoryated in a PI3-kinase dependent manner. The LY294002 sensitive hyperphosphorylated form of Sgk is similarly produced in cells treated with both serum and dexamethasone (data not shown). Using an in vitro transphosphorylation assay with the Sgktide peptide substrate (KKRNRRLSVA), we have previously shown that the immunoprecipitated hyperphosphorylated Sgk, such as produced in the mammary tumor cells, is enzymatically active [54].
MEK Inhibitor causes Sgk and ERK/MAPK to localize to the cytoplasm in serum treated cells
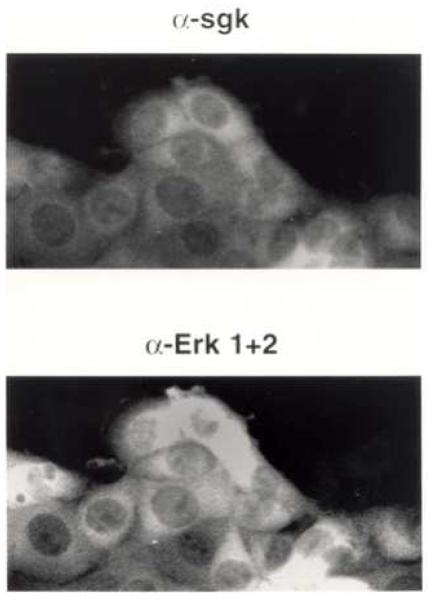
To further investigate the cellular properties of Sgk and Erk/MAPK localization, the effect of inhibiting Erk/MAPK activation on Sgk and Erk/MAPK localization was characterized by immunofluorescence microscopy in serum treated cells. Con8 cells were treated with PD98095, a selective MEK (MAPK kinase) inhibitor that prevents phosphorylation and subsequent activation of MAPK. Earlier in this study, we show that in serum-treated cells not exposed to this MEK inhibitor, both Sgk and Erk/MAPK co-localized to the nucleus (see Fig. 1). In contrast, in cells treated with PD98095, co-staining for both Sgk and Erk/MAPK showed both protein kinases to localize primarily to the cytoplasmic compartment (Fig. 9, upper and lower panels). Thus, the inhibition of Erk/MAPK activation prevents the nuclear import of both Sgk and Erk/MAPK, and further demonstrates that the subcellular localization of these two protein kinases is linked.
Fig. 9.
Effects of the PD 98095 MEK inhibitor on the subcellular localization of Sgk and Erk/MAPK. Con8 cells were treated with 10% serum for 18 hours and 50 uM PD 98095 for the last 8 hours. The subcellular distribution of Sgk and Erk/MAPK was examined by indirect immunofluorescence microscopy using affinity-purified rabbit polyclonal antibodies to Sgk (upper panel) or monoclonal anti-Erk 1 and Erk 2 antibodies (lower panel) as the primary antibodies respectively. The secondary antibodies were FITC-conjugated goat anti-rabbit and rhodamine-conjugated goat-anit-mouse, respectively.
Discussion
In this study we have documented that a new level of cross talk between steroid induced and serum/growth factor cascades is the regulated co-localization of Sgk and Erk/MAPK to the nucleus or the cytoplasmic compartment in a signal dependent manner. Both protein kinases localize primarily to the nucleus in serum treated cells and to the cytoplasmic compartment in glucocorticoid treated cells. The mutual interaction of Sgk and Erk/MAPK with the importin-alpha component of the nuclear transport machinery, in combination with the signal dependent localization of importin-alpha, provides a mechanistic basis to account for the observed co-localization of these two serine/threonine protein kinases. Furthermore, RNAi mediated knock down of importin-alpha demonstrated that this nuclear receptor protein is responsible for the stimulus-dependent nuclear localization of Sgk and Erk, and that the cytoplasmic localization of both protein kinases in the glucocorticoid treated cells appears to result from the loss of importin-alpha function. These results suggest that the cellular processes controlling the nuclear targeting of Sgk and Erk/MAPK play a critical role in deciding the specific set of responses to extracellular stimuli, and within a given cellular location Sgk and Erk/MAPK encounter functionally critical targets of their respective signaling cascades. For example, in serum treated cells, transport into the nucleus likely allows access of these protein kinases to potential nuclear targets, such as transcription factors or components of the cell cycle machinery. Consistent with this view, the nuclear targeting of Erk/MAPK is essential for cell cycle entry and phosphorylation of its substrate, the Elk-1 transcription factor [70]. Similarly, the forkhead transcription factor FKHRL1 has been shown to be phosphorylated by Sgk, which supports the notion that a specific set of nuclear proteins implicated in growth control and cell survival may be targeted by Sgk in serum stimulated cells [71].
The nuclear pore complex (NPC) controls the selective bidirectional nuclear/cytoplasmic trafficking of cellular proteins by a family of importin/exportin shuttling transport factors, whose regulated activities help establish a controlled barrier between the cytoplasmic and nuclear compartments [72-75]. Importin-alpha is an adapter protein that directs nuclear localization sequence driven nuclear import of protein cargoes through the nuclear pore complex. Results from the immunofluorescence studies of exogenous importin-alpha suggest that the serum and glucocorticoid signaling pathways regulate the nuclear import function of importin-alpha at the level of its subcellular localization. Consistent with our observations, the localization of the Drosophila form of importin-alpha is regulated in synchrony with the cell cycle in that it is localized in the cytoplasm in G1 and in the nucleus in S and G2/M phases of the Drosophila cell cycle [76]. We propose that the stimulus-dependent localization of importin-alpha directs the congruent nuclear/cytoplasmic compartmentalization of both Sgk and Erk/MAPK, and conceivably the cytoplasmic residing importin-alpha prevents the nuclear transport of Sgk and Erk/MAPK in glucocorticoid treated cells. A key future direction will be to uncover the precise glucocorticoid regulated gene product that controls the signal dependent nuclear/cytoplasmic distribution of importin-alpha.
GST-pull down assays revealed that both the full length and a truncated 106 amino acid carboxy-terminal form of importin-alpha, specifically interact with either Sgk or Erk/MAPK. The carboxy terminal fragment of this nuclear importer has an acidic rich region that is likely to be involved in binding to both kinases, but is devoid of the central array of arm repeats, which appear to be critical for binding of certain NLS containing proteins [77]. A diverse set of proteins involved in cellular activities ranging from protein export (such as components of nuclear transport machinery), gene expression (such as Pax and p300 coactivator) and mRNA biogenesis (such as methyltransferase) selectively interact with the carboxy end of importin-alpha [78-80]. These findings raise the possibility of importin-alpha acting as a molecular scaffold, allowing assembly of various proteins similar to the 14-3-3 regulatory proteins and PKA anchoring protein AKAP-79 [81, 82]. We have observed that wild type or kinase dead forms of Sgk can be co-immunoprecipitated with Erk/MAPK, suggesting that these protein kinases can reside in a common protein complex. Conceivably, the mutual interaction with importin-alpha may be to facilitate the regulated nuclear transport of Sgk and Erk/MAPK in the form of a large signaling complex that enters the nucleus as a unit, as noted in the case of the nuclear/cytoplasmic shuttling of the yeast Ste5 scaffold protein [83]. Alternatively, the protein complex may potentially serve a second function to tether Sgk and Erk/MAPK in close physical proximity. We are unable to detect MAPK bound to anti-Sgk immunoprecipitated complexes (data not shown). One explanation is that the anti-Sgk polyclonal antibody interferes with the binding of MAPK to Sgk. Nevertheless, the association of Sgk and MAPK in mammary epithelial tumor cells may indicate a functional association.
Strikingly, no associations between importin-alpha and Jnk (Fig. 8), which is a MAPK family member related to Erk/MAPK, or PKC ξ (data not shown), which is highly homologous to Sgk, were discernible, despite the fact that these signaling kinases exhibit regulated nuclear import [66, 84]. These results underscore the specificity of binding of importin-alpha to the Sgk and Erk/MAPK target proteins. Different isoforms of importin-alpha have been shown to display unique as well as overlapping substrate specificities [85]. Additional studies will be necessary to clarify whether other importin-alpha isoforms are also capable of interacting with Erk/MAPK besides importin-alpha 1, especially since these different isoforms display preferential binding to cellular proteins [85, 86].
Nuclear translocation of Erk/MAPK in response to proliferative stimuli has been documented in other cell systems [67]. A variety of mechanisms involving the existence of cytoplasmic anchors such as MEK, Erk specific phosphatases MKP-3 and PTP-SL, and the Erk/MAPK scaffolding protein MP-1 [70, 87] have been invoked to explain the cytoplasmic sequestration of Erk/MAPK. However, the cellular machinery that mediates the nuclear transport of this kinase has not been characterized. Previous studies have hinted at the possible involvement of an active transport process, based on the efficient nuclear import of GFP-Erk2/MAPK and the energy dependence of this process [67, 68, 88]. However, our results indicate for the first time a direct interaction between Erk2/MAPK and importin-alpha, thereby implicating this nuclear import protein in the regulated nuclear transport of MAPK family members in other cell systems. The Erk2/MAPK protein sequence does not contain any obvious NLS, although the 31 amino acid insertion sequence located close to the phosphorylation lip in the carboxy region of Erk/MAPK is suspected to harbor sequences resembling a bipartite NLS [89]. Also, alteration of residues 321 and 327 within the context of the full-length Erk/MAPK protein to alanines prevented the mitogen induced nuclear translocation, highlighting the importance of these residues in regulated transport of this kinase [88].
Erk/MAPK is hyperphosphorylated and active in serum-stimulated cells, while the hypophosphorylated non-active form of Erk/MAPK is produced in glucocorticoid treated cells, perhaps as a result of a glucocorticoid-induced broad range phosphatase, such as observed in primary human T cells [29], or in osteoblasts [90], or the actions of MAPK-specific phosphatases, such as MKP-3 [70] or HVH-1 [91]. Evidence demonstrating inhibition of Erk/MAPK and Jnk/MAPK members by glucocorticoids in other cell systems [35, 36], suggests the importance of functional cross-talk between the steroid hormone and MAPK signaling pathways in mediating specific cellular responses to glucocorticoids The PI 3-kinase-dependent hyperphosphorylation of Sgk was observed in both serum or glucocorticoid treated cells, regardless whether the kinase was nuclear in proliferating cells or cytoplasmic in growth inhibited cells. In this regard, a cytoplasmic form of Sgk is observed after hyperosmotic stress [55] and in terminally differentiated ovarian cells [92], which are biological scenarios in which the cells are not proliferating. Sgk expression has been shown to be elevated by a variety of extracellular cues and physiological conditions [93-100], and it will be interesting to determine how Sgk localization is regulated in a stimuli and tissue specific manner in these systems.
We have shown that Sgk shuttles between the nucleus and cytoplasm in synchrony with the cell cycle [56]. Intriguingly, the human Sgk gene is localized to a single chromosomal locus at band 6q23, which is a region frequently deleted in various human neoplasms [101]. Erk/MAPK activity and utilization can be correlated with normal and tumor cell proliferation in many systems. It is therefore tempting to speculate that functional interactions between importin-alpha and either Sgk or Erk/MAPK under normal conditions may somehow retard tumor progression, probably by selectively localizing these protein kinases to specific cellular compartments containing target proteins involved in growth control. Our current studies are directed at determining the physiological significance of the nuclear transport machinery that employs importin-alpha to control the nuclear import of a subset of protein kinases in a stimulus-dependent manner.
Acknowledgments
This study is dedicated to the memory of Dr. Anita C. Maiyar, the second author, who contributed significantly to this work and many other projects in the laboratory, and who will always be remembered. This work was supported by a National Institute of Health grant #DK-42799 awarded to G. L. Firestone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Beato M, Chavez S, Truss M. Transcriptional regulation by steroid hormones. Steroids. 1996;61:240–51. doi: 10.1016/0039-128x(96)00030-x. [DOI] [PubMed] [Google Scholar]
- [2].Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr. Rev. 1996;17:587–609. doi: 10.1210/edrv-17-6-587. [DOI] [PubMed] [Google Scholar]
- [3].Dupont J, Le Roith D. Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects. Mol. Pathol. 2001;54:149–54. doi: 10.1136/mp.54.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson GL, Vaillancourt RR. Sequential protein kinase reactions controlling cell growth and differentiation. Curr. Opin. Cell Biol. 1994;6:230–8. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- [5].Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270:16483–6. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- [6].Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997;9:180–6. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- [7].Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- [8].Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–89. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- [9].Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- [10].Zilliacus J, Wright AP, Carlstedt-Duke J, Gustafsson JA. Structural determinants of DNA-binding specificity by steroid receptors. Mol. Endocrinol. 1995;9:389–400. doi: 10.1210/mend.9.4.7659083. [DOI] [PubMed] [Google Scholar]
- [11].Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr. Rev. 1996;17:245–61. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- [12].Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure:function relationship. J. Steroid Biochem. Mol. Biol. 2005;94:383–94. doi: 10.1016/j.jsbmb.2004.12.046. [DOI] [PubMed] [Google Scholar]
- [13].Lu NZ, Cidlowski JA. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006;16:301–7. doi: 10.1016/j.tcb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- [14].McEwan IJ, Wright AP, Gustafsson JA. Mechanism of gene expression by the glucocorticoid receptor: role of protein-protein interactions. BioEssays. 1997;19:153–60. doi: 10.1002/bies.950190210. [DOI] [PubMed] [Google Scholar]
- [15].Yudt MR, Cidlowski JA. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol. Endocrinol. 2002;16:1719–26. doi: 10.1210/me.2002-0106. [DOI] [PubMed] [Google Scholar]
- [16].Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–17. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [17].Clark AR, Lasa M. Crosstalk between glucocorticoids and mitogen-activated protein kinase signalling pathways. Curr. Opin. Pharmacol. 2003;3:404–11. doi: 10.1016/s1471-4892(03)00073-0. [DOI] [PubMed] [Google Scholar]
- [18].Herrlich P. Cross-talk between glucocorticoid receptor and AP-1. Oncogene. 2001;20:2465–75. doi: 10.1038/sj.onc.1204388. [DOI] [PubMed] [Google Scholar]
- [19].Karin M, Chang L. AP-1--glucocorticoid receptor crosstalk taken to a higher level. J. Endocrinol. 2001;169:447–51. doi: 10.1677/joe.0.1690447. [DOI] [PubMed] [Google Scholar]
- [20].Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann. N. Y. Acad. Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- [21].Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol. Cell. Biol. 1997;17:3947–54. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rogatsky I, Logan SK, Garabedian MJ. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2050–5. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- [24].Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. Embo J. 1998;17:2008–18. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tsitoura DC, Rothman PB. Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J Clin Invest. 2004;113:619–27. doi: 10.1172/JCI18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Onda K, Nagashima M, Kawakubo Y, Inoue S, Hirano T, Oka K. Mitogen-activated protein kinase kinase 1/extracellular signal-regulated kinase (MEK-1/ERK) inhibitors sensitize reduced glucocorticoid response mediated by TNFalpha in human epidermal keratinocytes (HaCaT) Biochem. Biophys. Res. Commun. 2006;351:266–72. doi: 10.1016/j.bbrc.2006.10.032. [DOI] [PubMed] [Google Scholar]
- [27].Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br. J. Pharmacol. 2000;130:289–98. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Georgakopoulos A, Tsawdaroglou N. Insulin potentiates the transactivation potency of the glucocorticoid receptor. FEBS Lett. 1996;381:177–82. doi: 10.1016/0014-5793(96)00115-9. [DOI] [PubMed] [Google Scholar]
- [29].Paliogianni F, Hama N, Balow JE, Valentine MA, Boumpas DT. Glucocorticoid-mediated regulation of protein phosphorylation in primary human T cells. Evidence for induction of phosphatase activity. J. Immunol. 1995;155:1809–17. [PubMed] [Google Scholar]
- [30].Wakui H, Wright AP, Gustafsson J, Zilliacus J. Interaction of the ligand-activated glucocorticoid receptor with the 14-3-3 eta protein. J. Biol. Chem. 1997;272:8153–6. doi: 10.1074/jbc.272.13.8153. [DOI] [PubMed] [Google Scholar]
- [31].Widen C, Zilliacus J, Gustafsson JA, Wikstrom AC. Glucocorticoid receptor interaction with 14-3-3 and Raf-1, a proposed mechanism for cross-talk of two signal transduction pathways. J. Biol. Chem. 2000;275:39296–301. doi: 10.1074/jbc.M006943200. [DOI] [PubMed] [Google Scholar]
- [32].Alexander DB, Goya L, Webster MK, Haraguchi T, Firestone GL. Glucocorticoids coordinately disrupt a transforming growth factor alpha autocrine loop and suppress the growth of 13762NF-derived Con8 rat mammary adenocarcinoma cells. Cancer Res. 1993;53:1808–15. [PubMed] [Google Scholar]
- [33].Niu H, Hinkle DA, Wise PM. Dexamethasone regulates basic fibroblast growth factor, nerve growth factor and S100beta expression in cultured hippocampal astrocytes. Brain Res Mol Brain Res. 1997;51:97–105. doi: 10.1016/s0169-328x(97)00221-0. [DOI] [PubMed] [Google Scholar]
- [34].Ayroldi E, Zollo O, Macchiarulo A, Di Marco B, Marchetti C, Riccardi C. Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1. Mol. Cell. Biol. 2002;22:7929–41. doi: 10.1128/MCB.22.22.7929-7941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gonzalez MV, Gonzalez-Sancho JM, Caelles C, Munoz A, Jimenez B. Hormone-activated nuclear receptors inhibit the stimulation of the JNK and ERK signalling pathways in endothelial cells. FEBS Lett. 1999;459:272–6. doi: 10.1016/s0014-5793(99)01257-0. [DOI] [PubMed] [Google Scholar]
- [36].Gonzalez MV, Jimenez B, Berciano MT, Gonzalez-Sancho JM, Caelles C, Lafarga M, Munoz A. Glucocorticoids antagonize AP-1 by inhibiting the Activation/phosphorylation of JNK without affecting its subcellular distribution. J. Cell Biol. 2000;150:1199–208. doi: 10.1083/jcb.150.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J. Biol. Chem. 2002;277:10876–82. doi: 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- [38].Hansson A, Hehenberger K, Thoren M. Long-term treatment of Swiss 3T3 fibroblasts with dexamethasone attenuates MAP kinase activation induced by insulin-like growth factor-I (IGF-I) Cell Biochem. Funct. 1996;14:121–9. doi: 10.1002/cbf.656. [DOI] [PubMed] [Google Scholar]
- [39].Hiragun T, Peng Z, Beaven MA. Dexamethasone up-regulates the inhibitory adaptor protein Dok-1 and suppresses downstream activation of the mitogen-activated protein kinase pathway in antigen-stimulated RBL-2H3 mast cells. Mol. Pharmacol. 2005;67:598–603. doi: 10.1124/mol.104.008607. [DOI] [PubMed] [Google Scholar]
- [40].Rider LG, Hirasawa N, Santini F, Beaven MA. Activation of the mitogen-activated protein kinase cascade is suppressed by low concentrations of dexamethasone in mast cells. J. Immunol. 1996;157:2374–80. [PubMed] [Google Scholar]
- [41].Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J. Endocrinol. 2003;178:5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- [42].Webster MK, Guthrie J, Firestone GL. Suppression of rat mammary tumor cell growth in vitro by glucocorticoids requires serum proteins. Characterization of wild type and glucocorticoid-resistant epithelial tumor cells. J. Biol. Chem. 1990;265:4831–8. [PubMed] [Google Scholar]
- [43].Webster MK, Guthrie J, Firestone GL. Glucocorticoid growth suppression response in 13762NF adenocarcinoma-derived Con8 rat mammary tumor cells is mediated by dominant trans-acting factors. Cancer Res. 1991;51:6031–8. [PubMed] [Google Scholar]
- [44].Geradts J, Richards J, Edery M, Pang K, Larson L, Nandi S. Heterogeneity in the hormonal responsiveness of clones derived from the 13762NF rat mammary tumor. Cancer Res. 1986;46:1920–7. [PubMed] [Google Scholar]
- [45].Goya L, Maiyar AC, Ge Y, Firestone GL. Glucocorticoids induce a G1/G0 cell cycle arrest of Con8 rat mammary tumor cells that is synchronously reversed by steroid withdrawal or addition of transforming growth factor-alpha. Mol. Endocrinol. 1993;7:1121–32. doi: 10.1210/mend.7.9.8247014. [DOI] [PubMed] [Google Scholar]
- [46].Buse P, Woo PL, Alexander DB, Cha HH, Reza A, Sirota ND, Firestone GL. Transforming growth factor-alpha abrogates glucocorticoid-stimulated tight junction formation and growth suppression in rat mammary epithelial tumor cells. J. Biol. Chem. 1995;270:6505–14. doi: 10.1074/jbc.270.12.6505. [DOI] [PubMed] [Google Scholar]
- [47].Buse P, Woo PL, Alexander DB, Reza A, Firestone GL. Glucocorticoid-induced functional polarity of growth factor responsiveness regulates tight junction dynamics in transformed mammary epithelial tumor cells. J. Biol. Chem. 1995;270:28223–7. doi: 10.1074/jbc.270.47.28223. [DOI] [PubMed] [Google Scholar]
- [48].Woo PL, Cercek A, Desprez PY, Firestone GL. Involvement of the helix-loop-helix protein Id-1 in the glucocorticoid regulation of tight junctions in mammary epithelial cells. J. Biol. Chem. 2000;275:28649–58. doi: 10.1074/jbc.M910373199. [DOI] [PubMed] [Google Scholar]
- [49].Woo PL, Ching D, Guan Y, Firestone GL. Requirement for Ras and phosphatidylinositol 3-kinase signaling uncouples the glucocorticoid-induced junctional organization and transepithelial electrical resistance in mammary tumor cells. J. Biol. Chem. 1999;274:32818–28. doi: 10.1074/jbc.274.46.32818. [DOI] [PubMed] [Google Scholar]
- [50].Hernandez-Sotomayor SM, Carpenter G. Epidermal growth factor receptor: elements of intracellular communication. J. Membr. Biol. 1992;128:81–9. doi: 10.1007/BF00231881. [DOI] [PubMed] [Google Scholar]
- [51].Guan Y, Woo PL, Rubenstein NM, Firestone GL. Transforming growth factor-alpha abrogates the glucocorticoid stimulation of tight junction formation and reverses the steroid-induced down-regulation of fascin in rat mammary epithelial tumor cells by a Ras-dependent pathway. Exp. Cell Res. 2002;273:1–11. doi: 10.1006/excr.2001.5415. [DOI] [PubMed] [Google Scholar]
- [52].Webster MK, Goya L, Firestone GL. Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J. Biol. Chem. 1993;268:11482–5. [PubMed] [Google Scholar]
- [53].Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 1993;13:2031–40. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. Embo J. 1999;18:3024–33. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bell LM, Leong ML, Kim B, Wang E, Park J, Hemmings BA, Firestone GL. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J. Biol. Chem. 2000;275:25262–72. doi: 10.1074/jbc.M002076200. [DOI] [PubMed] [Google Scholar]
- [56].Buse P, Tran SH, Luther E, Phu PT, Aponte GW, Firestone GL. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. J. Biol. Chem. 1999;274:7253–63. doi: 10.1074/jbc.274.11.7253. [DOI] [PubMed] [Google Scholar]
- [57].Maiyar AC, Leong ML, Firestone GL. Importin-alpha mediates the regulated nuclear targeting of serum- and glucocorticoid-inducible protein kinase (Sgk) by recognition of a nuclear localization signal in the kinase central domain. Mol. Biol. Cell. 2003;14:1221–39. doi: 10.1091/mbc.E02-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Maiyar AC, Phu PT, Huang AJ, Firestone GL. Repression of glucocorticoid receptor transactivation and DNA binding of a glucocorticoid response element within the serum/glucocorticoid-inducible protein kinase (sgk) gene promoter by the p53 tumor suppressor protein. Mol. Endocrinol. 1997;11:312–29. doi: 10.1210/mend.11.3.9893. [DOI] [PubMed] [Google Scholar]
- [59].Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 1992;12:915–27. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Edelmann HM, Kuhne C, Petritsch C, Ballou LM. Cell cycle regulation of p70 S6 kinase and p42/p44 mitogen-activated protein kinases in Swiss mouse 3T3 fibroblasts. J. Biol. Chem. 1996;271:963–71. doi: 10.1074/jbc.271.2.963. [DOI] [PubMed] [Google Scholar]
- [61].Lenormand P, Pages G, Sardet C, L’Allemain G, Meloche S, Pouyssegur J. MAP kinases: activation, subcellular localization and role in the control of cell proliferation. Adv Second Messenger Phosphoprotein Res. 1993;28:237–44. [PubMed] [Google Scholar]
- [62].Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- [63].Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J. Biol. Chem. 2003;278:5871–82. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- [64].Guan Y, Rubenstein NM, Failor KL, Woo PL, Firestone GL. Glucocorticoids control beta-catenin protein expression and localization through distinct pathways that can be uncoupled by disruption of signaling events required for tight junction formation in rat mammary epithelial tumor cells. Mol. Endocrinol. 2004;18:214–27. doi: 10.1210/me.2003-0014. [DOI] [PubMed] [Google Scholar]
- [65].Herold A, Truant R, Wiegand H, Cullen BR. Determination of the functional domain organization of the importin alpha nuclear import factor. J. Cell Biol. 1998;143:309–18. doi: 10.1083/jcb.143.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mizukami Y, Yoshioka K, Morimoto S, Yoshida K. A novel mechanism of JNK1 activation. Nuclear translocation and activation of JNK1 during ischemia and reperfusion. J. Biol. Chem. 1997;272:16657–62. doi: 10.1074/jbc.272.26.16657. [DOI] [PubMed] [Google Scholar]
- [67].Adachi M, Fukuda M, Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. Embo J. 1999;18:5347–58. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Reiser V, Ammerer G, Ruis H. Nucleocytoplasmic traffic of MAP kinases. Gene Expr. 1999;7:247–54. [PMC free article] [PubMed] [Google Scholar]
- [69].Sweitzer TD, Love DC, Hanover JA. Regulation of nuclear import and export. Curr. Top. Cell. Regul. 2000;36:77–94. doi: 10.1016/s0070-2137(01)80003-0. [DOI] [PubMed] [Google Scholar]
- [70].Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. Embo J. 1999;18:664–74. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol. Cell. Biol. 2001;21:952–65. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Christophe D, Christophe-Hobertus C, Pichon B. Nuclear targeting of proteins: how many different signals? Cell. Signal. 2000;12:337–41. doi: 10.1016/s0898-6568(00)00077-2. [DOI] [PubMed] [Google Scholar]
- [73].Ferrigno P, Posas F, Koepp D, Saito H, Silver PA. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. Embo J. 1998;17:5606–14. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gorlich D. Transport into and out of the cell nucleus. Embo J. 1998;17:2721–7. doi: 10.1093/emboj/17.10.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? BioEssays. 2000;22:532–44. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [76].Kussel P, Frasch M. Pendulin, a Drosophila protein with cell cycle-dependent nuclear localization, is required for normal cell proliferation. J. Cell Biol. 1995;129:1491–507. doi: 10.1083/jcb.129.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Prieve MG, Guttridge KL, Munguia J, Waterman ML. Differential importin-alpha recognition and nuclear transport by nuclear localization signals within the high-mobility-group DNA binding domains of lymphoid enhancer factor 1 and T-cell factor 1. Mol. Cell. Biol. 1998;18:4819–32. doi: 10.1128/mcb.18.8.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bannister AJ, Miska EA, Gorlich D, Kouzarides T. Acetylation of importin-alpha nuclear import factors by CBP/p300. Curr. Biol. 2000;10:467–70. doi: 10.1016/s0960-9822(00)00445-0. [DOI] [PubMed] [Google Scholar]
- [79].Kovac CR, Emelyanov A, Singh M, Ashouian N, Birshtein BK. BSAP (Pax5)-importin alpha 1 (Rch1) interaction identifies a nuclear localization sequence. J. Biol. Chem. 2000;275:16752–7. doi: 10.1074/jbc.M001551200. [DOI] [PubMed] [Google Scholar]
- [80].Wen Y, Shatkin AJ. Cap methyltransferase selective binding and methylation of GpppG-RNA are stimulated by importin-alpha. Genes Dev. 2000;14:2944–9. doi: 10.1101/gad.848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Burack WR, Shaw AS. Signal transduction: hanging on a scaffold. Curr. Opin. Cell Biol. 2000;12:211–6. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- [82].Edwards AS, Scott JD. A-kinase anchoring proteins: protein kinase A and beyond. Curr. Opin. Cell Biol. 2000;12:217–21. doi: 10.1016/s0955-0674(99)00085-x. [DOI] [PubMed] [Google Scholar]
- [83].Mahanty SK, Wang Y, Farley FW, Elion EA. Nuclear shuttling of yeast scaffold Ste5 is required for its recruitment to the plasma membrane and activation of the mating MAPK cascade. Cell. 1999;98:501–12. doi: 10.1016/s0092-8674(00)81978-9. [DOI] [PubMed] [Google Scholar]
- [84].Neri LM, Martelli AM, Borgatti P, Colamussi ML, Marchisio M, Capitani S. Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3,4,5) trisphosphate synthesis precede PKC-zeta translocation to the nucleus of NGF-treated PC12 cells. FASEB J. 1999;13:2299–310. [PubMed] [Google Scholar]
- [85].Kohler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Gorlich D, Hartmann E. Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol. Cell. Biol. 1999;19:7782–91. doi: 10.1128/mcb.19.11.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mathe E, Bates H, Huikeshoven H, Deak P, Glover DM, Cotterill S. Importin-alpha3 is required at multiple stages of Drosophila development and has a role in the completion of oogenesis. Dev. Biol. 2000;223:307–22. doi: 10.1006/dbio.2000.9743. [DOI] [PubMed] [Google Scholar]
- [87].Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. Embo J. 1997;16:1901–8. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rubinfeld H, Hanoch T, Seger R. Identification of a cytoplasmic-retention sequence in ERK2. J. Biol. Chem. 1999;274:30349–52. doi: 10.1074/jbc.274.43.30349. [DOI] [PubMed] [Google Scholar]
- [89].Cobb MH, Goldsmith EJ. Dimerization in MAP-kinase signaling. Trends Biochem. Sci. 2000;25:7–9. doi: 10.1016/s0968-0004(99)01508-x. [DOI] [PubMed] [Google Scholar]
- [90].Hulley PA, Gordon F, Hough FS. Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA 15.4) by dexamethasone: role of protein phosphatases. Endocrinology. 1998;139:2423–31. doi: 10.1210/endo.139.5.6020. [DOI] [PubMed] [Google Scholar]
- [91].Zheng CF, Guan KL. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr protein phosphatase. J. Biol. Chem. 1993;268:16116–9. [PubMed] [Google Scholar]
- [92].Alliston TN, Gonzalez-Robayna IJ, Buse P, Firestone GL, Richards JS. Expression and localization of serum/glucocorticoid-induced kinase in the rat ovary: relation to follicular growth and differentiation. Endocrinology. 2000;141:385–95. doi: 10.1210/endo.141.1.7257. [DOI] [PubMed] [Google Scholar]
- [93].Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2514–9. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Imaizumi K, Tsuda M, Wanaka A, Tohyama M, Takagi T. Differential expression of sgk mRNA, a member of the Ser/Thr protein kinase gene family, in rat brain after CNS injury. Brain Res Mol Brain Res. 1994;26:189–96. doi: 10.1016/0169-328x(94)90090-6. [DOI] [PubMed] [Google Scholar]
- [95].Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr., Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–7. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- [96].Kumar JM, Brooks DP, Olson BA, Laping NJ. Sgk, a putative serine/threonine kinase, is differentially expressed in the kidney of diabetic mice and humans. J. Am. Soc. Nephrol. 1999;10:2488–94. doi: 10.1681/ASN.V10122488. [DOI] [PubMed] [Google Scholar]
- [97].Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial na+ channels. J. Biol. Chem. 1999;274:16973–8. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- [98].Wagner CA, Broer A, Albers A, Gamper N, Lang F, Broer S. The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a non-selective cation channel that is regulated by the serine/threonine kinase sgk-1. J Physiol. 2000;526(Pt 1):35–46. doi: 10.1111/j.1469-7793.2000.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4440–5. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Waldegger S, Klingel K, Barth P, Sauter M, Rfer ML, Kandolf R, Lang F. h-sgk serine-threonine protein kinase gene as transcriptional target of transforming growth factor beta in human intestine. Gastroenterology. 1999;116:1081–8. doi: 10.1016/s0016-5085(99)70011-9. [DOI] [PubMed] [Google Scholar]
- [101].Waldegger S, Erdel M, Nagl UO, Barth P, Raber G, Steuer S, Utermann G, Paulmichl M, Lang F. Genomic organization and chromosomal localization of the human SGK protein kinase gene. Genomics. 1998;51:299–302. doi: 10.1006/geno.1998.5258. [DOI] [PubMed] [Google Scholar]