Abstract
The purpose of this study was to investigate the role of dipeptidyl peptidase IV in regulating the effects of two of its substrates, neuropeptide Y1-36 and peptide YY1-36, on proliferation of and collagen production by preglomerular vascular smooth muscle and glomerular mesangial cells from spontaneously hypertensive and normotensive rats. In cells from hypertensive rats, neuropeptide Y1-36 and peptide YY1-36 stimulated [3H]-thymidine incorporation (cell proliferation index), cell number and [3H]-proline incorporation (index of collagen synthesis); and sitagliptin (dipeptidyl peptidase IV inhibitor) significantly enhanced most of these effects. Neuropeptide Y3-36 and peptide YY3-36 (products of dipeptidyl peptidase IV) had little effect on [3H]-thymidine incorporation, and sitagliptin did not enhance the effects of either peptide. BIBP3226 (Y1 receptor antagonist) blocked the effects of neuropeptide Y1-36 and peptide YY1-36 on [3H]-thymidine incorporation in the absence and presence of sitagliptin. Neuropeptide Y1-36 and peptide YY1-36 stimulated [3H]-thymidine and [3H]-proline incorporation and cell number in cells from normotensive rats; however, the effects were weak and mostly not affected by sitagliptin. Real-time PCR and Western blotting showed similar dipeptidyl peptidase IV mRNA and protein levels in cells from hypertensive versus normotensive rats, with greater levels in smooth muscle versus mesangial cells. Both cell types converted peptide YY1-36 to peptide YY3-36 in a concentration-dependent manner that was attenuated by sitagliptin, and dipeptidyl peptidase IV activity was greater in smooth muscle versus mesangial cells. Conclusion: Dipeptidyl peptidase IV inhibitors might entail a risk of renal dysfunction due to abnormal proliferation of cells in the preglomerular microcirculation and glomeruli.
Keywords: dipeptidyl peptidase IV, peptide YY1-36, neuropeptide Y1-36, preglomerular vascular smooth muscle cells, mesangial cells, spontaneously hypertensive rats, cell proliferation
INTRODUCTION
Inhibitors of dipeptidyl peptidase IV (DPPIV), for example sitagliptin, represent a novel class of antidiabetic drugs for treatment of type 2 diabetes that afford sustained reductions in HbA1c with a low risk of hypoglycemia and little effect on body weight.1 Because millions of patients will be taking DPPIV inhibitors for life, there is some urgency to more fully understand the long-term risks associated with DPPIV inhibition. The long-term risks of DPPIV inhibitors in the setting of hypertension are of particular interest because frequently this condition is a co-morbidity in type 2 diabetics.2
DPPIV metabolizes incretin hormones, such as gastric inhibitor peptide (GIP) and glucagon-like peptide-1 (GLP-1).3 Consequently, DPPIV inhibitors raise circulating levels of incretin hormones and thereby exert antidiabetic actions by increasing insulin release.3 However, there are at least 35 known peptide substrates for DPPIV,4, 5 and therefore inhibition of DPPIV increases levels of an array of biologically active peptides. Long-term increases in some of these peptide substrates of DPPIV may entail risks. For example, Brown et al.6 provide evidence that DPPIV inhibitors increase angioedema risk in patients treated with ACE inhibitors, most likely due to blockade of substance P metabolism.
With respect to other substrates for DPPIV that may entail risks, two peptides of particular importance are NPY1-36 and PYY1-36. Both of these peptides are members of the pancreatic polypeptide-fold (PP-fold) family,7 and the kidney (a major target organ for diabetes-induced damage) is likely exposed to high levels of these endogenous peptides. For example, renal sympathetic nerves release NPY1-36 in response to CNS-mediated activation of renal sympathetic tone,8 resulting in high local levels of NPY1-36 in sympathetically-innervated renal microvessels. Also, NPY1-36 is made by renal epithelial cells and released into the renal interstitium where it can affect vascular and glomerular cells.9 With regard to PYY1-36, fatty meals release this peptide into the systemic circulation from endocrine L-cells in the GI tract producing physiologically active levels of PYY1-36 in plasma,10, 11 and this circulating PYY1-36 would be delivered to the renal microcirculation and glomeruli via the blood stream. Type 2 diabetics, particular those with the metabolic syndrome, have increased renal sympathetic tone12 and often ingest fatty meals.13 Thus, diabetics likely would have high renal levels of both NPY1-36 and PYY1-36, and inhibition of DPPIV would increase these elevated levels.
Both NPY1-36 and PYY1-36 are potent endogenous agonists of Y1Rs.7 However, DPPIV is anchored to the cell surface and efficiently converts PYY1-36 to PYY3-36 and NPY1-36 to NPY3-36 by cleaving two amino acids from the N-termini of PYY1-36 and NPY1-36.3, 5 DPPIV could just as logically be named “NPY Converting Enzyme” because the kcat/Km of DPPIV for NPY1-36 is approximately 36-fold and 73-fold greater for NPY1-36 compared with GLP-1 and GIP, respectively.5 Whereas PYY1-36 and NPY1-36 are potent Y1R agonists, PYY3-36 and NPY3-36 are inactive at Y1Rs but are selective Y2R agonists.14 Thus, likely DPPIV inhibitors chronically increase Y1R activation in PGVSMCs and GMCs.
Because diabetic nephropathy entails hypercellarity of glomeruli and increased extracellular matrix production in glomeruli, a significant unanswered question is whether PYY1-36 and NPY1-36 stimulate proliferation of and collagen production by preglomerular microvascular smooth muscle (PGVSMCs) and glomerular mesangial cells (GMCs) and whether DPPIV modulates these responses. Accordingly the goals of the present study were to determine in PGVSMCs and GMCs: 1) the expression of DPPIV mRNA, the expression of DPPIV protein and DPPIV activity; 2) whether there is an interaction between PYY1-36 and DPPIV and NPY1-36 and DPPIV such that the effects of PYY1-36 and NPY1-36 on proliferation and collagen production are enhanced by inhibition of DPPIV in PGVMSCs and GMCs; and 3) whether the expression and function of DPPIV and actions of PYY1-36 and NPY1-36 differ in PGVSMCs and GMCs from genetically hypertensive versus normotensive animals.
METHODS
Animals; Isolation and Culture of PGVSMCs and GMCs; Quantitative Real Time PCR for DPPIV; Western Blotting for DPPIV; Assay for PYY3-36; Extraction of Sitagliptin; Assessment of Cell Proliferation and Collagen Production; and Statistical Analysis: Please see online supplement (http://hyper.ahajournals.org).
RESULTS
Expression of DPPIV mRNA and Protein in PGVSMCs and GMCs
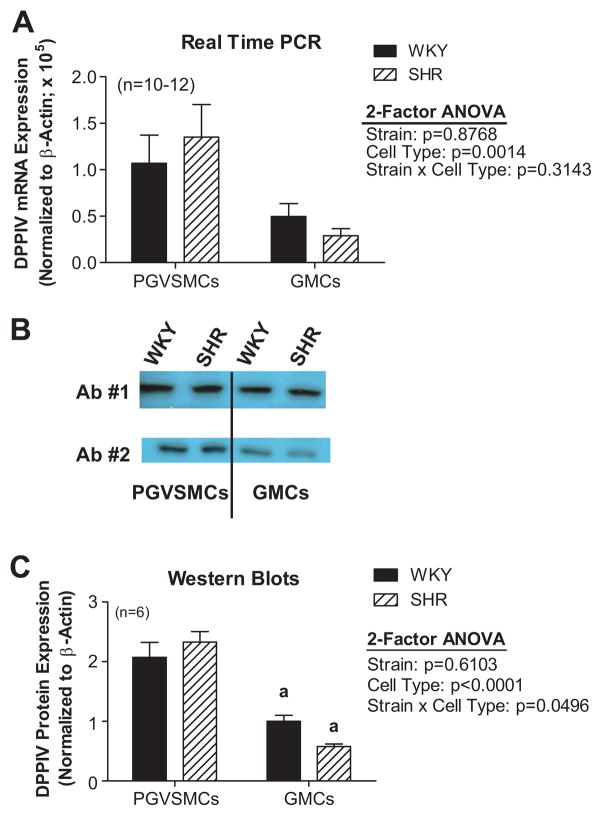
DPPIV mRNA was detected by quantitative real-time PCR in PGVSMCs and GMCs obtained from spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto rats (WKY). DPPIV mRNA expression was similar in SHR PGVSMCs compared with WKY PGVSMCs and was similar in SHR GMCs compared with WKY GMCs; however, overall DPPIV mRNA levels were lower in GMCs compared with PGVSMCs (Figure 1A). Western blots for DPPIV revealed a single band at 55 kDa in both cell types for both strains using two different antibodies (Figure 1B). This 55 kDa band is consistent with the datasheet for the DPP4 antibody 10940-1-AP and with a report by Bauvois.15 Bands were digitized and normalized to β-actin. DPPIV protein levels were similar in SHR versus WKY PGVSMCs and tended to be lower in SHR compared with WKY GMCs, although this did not achieve statistical significance (Figure 1C). Overall DPPIV protein levels were lower in GMCs compared with PGVSMCs.
Figure 1. (A) DPPIV mRNA levels in SHR and WKY PGVSMCs and GMCs.
DPPIV mRNA expression was determined by quantitative real time PCR and normalized to β-actin mRNA. (B) Western blotting for DPPIV protein using two different antibodies (Ab #1 and #2). A distinct and single band was observed at 55 kDa for both WKY and SHR PGVSMCs and GMCs. (C) DPPIV protein expression in SHR and WKY PGVSMCs and GMCs. DPPIV protein expression was quantified by densitometry analysis of Western blots and normalized to β-actin protein. Letter above bars (a) indicate significantly different (Fisher’s LSD test) from PGVSMCs.
Conversion of PYY1-36 to PYY3-36 in SHR PGVSMCs and GMCs
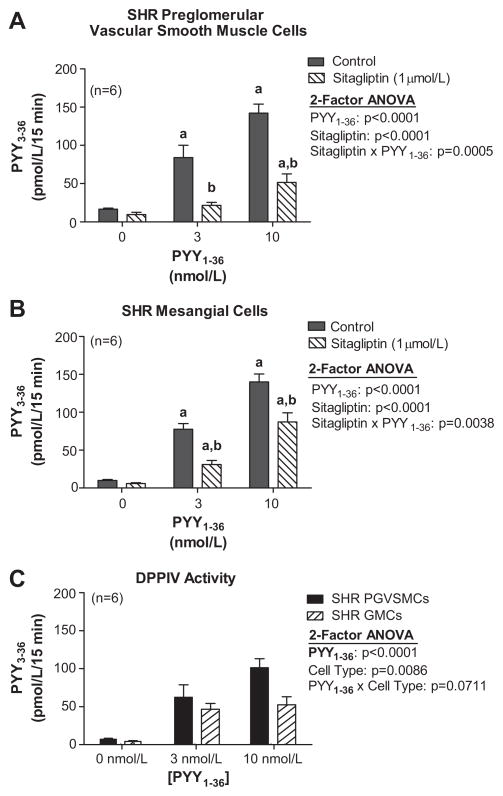
Confluent monolayers of cells were incubated in phosphate-buffered saline for 15 minutes with either 0, 3 or 10 nmol/L of PYY1-36 without or with sitagliptin, and the medium was assayed for PYY3-36. In both PGVSMCs (Figure 2A) and GMCs (Figure 2B), incubation with 3 and 10 nmol/L of exogenous PYY1-36 increased PYY3-36 medium levels to approximately 6-fold and 13-fold of basal, respectively. Sitagliptin significantly attenuated the increase in PYY3-36 levels in PGVSMCs and GMCs; however, sitaglipin did not abolish the PYY1-36-induced increase in PYY3-36 levels in either cell type. The sitagliptin-inhibitable PYY3-36 production (PYY3-36 levels in the absence of sitagliptin minus the average levels of PYY3-36 in the presence of sitagliptin) was greater in PGVSMCs compared with GMCs (Figure 2C).
Figure 2. Levels of PYY3-36 in the medium after incubation of SHR PGVSMCs (A) and GMCs (B) with PYY1-36.
Incubation was for 15 minutes at the indicated concentrations of PYY1-36 with or without sitagliptin. C: DPPIV activity as assessed by sigagliptin-inhibitable metabolism of PYY1-36 to PYY3-36 in SHR PGVSMCs and GMCs. Letters above bars indicate significantly different (Fisher’s LSD test) from basal (a) or from corresponding control (b).
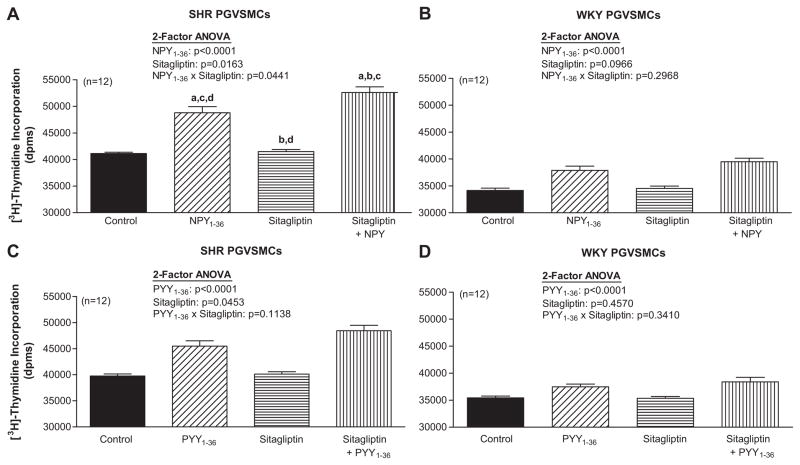
Effects of NPY1-36, PYY1-36 and Sitagliptin on [3H]-Thymidine Incorporation in SHR and WKY PGVSMCs
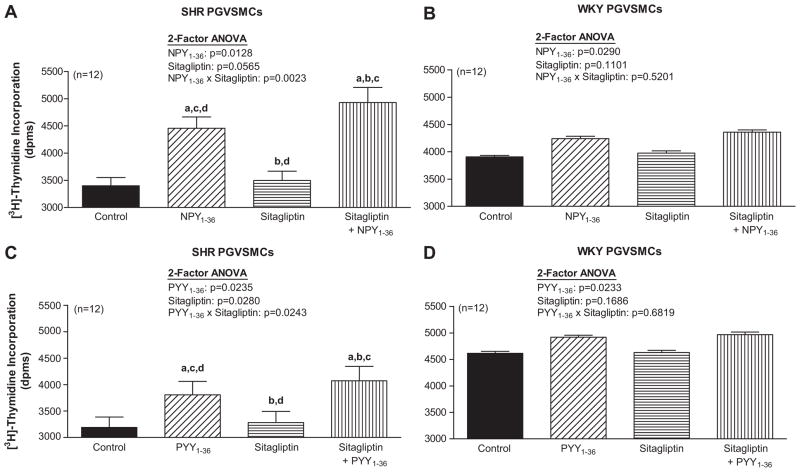
In SHR PGVSMCs, 2-factor ANOVA indicated a significant interaction between sitagliptin and NPY1-36 (Figure 3A) and sitagliptin and PYY1-36 (Figure 3C) on [3H]-thymidine incorporation. NPY1-36 and PYY1-36 stimulated [3H]-thymidine incorporation and this response was enhanced by sitagliptin. In WKY PGVSMCs, NPY1-36 (Figure 3B) and PYY1-36 (Figure 3D) significantly, but very mildly, increased [3H]-thymidine incorporation and these effects were not augmented by sitagliptin.
Figure 3. Effects of NPY1-36 (10 nmol/L; panels A and B) and PYY1-36 (10 nmol/L; C and D) in the absence or presence of sitagliptin (1 μmol/L) on [3H]-thymidine incorporation in SHR (A and C) and WKY (B and D) PGVSMCs.
Letters above bars indicate significantly different (Fisher’s LSD test) from control (a), peptide (b), sitagliptin (c) or sitagliptin plus peptide (d).
Effects of NPY1-36, PYY1-36 and Sitagliptin on Cell Number in SHR and WKY PGVSMCs
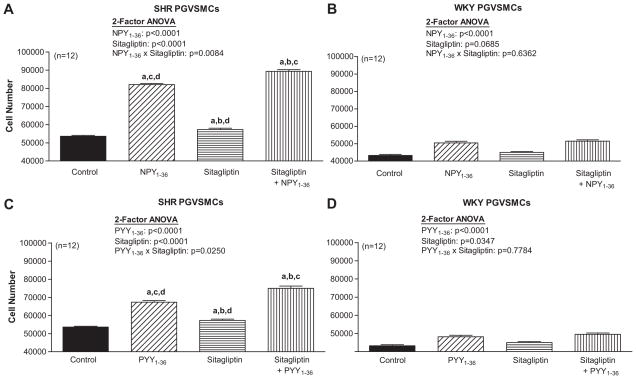
In SHR PGVSMCs, 2-factor ANOVA indicated a significant interaction between sitagliptin and NPY1-36 (Figure 4A) and sitagliptin and PYY1-36 (Figure 4C) on cell number. NPY1-36 and PYY1-36 increased cell number, and sitagliptin enhanced the growth effects of NPY1-36 and PYY1-36. In WKY PGVSMCs, NPY1-36 (Figure 4B) and PYY1-36 (Figure 4D) significantly, but very mildly, increased cell number; however these responses were not augmented by sitagliptin.
Figure 4. Effects of NPY1-36 (10 nmol/L; panels A and B) and PYY1-36 (10 nmol/L; C and D) in the absence or presence of sitagliptin (1 μmol/L) on cell number in SHR (A and C) and WKY (B and D) PGVSMCs.
Letters above bars indicate significantly different (Fisher’s LSD test) from control (a), peptide (b), sitagliptin (c) or sitagliptin plus peptide (d).
Effects of NPY1-36, PYY1-36 and Sitagliptin on [3H]-Proline Incorporation in SHR and WKY PGVSMCs
In SHR PGVSMCs, 2-factor ANOVA indicated a significant interaction between sitagliptin and NPY1-36 on [3H]-proline incorporation (Figure 5A). NPY1-36 stimulated [3H]-proline incorporation and this response was enhanced by sitagliptin. PYY1-36 also stimulated [3H]-proline incorporation in SHR PGVSMCs (Figure 5C); although, the interaction between sitagliptin and PYY1-36 was not statistically significant, there was a trend in this regard (p=0.1138). In WKY PGVSMCs, NPY1-36 (Figure 5B) and PYY1-36 (Figure 5D) significantly, but very mildly, increased [3H]-proline incorporation and these effects were not augmented by sitagliptin.
Figure 5. Effects of NPY1-36 (10 nmol/L; A and B) and PYY1-36 (10 nmol/L; C and D) in the absence or presence of sitagliptin (1 μmol/L) on [3H]-proline incorporation in SHR (A and C) and WKY (B and D) PGVSMCs.
Letters above bars indicate significantly different (Fisher’s LSD test) from control (a), peptide (b), sitagliptin (c) or sitagliptin plus peptide (d).
Effects of NPY1-36, PYY1-36 and Sitagliptin on [3H]-Thymidine Incorporation in SHR and WKY GMCs
In SHR GMCs, 2-factor ANOVA indicated a significant interaction between sitagliptin and NPY1-36 and sitagliptin and PYY1-36 on [3H]-thymidine incorporation (Figure S1A and S1C, respectively; please see http://hyper.ahajournals.org). NPY1-36 and PYY1-36 stimulated [3H]-thymidine incorporation and this response was enhanced by sitagliptin. In WKY GMCs, NPY1-36 and PYY1-36 significantly, but very mildly, increased [3H]-thymidine incorporation and these effects were not augmented by sitagliptin (Figure S1B and S1D, respectively; please see http://hyper.ahajournals.org).
Effects of NPY1-36, PYY1-36 and Sitagliptin on Cell Number in SHR and WKY GMCs
In SHR GMCs, 2-factor ANOVA indicated a significant interaction between sitagliptin and NPY1-36 and sitagliptin and PYY1-36 on cell number (Figure S2A and S2C, respectively; please see http://hyper.ahajournals.org). NPY1-36 and PYY1-36 increased cell number, and sitagliptin enhanced the growth effects of NPY1-36 and PYY1-36. In WKY PGVSMCs, NPY1-36 and PYY1-36 significantly, but very mildly, increased cell number; however these responses were not augmented by sitagliptin (Figure S2B and S2D, respectively; please see http://hyper.ahajournals.org)
Effects of NPY1-36, PYY1-36 and Sitagliptin on [3H]-Proline Incorporation in SHR and WKY GMCs
In SHR GMCs, 2-factor ANOVA indicated a significant interaction between sitagliptin and NPY1-36 on [3H]-proline incorporation (Figure S3A; please see http://hyper.ahajournals.org). NPY1-36 stimulated [3H]-proline incorporation and this response was enhanced by sitagliptin. PYY1-36 also stimulated [3H]-proline incorporation in SHR GMCs; although, the interaction between sitagliptin and PYY1-36 was not statistically significant, there was a trend in this regard (p=0.0708) (Figure S3C; please see http://hyper.ahajournals.org). In WKY GMCs, NPY1-36 and PYY1-36 significantly increased [3H]-proline incorporation and the effect of NPY1-36, but not PYY1-36, was significantly augmented by sitagliptin (Figure S3B and S3D, respectively; please see http://hyper.ahajournals.org).
Effects of NPY3-36, PYY3-36 and Sitagliptin on [3H]-Thymidine Incorporation in SHR and PGVSMCs and GMCs
In SHR PGVSMCs, neither NPY3-36 nor PYY3-36 affected [3H]-thymidine incorporation (Figure S4A and S4B, respectively; please see http://hyper.ahajournals.org) either in the absence or presence of sitagliptin. In SHR GMCs, NPY3-36 and PYY3-36 significantly, but very mildly, increased [3H]-thymidine incorporation and these effects were not augmented by sitagliptin (Figure S4C and S4D, respectively; please see http://hyper.ahajournals.org).
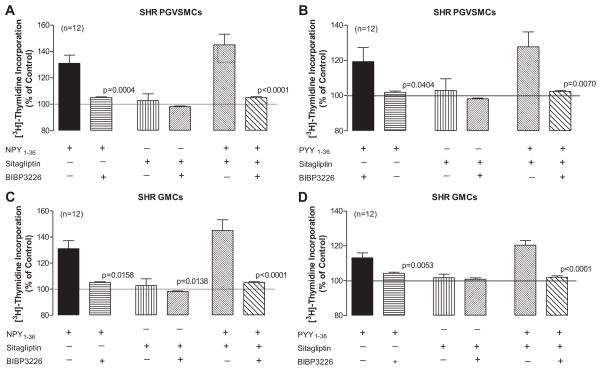
Effects of BIBP3226 on [3H]-Thymidine Incorporation Induced by NPY1-36, PYY1-36, Sitagliptin plus NPY1-36 and Sitagliptin plus PPY1-36 in SHR PGVSMCs and GMCs
In SHR PGVSMCs and GMCs BIBP3226 abolished the ability of both NPY1-36 and PYY1-36 to stimulate [3H]-thymidine incorporation (Figure 6). This complete inhibition by BIBP3226 was also observed for the combination effects of sitagliptin plus NPY1-36 and sitagliptin plus PYY1-36.
Figure 6. Effects of BIBP3226 (10 μmol/L) in SHR PGVSMCs (A and B) and GMCs (C and D) on [3H]-thymidine incorporation induced by NPY1-36 (10 nmol/L; A and C) and PYY1-36 (10 nmol/L; B and D) in the absence or presence of sitagliptin (1 μmol/L).
P-values in panels compare corresponding treatment groups with and without BIBP3226 (unpaired Student t-tests).
Effects of GLP-1 on Cell Number in SHR and WKY PGVSMCs
Because 1) GLP-1 is also an important substrate for DPPIV, 2) GLP-1 induces vasodilation16, 17, and 3) vasodilators often are associated with inhibition of vascular smooth muscle cell growth, we also examined the effects of GLP-1(7–36) on proliferation of SHR and WKY PGVSMCs in the absence and presence of sitagliptin. At concentrations from 1 to 100 nmol/L, GLP-1 had little, if any effect, on PDGF-induced proliferation of either SHR or WKY PGVSMCs, and there was no interaction between sitagliptin and GLP-1 in either SHR or WKY PGVSMCs (Figure S5; please see http://hyper.ahajournals.org).
DISCUSSION
The present results indicate that DPPIV message and protein are expressed in vascular and glomerular cellular elements and could therefore importantly influence the metabolism of NPY1-36 and PYY1-36. Because we do not have an assay that distinguishes NPY1-36 from NPY3-36 yet we do have an assay that detects PYY3-36 without cross-reacting with PYY1-36, in the present study we assessed the ability of DPPIV to metabolize PP-fold peptides by measuring the production of PYY3-36 from PYY1-36. Likely, both peptides are metabolized similarly by DPPIV, so the results for PYY1-36 metabolism should reflect the ability of DPPIV to metabolize NPY1-36. Indeed, our results show that the expression of DPPIV message and protein by PGVSMCs and GMCs is associated with the ability of these cell types to metabolize PYY1-36 to PYY3-36. Importantly, sitagliptin, a potent and selective inhibitor of DPPIV1, suppresses the conversion of PYY1-36 to PYY3-36 in both cell types. This finding confirms that at least a portion of the enzymatic activity that converts PYY1-36 to PYY3-36 in PGVSMCs and GMCs is due to DPPIV.
We hypothesize that inhibition of DPPIV may have adverse renal consequences because: 1) proliferation of PGVSMCs and GMCs mediate detrimental changes in renal structure and function;18 2) activation of Y1 receptors may stimulate growth of aortic vascular smooth muscle cells19 so it is conceivable that Y1 receptors could stimulate growth of PGVSMCs and GMCs; 3) NPY1-36 and PYY1-36, but not NPY3-36 and PYY3-36, activate Y1 receptors; 4) DPPIV is present in PGVSMCs and GMCs; and 5) inhibition of DPPIV reduces the metabolism PP-fold peptides in PGVSMCs and GMCs.
Importantly, the results of the present study demonstrate that NPY1-36 and PYY1-36 stimulate proliferation of PGVSMCs and GMCs. Although these effects of NPY1-36 and PYY1-36 are barely perceptible in PGVSMCs and GMCs obtained from normotensive rats, the ability of NPY1-36 and PYY1-36 to stimulate proliferation of PGVSMCs and GMCs is pronounced in cells from genetically-hypertensive animals. These findings underscore the potential for NPY1-36 and PYY1-36 to regulate cell growth in renovascular and glomerular cellular elements in genetically-susceptible animals.
Real-time PCR and Western blotting show similar DPPIV mRNA and protein levels in PGVSMCs and GMCs from SHR versus WKY rats, yet NPY1-36 and PYY1-36 have little effect on proliferation of WKY PGVSMCs and GMCs. Our previously published work demonstrates that Y1 and Y2 receptor expression is not different in freshly isolated preglomerular microvessels from SHR versus WKY.20 However, our previously published work does show that signaling via Gi-linked receptors is enhanced in the renal vasculature of SHR versus WKY.20 Inasmuch as Y1 receptors are Gi-coupled receptors, the most likely reason that NPY1-36 and PYY1-36 stimulate growth more in SHR versus WKY PGVSMCs and GMCs is that post-receptor signaling via the Gi pathway is enhanced in SHR PGVSMCs and GMCs.
The present study also demonstrates that the pro-growth effects of NPY1-36 and PYY1-36 in cells from genetically-susceptible rats are enhanced by inhibition of DPPIV; whereas inhibition of DPPIV appears to have little effect on the pro-growth effects of NPY1-36 and PYY1-36 in cells from genetically-non-susceptible rats. The enhancement of the pro-growth effects of NPY1-36 and PYY1-36 in cells from genetically-susceptible animals by inhibition of DPPIV is likely mediated by reducing the rate of metabolism of NPY1-36 and PYY1-36 to NPY3-36 and PYY3-36, respectively, leading to greater activation of Y1 receptors. The evidence for this conclusion is that unlike NPY1-36 and PYY1-36, NPY3-36 and PYY3-36 have little effect on cell growth even in cells from genetically-susceptible animals and the pro-growth effects of both NPY1-36 and PYY1-36 are abolished by the selective Y1-receptor antagonist BIBP3226.
In addition to accelerating cell proliferation, out experiments support the conclusion that both NPY1-36 and PYY1-36 stimulate collagen production by both PGVSMCs and GMCs, with the effect being more pronounced in cells from genetically-hypertensive animals. The ability of NPY1-36 to stimulate extracellular matrix production was significantly enhanced by DPPIV inhibition in SHR PGVSMCs and SHR and WKY GMCs. Although the interaction between sitagliptin and PYY1-36 did not reach statistical significance, there was a strong trend toward such an interaction in SHR PGVSMCs and GMCs.
Although the focus of the present study was NPY1-36 and PYY1-36, these peptides are not the only substrates for DPPIV that could enhance proliferation of PGVSMCs and GMCs. An important question, therefore, is whether the effects of sitagliptin observed in the present study can be attributed entirely to an interaction with the metabolism of NPY1-36 and PYY1-36. Importantly, in our experiments we used PDGF as our growth stimulant (i.e., we did not add serum). So the only pro-growth factors present in the medium were PDGF and NPY1-36 or PYY1-36. Since PDGF is not a substrate for DPPIV, we are reasonably certain that the effects of sitagliptin were mediated in our experimental paradigm via inhibition of NPY1-36 and PYY1-36 metabolism. This hypothesis is further supported by the complete blockade of the effect of sitagliptin by antagonism of Y1 receptors.
Nonetheless, in vivo sitagliptin certainly affects the metabolism of multiple peptides, and any adverse effects of sitagliptin on renal morphology in vivo could very well be due to enhancement of the levels of multiple pro-growth peptides. To address this issue, it will be necessary to examine in vitro the interaction of sitagliptin with all possible growth promoting peptides that are metabolized by DPPIV. The lack of an interaction between GLP-1 and sitagliptin on PDGF-induced proliferation of either SHR PGVSMCs or GMCs suggests that in vivo the pro-growth effects of DPPIV inhibitors mediated by increases in NPY1-36 and PYY1-36 are unlikely to be offset by increases in GLP-1. However, in vivo DPP4 inhibitors decrease glucagon secretion somewhat21 and glucagon may stimulate GMC proliferation22. Thus, it is conceivable that in vivo decreases in glucagon-driven promotion of GMC growth could offset some of the pro-growth effects due to enhanced Y1 receptor activation.
Perspective
In 2006, the FDA approved sitagliptin, the first DPPIV inhibitor to attain FDA approval for the treatment of type 2 diabetes. More recently other DPPIV inhibitors have been approved by the FDA including saxagliptin and linagliptin, and many other DPPIV inhibitors are in development. In all likelihood, DPPIV inhibitors will become widely employed to treat type 2 diabetics, many of whom will have hypertension, vascular disease and renal diseases as co-morbidities. A recent study by Kirino and co-workers23 shows that in DPPIV-deficient rats, streptozotocin-induced diabetes profoundly and chronically reduces creatinine clearance when compared with DPPIV-expressing rats. Moreover, case reports are now appearing linking DPPIV inhibitors to renal impairment in patients with type 2 diabetes.24 The present study demonstrates that cellular elements in the preglomerular renal microcirculation and glomeruli express DPPIV message, protein and activity. Also our results indicate that in cells from genetically-susceptible animals, proliferation of and extracellular matrix production by PGVSMCs and GMCs can be driven by NPY1-36 and PYY1-36 and that DPPIV inhibition enhances these effects of NPY1-36 and PYY1-36. Because in vivo kidneys are normally exposed to high levels of NPY1-36 and PYY1-36, long-term treatment with DPPIV inhibitors could enhance proliferation of and collagen production by PGVSMCs and GMCs in genetically-susceptible hypertensive patients. Long-term treatment with DPPIV inhibitors might therefore entail a risk of renal dysfunction due to abnormal proliferation of cells in the preglomerular microcirculation and glomeruli. Therefore, the present findings suggest vigilance as DPPIV inhibitors become widely employed in the treatment of type 2 diabetics with hypertension, vascular disease or renal dysfunction. The recent finding that DPPIV inhibition may alter the antihypertensive effects of ACE inhibitors25 further underscores this concern.
Novelty and Significance.
What Is New?
Preglomerular vascular smooth muscle cells (PGVSMCs) and glomerular mesangial cells (GMCs) from spontaneously hypertensive rats (SHR) express dipeptidyl peptidase IV (DPPIV) mRNA, protein and activity.
Neuropeptide Y1-36 (NPY1-36) and peptide YY1-36 (PYY1-36), which are high-affinity endogenous substrates for DPPIV, stimulate proliferation of and collagen synthesis by SHR PGVSMCs and GMCs.
In SHR PGVSMCs and GMCs, inhibition of DPPIV enhances the induction of proliferation and collagen production by NPY1-36 and PYY1-36.
What Is Relevant?
Because in vivo kidneys are normally exposed to high levels of NPY1-36 and PYY1-36, long-term treatment with DPPIV inhibitors could enhance proliferation of and collagen production by PGVSMCs and GMCs in genetically-susceptible hypertensive patients.
Summary
Long-term treatment with DPPIV inhibitors might entail a risk of renal dysfunction due to abnormal proliferation of cells in the preglomerular microcirculation and glomeruli, and recent case reports support this concern.
Acknowledgments
SOURCES OF FUNDING
The work was supported by the National Institutes of Health [DK091190, HL069846, DK068575 and DK079307].
Footnotes
Disclosures: NONE
References
- 1.Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006;60:1454–1470. doi: 10.1111/j.1742-1241.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds K, Wildman RP. Update on the metabolic syndrome: hypertension. Curr Hypertens Rep. 2009;11:150–155. doi: 10.1007/s11906-009-0026-5. [DOI] [PubMed] [Google Scholar]
- 3.McIntosh CH, Demuth HU, Pospisilik JA, Pederson R. Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents? Regul Pept. 2005;128:159–165. doi: 10.1016/j.regpep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci. 2005;108:277–292. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 5.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 6.Brown NJ, Byiers S, Carr D, Maldonado M, Warner BA. Dipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedema. Hypertension. 2009;54:516–523. doi: 10.1161/HYPERTENSIONAHA.109.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med. 2003;228:217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- 8.DiBona GF, Kopp UC. Neural control of renal function. Physiological Reviews. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 9.Haefliger JA, Waeber B, Grouzmann E, Braissant O, Nussberger J, Nicod P, Waeber G. Cellular localization, expression and regulation of neuropeptide Y in kidneys of hypertensive rats. Regul Pept. 1999;82:35–43. doi: 10.1016/s0167-0115(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 10.Anini Y, Fu-Cheng X, Cuber JC, Kervran A, Chariot J, Roz C. Comparison of the postprandial release of peptide YY and proglucagon-derived peptides in the rat. Pflugers Arch. 1999;438:299–306. doi: 10.1007/s004240050913. [DOI] [PubMed] [Google Scholar]
- 11.Fu-Cheng X, Anini Y, Chariot J, Castex N, Galmiche JP, Roze C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: neural regulation by proximal gut. Pflugers Arch. 1997;433:571–579. doi: 10.1007/s004240050316. [DOI] [PubMed] [Google Scholar]
- 12.Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- 13.Freire RD, Cardoso MA, Gimeno SGA, Ferreira SRG Japanese-Brazilian Diabetes Study G. Dietary fat is associated with metabolic syndrome in Japanese Brazilians. Diabetes Care. 2005;28:1779–1785. doi: 10.2337/diacare.28.7.1779. [DOI] [PubMed] [Google Scholar]
- 14.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 15.Bauvois B. A collagen-binding glycoprotein on the surface of mouse fibroblasts is identified as dipeptidyl peptidase IV. Biochemical Journal. 252:723–731. doi: 10.1042/bj2520723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz S-S, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways.[Erratum appears in Circulation. 2008 Jul 22;118(4):e81] Circulation. 117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 17.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys. 478:136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Dubey RK, Jackson EK, Rupprecht HD, Sterzel RB. Factors controlling growth and matrix production in vascular smooth muscle and glomerular mesangial cells. Curr Opin Nephrol Hypertens. 6:88–105. doi: 10.1097/00041552-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Pons J, Kitlinska J, Jacques D, Perreault C, Nader M, Everhart L, Zhang Y, Zukowska Z. Interactions of multiple signaling pathways in neuropeptide Y-mediated bimodal vascular smooth muscle cell growth. Can J Physiol Pharmacol. 86:438–448. doi: 10.1139/y08-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubinion JH, Mi Z, Zhu C, Gao L, Jackson EK. Pancreatic polypeptide-fold peptide receptors and angiotensin II-induced renal vasoconstriction. Hypertension. 2006;47:545–551. doi: 10.1161/01.HYP.0000197033.54756.83. [DOI] [PubMed] [Google Scholar]
- 21.Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4) Best Practice & Research Clinical Endocrinology & Metabolism. 23:479–486. doi: 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Li XC, Carretero OA, Shao Y, Zhuo JL. Glucagon receptor-mediated extracellular signal-regulated kinase 1/2 phosphorylation in rat mesangial cells: role of protein kinase A and phospholipase C. Hypertension. 47:580–585. doi: 10.1161/01.HYP.0000197946.81754.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K, Nakahori Y. Interrelationship of dipeptidyl peptidase IV (DPP IV) with the development of diabetes, dyslipidaemia and nephropathy: A streptozotocin-induced model using wild-type and DPP IV-deficient rats. J Endocrinol. 2008 doi: 10.1677/JOE-08-0424. JOE-08-0424. [DOI] [PubMed] [Google Scholar]
- 24.Lestner JM, Baburaj R, Edwards CMB. Renal impairment with sitagliptin: is there a need for active monitoring of potential renal toxicity? Br J Hosp Med. 2011;72:412–413. doi: 10.12968/hmed.2011.72.7.412. [DOI] [PubMed] [Google Scholar]
- 25.Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 56:728–733. doi: 10.1161/HYPERTENSIONAHA.110.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]