Abstract
Tumor metastasis is a complex phenomenon that is the culmination of effects of numerous cellular factors. We have shown that the EBV oncoprotein, latent membrane protein 1 (LMP1), is capable of inducing a wide range of such factors in cell culture, expression of which is also elevated in the LMP1-expressing tumor, nasopharyngeal carcinoma (NPC), a highly invasive neoplasm. Recently, the membrane-crosslinker protein ezrin has been implicated in tumor cell metastasis and malignant progression. In this study, we evaluated the possible role of LMP1 and ezrin in the pathophysiology of NPC. We show that C-terminal phosphorylation of ezrin is increased by expression of LMP1 in nasopharyngeal (NP) cells through a Protein Kinase C (PKC) pathway. LMP1 enhances organization of a ternary complex of CD44, ezrin and F-actin which is a prerequisite for ezrin phosphorylation. In NPC tissues, expression of phosphoezrin and LMP1 is directly correlated. Silencing of endogenously expressed ezrin suppresses LMP1-induced cell motility and invasiveness. Moreover inhibition of ezrin phosphorylation by PKC inhibitor suppresses migration and invasion of NP cells. These data demonstrate that phosphorylation of ezrin and its recruitment to the cell membrane linked to F-actin and CD44 is a process required for LMP1-stimulated cell motility and invasion of NP cells.
Keywords: latent membrane protein 1, Ezrin, nasopharyngeal carcinoma, cell migration
Introduction
From a worldwide perspective NPC is the most important tumor associated with EBV infection. NPC is latently infected with the virus, and monoclonal EBV episomes are present and expressed in the tumor cells in both endemic and sporadic forms of NPC regardless of geographic origin. The EBV protein LMP1 is detected in most NPC, which classically displays a type II latent gene expression profile that consists of EBNA1, LMP1, LMP2a, LMP2b and EBERs. Among them LMP1 the principal oncoprotein is considered to be a key factor in NPC development (Tsao SW et al., 2002; Lo et al., 2004).
Among head-and-neck cancers NPC is distinguished by its highly metastatic character and poor prognosis (Lo et al., 2004). LMP1 is known to elicit its oncogenic properties by constitutively activating multiple signaling pathways, such as NF-κB, c-Jun, AP-1, JAK3/STAT, p38MAPK and, recently identified, the PKC pathway (Yan et al., 2007). We have shown that LMP1 induces the expression of a series of cell-invasiveness and angiogenetic factors and their effects in nasopharyngeal (NP) cells, including matrix metalloproteinase 9 (MMP9) (Yoshizaki et al., 1998), MMP1, MMP3 (Kondo et al., 2005), c-Met and ets-1 (Horikawa et al., 2001), fibroblast growth factor 2 (FGF-2) (Wakisaka et al., 2002), vascular endothelial factor (VEGF) (Murono et al., 2001), hypoxia-inducible factor 1 (HIF 1α) (Wakisaka et al., 2004), MUC1 (Kondo et al., 2007), Siah 1 (Kondo et al., 2006), and Twist (Horikawa et al., 2007). Several of these factors are expressed in NPC tissues in association with LMP1 and are thought to be involved in invasion and metastasis of NPC (Yoshizaki et al., 2005).
The ability of primary tumor cells to migrate is an early contributor to tumor cell metastasis. Cell migration is a highly regulated process that shapes the organization of every tissue in the body (Hopkins et al., 2007). Recently, it has been reported that ezrin modulates remodeling of actin cytoskeleton and is implicated in tumor cell migration and progression of certain tumors (Khanna et al., 2004; Bruce et al., 2007; Fais et al., 2004).
Ezrin is a member of the ezrin/moesin/radixin (ERM) family that acts as linker between the plasma membrane and the actin cytoskeleton and generates propulsive forces driving cell migration (Hopkins et al., 2007). ERM proteins are highly homologous to one another and there is no clear functional difference among them (Martin et al., 2003). The ERM family exhibits a tissue-specific expression pattern; Ezrin is the most ubiquitous ERM protein in epithelial cells and thought to play an important role in progression of several cancers (Khanna et al., 2004; Auvinen et al., 2007). Moesin is enriched in endothelial cells and leukocytes, and radixin is abundant in liver (Doi et al., 1998). In the cytoplasm, ezrin exists in an autoinhibited conformation through an intramolecular interaction between its N-terminal and C-terminal domains or through formation of oligomers that include ezrin and other ERM proteins. This interaction masks membrane and F-actin binding sites. Cellular signals trigger the activation of ezrin, resulting in the unmasking of the functional binding sites (Auvinen et al., 2007, Louvet-Vallée, 2000). Several residues of ezrin have recently been found to be phosphorylated and in that state functionally active (Louvet-Vallée, 2000). Specifically, the phosphorylation of ezrin on its C-terminal threonine 567 unmasks its binding sites for actin and cell membrane in its conformationally activated form (Fievet et al., 2004). In this state ezrin moves to the cell membrane and tethers actin to it, which also affects the architecture and dynamics of the actin cytoskeleton. Basic to these functional effects is the phosphorylation of ezrin which occurs upon stimulation of cells with hepatocyte growth factor (HGF) or epidermal growth factor (EGF) or androgen, resulting in modification of cell morphology (Crepaldi et al., 1997; Chuan et al., 2006).
Central functions of ezrin are regulation of cell shape, motility, adhesion and signal transduction, all of which are important for tumor development and progression (Fais et al., 2004). Here we analyze phosphorylation and activation of ezrin induced by LMP1 and show that phosphoezrin localizes predominantly in the cell membrane linked to CD44 and that the depletion of ezrin by small interfering RNA significantly attenuates LMP1-stimulated cell motility and invasion. Also, in NPC tissues expression of phosphoezrin and LMP1 is directly correlated.
Results
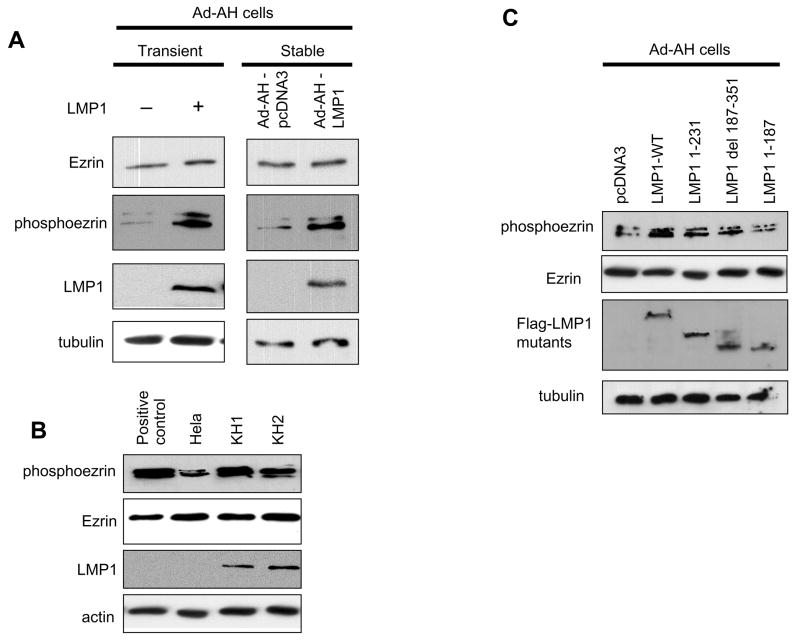
LMP1 phosphorylates ezrin at Thr 567 but does not induce its expression
To investigate whether LpMP1 affects the induction or the phosphorylation of ezrin, we used a nonmalignant EBV-negative nasopharyngeal cell line, Ad-AH. No effect on level of ezrin protein waps produced by expression of LMP1. However phosphorylation of ezrin detected with phosphoezrin antibody, which recognizes only protein phosphorylated on Thr567 in the C-terminal region, significantly increased in both transiently and stably transfected cells (Fig. 1A). Although phosphorylated moesin and radixin might be detected, ezrin is the most ubiquitous ERM protein in epithelial cells. We next investigated endogeneous levels of phosphoezrin in a set of type II latently EBV-infected cell lines, KH1 and KH2, which were derived by somatic-cell fusion of a latently EBV-infected type III lymphoblastoid KR4 cell line with Hela cells (Contreras-Brodin et al., 1991). Hela cells stimulated with EGF were used as a positive control for phosphorylation of ezrin (Baumgartner et al., 2006). In both type II cell lines, endogenous phosphoezrin levels were higher than in the parental Hela cells, which are EBV-negative (Fig. 1B).
Figure 1.
LMP1 increases phosphorylation of ezrin in nasopharyngeal epithelial cells. A, Immunoblot analyses of phosphoezrin and LMP1 in Ad-AH cells: transient (left) or stable (right) LMP1 transfectants. B, In type II latently EBV-infected lines, KH1 and KH2, phosphoezrin level was higher than in EBV-negative parental Hela cells. Hela cell stimulated with EGF (25 nM) served as positive control for phosphorylation. C, Ezrin is phosphorylated by both LMP1 CTAR1 and CTAR2. LMP1 1–231 retains CTAR1 only, LMP1 del 187–351 has CTAR2 only, and LMP1 1–187 has both CTAR1 and CTAR2 deleted.
To examine the viral mechanism of phosphorylation, we used mutants of expression plasmids of the principal LMP1 intracellular signaling domains, CTAR1 and CTAR2. LMP1 1–187, which has both CTAR1 and CTAR2 deleted, had no effect on the phosphorylation of ezrin. Both LMP1 1–231, which has CTAR1 only, and LMP1 del 187–351, which has CTAR2 only, induced phosphoezrin, but phosphorylation was weaker than when induced by LMP1-WT (Fig. 1C). These findings indicate that LMP1 can produce an increase in ezrin Thr567 phosphorylation in NP cells, and that LMP1 CTAR1 and CTAR2 both participate.
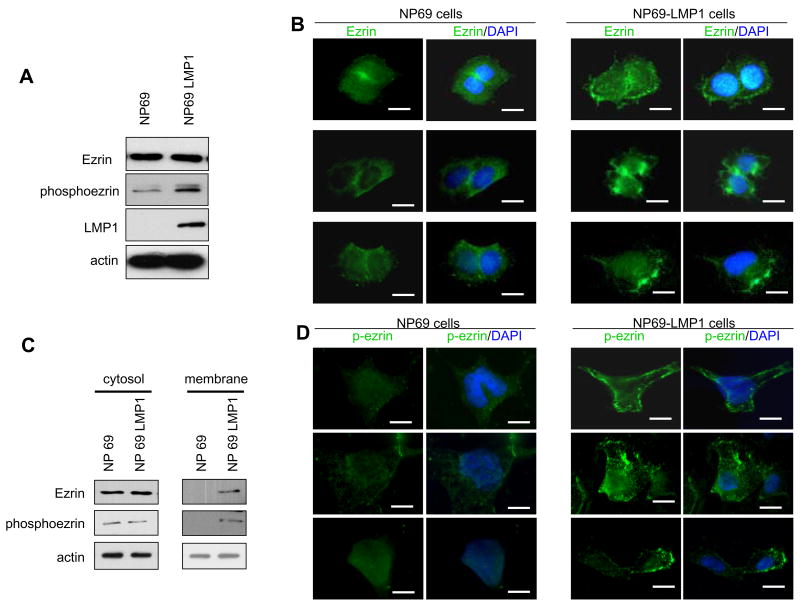
LMP1 modulates translocation of ezrin from the cytosol to the plasma membrane
C-terminal phosphorylated ezrin is known to be activated and recruited to the plasma membrane (Gautreau et al., 2002). We investigated the localization of endogenous ezrin phosphorylated by LMP1 by using another nonmalignant EBV-negative nasopharyngeal cell line, NP69. In LMP1-expressing NP69 cells, the level of phosphoezrin was increased compared with parental control NP69 cells (Fig. 2A); results were similar to those obtained with Ad-AH cells (Fig 1A). Fig. 2B shows that in control NP cells, ezrin is present in the cytosol and also in cell-cell contacts, which means that most of the ezrin exists in an inactive form. However in LMP1-expressing cells, ezrin was detected in plasma membrane. Subcellular fractionation verified this shift in ezrin distribution caused by LMP1: ezrin and phosphoezrin were mostly detected in the cytosol fraction, but only in the plasma membrane fraction in LMP1-expressing cells (Fig. 2C). Moreover, immunofluorescence analysis revealed phosphoezrin in plasma membrane (Fig. 2D). Thus LMP1 mediates translocation of ezrin from the cytoplasm to the cell membrane, which indicates that phosphorylated ezrin is activated and is positioned to function as a cross-linker protein between adhesion molecules and actin cytoskeleton.
Figure 2.
Localization of ezrin in NP69 cells. A, Immunoblot analysis of phosphoezrin in NP69 cells stably expressing LMP1. B, Immunofluorescence analyses. In control NP69 cells, most ezrin staining was localized at cell-cell contacts and cytoplasm, while in NP69-LMP1 expressing cells, ezrin was detected in cell membrane. B, Cells stained by p-ezrin antibody. C, Ezrin and phosphoezrin distribution in subcellular fractions. Equal amounts of proteins from membrane-enriched and cytosol fractions were probed with ezrin and phosphoezrin antibody. Ezrin and phosphoezrin were detected in membrane fraction only in LMP1-expressing cells. D, Immunofluorescence analyses of NP69 cells stained by phosphoezrin antibody. In NP69-LMP1 expressing cells, phosphoezrin was detected in plasma membrane. Bar=10 μm
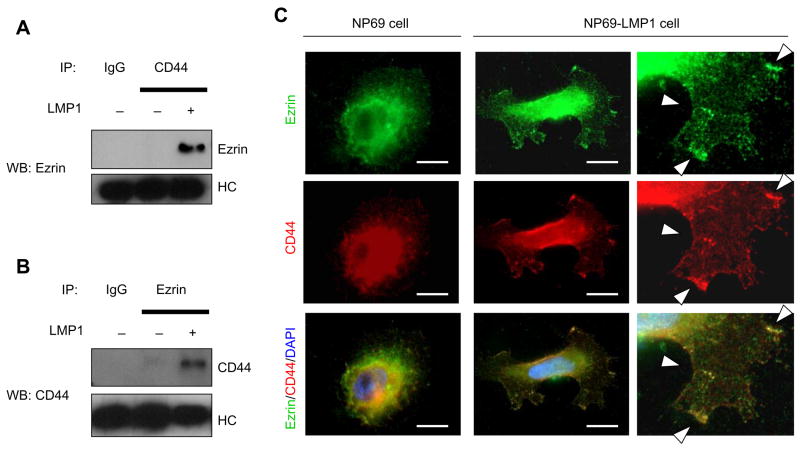
LMP1 modulates phosphorylation of ERM protein, which links to CD44
Phosphorylation of ezrin on Thr567 is reported to activate and disrupt intramolecular association and changes in ezrin localization; formation of actin-rich structures near the cell membrane are also produced by phosphorylation of ezrin on Thr567 (Fievet et al., 2004; Gautreau et al., 2002). To look for potential binding candidates for ezrin, we evaluated which membrane-anchored cell-adhesion molecule binds phosphorylated ezrin. LMP1 is reported to be associated with ICAM-1 and CD44 (Walter et al., 1995; Park et al., 2002). Fig. 3A shows that in LMP1-expressing cells ezrin could be coprecipitated by CD44 antibody. In addition CD44 could be coprecipitated by ezrin antibody in LMP1-exressing cells (Fig. 3B) Double-staining with CD44 and ezrin antibodies showed that both proteins were co-localized at the plasma membrane in LMP1-expressing cells (Fig. 3C). Interaction of ezrin with ICAM-1 was not detected by IP (data not shown). These data suggest that ezrin as modulated by LMP1 is recruited to the cell membrane and forms crosslinks between CD44 and actin cytoskeleton.
Figure 3.
Ezrin closslinks with CD44 in LMP1-expressing cells. A, Ezrin is immunoprecipitated from NP69 cells by CD44 antibody. LMP1 increases coprecipitation of CD44 and ezrin. B, CD44 is immunoprecipitated from NP69 cells by ezrin antibody. C, In LMP1-expressing NP69 cells, ezrin and CD44 colocalize in the cytosol and cell membrane. NP69 cells were immunostained for ezrin (green), CD44 (red) and nuclei (blue). Right panel is higher power view of lower right area in NP69 LMP1-expressing cells. Arrows indicate colocalization of ezrin with CD44. Bar=10 μm
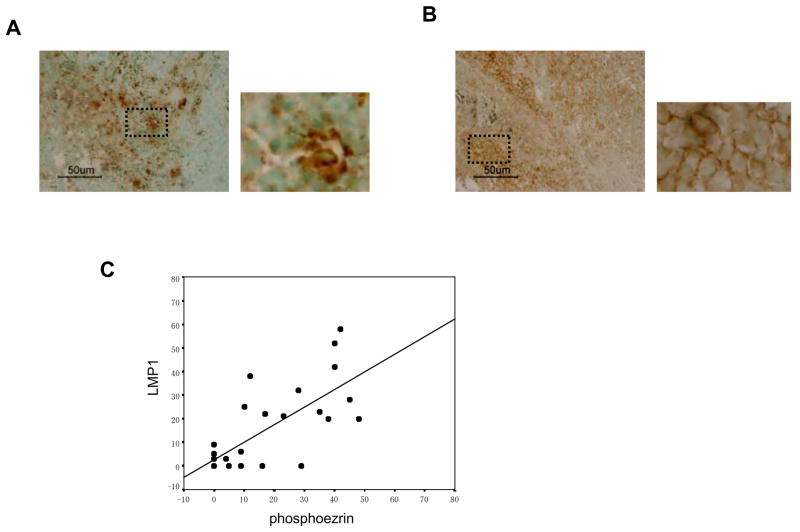
Expression of phosphoezrin correlates directly with LMP1-positivity in NPC tissue
Based on the results in the cell-culture models, we next tested whether phosphoezrin could be detected in LMP-positive NPC tissues (see details in Material and Methods). Immunohistochemical staining revealed phosphoezrin mainly in plasma membrane and cytoplasm of the tumor cells (Fig. 4A). LMP1 protein was also located in the membrane and cytoplasm (Fig. 4B). Expression scores for phosphoezrin and LMP1 were closely correlated (r=0.676; p<0.001) (Fig. 4C). These results support the conclusion that LMP1 induces the C-terminal phosphorylation of ezrin not only in cell culture but in tumor tissue in vivo.
Figure 4.
Expression of phosphoezrin and LMP1 proteins in NPC tissues. A, Detection of LMP1 in NPC by immunohistochemistry. LMP1 is detected mainly in cytoplasm and plasma membrane. B, Detection of phosphoezrin in NPC. phosphoezrin is visible in cytoplasm and plasma membrane. C, Expression scores for LMP1 and phosphoezrin in the 29 NPC specimens. Average percentages of tumor cells staining for LMP1 and p-ezrin are plotted. Expression scores (see Material and methods) for LMP1 and p-ezrin correlated significantly. Pearson correlation coefficient, r=0.676; p<0.001.
Ezrin plays a pivotal role in LMP1-stimulated cell morphology, motility and invasiveness
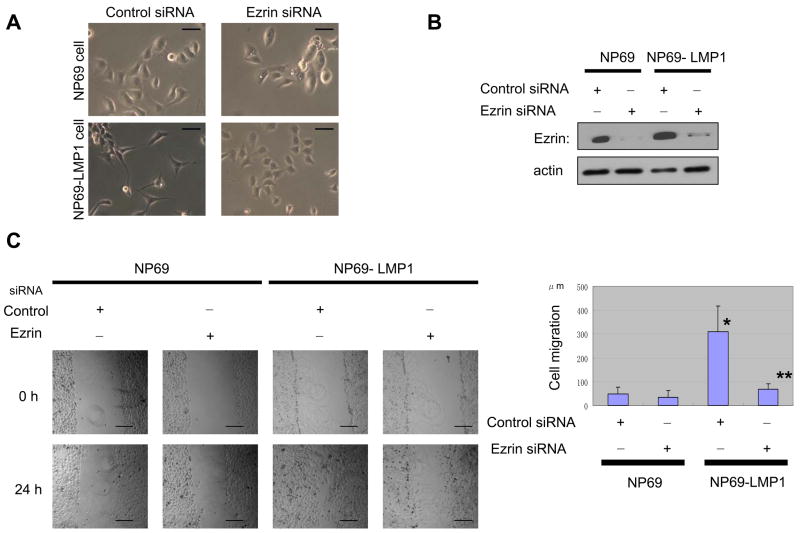
The regulation of cell shape, motility and invasiveness by ezrin in its phosphorylated state is well documented (Khanna et al., 2004; Chuan et al., 2006). We therefore studied the contribution of LMP1-indeced phosphoezrin to such properties by the use of siRNA targeting ezrin. LMP1-expressing NP cells characteristically have a spindle-like fibroblastic appearance whereas control cells have a cobble-stone-like morphology (Fig. 5A). The fibroblastic appearance disappears in LMP1-expressing cells transfected with ezrin siRNA. Expression of ezrin protein was barely detectable in these cells (Fig. 5B). Thus, ezrin is involved in control of NP cell shape.
Figure 5.
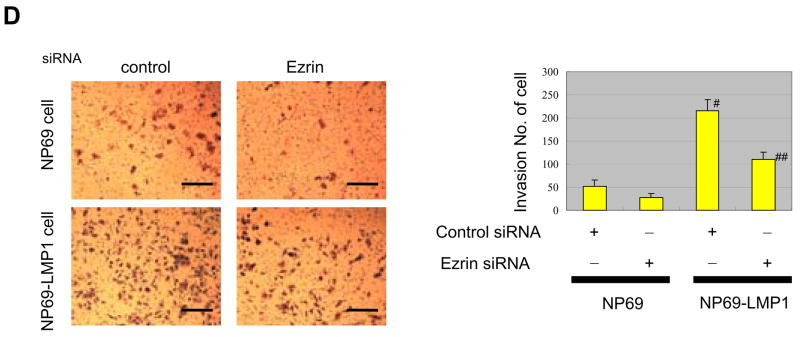
Ezrin contributes to LMP1-induced changes in cell phenotype. A, LMP1 induces fibroblast-like morphology in NP69 cells that disappears with ezrin siRNA. Bar, 50 μm. B, Reduction of ezrin protein level by expression of ezrin siRNA. C, Cell wound-migration assay. LMP1-enhanced cell migration is reduced by ezrin siRNA. Migration of NP69 cells was measured 24 h after scratching monolayer cultures at five locations in triplicate after 24 h. Each column represents five independent replicates, and error bars represent ± S.D. Difference was tested by paired t test. *indicates statistically significant (p=0.0003) compared with control NP69 cells. **indicates significant (p=0.0007) compared with LMP1-expressing NP69 +control siRNA cells. Bar=200 μm D, Invasiveness of cells in Matrigel assays. Cells on lower surface of filters were fixed and stained. Bar=50 μm. LMP1-enhanced invasiveness is suppressed by expression of ezrin siRNA. #, p=0.0011, compared with control NP69 cells. ##, p=0.0038, compared with LMP1-expressing NP69 +control siRNA cells
Next we assessed the role of ezrin in LMP1-stimulated cell invasiveness. LMP1 significantly enhanced both cell motility in wound-scratch assays (see Material and Methods) and invasiveness in Matrigel invasion assays. All these LMP1-induced phenotypes were significantly suppressed by ezrin siRNA (Fig. 6C, D). These data suggest that ezrin is a downstream target of LMP1-induced signaling and is essential for a critical step in the metastatic phenotype induced by LMP1.
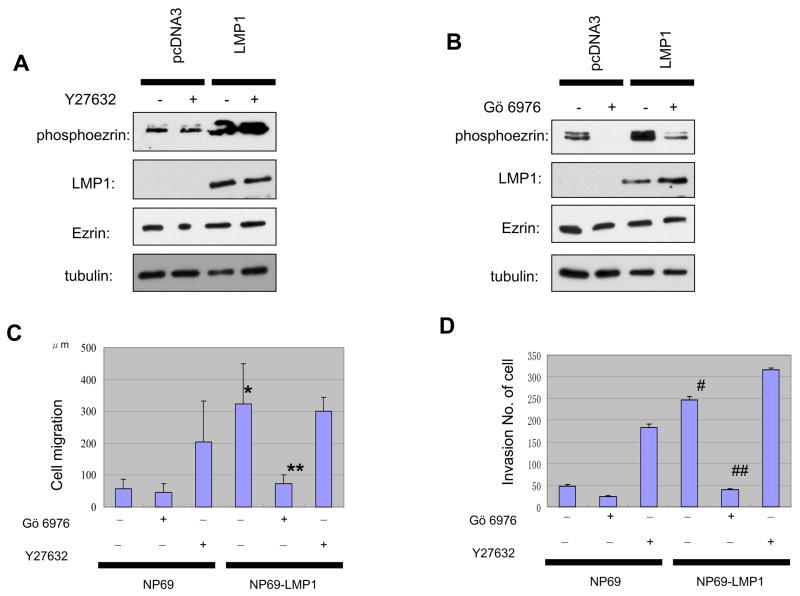
Figure 6.
Inhibition of PKC kinase but not ROCK kinase decreases phosphorylation of ezrin. A, ROCK inhibitor, Y27632, did not affect LMP1-induced ezrin phosphorylation. B, PKC inhibitor, Gö6976, decreases level of LMP1-induced phosphoezrin protein. C, LMP1-increased cell motility is suppressed by PKC inhibitor. Migration was measured as in the legend for Fig. 5. Each column represents five independent replicates, and error bars represent ± S.D. Differences were tested by paired t test. *indicates statistically significant (p=0.0005) compared with control NP69 cells. **indicates significant (p=0.001) regulation after PKC inhibitor treatment. D, LMP1-enhanced invasiveness of NP69 cells is decreased by PKC inhibitor. #, p=0.0012, compared with control NP69 cells. ##indicates significant effect (p=0.0039) of PKC inhibitor treatment in LMP1-expressing NP69 cells.
LMP1 enhances ezrin phosphorylation through PKC not Rho/ROCK pathways
Activation of ezrin involves phosphorylation of specific carboxyl-terminal threonine residues through several serine/threonine kinases known to phosphorylate ezrin on a C-terminal threonine (Louvet-Vallée, 2000; Fievet et al., 2004). Two major pathways have been implicated. One is the small GTP-binding protein Rho and the ROCK pathway (Tran et al., 2000). The other is the Protein Kinase C (PKC) pathway (Ng et al., 2001; Larsson et al., 2006). To examine which might account for the phosphorylation of ezrin in LMP1-expressing cells, we used two inhibitors. Y27632, an inhibitor of Rho-associated protein kinase (ROCK), had no effect on LMP1-stimulated phosphorylation of ezrin, suggesting that ezrin is not a substrate for Rho kinase in NP cells (Fig. 6A). In contrast, pretreatment with Gö6976, a PKC inhibitor, dramatically inhibited LMP1 stimulation of Thr567 phosphorylation (Fig. 6B). LMP1 has been reported to enhance PKC activity, which engages multiple signaling pathways of LMP1 (Yan et al., 2007; Zheng et al., 2007), and these data point to PKC as an activator of ezrin in LMP1-expressing cells.
Since LMP1 expression in NP cells is known to induce on invasive phenotype (Tsao et al., 2002, 4), we next examined with the use of the two inhibitors the contribution of phosphorylation state of ezrin to cell motility and invasiveness, in wound-scratch and Matrigel invasion assays. In LMP1-expressing NP69 cells, which have approximately five-fold increased cell motility and invasiveness compared with control cells (Fig. 6C, D) Gö6976 completely suppressed LMP1-enhanced cell motility and invasiveness, whereas Y27632 had no effect. Thus LMP1 induces PKC-mediated phosphorylation of ezrin, which leads to increases in effects on cell motility and invasiveness of NP cells. These data stress that ezrin phosphorylation is required for LMP1-stimulated cell motility and invasiveness.
Discussion
The oncogenic properties of LMP1, the principal EBV oncoprotein, are numerous and continue to be elaborated (Yoshizaki et al., 2005). The association of the virus and NPC from its inception is virtually universal, and it is likely but not strictly proven that EBV contributes etiologically to this distinctive malignancy. However in addition there is strong experimental and pathobiological evidence that LMP1 also affects late stages of oncogenesis through its ability to induce and activate a plethora of cellular factors that promote cell invasion and metastasis as well as angiogenesis. Initiation of this highly complex process is marked by increases in tumor cell motility. We have shown previously that LMP1 can induce matrix metalloproteinase 9 (MMP9) (Yoshizaki et al., 1998), MMP1, MMP3 (Kondo et al., 2005), c-Met and ets-1 (Horikawa et al., 2001), and Twist (Horikawa et al., 2007), all of which produce increases in cell motility and invasiveness.
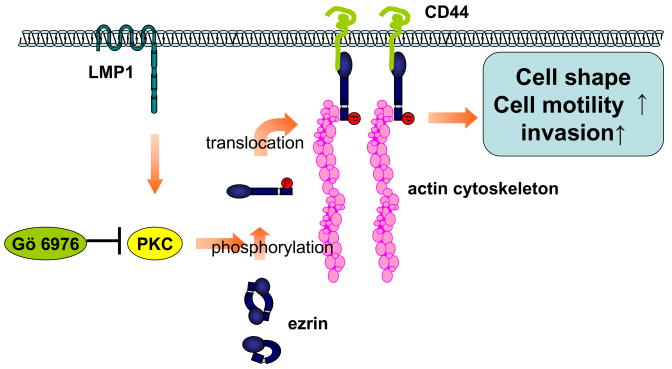
In this paper, we return to cell motility, but from a quite distinct perspective, namely, through the ERM family proteins typified by ezrin. We show here for the first time that in addition to HGF, EGF and androgen, the viral factor, LMP1, can enhance formation of a complex of CD44, ezrin and F-actin which permits phosphorylation of ezrin through PKC and results in increased cell motility and invasiveness which is neutralized by silencing expression of ezrin (Fig. 7). Moreover in NPC tissues, there is direct correlation between expression of phosphoezrin and LMP1. LMP1 activates multiple signaling cascades, NF-kB, MAPK, JNK, p38 and JAK/STAT, involved in cell immortalization, cell proliferation, and apoptosis (Zheng et al., 2007). Ezrin is essential for cortical cytoskeleton organization, cell shape, motility, adhesion and intracellular signaling and has been associated with malignant progression and metastasis in human neoplasias such as melanoma, lung and endometrial carcinoma, rhabdomyosarcoma, and osteosarcoma (Khanna et al., 2004; Bruce et al., 2007; Fais et al., 2004). Ezrin is highly expressed in most normal epithelial tissue as well as many cancers, but normal mesenchymal tissues have relatively low levels of ezrin (Bruce et al., 2007), suggesting that there are tissue-specific functions for ezrin. Ezrin overexpression in prostate cancer cells is alone not sufficient to promote cell invasiveness (Chuan et al., 2006). According to these studies, evaluating ezrin in the active state is more important in malignant behavior than the expression of total ezrin because of the ubiquitousness of ezrin in cancer as well as normal epithelial tissues.
Figure 7.
Scheme for proposed mechanism for LMP1-induced phosphorylation of ezrin. LMP1 enhances phosphorylation of ezrin in its C-terminal region through PKC pathway. Phosphorylated ezrin translocates from the cytosol to the plasma membrane and is linked to CD44 and actin cytoskeleton. Phosphorylated ezrin contributes to LMP1-induced changes in cell phenotype, cell motility and invasiveness in NPC.
Ezrin can exist both in inactive and in active forms, and phosphorylation of ezrin regulates its activities (Louvet-Vallée, 2000). Phosphorylation of the conserved C-terminal threonine residue within the actin-binding site of ezrin is the major mechanism for regulating conformational changes and functional activities of ezrin (Fievet et al., 2004). Several protein kinases are known to phosphorylate ezrin on the C-terminal threonine, including Rho/ROCK and PKC (Tran et al., 2000; Ng et al., 2001; Larsson et al., 2006). According to our findings, LMP1-induced PKC is required for phosphorylation of ezrin and its translocation from the cytosol to the cell membrane. PKC comprises a family of phospholipid-dependent serine/threonine kinases that regulates a large number of cellular processes, such as cell spreading and migration. PKC isoforms constitute a family of 10–15 members, and the different isoforms have unique effects in cellular regulation (Larsson et al., 2006). Among these isoforms, classical PKCα is known to bind and phosphorylate ezrin in fibrosarcoma cells (Ng et al., 2001). Overexpression of PKCα in MCF-10A human breast cells enhances cell motility (Sun et al., 1999). A recent study showed that LMP1 could activate the PKCα/PKCβ pathway (Yan et al., 2007; Zheng et al., 2007). Our data show that a selective PKCα, but not a Rho/ROCK, inhibitor Gö6976, which interacts with the ATP-binding site, decreased ezrin phosphorylation modulated by LMP1. Moreover inhibition of PKC markedly decreased LMP1-stimulated cell migration and invasion, consistent with previous reports (Chuan et al., 2006; Ng et al., 2001). However in a previous study, epithelial cells exposed to ROCK inhibitor exhibited increased cell motility and invasiveness (Hopkins et al., 2007), which was explained by an illusory enhancement of cell spreading, produced by stimulation of cell protrusiveness at the migrating front. Our data indicate that ezrin phosphorylation is required for LMP1-induced cell motility and invasiveness.
Once ezrin is phosphorylated and translocates to the plasma membrane, it interacts with a number of cell-surface adhesion molecules, including ICAM-1, -2, -3, CD43 and CD44 through its N-terminus and links to actin through its C-terminus (Martin et al., 2003). Our observations reveal that ezrin in its active state as modulated by LMP1 links to CD44, the major cell-surface hyaluronan receptor expressed in diverse cell types. Cellular functions that affect the ability of tumor cells to migrate within tissues, transmigrate through vessels and adhere to adjacent and distant organs are contributors to metastasis early in the process. CD44 itself can affect cell migration and metastasis (Martin et al., 2003). Further, PKC regulates the interaction between CD44 and ezrin as this complex regulates cell migration (Ng et al., 2001; Orian-Rousseau et al., 2002). PKC activation leads to dephosphorylation of S 325 and at the same time phosphorylation of S 291 in CD44 (Legg et al., 2002). Thus PKC activated by LMP1 may regulate cellular motility by controlling phosphorylation of both ezrin and CD44. Moreover, ezrin/CD44 complex is known to organize kinase signaling cascades, such as PI3k/AKT (Martin et al., 2003). We have previously shown that c-met induced by LMP1 is associated with cell motility in NPC (Horikawa et al., 2001). Since c-met can form a multimeric complex with HGF which induces phosphorylation of ezrin, we hypothesize that there may be signaling crosstalk between ezrin and c-met induced by LMP1.
We provide here novel data showing that downregulation of ezrin in LMP1-stimulated NP cells results in decreased cell motility and invasiveness. In other studies, Ezrin has been implicated in cytoskeleton-mediated cell motility via regulation of cell shape. Recent study shows that silencing of ezrin induces increased E-cadherin expression which is central to the process of epithelial cell-cell adhesion (Hiscox et al., 1999). Use of ezrin siRNA or dominant-negative ezrin has revealed that this protein is a key factor in the development of metastasis in some cancers (Khanna et al., 2004; Chuan et al., 2006). Our findings that silencing of expression of ezrin inhibits LMP1-induced cell motility and invasion and that detection of phosphoezrin correlates directly with LMP1-positivity in NPC, suggests perhaps a unique role for ezrin in NPC oncogenesis.
We provide here the first evidence that control of phosphorylation and relocation of ezrin by LMP1 regulates cell migration and invasiveness. Understanding of ezrin function and its binding partners in NPC could potentially provide much needed new targets for therapeutic agents that intercept metastasis of this highly invasive malignancy.
Material and Methods
Cell lines and drug treatment
Ad-AH cells, an EBV-negative human nasopharyngeal cell line, were kindly provided by Dr. Erik K. Flemington (Tulane University, New Orleans, LA) (Takimoto et al., 1998) and maintained in DMEM. NP69 and NP69LMP1, human immortalized nasopharyngeal epithelial cells, generous gifts of Dr. Sai Wai Tsao (University of Hong Kong, Hong Kong, China), were maintained in Keratinocyte-SFM (Tsao et al., 2002). KH-1 and KH-2 are EBV-positive type II cell lines derived by fusion of KR4 (an EBV-positive type III lymphoblastoid cell line) and Hela cells (human cervical carcinoma), kind gifts of Dr. Maria Masucci, (Karolinska Institute, Stockholm, Sweden) (Crepaldi et al., 1997).
Epidermal Growth Factor (EGF) was purchased from Sigma (St. Louis, Mo). Cells incubated with EGF (25 nM) for one hour. Inhibitors of Rho kinase Y-27632, PKCα Gö6976 were from Calbiochem, San Diego, CA. To evaluate the role of Rho kinase or PKC, cells were pretreated with either vehicle Y-27632 (20 μM) or Gö6976 (1 μM) for 30 min.
Plasmids and antibodies
pcDNA3-based LMP1 plasmid (Raab-Traub et al., 1987) and flag-tagged LMP1 mutants (LMP1 1–231, LMP1 del 187–351, LMP1 1–187) and LMP1 WT have been described (Miller, W. et al., 1997).
Rabbit monoclonal ezrin antibody and rabbit polyclonal phosphoezrin antibody (Thr-567) were purchased from Cell Signaling (Beverly, MA). Mouse CD44 antibody was from Sigma (St. Louis, Mo). Mouse LMP1 antibody was from DAKO (Glostrup, Denmark). Horseradish peroxidase-conjugated mouse or rabbit secondary antibody was from Amersham (Little Chalfont, United Kingdom)
Ezrin shRNA
For an ezrin shRNA-expressing cell line, retroviruses that encode ezrin shRNA were constructed by ligating ezrin oligonucleotide, (GGCUUUCCUUGGAGUGAAA) into a pSuper-retro vector (Oligoengine) (Zhang et al., 2006). Lipofectamine 2000 (Invitrogen) was used for transfection of NP69 cells, transfected cells were selected with puromycin (Stewart et al., 2003). A negative control cell line was generated by infecting cells with pSuper-puro construct targeting GFP (GCTACGTCCAGGAGGGCAC).
Western blot analysis
Extracts of cellular proteins were prepared as described previously (Wakisaka et al., 2002). Cell lysates were electrophoresed and transferred to nitrocellulose membrane. Protein concentration was determined by Bradfold assay (Bio-Rad). Membranes were blocked with nonfat dry milk in TBS and probed with primary antibody overnight, followed by corresponding secondary antibody. Antibody-bound proteins were visualized by the SuperSignal chemiluminescence system (Pierce, Rockford, IL ).
Immunoprecipitation
Whole-cell lysates were precleared with A/G PLUS-Agarose beads and incubated with CD44 (Santa Cruz Biotechnology) or ezrin antibody and then incubated with beads overnight. After intensive washing and centrifugation, immune complexes were separated by SDS/PAGE and probed with primary antibody by western blotting.
Immunofluorescence staining
Cells cultured on glass coverslips were fixed with 4% paraformaldehyde, washed with PBS, permeabilized with 0.5% Triton-X-100/PBS and blocked with 0.5% normal donkey serum. Cells were then incubated with primary antibodies to ezrin, phosphoezrin, and CD44 overnight and were the same as used for Western blotting.
Subcellular fractionation
Cellular fractions were obtained with a Qproteome Cell Compartment kit (Qiagen). Cytosolic and membrane proteins were obtained with specific extraction buffer according to the manufacturer’s instructions
Immunohistochemical analysis of NPC tissues
Primary NPC paraffin-embedded specimens from Kanazawa University Hospital (Ishikawa, Japan) Fukui Saiseikai Hospital (Fukui, Japan), Toyama City Hospital (Toyama, Japan) and Kurobe City Hospital (Toyama, Japan) were used for detection of LMP1 and phosphoezrin. Specimens were from 24 men and 5 women, ages 24 to 90 years (mean 56.3 years), and classified histologically as follows: 2 squamous cell carcinomas (WHO type I), 17 nonkeratinizing carcinomas (type II), and 10 undifferentiated carcinomas (type III): one stage IIA, four stage IIB, nine stage III, 12 stage IVA, and three stage IVB (5th TNM classification system of the International Union Against Cancer (1997)). Twenty-Two of 29 specimens were EBV-positive, 4 were equivocal, and 3 were negative on the basis of EBER staining. For complete details see supplementary table 1. Immunohistochemistry was done described before (Horikawa et al., 2001). Primary antibodies for LMP1 and phosphoezrin were the same as for Western blotting. Stained samples of phosphoezrin and LMP1 were evaluated as follows: using light microscopy, number of stained cells from 200 cells was carefully counted in the two areas in a 200x field without any clinicopathological knowledge of the patients. The percentage of positive cells obtained as an average of two counts was used as the expression score for both proteins.
Invasiveness and cell wound-migration assays
Matrigel was purchased from BD Bioscience and stored at −20°C. After dilution in serum-free medium, 50 μl of Matrigel suspension were inoculated onto the upper chamber. Matrigel invasiveness assays were carried out according to the manufacturer’s instructions. After 72 h, cells adherent to the lower surface of filters were fixed, stained, and scores calculated from counts of cells invading through the Matrigel-coated membrane. Cell wound-migration assay was done as described previously (Horikawa et al., 2001). Briefly, confluent cell monolayers were scratched with a micropipette tip, and spontaneous cell migration was monitored for 24 h. The distance to wound closure was measured in five independent sites per group. Migration was assessed by measuring distances between wound edges.
Statistical analysis
Pearson’s correlation coefficient was used to analyze correlations between the expression of LMP1 and phosphoezrin in NPC specimens. The differences in wound migration and invasiveness indices between NP69 cell clones were analyzed by the paired t test. Error bars indicate SD of the mean, and significance was defined at the level of p<0.05. All experiments were repeated at least three times.
Supplementary Material
Histopathologic analysis of primary NPC tumor tissues. Please refer to Material and Methods.
Acknowledgments
We thank Sai Wah Tsao and Erik K. Flemington respectively for the NP69SV40T cell line and the Ad-AH cell line. We thank Drs.Yojiro Kotake and Yuki Okada for helpful advice. This work was supported by NCI (CA 19014) (J.S.P.) and the Nakayama Foundation for Human Science (Tokyo, Japan) (K. E.)
References
- Auvinen E, Kivi N, Vaheri A. Regulation of ezrin localization by Rac1 and PIPK in human epithelial cells. Exp Cell Res. 2007;313:824–833. doi: 10.1016/j.yexcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW, et al. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci U S A. 2006;103:13391–6. doi: 10.1073/pnas.0605950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce B, Khanna G, Ren L, Landberg G, Jirström K, Powell C, et al. Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis. 2007;24:69–78. doi: 10.1007/s10585-006-9050-x. [DOI] [PubMed] [Google Scholar]
- Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J Biol Chem. 2006;281:29938–29948. doi: 10.1074/jbc.M602237200. [DOI] [PubMed] [Google Scholar]
- Contreras-Brodin BA, Anvret M, Imreh S, Altiok E, Klein G, Masucci MG. B cell phenotype-dependent expression of the Epstein-Barr virus nuclear antigens EBNA-2 to EBNA-6: studies with somatic cell hybrids. J Gen Virol. 1991;72:3025–3033. doi: 10.1099/0022-1317-72-12-3025. [DOI] [PubMed] [Google Scholar]
- Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T, et al. Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem. 1999;274:2315–2321. doi: 10.1074/jbc.274.4.2315. [DOI] [PubMed] [Google Scholar]
- Fais S. A role for ezrin in a neglected metastatic tumor function. Trends Mol Med. 2004;10:249–250. doi: 10.1016/j.molmed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautreau A, Louvard D, Arpin M. ERM proteins and NF2 tumor suppressor: the Yin and Yang of cortical actin organization and cell growth signaling. Curr Opin Cell Biol. 2002;14:104–109. doi: 10.1016/s0955-0674(01)00300-3. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112:3081–3090. doi: 10.1242/jcs.112.18.3081. [DOI] [PubMed] [Google Scholar]
- Hopkins AM, Pineda AA, Winfree LM, Brown GT, Laukoetter MG, Nusrat A. Organized migration of epithelial cells requires control of adhesion and protrusion through Rho kinase effectors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G806–817. doi: 10.1152/ajpgi.00333.2006. [DOI] [PubMed] [Google Scholar]
- Horikawa T, Sheen TS, Takeshita H, Sato H, Furukawa M, Yoshizaki T. Induction of c-Met proto-oncogene by Epstein-Barr virus latent membrane protein-1 and the correlation with cervical lymph node metastasis of nasopharyngeal carcinoma. Am J Pathol. 2001;59:27–33. doi: 10.1016/S0002-9440(10)61669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa T, Yang J, Kondo S, Yoshizaki T, Joab I, Furukawa M, et al. Twist and epithelial-mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res. 2007;67:1970–1978. doi: 10.1158/0008-5472.CAN-06-3933. [DOI] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- Kondo S, Wakisaka N, Schell MJ, Horikawa T, Sheen TS, Sato H, et al. Epstein-Barr virus latent membrane protein 1 induces the matrix metalloproteinase-1 promoter via an Ets binding site formed by a single nucleotide polymorphism: enhanced susceptibility to nasopharyngeal carcinoma. Int J Cancer. 2005;115:368–376. doi: 10.1002/ijc.20849. [DOI] [PubMed] [Google Scholar]
- Kondo S, Yoshizaki T, Wakisaka N, Horikawa T, Murono S, Jang KL, et al. MUC1 induced by Epstein-Barr virus latent membrane protein 1 causes dissociation of the cell-matrix interaction and cellular invasiveness via STAT signaling. J Virol. 2007;81:1554–1562. doi: 10.1128/JVI.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Seo SY, Yoshizaki T, Wakisaka N, Furukawa M, Joab I, et al. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 2006;66:9870–9877. doi: 10.1158/0008-5472.CAN-06-1679. [DOI] [PubMed] [Google Scholar]
- Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4:399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- Louvet-Vallée S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92:305–316. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003;46:165–186. doi: 10.1016/s1040-8428(02)00172-5. [DOI] [PubMed] [Google Scholar]
- Miller WE, Mosialos G, Kieff E, Raab-Traub N. Epstein-Barr virus LMP1 induction of the epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-kappaB activation. J Virol. 1997;71:586–94. doi: 10.1128/jvi.71.1.586-594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murono S, Inoue H, Tanabe T, Joab I, Yoshizaki T, Furukawa M, Pagano JS. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:6905–6910. doi: 10.1073/pnas.121016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20:2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Faller DV. Epstein-Barr virus latent membrane protein-1 induction by histone deacetylase inhibitors mediates induction of intercellular adhesion molecule-1 expression and homotypic aggregation. Virology. 2002;303:345–363. doi: 10.1006/viro.2002.1638. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N, Flynn K, Klein C, Pizza G, De Vinci C, Occhiuzzi L, et al. EBV associated malignancies. J Exp Pathol. 1987;3:449–456. [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XG, Rotenberg SA. Overexpression of protein kinase Calpha in MCF-10A human breast cells engenders dramatic alterations in morphology, proliferation, and motility. Cell Growth Differ. 1999;10:343–352. [PubMed] [Google Scholar]
- Takimoto T, Sato H, Ogura H, Tanaka S, Umeda R. The difference in tumorigenicity between Epstein-Barr virus (EBV) genome-positive and genome-negative epithelial hybrid cell lines derived from the human nasopharynx. Laryngoscope. 1988;98:1334–1338. doi: 10.1288/00005537-198812000-00010. [DOI] [PubMed] [Google Scholar]
- Tran Quang C, Gautreau A, Arpin M, Treisman R. Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. EMBO J. 2000;19:4565–4576. doi: 10.1093/emboj/19.17.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:473–487. doi: 10.1016/s1044579x02000901. [DOI] [PubMed] [Google Scholar]
- Tsao SW, Wang X, Liu Y, Cheung YC, Feng H, Zheng Z, et al. Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim Biophys Acta. 2002;1590:150–158. doi: 10.1016/s0167-4889(02)00208-2. [DOI] [PubMed] [Google Scholar]
- Wakisaka N, Murono S, Yoshizaki T, Furukawa M, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res. 2002;62:6337–6244. [PubMed] [Google Scholar]
- Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2004;24:5223–5234. doi: 10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Schirrmacher V, Mosier D. Induction of CD44 expression by the Epstein-Barr virus latent membrane protein LMP1 is associated with lymphoma dissemination. Int J Cancer. 1995;61:363–369. doi: 10.1002/ijc.2910610315. [DOI] [PubMed] [Google Scholar]
- Yan G, Luo W, Lu Z, Luo X, Li L, Liu S, et al. Epstein-Barr virus latent membrane protein 1 mediates phosphorylation and nuclear translocation of annexin A2 by activating PKC pathway. Cell Signal. 2007;19:341–348. doi: 10.1016/j.cellsig.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Yoshizaki T, Wakisaka N, Pagano JS. In: Epstein-Barr Virus: Chapter 12: Epstein-Barr virus, invasion and metastasis. Robertson Erle S., editor. Caister Academic Press; Norfolk: 2005. pp. 171–196. [Google Scholar]
- Yoshizaki T, Sato H, Furukawa M, Pagano JS. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci U S A. 1998;95:3621–3626. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hu MY, Wu WZ, Wang ZJ, Zhou K, Zha XL, et al. The membrane-cytoskeleton organizer ezrin is necessary for hepatocellular carcinoma cell growth and invasiveness. J Cancer Res Clin Oncol. 2006;132:685–697. doi: 10.1007/s00432-006-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li LL, Hu DS, Deng XY, Cao Y. Role of Epstein-Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol. 2007;4:185–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histopathologic analysis of primary NPC tumor tissues. Please refer to Material and Methods.