Abstract
The concept of “stabilization” of atmospheric CO2 concentration is re-examined in connection with climate-change mitigation strategies. A new “zero-emissions stabilization (Z-stabilization)” is proposed, where CO2 emissions are reduced to zero at some time and thereafter the concentration is decreased by natural removal processes, eventually reaching an equilibrated stable state. Simplified climate experiments show that, under Z-stabilization, considerably larger emissions are permissible in the near future compared with traditional stabilization, with the same constraint on temperature rise. Over longer time scales, the concentration and temperature decrease close to their equilibrium values, much lower than those under traditional stabilization. The smaller temperature rise at final state is essential to avoid longer-term risk of sea level rise, a significant concern under traditional stabilization. Because of these advantages a Z-stabilization pathway can be a candidate of practical mitigation strategies as treated in Part 2.
Keywords: CO2 emissions, carbon cycle, sea level rise, climate commitment, irreversible climate change, United Nations Framework Convention on Climate Change
Introduction
In the past few years, since the Intergovernmental Panel on Climate Change (IPCC) 4th Assessment Reports1–4) in 2007, discussion of practical strategies to mitigate climate change has become a central issue within international politics. At the Group of Eight (G8) Summits of 2007, 2008 and 2009, it was declared that the world’s total greenhouse gas (GHG) emissions should be reduced to 50% of current levels by 2050. Further, the G8 Declaration of 2009 stated that global mean temperature must not exceed 2 ℃ above preindustrial levels, in recognition of the scientific findings of the IPCC. Subsequently, at the 15th Conference of Parties (COP-15) of the United Nations Framework Convention on Climate Change (UNFCCC) held at Copenhagen in December 2009, the parties agreed to this temperature rise limit, again referring to the IPCC, although no consensus was reached regarding a common target for emissions reduction. A more stringent temperature restriction of 1.5 ℃ was noted in the Copenhagen Accord, originally raised by small island states presumably to avoid the threat of sea level rise. Discussions during the subsequent two years have illustrated the difficulties of agreeing an emissions reduction strategy that is acceptable for all nations.
It is well understood that the issue of climate change mitigation is a complex matter of climate science and socioeconomic policy issues, incorporating value judgments. Natural science alone cannot determine the appropriate threshold for global mean temperature rise, because the associated risk depends on factors such as the vulnerability of specific societies. Furthermore, strategies to mitigate temperature rise should balance potential losses due to climate change against the economic and cultural development of the worlds’s human society enabled by energy consumptions and CO2 emissions.
Thus, the overall role of climate science is limited, despite being the central starting point of the issue. So far, once the upper limit of global mean temperature rise is determined based on a wide range of considerations (2 ℃, for example), then GHG stabilization targets are determined at concentrations such that the equilibrium temperature rise becomes the upper limit and, by definition, concentrations are held constant after stabilization. The emissions pathways leading to stabilization from present levels are devised using integrated assessment models under certain evolutionary socioeconomic scenarios, such as the Representative Concentration Pathways (RCP) scenarios.5)
The present work is motivated by the question of whether such procedures are sufficient to determine appropriate emissions pathways or emissions reduction strategies within the full range of effective possibilities, the point raised in Ref. 6. We consider that existing procedures were restrictive and may therefore overlook emission-reduction strategies that deserve to be considered from the viewpoints of future evolution of climate change and associated risks, as well as practical socioeconomic application. Specifically, we first raise a question on the traditional stabilization concept and critically examine its limitations. In subsequent sections, we propose a new stabilization concept, in which CO2 emissions are reduced to zero at some time, typically in the next century, and; thereafter, CO2 concentration decreases by natural removal processes, finally reaching an equilibrium state, which we call “Zero-emissions stabilization (Z-stabilization).” Such a zero-emissions concept has been discussed as an alternative emissions pathway,7) or as a necessity for stabilizing global temperatures.8) In the present paper, we further clarify the implications of this concept as a long-term climate change mitigation strategy in contrast to the traditionally accepted method of stabilization; and also pay attention to a misleading concept, referred to as “committed climate change,” that is associated with the traditional stabilization. The evolution of CO2 concentration, temperature increase, and sea level rise associated with Z-stabilization is compared with those of a traditional stabilization pathway, which we call “Emissions-keeping stabilization (E-stabilization),” through projection calculations based on a simplified climate system model. From the results, we can infer that Z-stabilization is more advantageous than E-stabilization for avoiding long-term risks while meeting the short-term need for large emissions. Namely, by requiring zero-emissions not too far in the future, but within a feasible emissions reduction rate, larger emissions become permissible in the near future to meet societal need of fossil fuels to respond to energy demand in developing countries. Although this may lead to much higher CO2 concentration, being considerably higher than the concentration corresponding to the upper limit of temperature rise, the thermal inertia effect means that, for a limited period, it is possible to keep a lower temperature rise than the corresponding equilibrium value, thereby allowing an otherwise dangerous situation to be avoided. After zero-emission is achieved, the temperature will reduce as CO2 concentration is subject to natural removal processes, thereby approaching the final equilibrium state, which is set at a sufficiently low level to avoid long-term risks of ice sheet melting.
These few years, a number of studies have been published in the literature, closely related to the present work. One group of papers deals with the relationship between peak temperature and cumulative CO2 emissions, and showed that the former is determined by the latter for a number of emissions pathways. In most of those studies, a zero-emission (cessation of emissions) is included in emission pathways but not discussed clearly and explicitly. We discuss this problem later in this study citing individual literature, in comparison with the situation for traditional stabilization pathways. Another group of papers addresses the almost irreversible nature of climate change (temperature rise) after cessation of CO2 emissions under scenarios in which emissions show rapid growth, followed by cessation at a specified time (typically at 2100). These approaches use numerical models such as Earth System Models of Intermediate Complexity (EMICs) and full earth system models. The proposed Z-stabilization strategy is compared with the results of these studies and their implications are described in the discussion section by referring to specific works.
In Part 1, we consider CO2 emissions only because CO2 is a major component of GHGs. For qualitative discussion about the characteristics of pathways, this simplification may be permissible and gives a clearer view. In Part 2, we present a more realistic situation, including radiative forcings of other GHGs and aerosols; and present a new emissions pathway as a candidate for real application.
Examination of “stabilization” concept
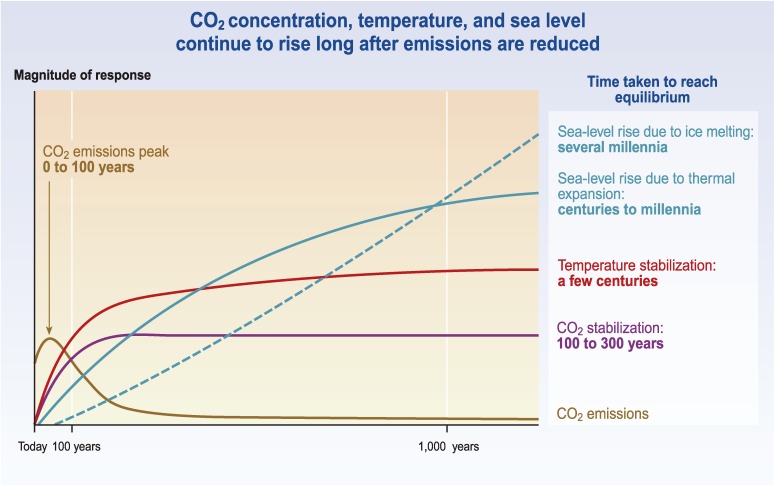
The concept or implication of “stabilization” of GHG concentrations, as it is usually understood by climate change researchers and policy makers, is illustrated in Fig. 1, which is taken from Fig. 5.2 in the Synthesis Report of the IPCC Third Assessment Report (TAR).9) As seen in Fig. 1, increasing atmospheric CO2 concentration levels off in a century or two and is then fixed at a constant level. So far, the word “stabilization” has been considered to refer to such a pathway (and its final state) as a straightforward interpretation of stabilization, stated in Article 2 of the UNFCCC.10) As shown in this figure, the emissions corresponding to this concentration pathway first increase but soon peak, and then decline rapidly to a very low level compared with “today’s” level. The quick decline of CO2 emissions does not continue, but rate of decline slows down after stabilization is attained and low level CO2 emissions continue over a millennium-long period.
Figure 1.
Schematic view of stabilized CO2 concentration and long-lasting climate change.
(Source) Fig. 5.2 of the Synthesis Report of the IPCC TAR.9)
Full legend: After CO2 emissions are reduced and atmospheric concentrations stabilize, surface air temperature continues to rise by a few tenths of a degree per century for a century or more. Thermal expansion of the ocean continues long after CO2 emissions have been reduced, and melting of ice sheets continues to contribute to sea-level rise for many centuries. This figure is a generic illustration for stabilization at any level between 450 and 1,000 ppm, and therefore has no units on the response axis. Responses to stabilization trajectories in this range show broadly similar time courses, but the impacts become progressively larger at higher concentrations of CO2.
What is hard to understand as the background mitigation strategy expressed in Fig. 1 is that there remain a small amount of CO2 emissions over a millennium-long period after the drastic reduction that is realized soon after the peak in emissions. Why does the reduction in emissions becomes slow after the greatly successful reduction achieved earlier in the scenario? For what purpose low level CO2 emissions continue over a very long time? Perhaps the reason is that it does not reflect any specific mitigation strategy, but that it is merely a result of inverse calculation from a pathway leading to stabilization of CO2 concentration in the atmosphere. The low level continuing emissions are needed to keep the CO2 concentration constant against natural uptake of CO2 working in the stabilized state, mostly uptake by the deep ocean. Further quantitative results of emissions pathways leading to stabilization are found in Fig. 3.13 of IPCC WG I TAR11) or Fig. 10.22 in IPCC WG I AR4.1) By referring to these data, we see that the near-constant level of emissions at the doubled CO2 concentration (550 ppm) is around 2 GtC y−1, which is a small but significant amount. We shall refer to this traditional stabilization process as “Emissions-keeping stabilization (E-stabilization).” Note that 1 GtC is equivalent to 3.7 GtCO2.
Figure 1 shows sea level rises due to thermal expansion of seawater and ice-sheet melting, both of which increase continually over a millennium time scale. These express the most important message of this figure, i.e., that sea level rises even after stabilization. Since the concept of “stabilization” in AR4 remains the same as that in TAR, it emphasizes sea level rise due to melting of the Greenland ice sheet as well as that due to thermal expansion of seawater as a long-term danger under the condition of sustained temperature rise (e.g., the last paragraph in SPM of the IPCC WG I AR41)).
Apparently, it is a questionable idea that low level anthropogenic emissions are held for many centuries to maintain the higher CO2 concentration and higher temperature against natural recovery effects. By noting these points, we are led to a naïve but attractive alternative. If we continue the efforts of reduction to make emissions sufficiently below the natural uptake level, then the net balance of CO2 in the atmosphere becomes negative, so the atmospheric concentration will decrease. In particular, if emissions reach zero not so far into the future, hopefully during the next century, then atmospheric CO2 concentration should decrease due to oceanic and biospheric uptakes and approach the final equilibrium state. In this state, atmospheric concentration is determined by the airborne fraction of the cumulative total of anthropogenic CO2 emissions. This final concentration is generally very low compared with the stabilization level now under discussion, i.e., 450–550 ppm, so that the equilibrium state is supposed to be safe from sea level rise associated with thermal expansion of seawater and ice-sheet melting. This final equilibrium state is a stable state similar to the preindustrial era, but with a different (higher) CO2 concentration due to anthropogenic addition of total carbon circulating in the climate system. This process, and the final stable state, can also be regarded as “stabilization” equally to the traditional concept, so we shall refer to this as “Zero-emissions stabilization (Z-stabilization).” Over the course of several previous reports, the present authors have advocated the need to extend the stabilization concept in this manner in order to avoid long-term risks while meeting the short-term need for relatively large emissions.12–15) This new concept of stabilization relaxes the limitation on emissions pathways posed by the temperature condition at the final equilibrium state in the E-stabilization scenario.
A recent study raised concern regarding “irreversible” climate changes following the cessation of CO2 emissions.16) The issue of irreversibility is further emphasized in the recent report by the US National Academies.17) The irreversibility discussed in such literature suggests that atmospheric CO2 concentration and temperature after the cessation of CO2 emissions will never return to preindustrial low levels but will remain at higher levels. However, unlike the E-stabilization case, in the Z-stabilization case the higher temperature is not constant but declines toward the final equilibrium, to some extent, which is the key to lessening the long-term risk of sea level rise, as will be discussed in the final section.
Projections based on a zero-emissions stabilization pathway
Setup of numerical experiments.
In order to test and verify characteristics of the proposed form of stabilization and to identify the potential for a new CO2 mitigation strategy based on the Z-stabilization pathway, we calculated climate projections for test case scenarios using a simplified climate system model.18) A brief description of the model and the values of parameters adopted in the present study are given in the Appendix. It should be noted that the equilibrium climate sensitivity—one of the key parameters containing uncertainties in the climate projection—is set at 3 ℃ as the best estimate.19) In climate change science, climate sensitivity is defined as a temperature rise at equilibrium state from the preindustrial level after doubling of CO2 concentration.
In the experiments in Part 1, we consider only CO2 as a GHG for the purpose of comparison of two types of stabilization pathways. In Part 2, we consider a more realistic situation including other GHGs. We designed a Z-stabilization pathway and a traditional E-stabilization pathway to allow comparison of projected climate changes. For the E-stabilization pathway, we adopted 450 ppm as the target concentration and devised an emissions pathway that continually increased atmospheric concentration towards this threshold (hereafter termed E450 emissions pathway). For the Z-stabilization pathway, we first chose 650 GtC as the cumulative CO2 emissions in the 21st century. The background to choosing this level is as follows: Since the IPCC TAR,11) several emissions scenarios have been investigated using stabilization levels of 450 ppm. By crudely estimating cumulative CO2 emissions, it was found that they are approximately 550 GtC in the 21st century. Since the Z-stabilization emissions pathways allow temporarily higher concentration than the corresponding E-stabilization target, higher overall CO2 emission is possible in the 21st century. Thus, we adopted 650 GtC as the total emissions in the 21st century and set the target date for zero emissions at 2160, the middle of the next century (subsequently termed Z650 emissions pathway). As will be seen subsequently, the highest temperature rise due to this emissions pathway is about 1.8 ℃, which is less than the current threshold of 2 ℃, whereas the E450 pathway indicates a final equilibrium temperature rise of slightly above 2 ℃. Although there is such a minor difference in the maximum temperature rise between the two pathways, this does not affect subsequent discussion of their comparison.
Results of projection experiment until the year 2200.
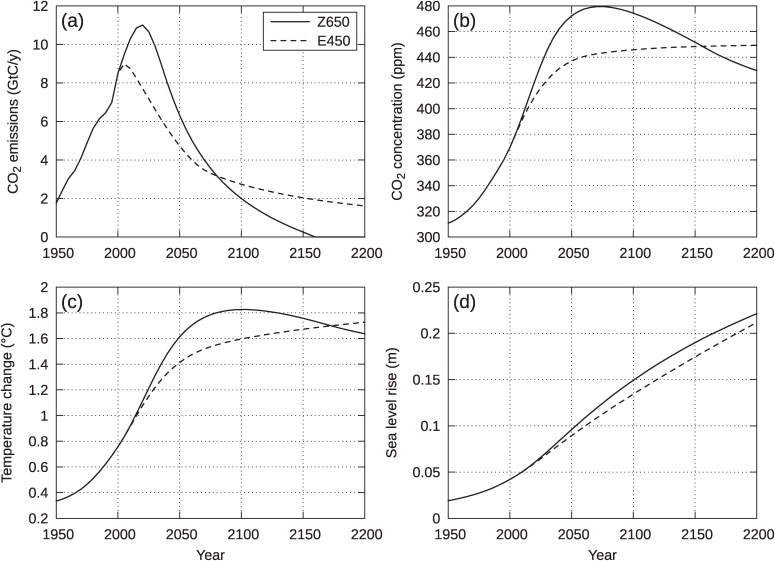
Figure 2(a–d) shows the emissions pathways (a), concentrations (b), global mean temperature rises from preindustrial level (c), and the sea level rises due to thermal expansion (d) for the two cases, Z650 and E450 (indicated by solid and dashed lines, respectively). As readily seen from Fig. 2(a), the emissions peak and the total emissions during the 21st century are significantly larger for Z650 than for E450. For the concentrations in Fig. 2(b), Z650 shows a peak-and-decline change, with the maximum concentration of about 480 ppm, which exceeds the 450 ppm limit corresponding to 2 ℃ temperature rise at the equilibrium. In contrast, the concentration of E450 stays below 450 ppm, although it increases steadily to approach the equilibrium value. It is interesting that the Z650 concentration decreases, intersecting the E450 projection at about 2150, close to the time when the cumulative amounts of the two scenarios become equal (the year 2185). Figure 2(c) shows that predicted temperature rises are similar to respective concentration changes. The most important point is that the maximum temperature increase of Z650 (at around 2100) remains around 1.8 ℃ (above the preindustrial level), which is well below the upper limit of 2 ℃, even though the concentration has a plateau exceeding 450 ppm for more than a century-long period, with the maximum at about 480 ppm. Thus, we confirm that the original objective to propose a new type of emissions pathway was actually achieved.
Figure 2.
Comparison between Z650 and E450 for CO2 emissions (a), CO2 concentration (b), global mean temperature rise (c), and sea level rise due to thermal expansion of sea water (d). Non-CO2 forcing is not included in the model.
In comparison, the temperature rise for E450 increases monotonically with increasing concentration to approach the targeted temperature rise of 2 ℃. However, at 2050, when the concentration reaches 440 ppm (94% of the increase above the preindustrial baseline), the temperature rise is about 1.4 ℃, which is 70% of the equilibrium, because of the large thermal inertia of the climate system. This delayed climate response is in accordance with results from intensive climate experiments by atmosphere–ocean general circulation models (AOGCMs), as documented in the IPCC WG I AR4.1) Even in the year 2200, when almost 150 years have elapsed after the near-stabilization, the temperature rise remains slightly above 80% of the equilibrium value. Figure 2(d) compares sea level rise due to thermal expansion for the two cases. For this component, differences between the two are very small (around 1 cm) with the Z650 case being larger for the period until 2200. The maximum values are approximately 22 cm for Z650 and 21 cm for E450.
Longer-term projections until 3000.
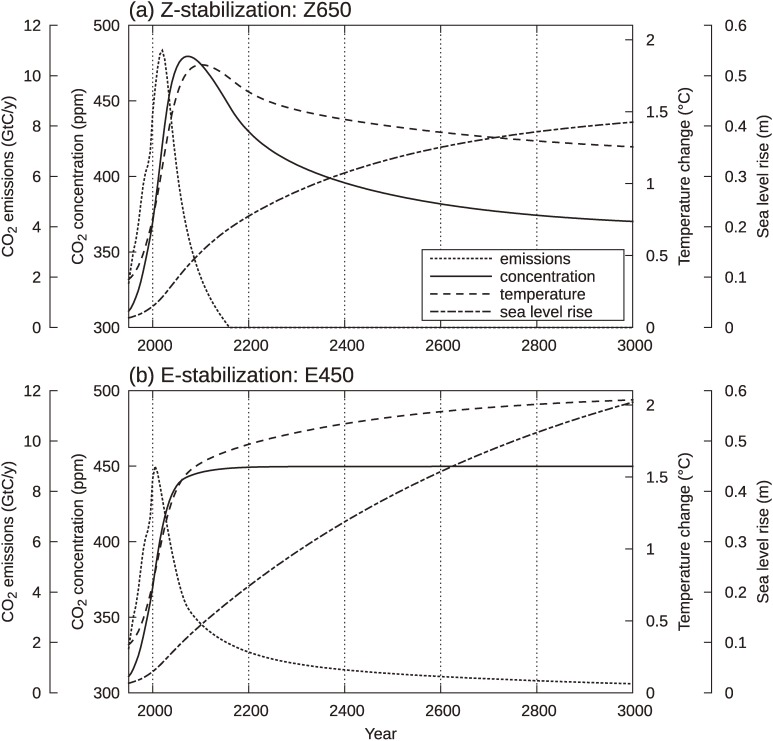
Figure 3 shows longer-term projections for the two scenarios. The four variables (emissions, concentration, temperature rise, and sea level rise) are shown in Fig. 3(a) for Z650 and Fig. 3(b) for E450 for the years 1950 to 3000. Note that we use the same climate system model without carbon cycle processes on geological time scales, which is valid for a few millennia. The concentration under Z650 increases to a maximum of 480 ppm at about 2075 and then decreases continuously after 2200 to become as low as 370 ppm at 3000, whereas the concentration in the E450 scenario is kept fixed at 450 ppm, by definition. In the year 3000, Z650 shows a concentration of 370 ppm, which is nearly the same as the value at 2000, irrespective of the additional emissions beyond 2000. The cause of the decrease below the present-day concentration can be understood as a restoring effect toward the preindustrial state, memorized by the large water masses in the deep ocean.20) As consequences of these concentration changes, the temperature rise of Z650 attains a temporal maximum of about 1.8 ℃ at about 2100, then decreases to reach around 1.3 ℃ at 3000. In contrast, the temperature for E450 continues to increase and slightly exceeds the 2 ℃ by the year 3000. This value is in accordance with the assumed climate sensitivity of 3 ℃.
Figure 3.
Long-term characteristics of Z650 (a) and E450 (b). Non-CO2 forcing is not included in the model. Sea level rise represents thermal expansion of sea water.
Some recent reports emphasized the irreversible nature of climate change caused by anthropogenic CO2 emissions.16,17) The above-described decrease in the CO2 concentration and temperature after their peaking do not conflict with the irreversibility discussed in the literature, in the sense that the values at 3000 (370 ppm and 1.3 ℃) are definitely much higher than preindustrial levels (280 ppm and 0 ℃), indicating that anthropogenic changes do not disappear on millennium time scales. Our argument here concerns the small restoring change after peaking, which makes an important difference in the persisting state regarding long-term accumulating influences.
The projections for Z650 and E450 differ in the extent of sea level rise, which is the most important issue over longer time scales. While sea level rise increases at an almost constant rate in E450, the rate of increase slows gradually in Z650. At the year 3000, sea level rises reach 58 cm and 41 cm for E450 and Z650, respectively. The differences between these two cases could increase substantially if the component of ice-sheet melting is taken into consideration.
Evolution of climate parameters and cumulative emissions.
Figure 3(b), which shows the four variables in the case of E450, is very similar to Fig. 1, a schematic picture of the E-stabilization, as it should be. From Fig. 3(b), we can see that, following stabilization, emissions decrease toward zero at the final equilibrium state but that the rate of decline is rather gradual, so that we infer that the cumulative total emissions after 2100 until the equilibration are much greater than those in the 21st century. The point can be seen more clearly in Fig. 4(a), which shows the cumulative emissions since the preindustrial era for the two cases, Z650 (solid line) and E450 (dashed line). Note that the model we used calculated prior total emissions until 2000 as 410 GtC from the observed concentration data. Figure 4(a) indicates that the cumulative emissions during the 21st century are about 520 GtC and 650 GtC for E450 and Z650, respectively. The latter is larger by 130 GtC, but this difference between the two is not very large, if we compare it with the difference after 2100. After 2100, the two cumulative emissions evolve quite differently. In E450, the cumulative emissions between 2100 and 3000 total approximately 800 GtC, which is much larger than the amount during the 21st century (520 GtC), whereas in Z650, the total emissions after 2100 is only 50 GtC, due to the cessation of emissions at 2160.
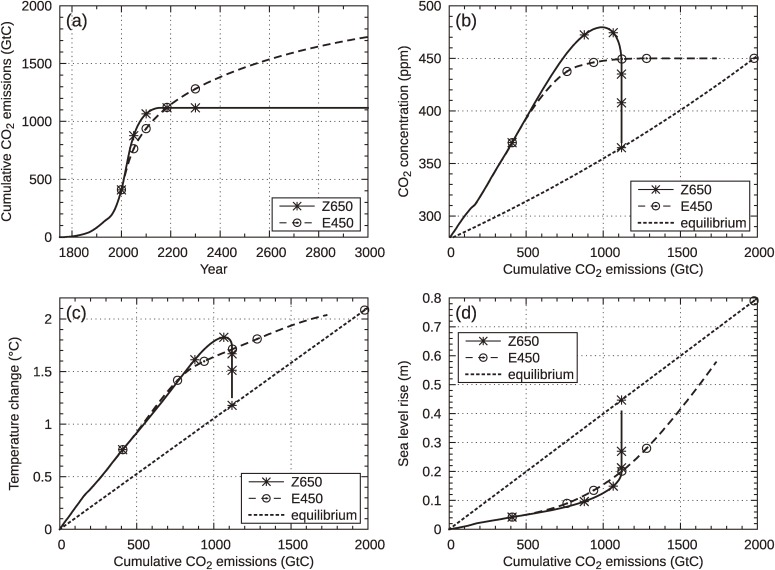
Figure 4.
Cumulative CO2 emissions of Z650 and E450 (a), and their pathways in terms of CO2 concentration (b), temperature change (c), and sea level rise due to thermal expansion of sea water (d) as functions of cumulative amount of CO2 emissions. Equilibrium states corresponding to transient cumulative emissions are shown by dotted line. Values are marked at specific years: 2000, 2050, 2100, 2185 and 2300, as well as at the final equilibrium state on the dotted line. Non-CO2 forcing is not included in the model.
In order to understand the relationships between cumulative emissions and concentration, temperature rise and sea level rise, we shall see the evolutions of these variables as functions of cumulative emissions, instead of time, for the two pathways (Note that the amount of cumulative emissions is a non-decreasing function of time for each pathway, unless negative emissions occur). Figure 4(b), (c) and (d) show the evolution of CO2 concentrations, temperature rises and sea level rises with increase of cumulative emissions, respectively. In each panel, the Z650 (solid line) and E450 (dashed line) cases are shown together. The dotted line in each figure expresses the value of each variable at the final equilibrium state, corresponding to total cumulative emissions. Small circles and asterisks attached to the lines indicate the positions at the years 2000, 2050, 2100, 2185, 2300 and ∞ (the final state). The two cumulative emissions of Z650 and E450 coincide in the year 2185 (see Fig. 4(a)). Figure 4(b) clearly depicts the different outcomes of the two pathways: After crossing at about 2185, the two lines become perpendicular. The Z650 line keeps the total emissions unchanged but the concentration decreases toward equilibrium by the action of the carbon cycle to redistribute the originally atmospheric CO2 to other reservoirs. In contrast, in the E450 scenario, the atmospheric concentration is kept at 450 ppm but emissions continue to fill up the other reservoirs, which are not yet equilibrated with the 450 ppm atmosphere. Corresponding to the concentrations the temperature rises in Fig. 4(c) show similar characteristics except that in E450, the temperature rise continues to increase following concentration stabilization due to the thermal inertia of oceans. It is interesting that the two temperature rises almost coincide at 2185, when cumulative emissions become equal. However, after 2185, the temperature for Z650 drops by approximately 0.5 ℃, while that for E450 rises by a further 0.5 ℃. In this way, although the temperature increases of the two pathways are similar for a century or two, they later diverge by more than 1 ℃.
A number of recent studies suggested cumulative emissions as a good measure of peak temperature rise.16,17,21–25) However, as demonstrated above, the peak temperature rises for Z650 and E450 (1.8 ℃ and 2.1 ℃) are of similar magnitude, differing by only 0.3 ℃, whereas the corresponding cumulative emissions are about 1,100 and 2,000 GtC, with about a factor-2 difference. The origin is, of course the difference of functional forms of the two pathways. In almost all studies incorporating cumulative emissions (except for Refs. 21 and 25), functional forms with exponential decrease or similar rapid decrease are assumed, which can be regarded as zero-emission pathways, although this was not further explored by the respective authors. It should be noted that the validity of a simple relationship between cumulative emissions and peak temperature increases is limited to a class of emission pathways with similar functional forms, particularly (effectively) zero-emission pathways, which generally have maxima in concentration pathways (over-shoot scenario). The relationship does not hold if stabilization pathways are included. Since emissions pathways (functional forms) are more or less similar for the two types during the 21st century, the relation between cumulative emissions and peak temperatures holds for a century or two. However, in the E-stabilization case, small but continuing emissions to keep CO2 concentrations constant result in much larger cumulative emissions with the lapse of time so that the simple relation does no longer hold later as seen in Fig. 4(c), where the two trajectories run close until 2185 but diverge after the crossing.
In view of the current situation, in which discussions of mitigation policies still concern with stabilization (of concentration) as the target, care must be taken to avoid confusion in mentioning cumulative emissions. Figure 4(d) shows projected sea level rise. Here, once again, we can see a critical difference between the two pathways Z650 and E450, namely 45 cm vs. 80 cm at the final equilibrium state. We can confirm that the warning message contained in Fig. 1 has a different degree of seriousness in Z-stabilization.
Two types of stabilization and “commitment”
In this Part 1, we first questioned the concept (or implication) of “stabilization,” which is usually accepted within the climate change research community, and then proposed a different type of stabilization as an alternative basis for climate change mitigation strategy. In connection with stabilization in the traditional sense (E-stabilization, in this paper), climate changes that take place in the long-term future under conditions of constant CO2 concentration are often called “climate change commitment” or “committed climate change” (see Box TS 9 of the IPCC WG I AR41)). The origin of this wording is likely intended to distinguish transient temperature rise at the time when CO2 concentration first reaches the targeted stabilization level from the final equilibrium temperature rise to be reached long after. In this case, the transient temperature (rise) is called “realized temperature (rise),” while the final equilibrium temperature (rise) is called “committed temperature (rise).” Such wording can be traced back to Ref. 26 and the first IPCC Report.27) Similar terms, such as “unrealized warming”,28) and “residual warming”29) were used to refer to the same quantity, as noted by Ref. 30.
Serious misunderstandings often arise from the use of the word commitment in the way described in Refs. 13 and 31. Recently, different types of warming commitment have been defined and used.32–34) We consider that “commitment” in such many ways will lead to further confusion, and that even the historically longest use of the word is misleading as discussed subsequently. In the following discussion, we shall assume that “stabilization of GHG concentration” refers to holding the radiative forcing of all compositions constant, as usually assumed in similar discussions. In the IPCC WG I AR4,1) temperature rises that occur after the GHG concentration is stabilized are referred to as committed temperature rise. Then, for example in the case of stabilization at the year 2000, the temperature rise after 2000 under the constant GHG concentration is described as if it were “inevitable”, because it is due to previous emissions. The statement “even if the concentrations of greenhouse gases in the atmosphere had been stabilized in the year 2000, we have already committed to further global warming of another half degree”,35) would be taken to imply that further warming is inevitable.
However, the “committed” temperature rise at 2000 is not inevitable. If all anthropogenic GHG emissions ceased in 2000, their concentrations in the atmosphere are no longer constant but would decrease by natural removal processes, so that the temperature will not rise as “committed” even though the climate system (ocean) tends to catch up with the equilibrium temperature. The misunderstanding of “committed” as “inevitable” may be very serious if the word commitment is used to refer to long-term climate changes associated with stabilization scenarios at high CO2 concentration levels, for example, 550 ppm. Committed change is often taken to mean that once we stabilize the concentration at this level, with a temperature rise of approximately 3 ℃ (assuming the best estimate of the climate sensitivity), a significant further and probably dangerous sea level rise is inevitable, due to thermal expansion and ice-sheet melting; an important point that Fig. 1 indicates, giving rise to some alarm.
In view of the above problem, we shall consider which climate-related outcomes may actually be inevitable. In the case of Z-stabilization, after the time when the emissions reach zero, the cumulative total CO2 at that time remains in the climate system for a long period and is partitioned between atmosphere, ocean and terrestrial ecosystems at definite rates under the equilibrium state. In this case, climate changes, including sea level rise due to the constant temperature rise at the equilibrium CO2 concentration, is actually inevitable. Ref. 16 raised a concern about these “irreversible” climate changes. We consider that the temperature rise at the equilibrium state for an increased CO2 content in the climate system due to previously emitted total anthropogenic CO2 (termed “zero-emissions commitment” in the IPCC WG I AR4 (Box TS9)),1) should instead be termed “committed temperature rise.”
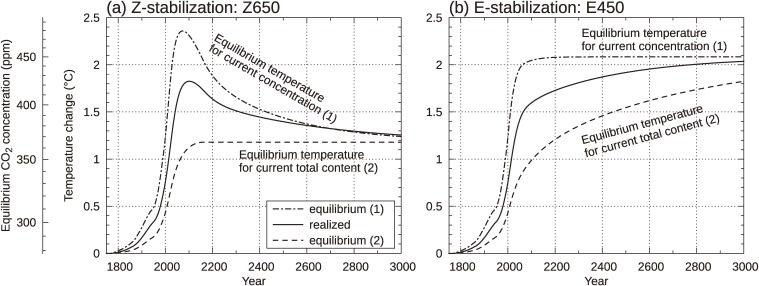
Figure 5(a) and (b) show three temperature rises: the equilibrium temperature rise corresponding to current atmospheric CO2 concentration or “committed temperature rise” in the sense of the IPCC AR4 TS Box 9 (indicated by a chain line); the actually realized temperature increase (transient response of the climate system to increasing CO2; solid line); and the temperature rise to be realized at the final equilibrium state for current total CO2 content in the climate system (“zero-emissions commitment” in Ref. 1 or truly committed temperature rise; dashed line). Assuming 3 ℃ as climate sensitivity, the CO2 concentration corresponding to temperature rise is also shown together, as the vertical axis. From Fig. 5(b) for the E-stabilization case, one can see the difference between the two committed temperature rises and understand that the use of “commitment” (as in the existing literature) to refer to the outcome (temperature rise) of hypothetically maintaining GHG concentration at current level is quite different from truly inevitable changes due to the previous emissions. Therefore, we must keep in mind that a stabilization target (CO2 concentration and temperature) can be revised to any lower levels above the lowest limit, the truly committed values due to previous emissions (shown by dashed lines). To be specific, at 2100 the concentration has already almost reached the target level of 450 ppm (Fig. 5(b); also see Fig. 2), in which the equilibrium temperature rise is 2.1 ℃, while the actually realized temperature rise is about 1.6 ℃. By using the word “commitment” in the same way as IPCC AR4, we express the situation as follows: In year 2100, although the observed temperature rise is still 1.6 ℃, committed global warming reaches 2.1 ℃ corresponding to the stabilization concentration. But actually, it is still possible to revise the stabilization level below 450 ppm down to the lowest limit of about 350 ppm (Fig. 5(b)), resulting in a final equilibrium temperature rise of approximately 1.0 ℃, both concentration and temperature rise being lower than the current levels. Thus, this diagram helps understanding that stabilization levels could be revised to lower ones. Similar differences between the two committed temperature rises, responding to the currently realized and the final equilibrium concentrations, are also found for the case of the Z-stabilization Z650 in Fig. 5(a), but decrease in the concentration and temperature down to the committed (truly inevitable) level is inherently realized because of the zero emissions.
Figure 5.
Three temperature increases for Z650 (a) and E450 (b): The equilibrium temperature rise for current atmospheric CO2 concentration (chain line), which is the same as committed temperature rise in the IPCC WG I AR4, the actually realized temperature rise (solid line), and the equilibrium temperature rise for current total CO2 content in the climate system (dashed line), which is truly committed and called zero-emissions commitment in the AR4 Technical Summary. Non-CO2 forcing is not included in the model. The additional vertical axis indicates CO2 concentrations; the chain line expresses the concentration pathway when it is read referring to the concentration scale.
Discussion and conclusion
Comparison with other studies regarding persistence of temperature rise.
Recently, the “irreversibility” of global warming has been investigated and emphasized in some studies we have cited. The central notion is that anthropogenic CO2 emitted into the atmosphere results in increased total carbon loading in the atmosphere–ocean–land system, which is then conserved so that atmospheric CO2 concentration level and temperature rise will persist over millennia time scales, making the global environment different from the present; this era with a new environmental state is named the “Anthropocene,” contrasting to the “Holocene” before the anthropogenic interference.36,37) In order to investigate the persistence of temperature rise, numerical experiments were performed using earth system models to include carbon cycle processes, mostly by EMICs, under the condition that CO2 emissions grow rapidly to reach a high level and that emissions are then instantaneously shut off to zero; thereafter, the atmospheric CO2 concentration and temperature increase both evolve under conditions of no further anthropogenic emissions. The results of such experiments are described in Chapter 10 of the IPCC WG I AR4 as well as in Ref. 34, where the results of 5 experiments using different EMICs are shown and compared. In each experiment, increasing emissions were introduced, to reach 750 ppm at 2100 and then the emissions were shut off; the projection was continued until 3000. The results of temperature changes (shown in Fig. 10.35 in the AR4) diverge, perhaps due to differing climate sensitivity of the models but, except for one case, the projected temperature increases at 3000 are all within the range 1–2 ℃. Tracing the evolutions of the temperature increases, we see that the differences between the peaks and the final states (3000) are almost the same magnitude, about 0.5 ℃ for 4 cases with peak temperature of 2–2.5 ℃. In the case with peak temperature about 1.3 ℃, the difference is smaller, about 0.3 ℃. Another recent paper16) presented the results of similar projections using Bern2.5CC EMIC,34,38) which treated several pathways with emissions to reach 450–1200 ppm at 2100. In this work, for the cases with peak temperature increase of 1–3 ℃ (concentration 450–750 ppm), the differences between the peak and the value projected at 3000 are about 0.5 ℃, although for the lowest and highest concentrations, differences are slightly lower and higher. Thus, we confirm that the present result of Z650, with peak temperature 1.8 ℃ at about 2100 and 1.3 ℃ at 3000, is consistent with those projections assuming a sudden cessation of emissions.
An exception is the case reported by Lowe et al. (2009).39) In that work, a full-scale earth system model was used instead of EMICs, but the integrations were performed for a short period of 100 years after cessation of CO2 emissions. In that case, for such a short period, the temperature rise does not show discernible decreasing trend. The CO2 concentration decreased very little, in contrast to other studies described above. The origin of this unique feature is attributable to return emissions from land ecosystems under warmer climate, especially due to the so-called Amazon dieback. However, this particular feature is limited only to this model among several full-scale earth system models and remains uncertain.40) The differences in carbon budget and temperature rise between this work and our results are further described in the Appendix.
Thus, the result of the present work is basically the same as those of recent studies emphasizing the irreversibility of temperature rise. Small decreases of temperature rise around 0.5 ℃, taking place over a millennium-long period may be regarded as negligible compared with their respective peaks in a range of 2–3 ℃, and this situation can be regarded as “irreversible.” In our view, however, this decrease of temperature rise, which is surely assured by the zero-emissions scenario, can be taken into account within strategies for avoiding long-term risks, particularly regarding melting of the Greenland ice sheet. It is suggested that the equilibrium ice sheet volume of Greenland as well as time to approach the final state depend sensitively on summer mean temperature rise in the vicinity of 3 ℃,41–44) which corresponds to around 2 ℃ global mean temperature rise, so that it is inferred that the difference between an increase of 2 ℃ or 1.5 ℃ will significantly influence sea level rise when this temperature lasts for a long period. (Note, however, that the long time scale of climate restoration processes might be critical if the ice flow of Greenland is very rapid as inferred from some recent observations cited in Ref. 1.) On the other hand, studies that emphasize irreversibility must take account of the concern regarding the persistence of some potentially challenging outcomes such as frequent occurrence of mega-drought after stabilization, but not necessarily concerned about the accumulation of longer-term small effects like ice-sheet melting.
Feasibility and need for zero-emission strategy.
Reducing CO2 emissions to zero plays a key role in this two-part paper. It is natural to question whether such an ambitious target could be realized and whether it is appropriate to consider climate change mitigation strategies on this basis. There are several reasons to include zero emissions as one possible and realistic measure in emissions reduction strategies. First, in current climate change mitigation discussions, the need to reduce emissions by around 80% for the stabilization of CO2 concentration at a reasonable level (450–550 ppm) has become understood and accepted as the target to be achieved by approximately the end of the 21st century. Further, by recognizing this need, the target of 80% reduction of CO2 emissions in the developed countries by 2050 has become part of the international political agenda and is currently subject to serious global discussions on the technological and socioeconomic implications of implementing this target. Under these circumstances, it is quite natural to include the zero emissions in climate change mitigation strategies as an extension of emissions reduction scenarios, next to that of stabilization. Here, note that “zero emissions” does not refer to exactly zero but to emissions sufficiently below the natural uptake level, which is equal to emissions under the stabilization, estimated at 1–1.5 GtC y−1 for stabilization levels in the range 450–550 ppm.
Secondly, to our knowledge, no arguments have been presented to demonstrate the existence of a particular barrier to reducing CO2 emissions below such levels. This is quite understandable, considering that most CO2 emissions derive from consumption of fossil fuels that can be substituted by other, carbon-free sources, such as renewable energy, and also that emerging carbon capture and sequestration (CCS) technology is expected to provide a powerful means to prevent the release of CO2 to the atmosphere. The CCS technology combined with extensively utilizing biomass energy or even direct CO2 removal from the atmosphere might enable negative emissions. There may be concern about the small level of emissions associated with “essential use,” as in the case of the regulation of CFC emissions. If the amount is 1.0 GtC y−1 (or 12% of the current level) and if it lasts for 200 years more (until 2360), the total emissions add 200 GtC to that projected under Z650. Referring to Fig. 4c, we see that the final temperature rise is still less than 1.5 ℃. In any case, “zero emissions” are considered to be a certain non-zero level but sufficiently below the natural uptake level, which could be resulted from the balance between reduced emissions and negative emissions.
Besides technological feasibility, we consider that mitigation strategies incorporating zero-emissions must be examined more seriously as an alternative to the traditional E-stabilization scenario. Some recent papers23,24) considered virtual-zero-emissions or limited cumulative emissions in order to maintain CO2 (generally GHG) concentration at low levels, although they do not mention that zero emissions and subsequent natural restoration effects are the key to maintaining the low concentrations. One previous study23) raised problems associated with stabilization (E-stabilization) and proposed CO2 emissions pathways in which emissions are reduced at a constant percentage rate at the declining stage of emissions, as a means to replace current emissions pathways aiming to achieve stabilization. Since, in such an exponentially decreasing emissions pathway, the cumulative emissions total (at an infinite time in the future) is limited to a definite value, such pathways are termed “containment scenarios.”23) As noted previously, almost all studies that incorporate cumulative emissions address such pathways. It is clear that such pathways are simply a form of zero-emission, although no particular interest was shown in their nature or merit compared with the traditional stabilization.
In addition, the United Kingdom Climate Change Committee (UKCCC) assessed potential temperature increases under a variety of emissions pathways to determine the most appropriate options for climate change mitigation.45) That study investigated CO2 emissions with pathways very similar to those in the present work. In the least-emissions pathways with exponentially decreasing functional forms, similar to those treated in Ref. 23, the CO2 emissions are terminated when the magnitude becomes practically zero (typically less than 0.1 GtC y−1), mostly during the 22nd century, whereas for other CO2 emissions pathways, a constant value (1.4 GtC y−1) is assumed to continue after the time when the emissions decrease to this value. In addition, other GHG emissions are assumed to continue over the whole period until 2300.
Quite recently, the Committee on Stabilization Targets for Atmospheric Greenhouse Gas Concentrations of the US Academies published its report,17) in which it appears that the term “stabilization target” refers to the traditional E-stabilization without zero-emissions. However, the report mentioned that cumulative total CO2 emission is a good measure of temperature rise, in reference to the works mentioned previously. Thus, the cumulative emissions calculations in that report should be treated similarly to other works, i.e., a definite amount until the time of zero emissions.
Thus, quite a few studies already incorporate zero-CO2 emission scenarios as part of climate change mitigation measures. Moreover, as mentioned previously in some recent scenario studies, even negative emissions are taken into account (e.g., Category I scenarios in the IPCC WG III AR43)). If one considers that a considerable amount of negative emissions (e.g., biomass energy use combined with CCS) are feasible from technological and socioeconomic viewpoints in the 21st century to make the net world CO2 emissions negative, “zero-emissions” in this work should be taken as an illustrative case where net anthropogenic CO2 emissions are small compared with natural uptake. What we have shown is that keeping such low level emissions in the next century and beyond will make more emissions in this century permissible while it lessens the long-term risks of sea level rise compared with traditional stabilization scenarios with the same temperature rise constraint.
Nevertheless, in many official discussions related to policy, emissions reduction is considered in connection with the IPCC WG III AR4,3) in which stabilization (E-stabilization) plays a central role and temperature rise constraint is connected with target concentrations and temperatures, as if this were self evident. This viewpoint persists and strongly influences stabilization scenarios, not only in the IPCC WG III AR4 but also in recent activity on the production of RCP for the next IPCC report for climate change assessment. Restriction due to E-stabilization, which we have criticized in this Part 1, mainly from the perspective of long time scales (several centuries to millennia) in connection with sea level rise, also involves significant difficulties over shorter time scales, such as enforcing unnecessarily stringent CO2 reductions during the 21st century in order to avoid greater temperature increases that would occur many centuries later, at the targeted stabilization state, as will be discussed in Part 2.
Under these circumstances, it is apparent that we must look beyond the traditional stabilization concept for the most suitable emissions pathway as a climate change mitigation strategy,6) and that this process should be guided by the climate science. To this end, it might be necessary to clarify the implication of “stabilization” in Article 2 of the UNFCCC.
Acknowledgments
This paper is closely based on presentations at the 1st and 2nd Canon Institute for Global Studies (CIGS, Tokyo) Symposia, held October 27, 2009 and September 16, 2011, respectively. We are grateful to the participants for productive discussions. In particular, we express our gratitude to Prof. Sir Brian Hoskins of the Imperial College London and Prof. Susan Solomon of Massachusetts Institute of Technology, invited lecturers at the 1st and 2nd Symposia, respectively, who offered valuable advice and comments. We also thank Dr. Kuno Strassmann of the University of Bern, Dr. Seita Emori of the National Institute for Environmental Studies in Japan, and Dr. Kooiti Masuda of the Japan Agency for Marine–Earth Science and Technology, for their constructive comments and suggestions on an early draft of this paper. We are much obliged to Prof. Tetsuo Yuhara and Dr. Fengjun Duan of CIGS, for their continued interest in this work and encouragement during preparation of the draft. Finally, we thank three reviewers for their valuable and constructive comments, which contributed greatly to improvement of the original draft.
Appendix: Brief description of the model
The simplified climate system model used in this study is based on the Nonlinear Impulse response model of the coupled Carbon cycle–Climate System (NICCS), developed at the Max Planck Institute for Meteorology.46) Details and modifications to the base formulation are described in Ref. 18.
The model calculates temporal changes in atmospheric concentration for a given pathway of annual CO2 emissions from all over the world, which is specified by 5-yearly time series. The excess carbon is partitioned into the atmosphere and the upper ocean, considering the chemistry governing ocean CO2 uptake. Processes of further uptake into the intermediate and deep ocean are modeled on the impulse-response function, represented by a sum of four decaying exponentials plus one constant, corresponding to the equilibrium airborne fraction for initial pulse CO2 emissions. Parameters used in this study are given in Table A1. Note that the equilibrium airborne fraction depends on the cumulative total of CO2 emissions due to the nonlinear CO2 chemistry in the ocean. The response parameters are adjusted in this study so that the equilibrium airborne fraction is about 0.2 for cumulative emissions of 1000–2000 GtC, in accordance with a recent review study.47) A part of the atmospheric carbon is stored in the terrestrial biosphere through CO2 fertilization, based on Ref. 48. Processes of a return of terrestrial carbon to the atmosphere due to respiration are also modeled with the impulse-response function represented by a sum of four decaying exponentials. Parameters of this terrestrial uptake are given in Table A2.
Table A1.
Response function parameters for ocean CO2 uptake
| i | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Ai | 0.170 | 0.273 | 0.253 | 0.209 | 0.095 |
| τi (y) | ∞ | 236.5 | 59.52 | 12.17 | 1.271 |
The response function is formulated as R(t)=∑i=04Aiexp(-t/τi), where Ai, τi, and t are amplitude, time constant, and time, respectively. The function represents excess carbon remaining in the atmosphere after ocean uptake for given impulse emissions.
Table A2.
Response function parameters for the return flux of terrestrial CO2 uptake
| i | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Ai (y−1) | 0.702 | 0.0134 | −0.718 | 0.00293 |
| τi (y) | 2.86 | 20 | 2.18 | 100 |
The response function is formulated as R(t)=∑i=14Aiexp(-t/τi). This function is used in the second term of the terrestrial carbon uptake, b(t)=δb(t)-∫0tδb(t’)R(t-t’)dt', where b represents net primary production (NPP). The first term is an additional NPP by CO2 fertilization, parameterized as δb(t)=b0βln(C(t)/C0), where β and C are fertilization factor (0.287) and atmospheric CO2 concentration, respectively. Subscript 0 represents a preindustrial value (60 GtC y−1 for b0 and 278 ppm for C0).
In addition to the original formulation of this simplified carbon cycle, we developed an inversion procedure to calculate CO2 emissions for a given concentration pathway. Since the model requires historical emissions from the preindustrial state, we applied this inversion procedure to observed CO2 concentration from 1750 through 1995. The calculated carbon budget during this period is consistent with the current scientific understanding, summarized in Tables 5.1 and 7.1 of the IPCC WG I AR4.1) The cumulative total emissions until 1995 amount to 380 GtC. The airborne fraction for this CO2 is about 47% at 1995, while the oceanic and terrestrial uptake fractions are about 33% and 20%, respectively.
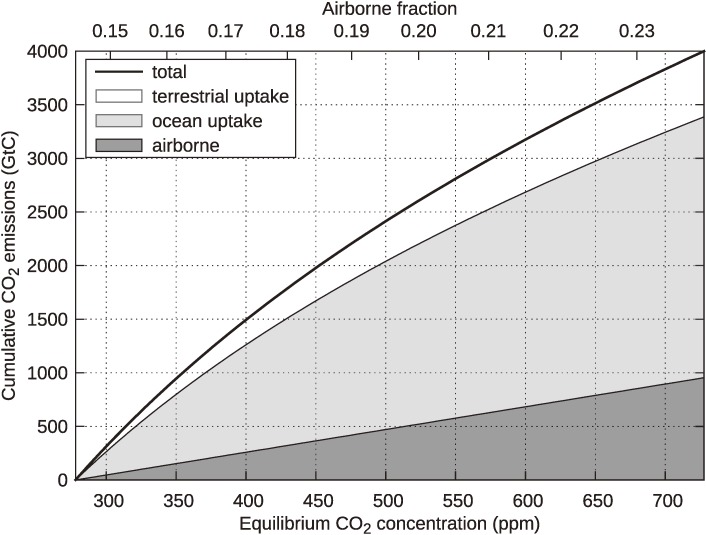
In the model, the equilibrium state of the carbon cycle is directly evaluated from a given atmospheric CO2 concentration, and the relationship between cumulative emissions and equilibrium contents in the three reservoirs is obtained as shown in Fig. A1. Although the equilibrium carbon in the terrestrial biosphere increases with an increase in cumulative emissions, its value may be overestimated, particularly for large emissions, because the model does not include any feedback processes that accelerate the respiration of organic materials due to the fertilization effect with an increase in temperature. Processes relevant to ocean CO2 uptake in the model do not depend on temperature either, which may somewhat overestimate oceanic carbon content.
Figure A1.
Relationship between cumulative CO2 emissions and equilibrium CO2 concentrations. Excess carbon within the atmosphere, ocean, and terrestrial biosphere are illustrated by vertical partitions with different shading densities.
Climate response to temporal changes in atmospheric CO2 concentration is represented by a convolution with CO2 forcing and impulse-response function. Two climate variables are considered in this study: surface temperature and sea level rise due to thermal expansion of seawater. Radiative forcing is proportional to the logarithm of atmospheric CO2 concentration, where the equilibrium climate sensitivity, defined as a temperature rise (or a sea level rise) at equilibrium state from the preindustrial level after doubling CO2 concentration, is used as a proportional constant. Parameters of this response model are given in Table A3. Since the climate sensitivity involves considerable uncertainties about climate feedbacks to CO2 forcing, an uncertainty range of surface warming should be taken into account, such as factors of 0.67–1.5 to the model output, assuming the best estimate of climate sensitivity (3 ℃). These factors correspond to the likely range of sensitivity, 2–4.5 ℃ (>66% probability), by the current comprehensive assessment.1) Transient warming and the thermal expansion of seawater represented by the response-function parameters are also subject to uncertainties associated with oceanic heat uptake.
Table A3.
Response function parameters for surface temperature and sea level rise due to thermal expansion of seawater
| Surface temperature | Sea level rise | |||
|---|---|---|---|---|
| i | 1 | 2 | 1 | 2 |
| Ai | 0.290 | 0.710 | 0.963 | 0.037 |
| τi (y) | 400 | 12 | 800 | 25 |
The climate response is formulated as x(t)=S∫0tlog2(C(t)/C0)∑i=12(Ai/τi)exp(-(t-t’)/τi)dt', where S is a sensitivity parameter (3 ℃ for the surface temperature rise, i.e., the best estimate of the equilibrium climate sensitivity; and 1.14 m for the sea level rise).
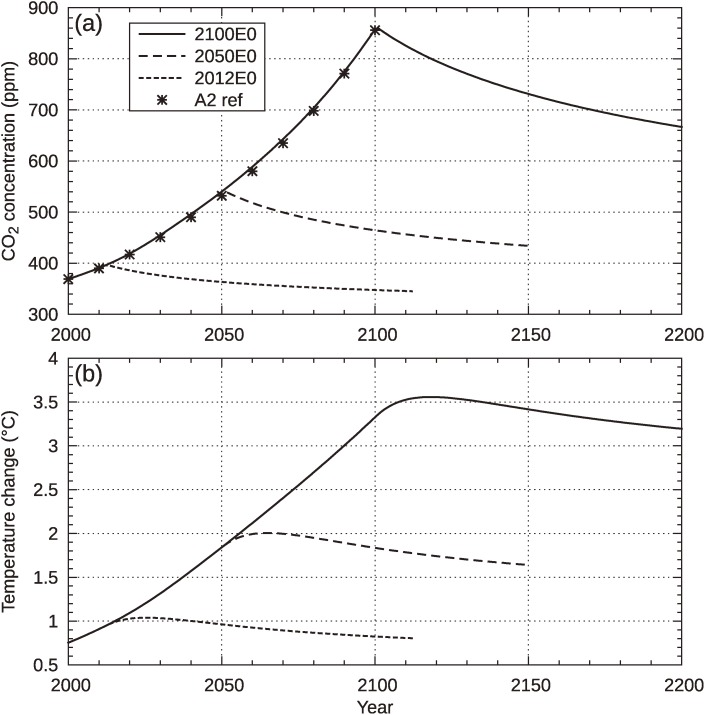
These uncertainties about climate–carbon cycle feedbacks and climate responses require considerable further investigation. Here, we present a brief comparison of our model and two other models, HadCM3LC50) and Bern2.5CC,34,38) by conducting idealized emission-shutting-off experiments, as described in Ref. 39. The experiments were conducted first following the SRES49) A2 scenario (CO2 only), then suddenly shutting off the emissions at 2012, 2050 and 2100, followed by a zero-emission period of 100 years. Figure A2 shows the evolutions of the CO2 concentration and surface temperature for these three emissions pathways. The tendency for increasing concentration in the SRES A2 until 2100 is almost identical to that of the reference data provided by IPCC WG I TAR,11) particularly those of the ISAM reference (see Fig. A2). After the cessation of emissions, concentrations decline at different rates, depending on the time of the cessation. Although the temperature response is delayed due to the thermal inertia of oceans, the temperature begins to decline within a few decades in all of the three pathways.
Figure A2.
Similar to Fig. 1 of Ref,39) but CO2 concentrations and temperature changes indicated by the present model. The experiments were conducted with the SRES A2 scenario (CO2 only), followed by zero emissions of 100 years from 2012, 2050 and 2100, labeled as 2012E0, 2050E0 and 2100E0, respectively. Asterisk markers show reference concentrations taken from the ISAM reference in Ref. 11.
These responses are model-dependent, and cooling after zero emissions does not appear in the case of HadCM3LC, one of the full-scale earth system models used in Ref. 39. Table A4 compares natural CO2 uptake during the 100 years after the cessation of CO2 emissions between the present model and the HadCM3LC model. The latter is characterized by very low rates of decline in the CO2 concentration, which is mainly explained by the evolution of its terrestrial biosphere changing, with warming, from a carbon sink to a source. The oceanic CO2 uptake in HadCM3LC is also smaller than that in the present model, despite their qualitative similarity. Table A5 shows the responses of CO2 concentration and temperature for the case of cessation in 2050, estimated by the two models; and a comparable experiment by the Bern2.5CC model,34,38) one of the representative EMICs. The declining concentration in the present model is more than twice that in HadCM3LC, and the temperature response shows a different sign between the two models. In contrast, Bern2.5CC shows similar responses to the present model.
Table A4.
Comparison of natural CO2 uptake in the 100 years after cessation of CO2 emissions in the 2012E0, 2050E0 and 2100E0 experiments. Results from the HadCM3LC model are taken from Ref. 39
| HadCM3LC (GtC) | Present model (GtC) | |
|---|---|---|
| 2012E0 land | 23 | 31.3 |
| ocean | 54 | 78.5 |
| 2050E0 land | −50 | 69.5 |
| ocean | 132 | 160.6 |
| 2100E0 land | −76 | 129.1 |
| ocean | 235 | 286.1 |
Table A5.
Comparison of climate responses in the 100 years after cessation of CO2 emissions in 2050E0. Results from the HadCM3LC model are taken from Ref. 39, and results from the Bern2.5CC model34,38) are taken from a similar experiment, described in Ref. 16
| Concentration (ppm) | Temperature (℃) | |
|---|---|---|
| HadCM3LC | −40 | +0.2 |
| Bern2.5CC | −100 | −0.1 to −0.2 |
| the present model | −106 | −0.2 |
This comparison helps explain the behavior of the present model in a broad range of uncertainties related to the carbon cycle and slow climate responses associated with terrestrial ecosystem and oceanic heat uptake. The results obtained from our simplified model should be considered as an illustrative case and further examined for better understanding of climate restoration by zero emissions in light of upcoming earth system modeling studies.
References
- 1).IPCC WG1 (2007) Climate change 2007: The physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds. Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M. and Miller, H.L.). Cambridge University Press, Cambridge. [Google Scholar]
- 2).IPCC WG2 (2007) Climate change 2007: Impacts, adaptation and vulnerability. Contribution of Working Group II to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds. Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J. and Hanson, C.E.). Cambridge University Press, Cambridge. [Google Scholar]
- 3).IPCC WG3 (2007) Climate change 2007: Mitigation of climate change. Contribution of Working Group III to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds. Metz, B., Davidson, O.R., Bosch, P.R., Dave, R. and Meyer, L.A.). Cambridge University Press, Cambridge. [Google Scholar]
- 4).IPCC (2007) Climate change 2007: Synthesis report. Contribution of Working Groups I, II and III to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds. Core Writing Team, Pachauri, R.K. and Reisinger, A.). Intergovernmental Panel on Climate Change, Geneva. [Google Scholar]
- 5).Moss, R., Babiker, M., Brinkman, S., Calvo, E., Carter, T., Edmonds, J., Elgizouli, I., Emori, S., Erda, L., Hibbard, K., Jones, R., Kainuma, M., Kelleher, J., Lamarque, J.F., Manning, M., Matthews, B., Meehl, J., Meyer, L., Mitchell, J., Nakicenovic, N., O’Neill, B., Pichs, R., Riahi, K., Rose, S., Runci, P., Stouffer, R., van Vuuren, D., Weyant, J., Wilbanks, T., van Ypersele, J.P. and Zurek, M. (2008) Towards new scenarios for analysis of emissions, climate change, impacts, and response strategies. Intergovernmental Panel on Climate Change, Geneva. [Google Scholar]
- 6).Science Council of Japan (2008) International professional meeting on global warming issues, chairperson’s summary. 23–24 June 2008 at Sapporo (http://www.scj.go.jp/ja/member/iinkai/chikyu/).
- 7).Kheshgi H.S., Smith S.J., Edmonds J.A. (2005) Emissions and atmospheric CO2 stabilization: long-term limits and paths. Mitigation and Adaptation Strategies for Global Change 10, 213–220 [Google Scholar]
- 8).Matthews H.D., Caldeira K. (2008) Stabilizing climate requires near-zero emissions. Geophys. Res. Lett. 35, L04705 doi:10.1029/2007GL032388 10.1029/2007GL032388 [DOI] [Google Scholar]
- 9).IPCC (2001) Climate change 2001: Synthesis report. A contribution of Working Groups I, II, and III to the third assessment report of the Integovernmental Panel on Climate Change (eds. Watson, R.T. and the Core Writing Team). Cambridge University Press, Cambridge. [Google Scholar]
- 10).United Nations (1992) United Nations Framework Convention on Climate Change. http://www.unfccc.int/ [DOI] [PubMed]
- 11).IPCC WG1 (2001) Climate change 2001: The scientific basis. Contribution of Working Group I to the third assessment report of the Intergovernmental Panel on Climate Change (eds. Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K. and Johnson, C.A.). Cambridge University Press, Cambridge. [Google Scholar]
- 12).Matsuno, T. (2005) A comment. In Science on Sustainability 2006. Report of the Research on the Scientific Basis for Sustainability (eds. Kitagawa, M. and Yamamoto, R.), E-Square Inc., pp. 46–47 (in Japanese). [Google Scholar]
- 13).Matsuno T. (2007) The turning point of climate change research from the warning to response to the problem. Kagaku (Science) 77, 730–736(in Japanese) [Google Scholar]
- 14).Matsuno, T. (2007) Projection of the future climate change for decades, a century and beyond. In Proceedings of CAETS 2007 TOKYO (17th Convocation of the International Council of Academies of Engineering and Technological Sciences, 23–26 October 2007, Tokyo). The Engineering Academy of Japan, Tokyo, Japan, pp. 34–37. [Google Scholar]
- 15).Maruyama, K. (2008) Solutions of global warming. Special Edition of Denki Shimbun (the Electric Daily News), March 2008, 23 (in Japanese). [Google Scholar]
- 16).Solomon S., Plattner G.K., Knutti R., Friedlingstein P. (2009) Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. U.S.A. 106, 1704–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).National Research Council (2011) Climate stabilization targets: Emissions, concentrations, and impacts over decades to millennia. National Academies Press, Washington, DC. [Google Scholar]
- 18).Tsutsui J. (2011) SEEPLUS: A simple online climate model. Journal of Japan Society of Civil Engineers, Ser. G (Environmental Research) 67, 134–149 [Google Scholar]
- 19).Knutti R., Allen M.R., Friedlingstein P., Gregory J.M., Hegerl G.C., Meehl G.A., Meinshausen M., Murphy J.M., Plattner G.K., Raper S.C.B., Stocker T.F., Stott P.A., Teng H., Wigley T.M.L. (2008) A review of uncertainties in global temperature projections over the twenty-first century. J. Clim. 21, 2651–2663 [Google Scholar]
- 20).Matsuno, T. and Tanabe, K. (1995) Some considerations regarding carbon budgets based on simple ocean models. In Proceedings of the Tsukuba Global Carbon Cycle Workshop—Global Environment Tsukuba ’95—. Center for Global Environmental Research, National Institute for Environmental Studies, CGER-I018-95, pp. 21–29. [Google Scholar]
- 21).Matthews H.D., Gillett N.P., Stott P.A., Zickfeld K. (2009) The proportionality of global warming to cumulative carbon emissions. Nature 459, 829–832 [DOI] [PubMed] [Google Scholar]
- 22).Raupach M.R., Canadell J.G., Ciais P., Friedlingstein P., Rayner P.J., Trudinger C.M. (2011) The relationship between peak warming and cumulative CO2 emissions, and its use to quantify vulnerabilities in the carbon-climate-human system. Tellus B 63, 145–164 [Google Scholar]
- 23).Allen M.R., Frame D.J., Huntingford C., Jones C.D., Lowe J.A., Meinshausen M., Meinshausen N. (2009) Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 458, 1163–1166 [DOI] [PubMed] [Google Scholar]
- 24).Zickfeld K., Eby M., Matthews H.D., Weaver A.J. (2009) Setting cumulative emissions targets to reduce the risk of dangerous climate change. Proc. Natl. Acad. Sci. U.S.A. 106, 16129–16134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Meinshausen M., Meinshausen N., Hare W., Raper S.C.B., Frieler K., Knutti R., Frame D.J., Allen M.R. (2009) Greenhouse-gas emission targets for limiting global warming to 2℃. Nature 458, 1158–1162 [DOI] [PubMed] [Google Scholar]
- 26).Ramanathan V. (1988) The greenhouse theory of climate change: A test by an inadvertent global experiment. Science 240, 293–299 [DOI] [PubMed] [Google Scholar]
- 27).IPCC WG1 (1990) Climate change: The IPCC scientific assessment. Report prepared for Intergovernmental Panel on Climate Change by Working Group I (eds. Houghton, J.T., Jenkins, G.J. and Ephraums, J.J.). Cambridge University Press, Cambridge. [Google Scholar]
- 28).Hansen J., Russell G., Lacis A., Fung I., Rind D., Stone P. (1985) Climate response times: Dependence on climate sensitivity and ocean mixing. Science 229, 857–859 [DOI] [PubMed] [Google Scholar]
- 29).Wigley T.M.L., Schlesinger M.E. (1985) Analytical solution for the effect of increasing CO2 on global mean temperature. Nature 315, 649–652 [Google Scholar]
- 30).Wigley T.M.L. (2005) The climate change commitment. Science 307, 1766–1769 [DOI] [PubMed] [Google Scholar]
- 31).Matthews H.D., Weaver A.J. (2010) Committed climate warming. Nat. Geosci. 3, 142–143 [Google Scholar]
- 32).Friedlingstein P., Solomon S. (2005) Contributions of past and present human generations to committed warming caused by carbon dioxide. Proc. Natl. Acad. Sci. U.S.A. 102, 10832–10836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Hare B., Meinshausen M. (2006) How much warming are we committed to and how much can be avoided? Clim. Change 75, 111–149 [Google Scholar]
- 34).Plattner G.K., Knutti R., Joos F., Stocker T.F., von Bloh W., Brovkin V., Cameron D., Driesschaert E., Dutkiewicz S., Eby M., Edwards N.R., Fichefet T., Hargreaves J.C., Jones C.D., Loutre M.F., Matthews H.D., Mouchet A., Muller S.A., Nawrath S., Price A., Sokolov A., Strassmann K.M., Weaver A.J. (2008) Long-term climate commitments projected with climate-carbon cycle models. J. Clim. 21, 2721–2751 [Google Scholar]
- 35).Meehl G.A., Washington W.M., Collins W.D., Arblaster J.M., Hu A., Buja L.E., Strand W.G., Teng H. (2005) How much more global warming and sea level rise? Science 307, 1769–1772 [DOI] [PubMed] [Google Scholar]
- 36).Crutzen P.J. (2002) Geology of mankind. Nature 415, 23. [DOI] [PubMed] [Google Scholar]
- 37).Crutzen P.J., Steffen W. (2003) How long have we been in the Anthropocene era? Clim. Change 61, 251–257 [Google Scholar]
- 38).Joos F., Prentice I.C., Sitch S., Meyer R., Hooss G., Plattner G.K., Gerber S., Hasselmann K. (2001) Global warming feedbacks on terrestrial carbon uptake under the Intergovernmental Panel on Climate Change (IPCC) emission scenarios. Global Biogeochem. Cycles 15, 891–907 [Google Scholar]
- 39).Lowe J.A., Huntingford C., Raper S.C.B., Jones C.D., Liddicoat S.K., Gohar L.K. (2009) How difficult is it to recover from dangerous levels of global warming? Environ. Res. Lett. 4, 014012 doi:10.1088/1748-9326/4/1/014012 10.1088/1748-9326/4/1/014012 [DOI] [Google Scholar]
- 40).Friedlingstein P., Cox P., Betts R., Bopp L., von Bloh W., Brovkin V., Cadule P., Doney S., Eby M., Fung I., Bala G., John J., Jones C., Joos F., Kato T., Kawamiya M., Knorr W., Lindsay K., Matthews H.D., Raddatz T., Rayner P., Reick C., Roeckner E., Schnitzler K.-G., Schnur R., Strassmann K., Weaver A.J., Yoshikawa C., Zeng N. (2006) Climate–carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J. Clim. 19, 3337–3353 [Google Scholar]
- 41).Letréguilly A., Huybrechts P., Reeh N. (1991) Steady-state characteristics of the Greenland ice sheet under different climates. J. Glaciol. 37, 149–157 [Google Scholar]
- 42).Saito F., Abe-Ouchi A. (2005) Sensitivity of Greenland ice sheet simulation to the numerical procedure employed for ice-sheet dynamics. Ann. Glaciol. 42, 331–336 [Google Scholar]
- 43).Greve R. (2000) On the response of the Greenland ice sheet to greenhouse climate change. Clim. Change 46, 289–303 [Google Scholar]
- 44).Ridley J., Gregory J.M., Huybrechts P., Lowe J. (2010) Thresholds for irreversible decline of the Greenland ice sheet. Clim. Dyn. 35, 1049–1057 [Google Scholar]
- 45).United Kingdom Climate Change Committee (2008) Part I: The 2050 target. In Building a low-carbon economy—the UK’s contribution to tackling climate change, The Stationary Office, London. (http://www.theccc.org.uk/reports/building-a-low-carbon-economy).
- 46).Hooss G., Voss R., Hasselmann K., Maier-Reimer E., Joos F. (2001) A nonlinear impulse response model of the coupled carbon cycle-climate system (NICCS). Clim. Dyn. 18, 189–202 [Google Scholar]
- 47).Archer D., Brovkin V. (2008) The millennial atmospheric lifetime of anthropogenic CO2. Clim. Change 90, 283–297 [Google Scholar]
- 48).Joos F., Bruno M., Fink R., Siegenthaler U., Stocker T.F., LeQuere C., Sarmiento J.L. (1996) An efficient and accurate representation of complex oceanic and biospheric models of anthropogenic carbon uptake. Tellus B 48, 397–417 [Google Scholar]
- 49).Nakicenovic, N. and Swart, R. (eds.) (2000) Emissions scenarios. A special report of working group III of the Intergovernmental Panel on Climate Change. Cambridge University Press, UK. [Google Scholar]
- 50).Cox P.M., Betts R.A., Jones C.D., Spall S.A., Totterdell I.J. (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408, 184–187 [DOI] [PubMed] [Google Scholar]