Abstract
Background
Because depression is a multidimensional construct and few studies have compared the relative importance of its facets in predicting cardiovascular risk, we evaluated the utility of depressive symptom clusters in predicting the 5-year incidence of coronary artery calcification (CAC).
Methods and Results
Participants were 2,171 middle-aged adults (58% female, 43% black) from the Coronary Artery Risk Development in Young Adults (CARDIA) Study who were free of cardiovascular disease. Depressive symptom clusters (z scores) were measured by questionnaires in 2000–2001, and CAC was measured by electron beam computed tomography in 2000–2001 and 2005–2006. There were 243 (11%) cases of incident CAC, defined as the absence of CAC at baseline and the presence of CAC at follow-up. Total depressive symptoms (OR = 1.16, 95% CI: 1.02–1.33, p = .03) and the depressed affect cluster (OR = 1.17, 95% CI: 1.03–1.33, p = .02) predicted incident CAC; however, the somatic, interpersonal distress, low positive affect, and pessimism clusters did not. The depressed affect-incident CAC relationship was independent of age, sex, race, education, and antidepressant use; was similar across gender and racial groups; and was partially accounted for by tobacco use and mean arterial pressure.
Conclusions
In contrast to recent results indicating that the somatic cluster is the most predictive of cardiovascular outcomes, we found that the prospective association between depressive symptoms and incident CAC was driven by the depressed affect cluster. Our findings raise the possibility that there may not be one facet of depression that is the most cardiotoxic across all contexts.
Keywords: atherosclerosis, cardiovascular disease risk factors, coronary artery calcification, depression, epidemiology
Introduction
Three decades of epidemiologic evidence indicate that depression is an independent risk factor for coronary artery disease (CAD).1 The prospective association between depression and CAD is strong and consistent; it is comparable in strength to that of known cardiovascular risk factors2 and has been detected in men and women and in various age and racial/ethnic groups.1 Despite these findings, few clinical trials have examined the effect of depression treatments on subsequent cardiovascular events,3–6 and results for the CAD outcomes in the larger trials have been generally disappointing.3,6 For instance, the Enhancing Recovery in Coronary Heart Disease (ENRICHD) Patients trial, in which nearly 2,500 cardiac patients with depression and/or low social support were randomized to cognitive-behavioral therapy or usual care, did not observe a group difference for the outcome of recurrent myocardial infarction or death.3
Depression is typically conceptualized as a multidimensional construct or disorder consisting of affective, cognitive, behavioral, and somatic symptoms.7 Given its multifaceted nature, it is surprising that little research has compared the relative importance of depressive symptom clusters in predicting CAD risk. Moreover, findings of the few prospective studies examining symptom clusters are conflicting, as some have observed that the somatic symptoms are the most predictive of CAD-related outcomes,8–10 whereas others have reported similar results for the cognitive symptoms of hopelessness11 and pessimism.12,13 Thus, a key remaining question is: are particular depressive symptom clusters stronger predictors of CAD risk than are others? The answer could have significant clinical implications. Although other reasons for the lack of a cardiovascular benefit observed in the ENRICHD trial have been offered, a possible explanation is that the most cardiotoxic aspects of depression were not sufficiently addressed.
Our primary objective was to compare the relative importance of depressive symptom clusters in predicting 5-year incidence of advanced subclinical coronary atherosclerosis, defined as the onset of any coronary artery calcification (CAC). CAC, a computed tomographic measure of the extent of calcified lesions,14 is a strong and independent predictor of cardiovascular events among healthy adults.15 In addition, measures of CAC incidence and progression predict cardiac events.16 To date, only two studies have examined the association between depression and CAC incidence or progression,17,18 and none has evaluated depressive symptom clusters as predictors. Our secondary objective was to explore whether potential mediators19 or confounders account for any observed relationships. We examined Year 15 and Year 20 data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, a prospective cohort investigation of cardiovascular disease and its risk factors. The CARDIA study afforded an ideal opportunity to achieve our aims, given that a multidimensional measure of depression, evaluations of several potential mediators/confounders, and repeated assessments of CAC were obtained from a large sample of middle-aged black and white community members.
Methods
Participants
Participants were 2,171 middle-aged adults from the CARDIA study who were free of cardiovascular disease and CAC in 2000–2001. The original sample consisted of 5,115 black and white community members aged 18–30 years who were recruited from four metropolitan areas in 1985–1986. Descriptions of the recruitment and examination procedures are available elsewhere (www.cardia.dopm.uab.edu). Institutional review board approval was obtained at each site, and participants provided written informed consent.
Of the original sample, 3,672 and 3,549 attended the Year 15 (2000–2001) and Year 20 (2005–2006) examinations. CAC data were obtained from 3,043 persons at Year 15 and 3,139 persons at Year 20. We selected all participants with CAC data at Year 15 and Year 20 (N = 2,486). We excluded participants who answered “yes” or “not sure” when asked about their history of heart attack, angina, peripheral vascular disease, or a stroke/transient ischemic attack at Year 15 (n = 79). Participants with CAC at Year 15 were also excluded (n = 224). Finally, we excluded participants with missing data for depressive symptoms, age, sex, or race (n = 12), leaving a final sample of 2,171 adults. Participants with clinically significant depressive symptoms differed in the expected directions from those without such symptoms on every variable except age and low density lipoprotein (LDL) cholesterol (Table 1). Compared to those excluded at Year 15 (n = 1,501), participants in our sample were more likely to be female (p < .01) and white (p < .01) and had a higher education level (p < .01). No age difference was observed (p = .64).
Table 1.
Characteristics of Participants
| Variable | Nondepressed (n = 1,691–1,848) | Depressed (n = 279–323) | p value |
|---|---|---|---|
| Age, years | 40.3 (3.6) | 39.9 (3.6) | .07 |
| Female, % | 56.3 | 66.9 | <.01 |
| Black, % | 39.7 | 65.0 | <.01 |
| Education, years | 15.3 (2.5) | 14.3 (2.4) | <.01 |
| Antidepressant Use, % | 4.9% | 17.3% | <.01 |
| Mean Arterial Pressure, mmHg | 86.2 (11.1) | 89.0 (11.9) | <.01 |
| LDL Cholesterol, mg/dL | 112.5 (30.0) | 112.6 (32.8) | .97 |
| Glucose ≥ 126 mg/dL, % | 1.7% | 3.8% | .02 |
| Body Mass Index, kg/m2 | 27.9 (5.8) | 29.3 (6.7) | <.01 |
| Tobacco Use, % | 17.6% | 34.7% | <.01 |
| Physical Activity Level, exercise units | 363.0 (277.4) | 296.2 (255.0) | <.01 |
| C-reactive Protein, mg/L | 2.0 (2.2) | 2.5 (2.3) | <.01 |
Note. Continuous variables are presented as mean ± SD, and categorical variables are presented as percentage. Individuals with a CES-D score <16 were classified as nondepressed, and those with a CES-D score ≥16 were classified as depressed. Sample sizes vary slightly across variables due to missing data. Independent-samples t tests and chi-square test were conducted to compare groups on continuous and categorical variables, respectively. LDL = low-density lipoprotein.
Measures and Procedures
Depressive Symptoms
Past-week depressive symptom severity was assessed at Year 15 using the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D).20 We computed a continuous CES-D Total variable, as well as a dichotomous CES-D Group variable to identify persons with (≥ 16) and without (< 16) clinically significant symptoms. The CES-D has good internal consistency, test-retest reliability, and construct validity,20 and a recent meta-analysis confirmed its four-factor structure.21 We computed the following CES-D subscale scores by summing the items that loaded on each factor: depressed affect (e.g., sadness and loneliness), somatic symptoms (e.g., poor appetite and sleep disturbance), interpersonal distress (e.g., feeling disliked), and (lack of) positive affect (e.g., happiness and life satisfaction). Because depressed individuals have negative expectations about the future,22 we conceptualized a widely used scale of dispositional pessimism, the revised Life Orientation Test (LOT-R),23 as a measure of this cognitive symptom of depression. The LOT-R has good internal consistency, test-retest reliability, and construct validity.23 Lower scores are indicative of greater pessimism.
Incident Coronary Artery Calcification
At Years 15 and 20, participants underwent two scans using an electron beam (Imatron C-150; GE Medical Systems) or a multidetector (GE Lightspeed; GE Medical Systems or S4+ Volume Zoom; Siemens) CT scanner. The scans were 1–2 minutes apart and generated 45–55 consecutive images (2.5- to 3.0-mm thickness) from the root of the aorta to the apex of the heart, including the main coronary arteries (left main, circumflex, left anterior descending, and right). Each pair of scans was analyzed using specially designed software that identified candidate calcified lesions.24 A trained cardiovascular radiologist then reviewed each candidate lesion to determine whether it was correctly identified and gave each participant a CAC score. Scores were dichotomized into the presence (Agatston score > 0) or absence (Agatston score = 0) of calcification. Another blinded expert investigator reviewed a subsample of scan pairs. Inter-rater agreement was high, with 95% and 82% and agreement for concordant and discordant scan pairs, respectively. We defined incident CAC as the absence of CAC at Year 15 and the presence of CAC at Year 20, an outcome that reflects the development of type Vb lesions in the coronary arteries.14 We decided to examine CAC incidence instead of CAC progression as our primary outcome because the importance of biological and psychosocial factors may vary by stage in the natural history of CAD.1,25 We do report exploratory analyses for CAC progression.
Covariates
We examined self-reported age, sex (male = 0, female = 1), race (white = 0, black = 1), education, and current antidepressant use (0 = no, 1 = yes) as potential confounders from Year 15. Candidate mediators/confounders from Year 15 were: (1) cardiovascular risk factors – mean arterial pressure (MAP), LDL cholesterol, fasting glucose, and body mass index (BMI), (2) health behaviors – tobacco use and physical activity, and (3) C-reactive protein (CRP). These factors were examined as potential mediators because they are among the putative mechanisms underlying the depression-CVD relationship.2,19 However, because these factors were measured concurrently with depressive symptoms, it is also possible that they could be confounders. The last two of a set of three blood pressure readings were used to compute MAP. LDL cholesterol was derived from the Friedewald equation. Fasting glucose was assessed using a hexokinase ultraviolet method, and CRP was measured by high-sensitivity nephelometry-based methods. Because the glucose variable was positively skewed, we created a dichotomous variable (< 126, ≥ 126 mg/dL). BMI was calculated from measured height and weight. Participants were coded as using tobacco if they reported current use of cigarettes, cigars, snuff, or a pipe. The Physical Activity History was administered to assess physical activity level over the past year.26
Data Analysis
We evaluated the internal consistency of each depressive symptom measure (Cronbach’s alphas) and their interrelationships (Pearson correlations), and we tested for gender differences (independent samples t tests). To determine which depressive symptom clusters were the most predictive of incident CAC, we performed logistic regression analyses in which age, sex, and race were entered as covariates (demographics-adjusted models). The following predictors were examined in separate models: CES-D Total, CES-D Group, depressed affect subscale, somatic symptoms subscale, interpersonal distress subscale, positive affect subscale, and LOT-R. To facilitate comparisons of effect sizes, continuous predictors were converted to z scores. We then entered into the same model the depressive symptom measures that were significant or fell just short of significance in the demographics-adjusted models (simultaneous entry model). Because the potential for misclassification is increased with smaller CAC scores, we also performed sensitivity analyses using an alternative definition of CAC incidence (presence: Year 20 score > 10, absence: Year 20 score ≤ 10).
We also conducted four sets of exploratory analyses. In the first set, education and antidepressant use were added to the demographics-adjusted models to evaluate the influence of these potential confounders. The second set examined the individual items (z scores) of the significant depressive symptom measures in our primary analyses as predictors of incident CAC in separate demographics-adjusted models. In the third set, the depressive symptom measure x gender and x race interaction terms were tested in separate demographics-adjusted models to ascertain whether the predictive utility of these measures varied across groups. The fourth set evaluated whether the same pattern of results was observed after substituting CAC progression for CAC incidence. A total of 2,352 individuals were available for the progression analyses, given that those with CAC at Year 15 were not excluded. Agatston scores were used to create two CAC progression groups: no progression (Year 20 score ≤ Year 15 score) and progression (Year 20 score > Year 15 score).
Finally, to quantify the effect of potential mediators/confounders on any observed relationships, other covariates were added one at a time to the demographic-adjusted model that included the significant depressive symptom measures. Potential mediators/confounders were MAP, LDL cholesterol, fasting glucose, BMI, tobacco use, physical activity level, and CRP. The change in the effect size was computed as (Bmediator/confounder − Bdemographics)/Bdemographics × 100, where Bmediator/confounder is the unstandardized coefficient for the depressive symptom measure from the model with the selected mediator/confounder and the demographic factors, and Bdemographics is the unstandardized coefficient for the same variable from the model with the demographic factors only. Sobel tests were conducted to assess whether the potential mediators/confounders partially accounted for any observed associations. In the final analysis, all potential mediators/confounders were simultaneously entered into the same model. These analyses were performed on the participants who had useable data for the selected mediators/confounders. For analyses involving CRP, we excluded individuals with CRP ≥ 10 mg/L (n = 137) because levels above this value are likely indicative of acute inflammation rather than chronic low-grade inflammation predictive of CVD risk.27 All data were analyzed using PASW Statistics 18 software, and significance was determined at p < .05. Because CES-D Total was a predictor of incident CAC, we did not adjust the significance level of the tests of the subscales for multiple comparisons.
Results
Descriptive Statistics
As is shown in Table 2, the mean CES-D total score fell in the subclinical range; however, 323 (15%) participants had scores indicative of clinically significant symptoms. Women had higher CES-D Total, depressed affect subscale, and somatic symptoms subscale scores than men. Aside from the interpersonal distress subscale, each depressive symptom measure had adequate internal consistency (Cronbach’s α ≥ .70). The CES-D subscales and the LOT-R were moderately correlated with each other in the expected directions. There were 243 (11%) cases of incident CAC, which included 53 black men, 39 black women, 107 white men, and 44 white women. Among the incident cases, CAC scores at Year 20 ranged from 1 to 759 Agatston units (mean = 21, median = 9).
Table 2.
Descriptive Statistics for and Correlations among the Depressive Symptom Measures
| Measure (possible range) | All (N = 2171) M ± SD |
Men (n = 915) M ± SD |
Women (n = 1256) M ± SD |
Cronbach’s α | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. CES-D Total (0–60) | 8.6 ± 7.4 | 8.0 ± 6.6 | 9.0 ± 7.9* | .88 | --- | |||||
| 2. Depressed Affect (0–21) | 1.9 ± 2.8 | 1.6 ± 2.5 | 2.1 ± 3.0* | .86 | .88 | --- | ||||
| 3. Somatic Symptoms (0–21) | 3.0 ± 2.8 | 2.8 ± 2.6 | 3.2 ± 3.0* | .73 | .84 | .69 | --- | |||
| 4. Interpersonal Distress (0–6) | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.4 ± 0.8 | .59 | .53 | .43 | .41 | --- | ||
| 5. Positive Affect (0–12) | 8.8 ± 2.8 | 8.8 ± 2.7 | 8.7 ± 2.9 | .76 | −.74 | −.49 | −.40 | −.25 | --- | |
| 6. LOT-R (0–24) | 17.1 ± 3.6 | 17.0 ± 3.5 | 17.2 ± 3.6 | .78 | −.49 | −.38 | −.35 | −.25 | .48 | --- |
Note. N = 2171. CES-D = Center for Epidemiologic Studies Depression Scale. LOT-R = Life Orientation Test-Revised.
p < .05.
Depressive Symptoms Clusters as Predictors of Incident Coronary Artery Calcification
Logistic regression analyses adjusting for demographic factors (Table 3) revealed that the CES-D Total (p = .03) was a predictor of incident CAC, along with age (OR = 1.08, 95% CI: 1.04–1.13, p < .001) and female sex (OR = 0.32, 95% CI: 0.24–0.42, p < .001). Race, however, was not a predictor (OR = 0.85, 95% CI: 0.64–1.13, p = .27). A 1-SD increase in the CES-D total score was associated with a 16% increase in the odds of developing CAC. Using the CES-D Group variable, it was found that persons with clinically significant symptoms (CES-D ≥ 16) had a 61% greater odds of CAC onset than those with scores < 16 (CAC incidence rate = 13.6% vs. 10.8%, OR = 1.61, 95% CI: 1.11–2.32, p = .01).
Table 3.
Logistic Regression Analyses Predicting 5-Year Incidence of Coronary Artery Calcification
| Demographics-Adjusted Modelsa | Simultaneous Entry Modelb | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| CES-D Total | 1.16* (1.02–1.33) | --- |
| Depressed Affect Subscale | 1.17* (1.03–1.33) | 1.16 (0.97-1.38) |
| Somatic Symptoms Subscale | 1.13† (0.99–1.30) | 1.02 (0.85-1.23) |
| Interpersonal Distress Subscale | 1.05 (0.92–1.20) | --- |
| Positive Affect Subscale | 0.91 (0.79–1.04) | --- |
| LOT-R | 0.95 (0.83–1.09) | --- |
Note. N = 2171. CES-D = Center for Epidemiologic Studies Depression Scale. LOT-R = Life Orientation Test-Revised.
Adjusted for age, sex, and race.
Adjusted for age, sex, race, and either the CES-D depressed affect or somatic symptoms subscales.
p < .05.
p < .10.
Separate logistic regression analyses for each depressive symptom measure indicated that the CES-D depressed affect subscale (p = .02) – but not the somatic symptoms subscale (p = .08), interpersonal distress subscale (p = .44), positive affect subscale (p = .15), or the LOT-R (p = .44) – predicted incident CAC (Table 3). Thus, the relationship observed between the CES-D Total and CAC incidence was largely due to the depressed affect subscale, although the somatic symptoms subscale also appears to contribute to this effect. Supporting this idea, a 1-SD increase in the depressed affect score was associated with a similar elevation in the odds of CAC onset as a 1-SD increase in the total score (17% vs. 16%). When the CES-D depressed affect and somatic symptoms subscales were entered simultaneously into the same model, the pattern of results was comparable (Table 3). Although the depressed affect subscale fell short of significance (p = .11), its effect size was reduced by only 8%. In contrast, the effect size of the somatic symptoms subscale was reduced by 84% (p = .84).
A similar pattern of results was observed in sensitivity analyses with incident CAC defined as a Year 20 score > 10 (111 cases); CES-D Total (OR = 1.34, 95% CI: 1.12–1.59, p = .001), CES-D Group (OR = 1.97, 95% CI: 1.20–3.24, p = .01), the depressed affect subscale (OR = 1.31, 95% CI: 1.11–1.54, p = .001), the somatic symptoms subscale (OR = 1.29, 95% CI: 1.07–1.54, p = .01), and the positive affect subscale (OR = 0.79, 95% CI: 0.66–0.96, p = .02) were predictors in demographic-adjusted models. Once again, the effect sizes of the somatic symptoms (OR = 1.11, 95% CI: 0.85–1.44, p = .45) and the positive affect (OR = 0.88, 95% CI: 0.70–1.12, p = .31) subscales were reduced to a greater extent in the simultaneous entry model than that of the depressed affect subscale (OR = 1.20, 95% CI: 0.93–1.57, p = .17).
In the first set of exploratory analyses, we found that adding the potential confounders of education and antidepressant use as covariates had little effect on the results. Of the depressive symptom measures, only CES-D Group (OR = 1.53, 95% CI: 1.05–2.33, p = .03) and the depressed affect subscale (OR = 1.15, 95% CI: 1.01–1.32, p = .04) were predictors of incident CAC in these models, although the CES-D Total fell just short of significance (OR = 1.13, 95% CI: 0.99–1.30, p = .08). The second set of exploratory analyses indicated that the blues item (“I felt that I could not shake off the blues even with the help of my family or friends”), the depressed item (“I felt depressed”), and the failure item (“I thought my life had been a failure”) predicted incident CAC. The odds ratios for blues, depressed, and failure items were 1.23 (95% CI: 1.09–1.38, p = .001), 1.19 (95% CI: 1.04–1.34, p = .008), and 1.20 (95% CI: 1.07–1.35, p = .002), respectively. In contrast, the other depressed affect items (i.e., fearful, lonely, crying, and sad items) did not predict incident CAC (all ps > .21).
In the third set of exploratory analyses, we tested the depressive symptom measure x gender and x race interaction terms. None of these variables, including the depressed affect x gender (p = .47) and depressed affect x race (p = .35) interactions, was significant (all ps > .07), demonstrating that the predictive utility of the depressed affect subscale was similar across these groups. The odds ratios for the depressed affect subscale was 1.21 (95% CI: 1.02–1.44, p = .03) among women and 1.12 (95% CI: 0.93–1.35, p = .22) among men and was 1.23 (95% CI: 1.04–1.46, p = .02) among black adults and 1.10 (95% CI: 0.91–1.34, p = .31) among white adults. Finally, the fourth set of exploratory analyses revealed that the pattern of results was similar when CAC progression (an increase in CAC from Year 15 to Year 20; 431 cases) was the outcome. In separate models adjusted for demographic factors and CAC at Year 15, CES-D Total (OR = 1.18, 95% CI: 1.04–1.34, p = .01), CES-D Group (OR = 1.59, 95% CI: 1.11–2.30, p = .01), the depressed affect subscale (OR = 1.19, 95% CI: 1.06–1.35, p = .01), and the somatic symptoms subscale (OR = 1.15, 95% CI: 1.01–1.31, p = .04) predicted CAC progression. As with incident CAC, the effect size of the somatic symptoms subscale was substantially reduced in simultaneous entry model (OR = 1.03, 95% CI: 0.85–1.15, p = .78), while the effect size of the depressed affect subscale was not (OR = 1.18, 95% CI: 0.98–1.42, p = .09).
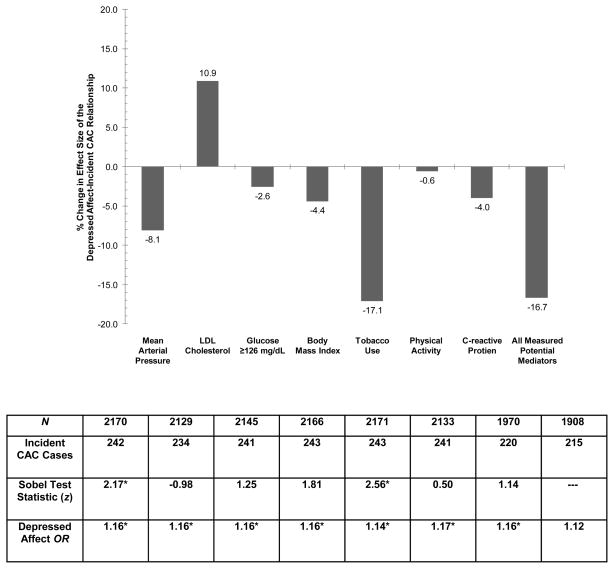
Figure 1 displays the results of analyses examining potential mediators/confounders of the relationship between the CES-D depressed affect subscale and incident CAC. Only tobacco use and MAP reduced the depressed affect-incident CAC effect size by more than 5%, with tobacco use bringing about a decline more than twice the size of any other factor. In contrast, LDL cholesterol appears to be acting as a suppressor variable. Sobel tests confirmed these descriptive results, as only tobacco use (p = .01) and mean arterial pressure (p = .03) partially accounted for the observed association (all other ps ≥ .07). When all potential mediators/confounders were entered simultaneously into the model, they accounted for 16.7% of the depressed affect-incident CAC relationship. As is shown in Figure 1, the depressed affect-incident CAC relationship remained significant after individually adjusting for each factor and fell short of significance after simultaneously adjusting for all seven factors (OR = 1.12, 95% CI: 0.97–1.29, p = .11). The independent predictors of incident CAC in the model with all factors were age (OR = 1.08, 95% CI: 1.04–1.13, p = .001), female sex (OR = 0.33, 95% CI: 0.24–0.46, p < .001), black race (OR = 0.67, 95% CI: 0.49–0.93, p = .02), LDL cholesterol (OR = 1.01, 95% CI: 1.00–1.01, p = .004), BMI (OR = 1.06, 95% CI: 1.03–1.09, p < .001), and tobacco use (OR = 1.62, 95% CI: 1.14–2.29, p = .01).
Figure 1.
Logistic regression analyses examining potential mediators of the relationship between the depressed affect subscale of the Center for Epidemiologic Studies-Depression Scale (CES-D) and incident coronary artery calcification (CAC). The y-axis represents the percent change in the effect size of the depressed affect-incident CAC relationship after the inclusion of each potential mediator in the demographic-adjusted model. LDL = low-density lipoprotein. *p < .05.
Discussion
Our primary objective was to compare the relative importance of depressive symptom clusters in predicting the 5-year incidence of advanced subclinical coronary atherosclerosis, defined as the development of calcified lesions. In a large and racially diverse sample of middle-aged community members, we observed that the relationship between elevated depressive symptoms and incident CAC was driven largely by the depressed affect cluster. Specifically, a 1-SD increase in the CES-D depressed affect subscale was associated with a 17% greater odds of developing CAC. The observed relationship was independent of age, sex, race, education, and antidepressant use and was similar in magnitude across gender and racial groups. The depressed affect-incident CAC relationship fell short of significance after adjustment for all potential mediators/confounders (i.e., cardiovascular risk factors, health behaviors, and CRP), with tobacco use accounting for one sixth of this association. Exploratory analyses revealed that two items assessing depressed mood and one item assessing sense of failure predicted incident CAC. A similar pattern of results was observed for an alternative definition of incident CAC and for CAC progression, suggesting that the present findings are robust. Measures of CAC change over time might have clinical relevance beyond a single CAC score, given that CAC incidence/progression may reflect more recent disease activity while a CAC score is an index of atherosclerotic burden at one point in time.16 Supporting this notion is an emerging literature showing that CAC incidence/progression predicts cardiac events.16
Our findings suggest that the negative affective elements of depression – especially depressed mood and sense of failure – may be the most cardiotoxic among middle-aged adults free of cardiovascular disease, which contrasts with past findings. Results of recent studies examining depressive symptoms clusters suggest that the somatic symptoms may be the most predictive of CAD risk markers.8–10 These findings are paralleled by those of studies examining cardiovascular prognosis. Both de Jonge et al.28 and Martens et al.29 observed that elevated scores on the somatic-affective subscale of the Beck Depression Inventory, but not on the cognitive-affective subscale, predicted cardiovascular events or death among post-myocardial infarction patients. Comparable results have been reported in other prognostic studies;30,31 however, there are notable exceptions.32–34 To our knowledge, the only other study to favor the depressed affect cluster was conducted by Barefoot and colleagues,32 who found that only hopelessness and the negative affective symptoms of depression predicted cardiovascular mortality among CAD patients. Although our study is the first to examine depressive symptom clusters in relation to CAC incidence, Hamer et al.35 found that persistent cognitive symptoms of depression were positively associated with prevalent CAC, and two other studies have reported that global depression measures are predictive of CAC progression.17,18
What factors might explain why the depressed affect cluster was the strongest predictor of incident CAC? One possibility is that depressed affect may be more strongly associated with some of the putative behavioral or physiologic mechanisms underlying the depression-CVD relationship.19 For instance, smoking may be an effective, albeit unhealthy, strategy for attenuating negative affect in the short-term.36 It is also possible that a sense of failure reflects low perceived self-efficacy, which has been shown to predict adherence to cardiovascular medications and lifestyle recommendations.37 Finally, central serotonergic dysfunction is considered one of the neurobiological underpinnings of the depressed mood component of major depression.38 Other evidence suggests that serotonin also plays a role in regulating platelet function,39 and abnormalities in platelet function may contribute to atherosclerotic progression and cardiovascular events.40 Another related possibility is that the negative affective elements may have a stronger connection than the other clusters to a third factor that contributes to both depression and atherosclerotic progression, such as genetic variants related to serotonergic function.41
Methodological factors could also account for our results and should not be ignored. Because over half of the CES-D items are affective in nature, the depressed affect cluster may have been the strongest predictor simply because the CES-D provided the most comprehensive assessment of this set of symptoms. Moreover, the depressed affect cluster may have had greater reliability or variability than the other clusters, which could have resulted in greater predictive utility. This does not appear to be the case here, given that the CES-D subscales and the LOT-R had adequate internal consistency and exhibited comparable variability. Evidence also suggests that older adults tend to report more somatic symptoms and fewer cognitive symptoms than younger or middle-aged adults,42 possibly due to a reporting bias or a true age-related difference in the phenomenology of depression. Thus, the predictive utility of a given depressive symptom cluster may depend on the age of the participants, with the somatic symptoms cluster being favored in older samples as a result of increased variability and/or improved sensitivity. Importantly, these statistical advantages may not be present in middle-aged samples, like the current one. A final possibility is that particular elements of depression may predict different aspects of the atherosclerotic process, thereby producing inconsistent results across outcomes.
Our secondary objective was to examine whether potential mediators/confounders explained the observed relationship. In separate models, tobacco use and MAP accounted for 17% and 8% of the depressed affect-incident CAC association, respectively. When included in the same model, tobacco use, but not MAP, predicted incident CAC, suggesting that tobacco use is a key mediator/confounder. Further supporting this idea is the observation that tobacco use accounted for as much of the depressed affect-CAC relationship as all potential mediators/confounders combined. It is more plausible that tobacco use is operating as a mediator (versus a confounder) in our study, given that the bulk of the evidence indicates that depression precedes and predicts smoking initiation.43 However, some have detected links between smoking and future depression, and others have proposed that genetic factors explain the depression-smoking relationship.43 Our results corroborate previous findings indicating that smoking accounts for 11–17% of the deleterious effect of depressive symptoms on cardiovascular prognosis.44,45 The candidate mediators (poor medication/lifestyle adherence and abnormal platelet function) and third factors (serotonergic genetic variants) proposed earlier might explain a portion of the depressed affect-incident CAC relationship. Future studies are needed to evaluate these possibilities, given that measures of these factors are not available in this study.
In addition to our investigation’s strengths (e.g., multidimensional depression measure, repeated CAC assessments, and large and diverse sample), there are limitations. First, the CES-D does not yield a clinical diagnosis nor does it represent all facets of the construct of depression (e.g., some cognitive and behavioral symptoms are not adequately assessed). Second, depressive symptoms and potential mediators/confounders were measured concurrently, which precluded us from establishing the directionality of these associations. Third, because our sample consisted of black and white middle-aged adults, our findings may not extend to other racial groups or older individuals.
To conclude, we found that negative affective elements of depression, particularly depressed mood and sense of failure, were the strongest predictors of incident CAC in a large and diverse sample of middle-aged community members. Ultimately, this line of research could have important theoretical and clinical implications. Elucidating the cardiotoxic aspects of depression could help to (a) pinpoint the mechanisms underlying the depression-CVD relationship, (b) identify a subpopulation of depressed persons at greatest risk for CVD in which early intervention is warranted, and (c) develop focused depression interventions specifically designed to reduce CVD risk, which may yield more pronounced cardiovascular benefits than those observed in past trials.3,6 However, given the inconsistent results across studies, there is a need for future investigations in this area, including re-analysis of completed projects in which a multidimensional depression measure was administered. As the search for the cardiotoxic aspects of depression continues, we urge researchers to consider methodological explanations for their findings and to remain open to the possibility that a single facet of depression may not be the most noxious across all contexts.
Acknowledgments
Funding Sources: Work on this manuscript was supported by the following contracts: University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC-48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern University, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; Harbor-UCLA Research Education Institute, Computed Tomography Reading Center (Year 15 Exam), N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; New England Medical Center (Year 20 Exam), N01-HC-45204 from the National Heart, Lung and Blood Institute.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 2.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 4.Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, Albanese G, Kronish I, Hegel M, Burg MM. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: Coronary Psychosocial Evaluation Studies randomized controlled trial. Arch Intern Med. 2010;170:600–608. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, McLvor M. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 6.van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, Schins A, Tulner D, van den Berg MP, Ormel J. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 7.Davidson KW, Rieckmann N, Rapp MA. Definitions and distinctions among depressive syndromes and symptoms: implications for a better understanding of the depression-cardiovascular disease association. Psychosom Med. 2005;67 (Suppl 1):S6–9. doi: 10.1097/01.psy.0000162257.19266.fc. [DOI] [PubMed] [Google Scholar]
- 8.Deverts DJ, Cohen S, Dilillo VG, Lewis CE, Kiefe C, Whooley M, Matthews KA. Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2010;72:734–741. doi: 10.1097/PSY.0b013e3181ec4b98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everson SA, Goldberg DE, Kaplan GA, Cohen RD, Pukkala E, Tuomilehto J, Salonen JT. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom Med. 1996;58:113–121. doi: 10.1097/00006842-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kubzansky LD, Sparrow D, Vokonas P, Kawachi I. Is the glass half empty or half full? A prospective study of optimism and coronary heart disease in the normative aging study. Psychosom Med. 2001;63:910–916. doi: 10.1097/00006842-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Matthews KA, Raikkonen K, Sutton-Tyrrell K, Kuller LH. Optimistic attitudes protect against progression of carotid atherosclerosis in healthy middle-aged women. Psychosom Med. 2004;66:640–644. doi: 10.1097/01.psy.0000139999.99756.a5. [DOI] [PubMed] [Google Scholar]
- 14.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 15.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 16.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Matthews KA, Chang YF, Sutton-Tyrrell K, Edmundowicz D, Bromberger JT. Recurrent major depression predicts progression of coronary calcification in healthy women: Study of Women’s Health Across the Nation. Psychosom Med. 2010;72:742–747. doi: 10.1097/PSY.0b013e3181eeeb17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen I, Powell LH, Matthews KA, Cursio JF, Hollenberg SM, Sutton-Tyrell K, Bromberger JT, Everson-Rose SA. Depressive symptoms are related to progression of coronary calcium in midlife women. Am Heart J. 2011;161:1186–1191. doi: 10.1016/j.ahj.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–261. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 21.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 22.Alloy LB, Ahrens AH. Depression and pessimism for the future: biased use of statistically relevant information in predictions for self versus others. J Pers Soc Psychol. 1987;52:366–378. doi: 10.1037//0022-3514.52.2.366. [DOI] [PubMed] [Google Scholar]
- 23.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 24.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 25.Scheier MF, Bridges MW. Person variables and health: personality predispositions and acute psychological states as shared determinants for disease. Psychosom Med. 1995;57:255–268. doi: 10.1097/00006842-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of Short Physical Activity History: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil Prev. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SS, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 28.de Jonge P, Ormel J, van den Brink RH, van Melle JP, Spijkerman TA, Kuijper A, van Veldhuisen DJ, van den Berg MP, Honig A, Crijns HJ, Schene AH. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry. 2006;163:138–144. doi: 10.1176/appi.ajp.163.1.138. [DOI] [PubMed] [Google Scholar]
- 29.Martens EJ, Hoen PW, Mittelhaeuser M, de Jonge P, Denollet J. Symptom dimensions of post-myocardial infarction depression, disease severity and cardiac prognosis. Psychol Med. 2010;40:807–814. doi: 10.1017/S0033291709990997. [DOI] [PubMed] [Google Scholar]
- 30.Hoen PW, Conradi HJ, Denollet J, Martens EJ, de Jonge P. Interview-based ratings of somatic and cognitive symptoms of depression and their impact on cardiovascular prognosis. Psychother Psychosom. 2010;79:319–320. doi: 10.1159/000319528. [DOI] [PubMed] [Google Scholar]
- 31.Linke SE, Rutledge T, Johnson BD, Vaccarino V, Bittner V, Cornell CE, Eteiba W, Sheps DS, Krantz DS, Parashar S, Bairey Merz CN. Depressive symptom dimensions and cardiovascular prognosis among women with suspected myocardial ischemia: a report from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Arch Gen Psychiatry. 2009;66:499–507. doi: 10.1001/archgenpsychiatry.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barefoot JC, Brummett BH, Helms MJ, Mark DB, Siegler IC, Williams RB. Depressive symptoms and survival of patients with coronary artery disease. Psychosom Med. 2000;62:790–795. doi: 10.1097/00006842-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Davidson KW, Burg MM, Kronish IM, Shimbo D, Dettenborn L, Mehran R, Vorchheimer D, Clemow L, Schwartz JE, Lesperance F, Rieckmann N. Association of anhedonia with recurrent major adverse cardiac events and mortality 1 year after acute coronary syndrome. Arch Gen Psychiatry. 2010;67:480–488. doi: 10.1001/archgenpsychiatry.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen SS, Denollet J, Daemen J, van de Sande M, de Jaegere PT, Serruys PW, Erdman RA, van Domburg RT. Fatigue, depressive symptoms, and hopelessness as predictors of adverse clinical events following percutaneous coronary intervention with paclitaxel-eluting stents. J Psychosom Res. 2007;62:455–461. doi: 10.1016/j.jpsychores.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Hamer M, Kivimaki M, Lahiri A, Marmot MG, Steptoe A. Persistent cognitive depressive symptoms are associated with coronary artery calcification. Atherosclerosis. 2010;210:209–213. doi: 10.1016/j.atherosclerosis.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 37.Clark NM, Dodge JA. Exploring self-efficacy as a predictor of disease management. Health Health Educ Behav. 1999;26:72–89. doi: 10.1177/109019819902600107. [DOI] [PubMed] [Google Scholar]
- 38.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 39.Bruce EC, Musselman D. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;67:S34–S36. doi: 10.1097/01.psy.0000164227.63647.d9. [DOI] [PubMed] [Google Scholar]
- 40.Goldschmidt PJ, Lopes N, Crawford LE. Atherosclerosis and coronary artery disease. In: Michelson AD, editor. Platelets. San Diego: Academic Press; 2002. [Google Scholar]
- 41.McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, Lesperance F. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y, Pilkonis PA, Frank E, Thase ME, Reynolds CF. Differential functioning of the Beck Depression inventory in late-life patients: use of item response theory. Psychol Aging. 2002;17:379–391. doi: 10.1037//0882-7974.17.3.379. [DOI] [PubMed] [Google Scholar]
- 43.Freedland KE, Carney RM, Skala JA. Depression and smoking in coronary heart disease. Psychosom Med. 2005;67:S42–S46. doi: 10.1097/01.psy.0000162255.55629.9c. [DOI] [PubMed] [Google Scholar]
- 44.Brummett BH, Babyak MA, Siegler IC, Mark DB, Williams RB, Barefoot JC. Effect of smoking and sedentary behavior on the association between depressive symptoms and mortality from coronary heart disease. Am J Cardiol. 2003;92:529–532. doi: 10.1016/s0002-9149(03)00719-7. [DOI] [PubMed] [Google Scholar]
- 45.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]